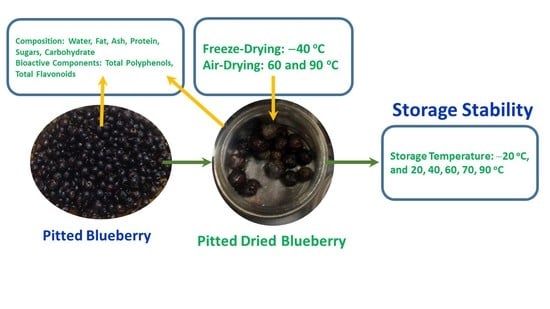
Polyphenol and Flavonoid Stability of Wild Blueberry (Sideroxylon mascatense) during Air- and Freeze-Drying and Storage Stability as a Function of Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Būt Berry (Sideroxylon mascatense)
2.3. Drying Methods
2.4. Chemical Composition
2.5. Sugar Analysis
2.6. Titratable Acidity and pH
2.7. Preparation of Fruit Extract for Phytochemical Analysis
2.8. Total Phenolic Content (TPC)
2.9. Total Flavonoid Content (TFC)
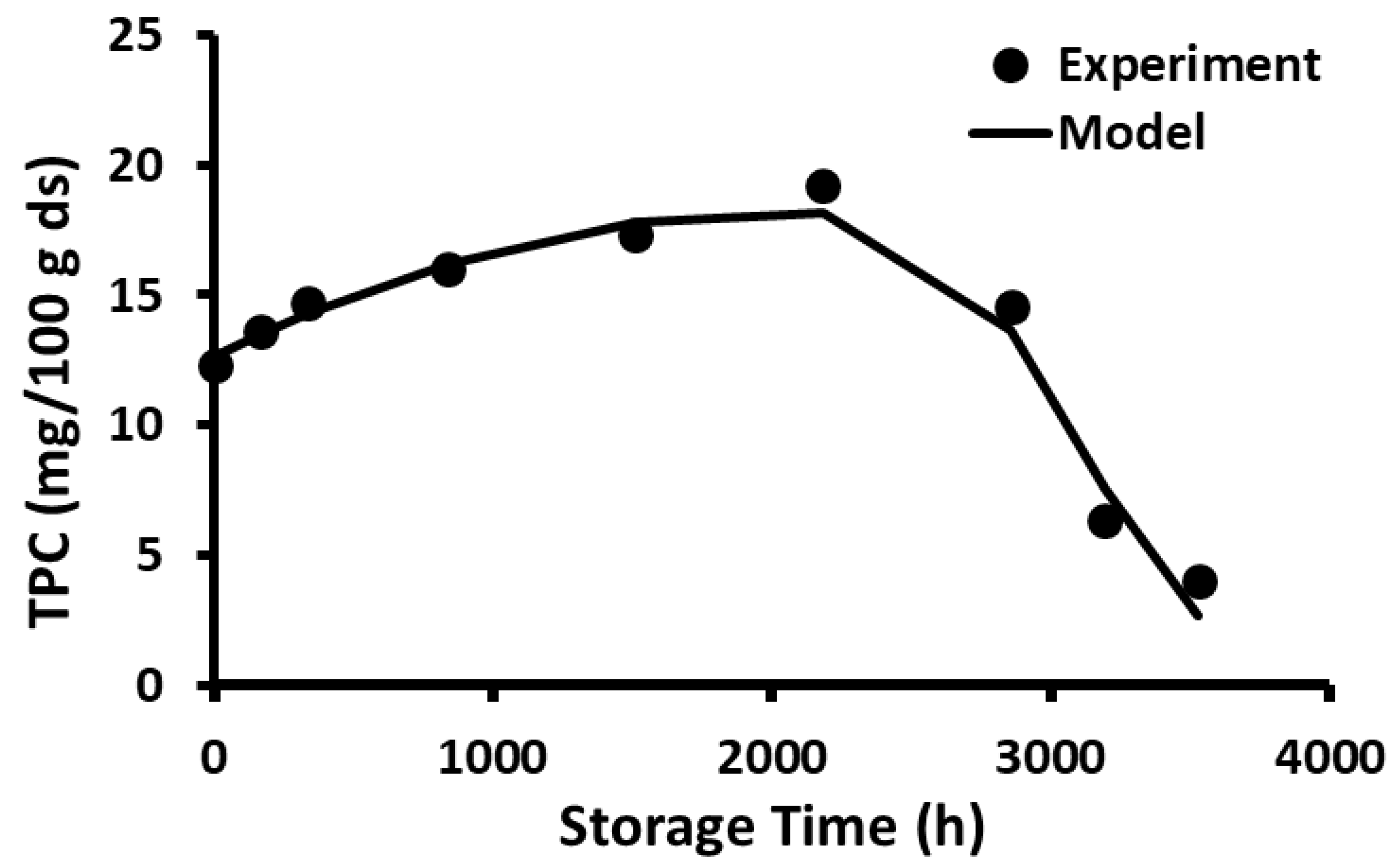
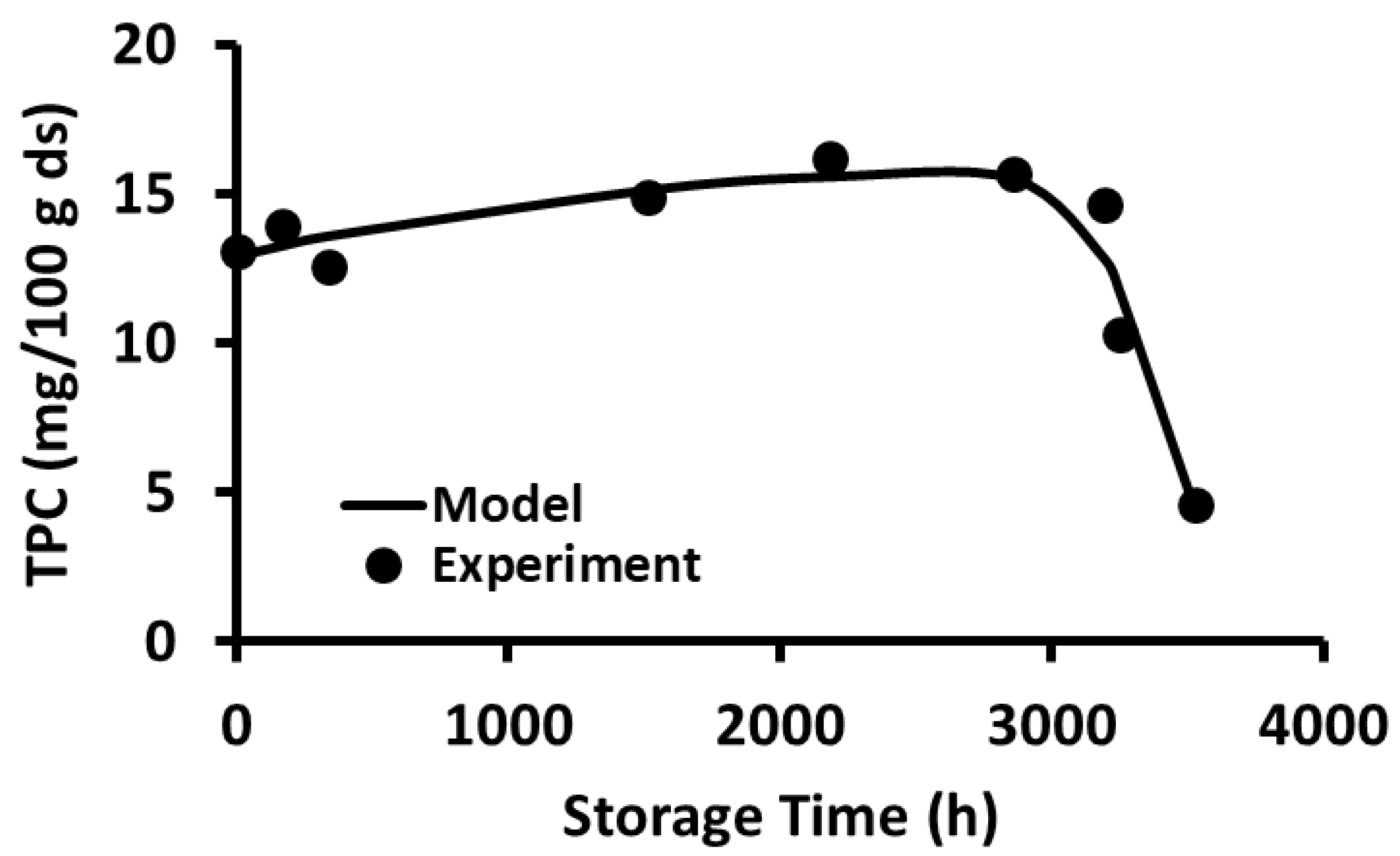
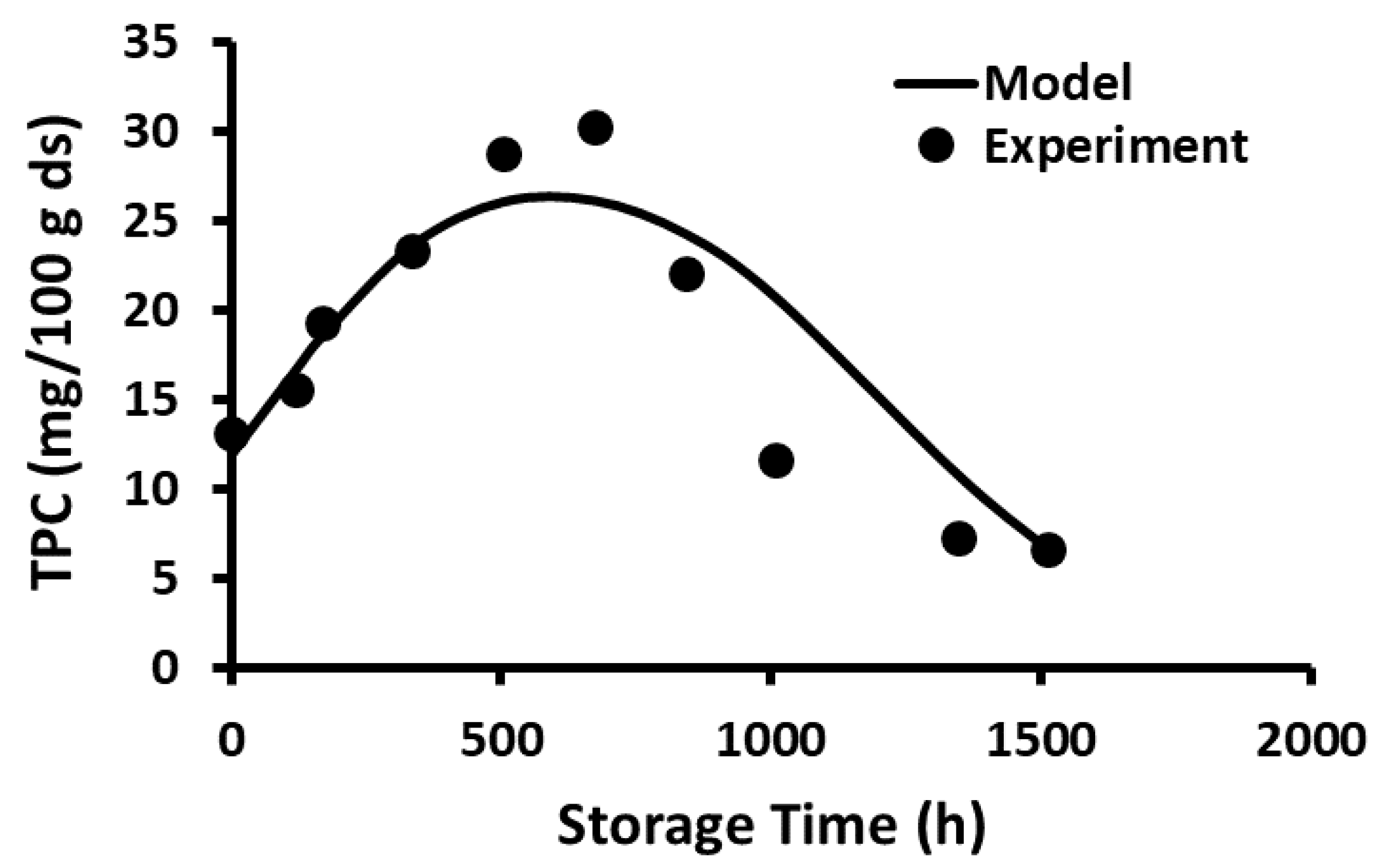
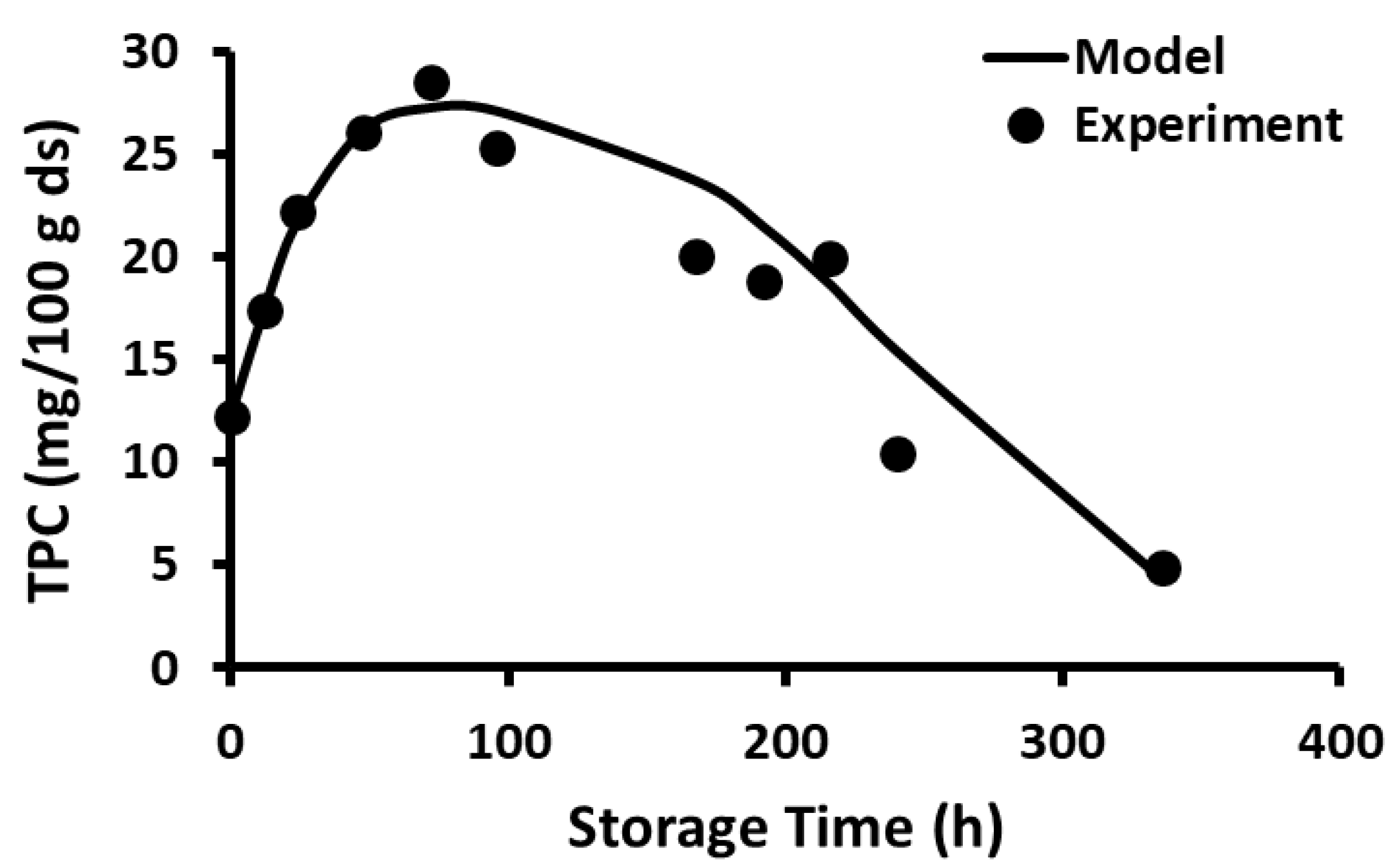
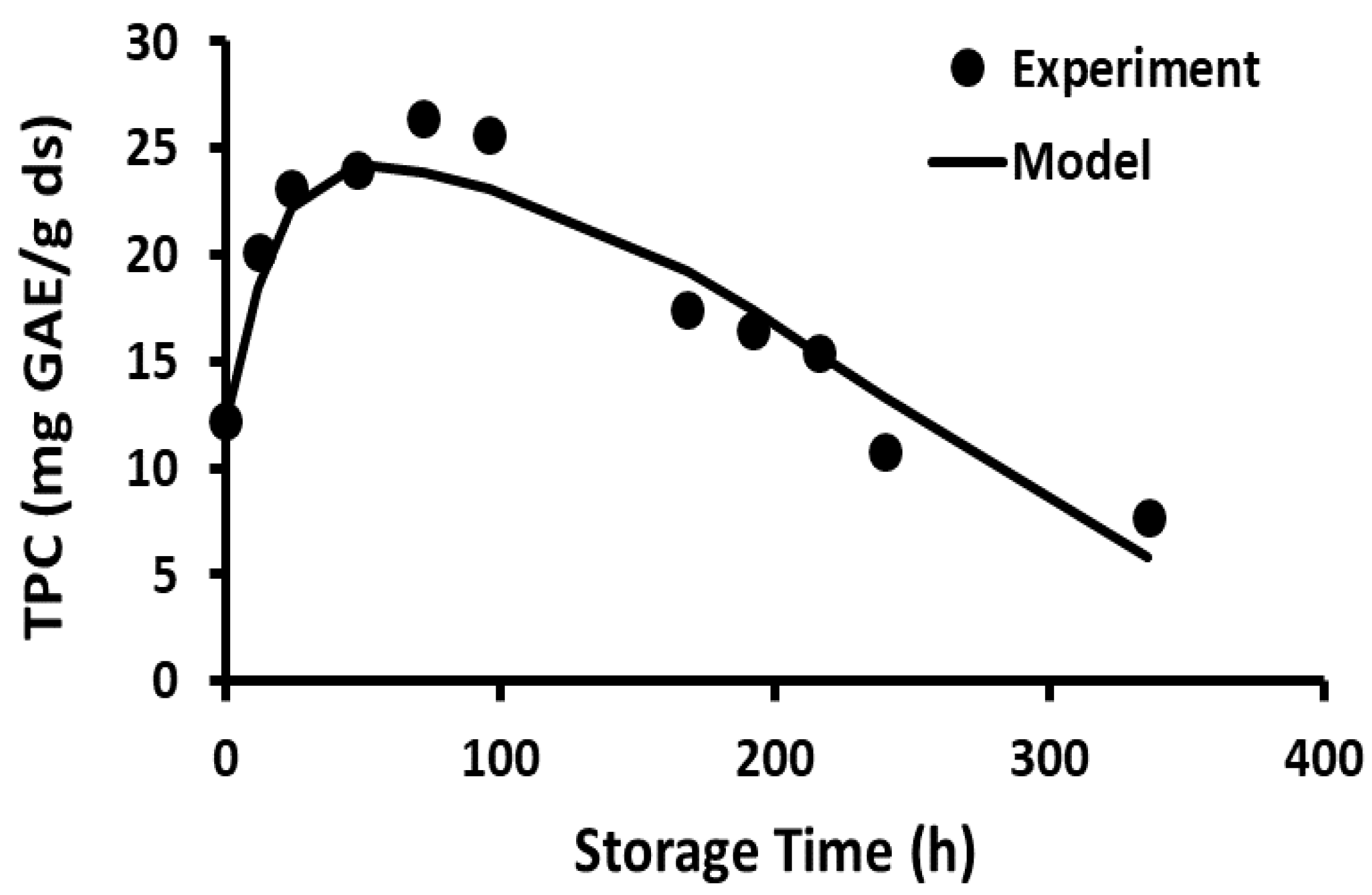
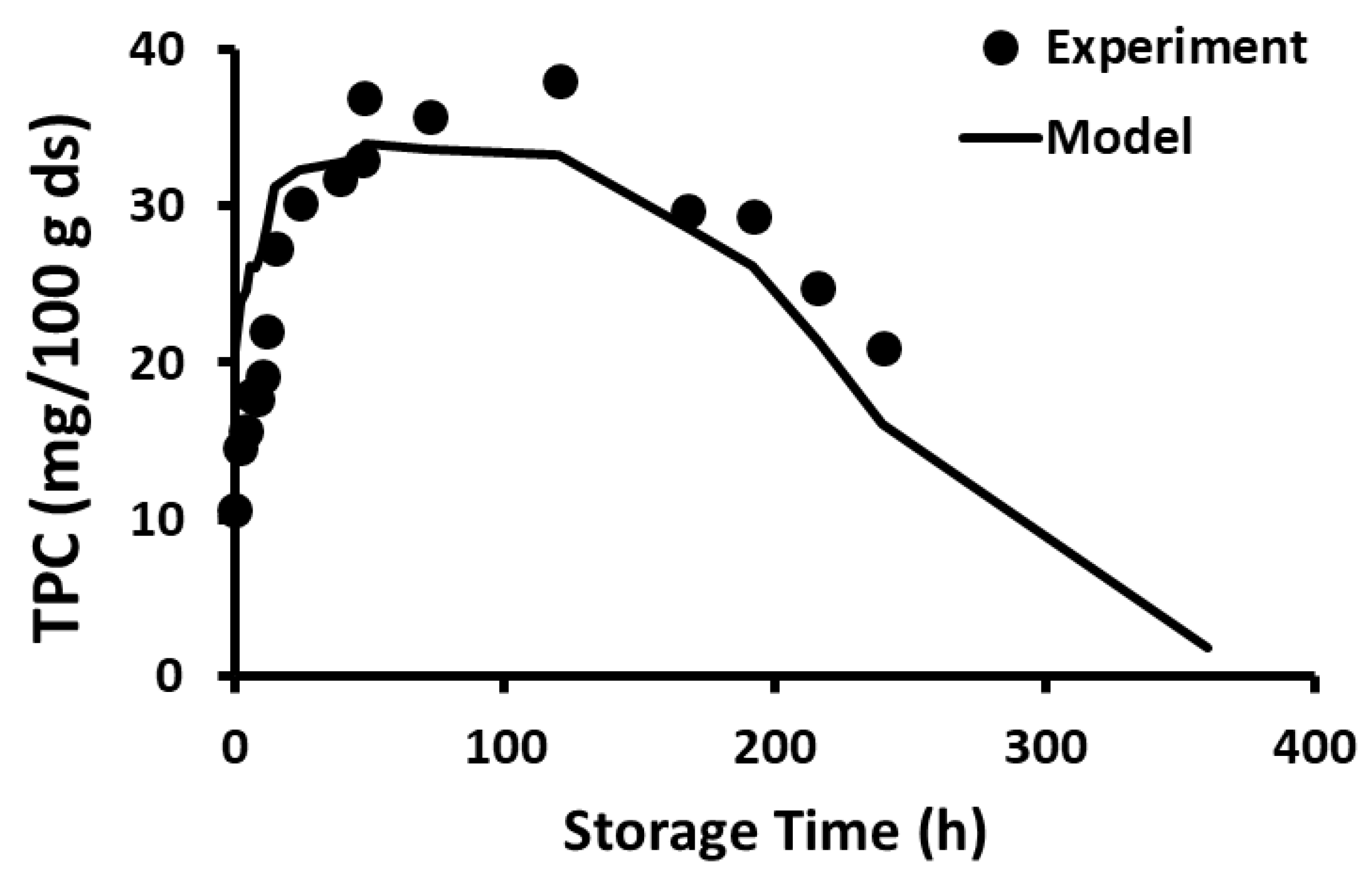
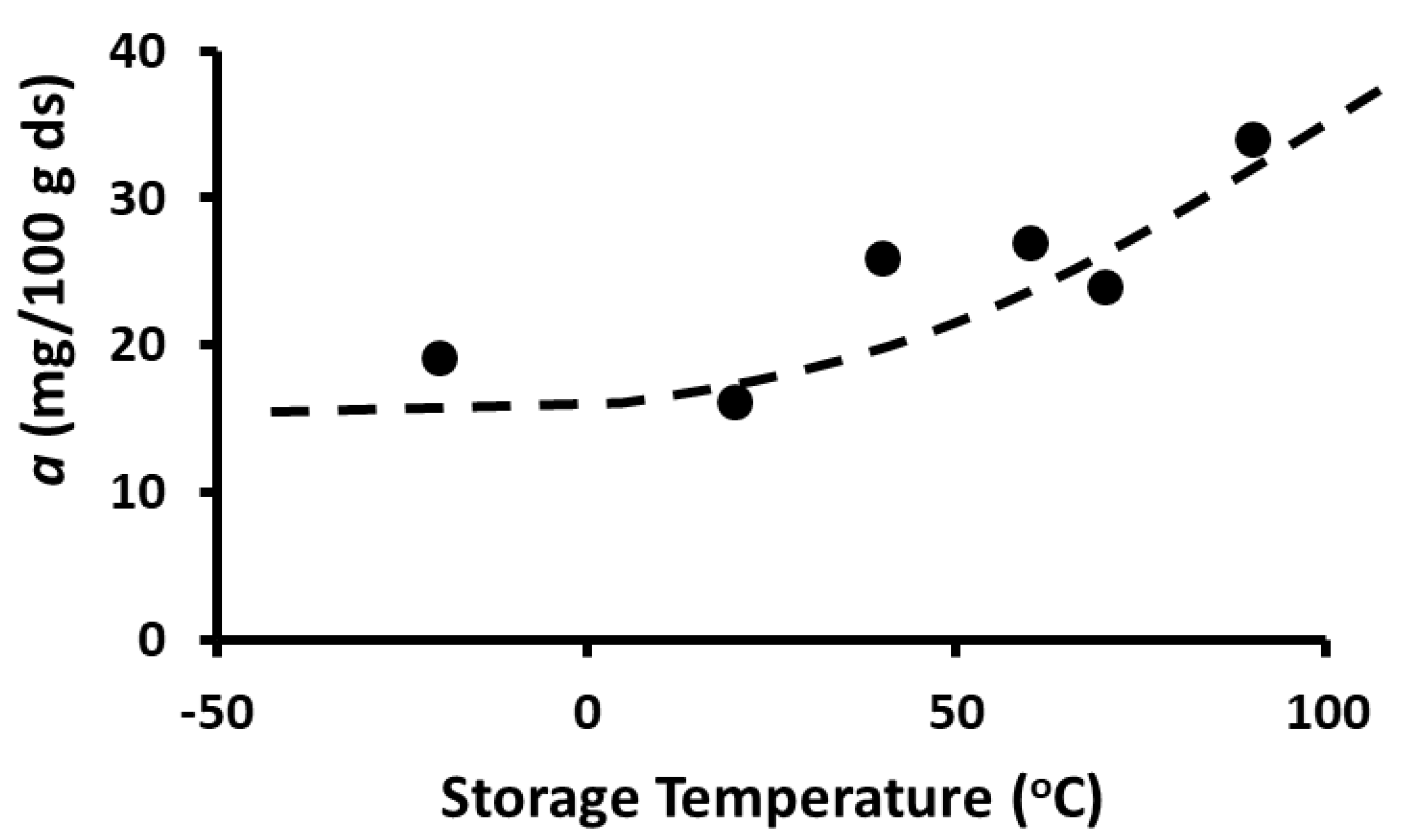
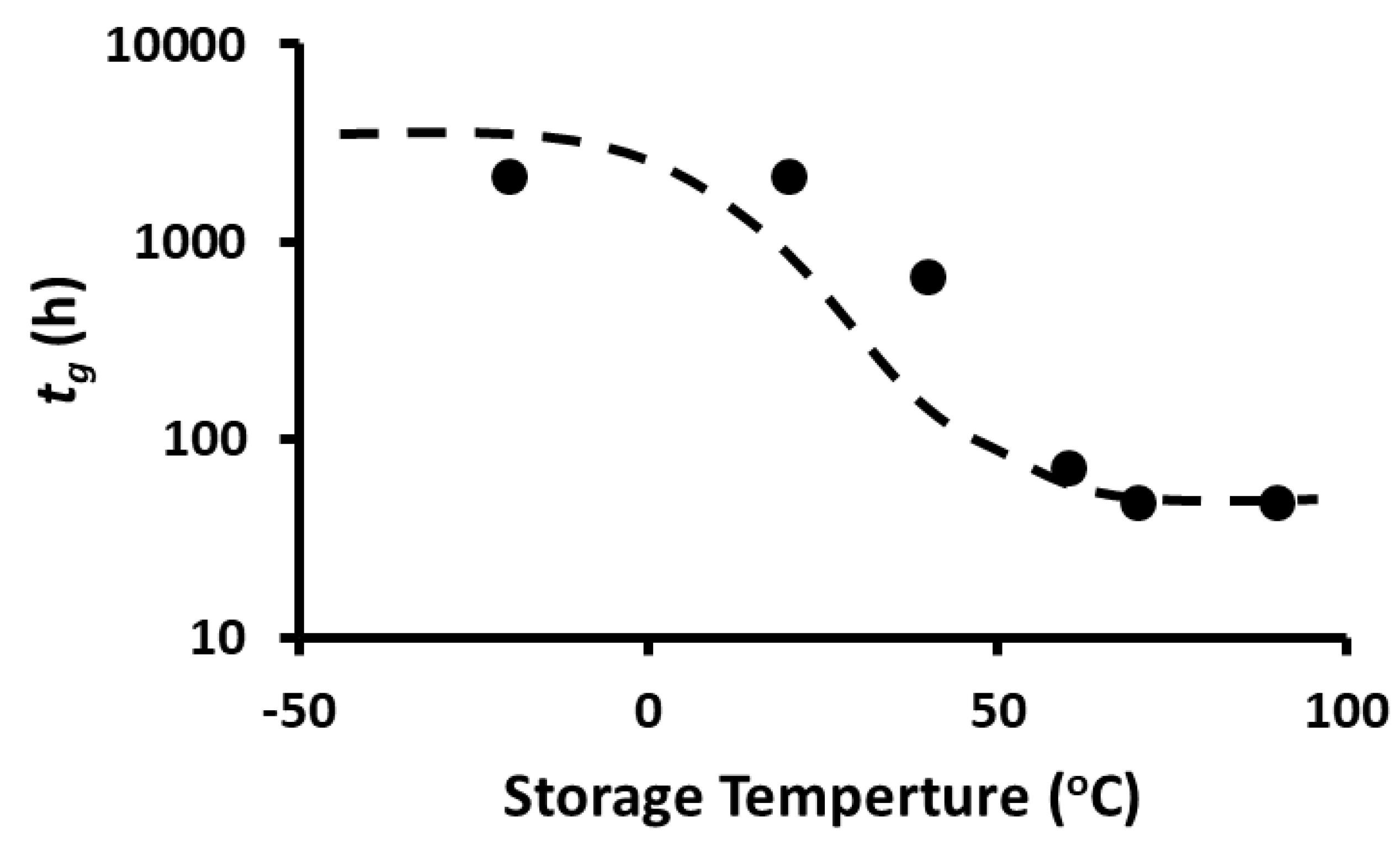
2.10. Storage Stability of Polyphenols
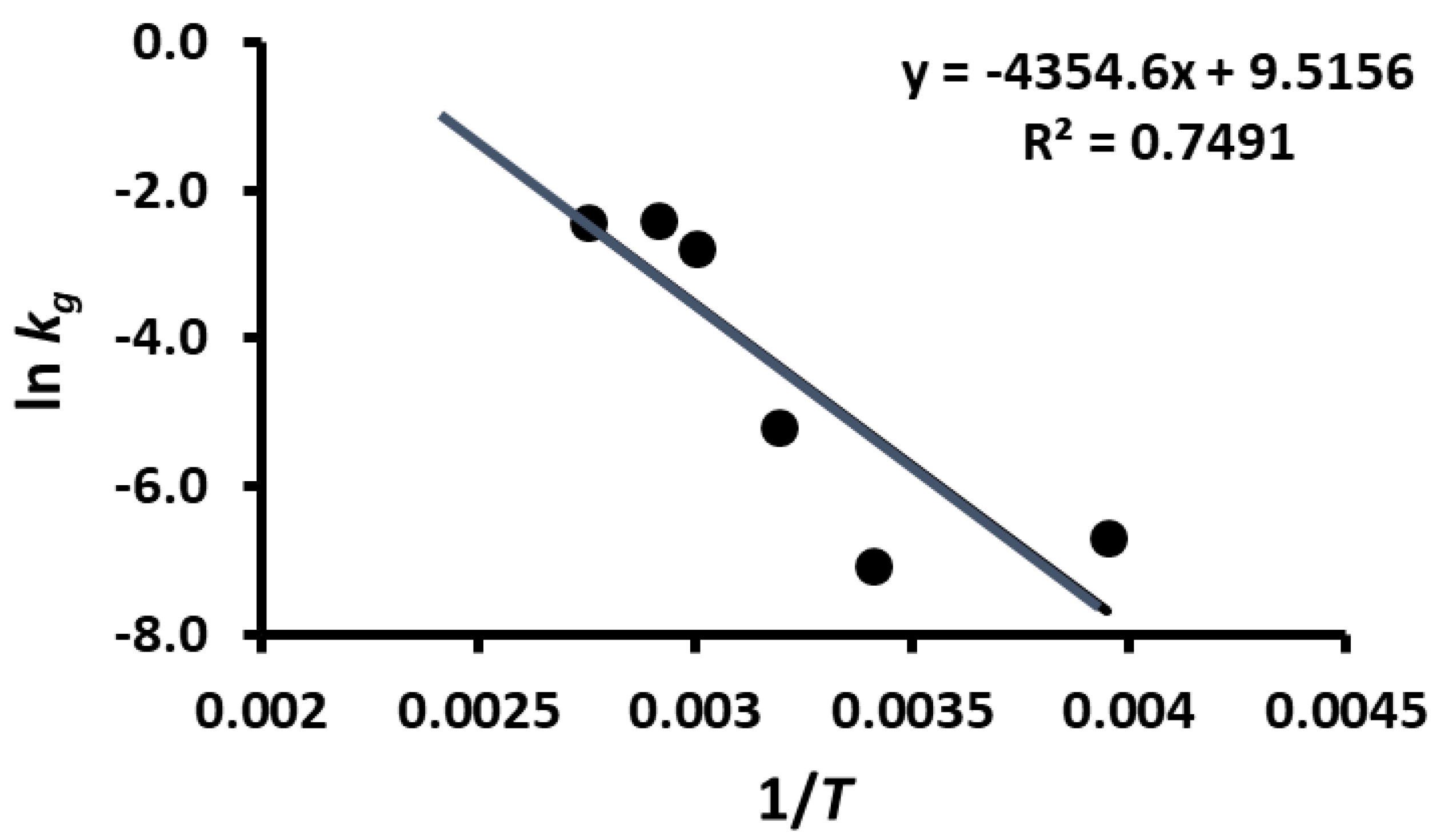
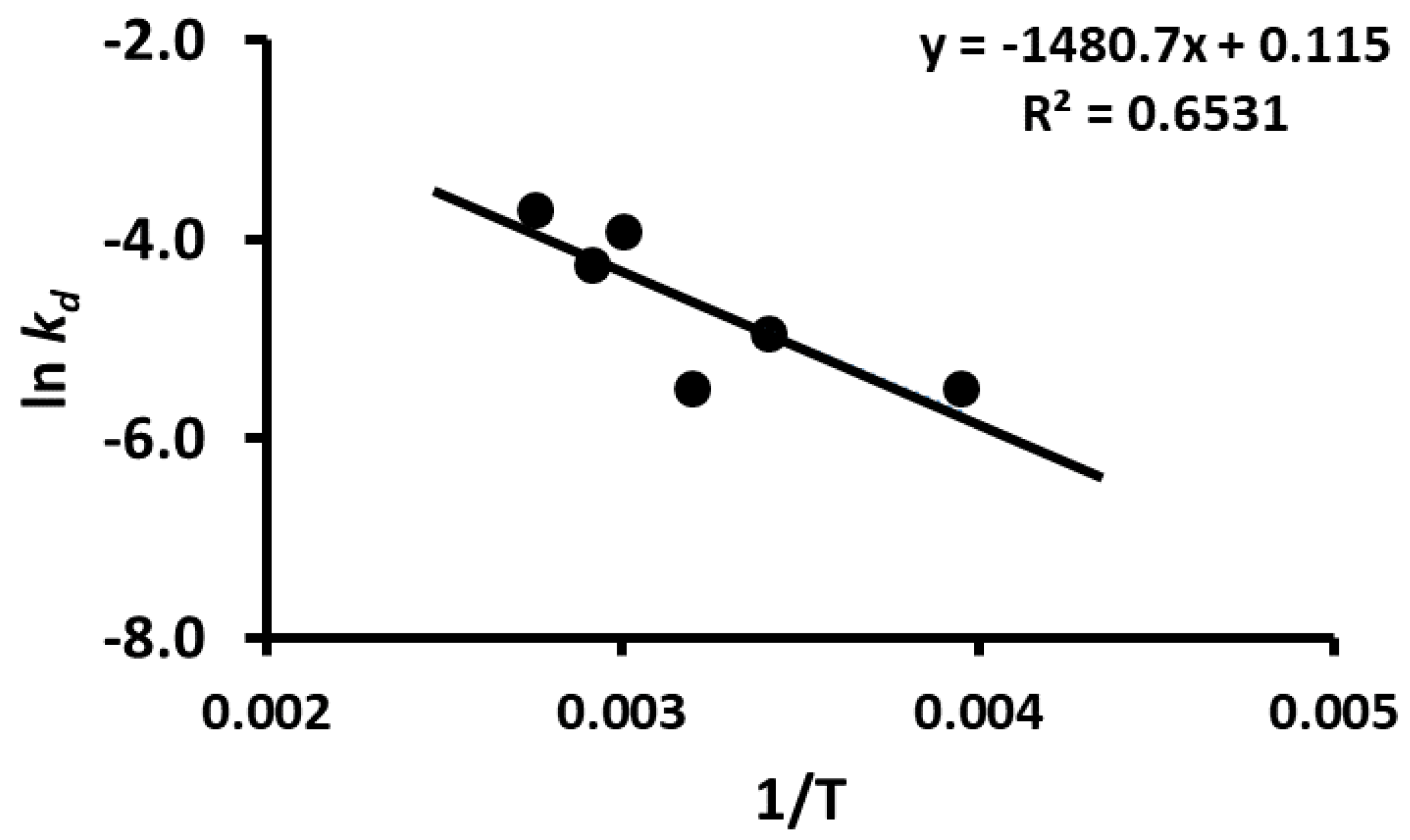
2.11. Storage Stability Prediction Model
2.12. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Sugars
3.3. Titratable Acidity and pH
3.4. Total Polyphenol and Flavonoid Content for Fresh and Dried Blueberries
3.5. Stability of Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hopkins, E.; Al-Yahyai, R. Sideroxylon mascatense: A new crop for high elevation arid climates. J. Agric. Mar. Sci. 2020, 25, 02–08. [Google Scholar] [CrossRef]
- Al-Yahyai, R.; Al-Nabhani, H. Botanical description and phenological cycles of Monotheca buxifolia. In Proceedings of the International Symposium on Asian Plants with Unique Horticultural Potential, International Society for Horticultural Science, Seoul, Republic of Korea, 13–19 August 2006. [Google Scholar]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Krewer, G. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Al Hasani, S.; Al-Attabi, Z.H.; Waly, M.; Rahman, M.S.; Tamimi, Y. Antioxidant and antitumor properties of wild blueberry (Sideroxylon mascatense): Effects of drying methods. Int. J. Nutr. Pharmacol. Neurol. Dis. 2021, 11, 71–79. [Google Scholar]
- Jan, S.; Khan, M.R.; Rashid, U.; Bokhari, J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of Monotheca Buxifolia fruit. Osong Public Heal. Res. Perspect. 2013, 4, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Raihana, A.N.; Marikkar, J.; Amin, I.; Shuhaimi, M. A Review on Food Values of Selected Tropical Fruits’ Seeds. Int. J. Food Prop. 2015, 18, 2380–2392. [Google Scholar] [CrossRef]
- Jimenez-Garcia, S.N.; Guevara-Gonzalez, R.G.; Miranda-Lopez, R.; Feregrino-Perez, A.A.; Torres-Pacheco, I.; Vazquez-Cruz, M.A. Functional properties and quality characteristics of bioactive compounds in berries: Biochemistry, biotechnology, and genomics. Int. Food Res. J. 2013, 54, 1195–1207. [Google Scholar] [CrossRef]
- Reque, P.M.; Steffens, R.S.; Da Silva, A.M.; Jablonski, A.; Flores, S.H.; Rios, A.D.O.; De Jong, E.V. Characterization of blueberry fruits (Vaccinium spp.) and derived products. Food Sci. Technol. 2014, 34, 773–779. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Mujumdar, A. Berry drying: Mechanism, pretreatment, drying technology, nutrient preservation, and mathematical models. Food Eng. Rev. 2019, 11, 61–77. [Google Scholar] [CrossRef]
- Pateiro, M.; Vargas-Ramella, M.; Franco, D.; Gomes da Cruz, A.; Zengin, G.; Kumar, M.; Dhama, K.; Lorenzo, J.M. The role of emerging technologies in the dehydration of berries: Quality, bioactive compounds, and shelf life. Food Chem. 2022, 16, 100465. [Google Scholar] [CrossRef]
- Zielinska, M.; Michalska, A. Microwave-assisted drying of blueberry (Vaccinium corymbosum L.) fruits: Drying kinetics, polyphenols, anthocyanins, antioxidant capacity, colour and texture. Food Chem. 2016, 212, 671–680. [Google Scholar] [CrossRef]
- Chen, W.; Guo, Y.; Zhang, J.; Zhang, X.; Meng, Y. Effect of different drying processes on the physicochemical and antioxidant properties of thinned young apple. Int. J. Food Eng. 2015, 11, 207–219. [Google Scholar] [CrossRef]
- Raveendran, D.; Bhagwat, M.; Chidanand, V.D.; Anandakumar, S.; Sunil, C.K. Highlight on drying fruit slices with better retention of bioactive compounds. J. Food Process. Eng. 2022, 45, e14048. [Google Scholar] [CrossRef]
- Bustos, M.C.; Rocha-Parra, D.; Sampedro, I.R.; Pascual-Teresa, S.D.; Leon, A.E. The influence of different air-drying conditions on bioactive compounds and antioxidant activity of berries. J. Agric. Food Chem. 2018, 66, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Fracassetti, D.; Del Bo’, C.; Simonetti, P.; Gardana, C.; Klimis-Zacas, D.; Ciappellano, S. Effect of time and storage temperature on anthocyanin decay and antioxidant activity in wild blueberry (Vaccinium angustifolium) powder. J. Agric. Food Chem. 2013, 61, 2999–3005. [Google Scholar] [CrossRef] [PubMed]
- Lavefve, L.; Brownmiller, C.; Howard, L.; Reeves, D.; Sean, H.; Adams, S.H.; Chen, J.; Eva, C.; Diaz, E.V.; Mauromoustakos, A. Changes in Polyphenolics during Storage of Products Prepared with Freeze-Dried Wild Blueberry Powder. Foods 2020, 9, 466. [Google Scholar] [CrossRef]
- Rehman, J.; Khan, I.U.; Farid, S.; Kamal, S.; Aslam, N. Phytochemical screening and evaluation of in-vitro antioxidant potential of Monotheca buxifolia. J. Biotechnol. Pharm. Res. 2013, 4, 54–60. [Google Scholar]
- Su, M.S.; Chien, P.J. Antioxidant activity, anthocyanins, and phenolics of rabbiteye blueberry (Vaccinium ashei) fluid products as affected by fermentation. Food Chem. 2007, 104, 182–187. [Google Scholar] [CrossRef]
- Zlabur, J.S.; Mikulec, N.; Dozdor, L.; Duralija, B.; Galic, A.; Voca, S. Preservation of biologically active compounds and nutritional potential of quick-frozen berry fruits of the genus Rubus. Processes 2021, 9, 1940. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Fu, Y.; Zhou, X.; Chen, S.; Sun, Y.; Shen, Y.; Ye, X. Chemical composition and antioxidant activity of Chinese wild raspberry (Rubus hirsutus Thunb.). LWT—Food Sci. Technol. 2015, 60, 1262–1268. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Peleg, M. A model of microbial growth and decay in a closed habitat based on combined Fermi’s and the logistic equations. J. Sci. Food Agric. 1996, 71, 225–230. [Google Scholar] [CrossRef]
- Corradini, M.G.; Peleg, M. Linear and non-linear kinetics in the synthesis and degradation of acrylamide in foods and model systems. Crit. Rev. Food Sci. Nutr. 2006, 46, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Sherrod, P.H. NLREG: Nonlinear Regression Analysis Program, 2015, Version 6.6; NLREG: Brentwood, TN, USA, 2015. [Google Scholar]
- Brauch, J.; Buchweitz, M.; Schweiggert, R.; Carle, R. Detailed analyses of fresh and dried maqui (Aristotelia chilensis (Mol.) Stuntz) berries and juice. Food Chem. 2016, 190, 308–316. [Google Scholar] [CrossRef]
- Marjanovic-Balaban, Z.; Grujic, S.; Jasic, M.; Vujadinovic, D. Testing of chemical composition of wild berries. In Proceedings of the Third International Scientific Symposium “Agrosym 2012”, Jahorina, Bosnia and Herzegovina, 15–17 November 2012. [Google Scholar]
- Rodriguez, K.; Ah-Hen, K.S.; Vega-Galvez, A.; Vasquez, V.; Quispe-Fuentes, I.; Rojas, P.; Lemus-Mondaca, R. Changes in bioactive components and antioxidant capacity of maqui, Aristotelia chilensis [Mol] Stuntz, berries during drying. LWT—Food Sci. Technol. 2016, 65, 537–542. [Google Scholar] [CrossRef]
- Viljakainen, S.; Visti, A.; Laakso, S. Concentrations of organic acids and soluble sugars in juices from nordic berries. Acta Agric. Scand.-B Soil Plant Sci. 2002, 52, 101–109. [Google Scholar] [CrossRef]
- Calin-S’anchez, A.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, A.A.; Figiel, A. Comparison of traditional and novel drying techniques and its effect on quality of fruits, vegetables and aromatic herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Reque, P.M.; Steffens, R.S.; Jablonski, A.; Flores, S.H.; Rios, A.D.O.; De Jong, E.V. Cold storage of blueberry (Vaccinium spp.) fruits and juice: Anthocyanin stability and antioxidant activity. J. Food Compos. Anal. 2014, 33, 111–116. [Google Scholar] [CrossRef]
- Drozdz, P.; Seziene, V.; Pyrzynska, K. Phytochemical Properties and Antioxidant Activities of Extracts from Wild Blueberries and Lingonberries. Plant Foods Hum. Nutr. 2017, 72, 360–364. [Google Scholar] [CrossRef]
- Vollmannova, A.; Musilova, J.; Toth, T.; Arvay, J.; Bystricka, J.; Medvecky, M.; Daniel, J. Phenolic compounds, antioxidant activity and Cu, Zn, Cd and Pb content in wild and cultivated cranberries and blueberries. J. Environ. Anal. Chem. 2014, 94, 1445–1451. [Google Scholar] [CrossRef]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Harbourne, N.; Jacquier, J.C.; O’Riordan, D. Optimisation of the extraction and processing conditions of chamomile (Matricaria chamomilla L.) for incorporation into a beverage. Food Chem. 2009, 115, 15–19. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willow; methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Shahidi, F. Nutraceuticals and functional foods: Whole versus processed foods. Trends Food Sci. Technol. 2009, 20, 376–387. [Google Scholar] [CrossRef]
- Davey, M.W.; Montagu, M.V.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Rahman, N.F.A.; Shamsudin, R.; Ismail, A.; Shah, N.N.A.K.; Varith, J. Effects of drying methods on total phenolic contents and antioxidant capacity of the pomelo (Citrus grandis (L.) Osbeck) peels. Innov. Food Sci. Emerg. Technol. 2018, 50, 217–225. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Effect of vacuum-drying, hot air-drying and freeze-drying on polyphenols and antioxidant capacity of lemon (Citrus limon) pomace aqueous extracts. Int. J. Food Sci. Technol. 2017, 52, 880–887. [Google Scholar] [CrossRef]
- Krapfenbauer, G.; Kinner, M.; GoSsinger, M.; Schonlechner, R.; Berghofer, E. Effect of thermal treatment on the quality of cloudy apple Juice. J. Agric. Food Chem. 2006, 54, 5453–5460. [Google Scholar] [CrossRef]
- Pimpao, R.C.; Dew, T.; Oliveira, P.B.; Williamson, G.; Ferreira, R.B.; Santos, C.N. Analysis of phenolic compounds in Portuguese wild and commercial berries after multienzyme hydrolysis. J. Agric. Food Chem. 2013, 61, 4053–4062. [Google Scholar] [CrossRef]
- Maatta-Riihinen, K.R.; Kamal-Eldin, A.; Mattila, P.H.; Gonzalez-Paramas, A.M.; Torronen, A.R. Distribution and contents of phenolic compounds in eighteen scandinavian berry species. J. Agric. Food Chem. 2004, 52, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Favell, D.J. A comparison of the vitamin C content of fresh and frozen vegetables. Food Chem. 1998, 62, 59–64. [Google Scholar] [CrossRef]
- Arroyo-Lopez, F.N.; Bautista-Gallego, J.; Duran-Quintana, M.C.; Rodriguez-Gomez, F.; Romero-Barranco, C.; Garrido-Fernandez, A. Improvement of the storage process for cracked table olives. J. Food Eng. 2009, 42, 581–586. [Google Scholar] [CrossRef]
- Moldovan, B.; Popa, A.; David, L. Effects of storage temperature on the total phenolic content of Cornelian Cherry (Cornus mas L.) fruits extracts. J. Appl. Bot. Food Q. 2016, 89, 208–2011. [Google Scholar]
- Kirca, A.; Ozkan, M.; Cemeroglu, B. Effects of temperature, solid content and pH on the stability of black carrot anthocyanins. Food Chem. 2007, 101, 212–218. [Google Scholar] [CrossRef]
| Component | Fresh | Freeze-Dried −40 °C | Air-Dried 60 °C | Air-Dried 90 °C |
|---|---|---|---|---|
| Moisture 1 | 64.5 | 11.9 | 10.9 | 8.6 |
| Moisture 2 | 181.7 ± 5.4 | 13.54 ± 0.09 | 12.17 ± 0.31 | 9.43 ± 0.09 |
| Ash 2 | 2.8 ± 0.1 a | 2.78 ± 0.01 a | 3.19 ± 0.14 bc | 3.34 ± 0.14 cd |
| Protein 2 | 1.9 ± 0.5 a | 4.71 ± 0.09 c | 4.82 ± 0.11 c | 4.92 ± 0.92 c |
| Fat 2 | 5.0 ± 1.3 a | 3.56 ± 0.35 ac | 3.07 ± 0.64 cd | 2.41 ± 0.19 d |
| Carbohydrates 2 | 90.30 | 88.95 | 90.01 | 89.33 |
| Glucose 2 | 37.4 ± 0.6 a | 40.82 ± 0.68 bc | 45.99 ± 0.82 cd | 41.01 ± 1.21 ad |
| Fructose 2 | 31.5 ± 0.2 a | 30.84 ± 0.61 a | 32.84 ± 0.41 a | 30.32 ± 0.82 a |
| Titratable Acidity 3 | 0.13 ± 0.06 a | 0.10 ± 0.00 a | 0.17 ± 0.06 a | 0.10 ± 0.00 a |
| pH | 4.91 ± 0.01 a | 6.35 ± 0.02 b | 5.42 ± 0.02 c | 5.43 ± 0.02 c |
| Component | Fresh | Freeze-Dried −40 °C | Air-Dried 60 °C | Air-Dried 90 °C |
|---|---|---|---|---|
| Moisture 1 | 32.9 | 3.18 | 3.19 | 3.07 |
| Moisture 2 | 49.0 ± 1.7 | 3.29 ± 0.06 | 3.31 ± 0.08 | 3.16 ± 0.60 |
| Ash 2 | 1.4 ± 0.2 a | 1.33 ± 0.11 a | 1.27 ± 0.1 a | 1.38 ± 0.03 a |
| Protein 2 | 6.2 ± 0.7 a | 7.70 ± 0.30 b | 6.99 ± 0.27 ac | 5.83 ± 0.49 ad |
| Fat 2 | 9.6 ±1.5 a | 17.15 ± 0.21 b | 15.56 ± 0.16 c | 14.27 ± 0.16 d |
| Carbohydrates 2 | 82.80 | 73.82 | 76.18 | 78.52 |
| Titratable Acidity 3 | 0.2 ± 0.01 a | 0.13 ± 0.06 a | 0.17 ± 0.06 a | 0.15 ± 0.05 a |
| pH | 4.4 ± 0.2 a | 7.32 ± 0.03 b | 5.04 ± 0.03 c | 5.91 ± 0.05 d |
| Drying Methods | |||||
|---|---|---|---|---|---|
| Bioactive Compounds | Material | Fresh | Freeze-Dried −40 °C | Air-Dried 60 °C | Air-Dried 90 °C |
| TPC (mg GAE/g ds) | Flesh | 2.009 ± 0.012 a | 2.317 ± 0.005 b | 2.486 ± 0.004 c | 2.638 ± 0.033 d |
| Seed | 0.069 ± 0.001 a | 0.117 ± 0.002 b | 0.623 ± 0.007 c | 0.632 ± 0.002 c | |
| TFC (mg CE/g ds) | Flesh | 0.199 ± 0.001 a | 0.395 ± 0.004 b | 0.195 ± 0.000 c | 0.186 ± 0.003 d |
| Seed | 0.056 ± 0.001 a | 0.089 ± 0.000 b | 0.044 ± 0.000 c | 0.039 ± 0.000 d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Hasani, S.; Al-Attabi, Z.; Waly, M.; Al-Habsi, N.; Al-Subhi, L.; Shafiur Rahman, M. Polyphenol and Flavonoid Stability of Wild Blueberry (Sideroxylon mascatense) during Air- and Freeze-Drying and Storage Stability as a Function of Temperature. Foods 2023, 12, 871. https://doi.org/10.3390/foods12040871
Al Hasani S, Al-Attabi Z, Waly M, Al-Habsi N, Al-Subhi L, Shafiur Rahman M. Polyphenol and Flavonoid Stability of Wild Blueberry (Sideroxylon mascatense) during Air- and Freeze-Drying and Storage Stability as a Function of Temperature. Foods. 2023; 12(4):871. https://doi.org/10.3390/foods12040871
Chicago/Turabian StyleAl Hasani, Shaima, Zahir Al-Attabi, Mostafa Waly, Nasser Al-Habsi, Lyutha Al-Subhi, and Mohammad Shafiur Rahman. 2023. "Polyphenol and Flavonoid Stability of Wild Blueberry (Sideroxylon mascatense) during Air- and Freeze-Drying and Storage Stability as a Function of Temperature" Foods 12, no. 4: 871. https://doi.org/10.3390/foods12040871
APA StyleAl Hasani, S., Al-Attabi, Z., Waly, M., Al-Habsi, N., Al-Subhi, L., & Shafiur Rahman, M. (2023). Polyphenol and Flavonoid Stability of Wild Blueberry (Sideroxylon mascatense) during Air- and Freeze-Drying and Storage Stability as a Function of Temperature. Foods, 12(4), 871. https://doi.org/10.3390/foods12040871