Improving Pea Protein Emulsifying Capacity by Glycosylation to Prepare High-Internal-Phase Emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Synthesis of PPI–MD Conjugates via Glycosylation
2.2.2. Preparation of High-Internal-Phase Emulsions (HIPEs)
2.3. Methodology
2.3.1. Conjugate Solubility
2.3.2. HIPE Physical Stability
2.3.3. HIPE Textural Properties
2.3.4. HIPE Microstructural Properties
2.4. Statistical Analysis
3. Results and Discussion
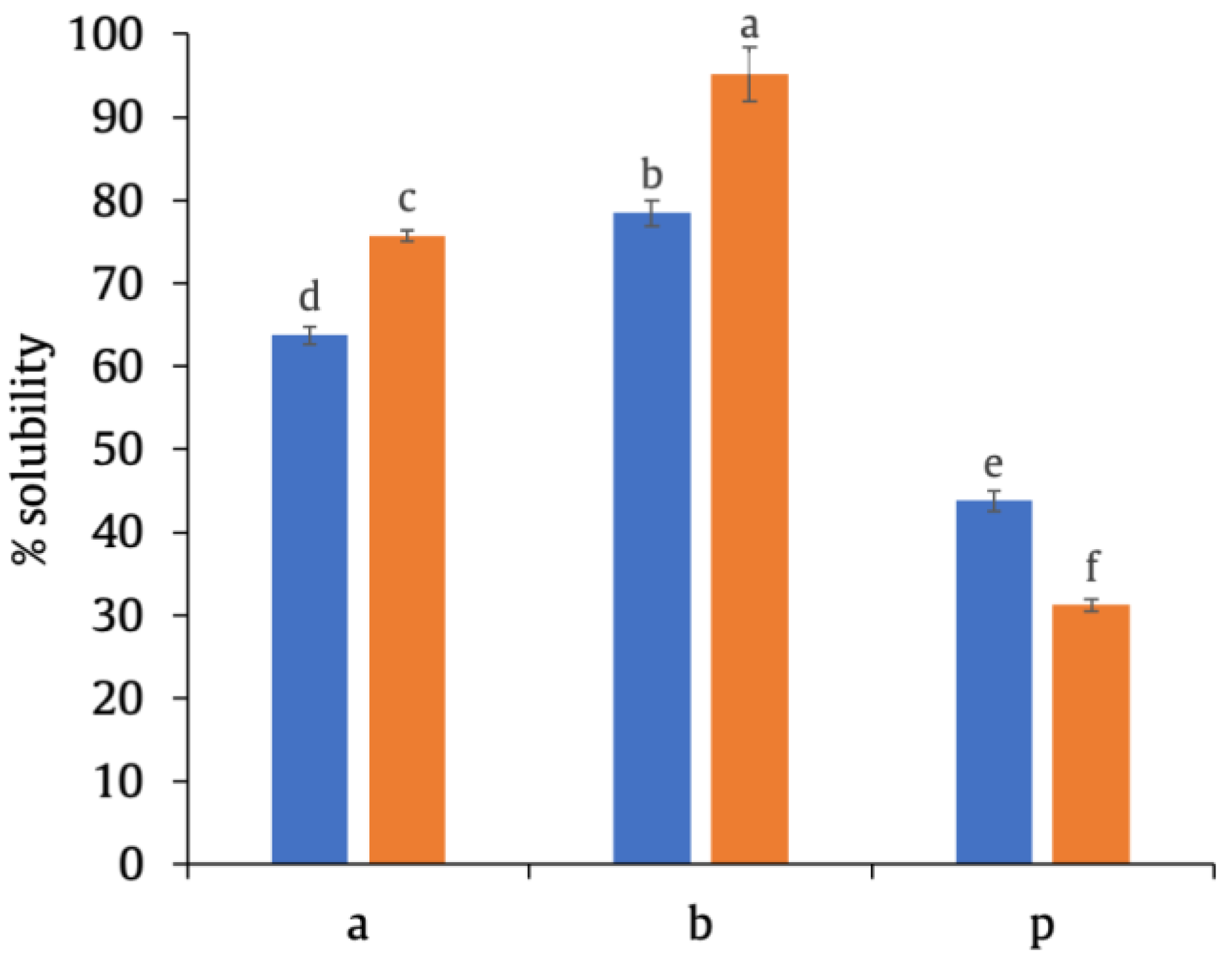
3.1. Conjugate Solubility
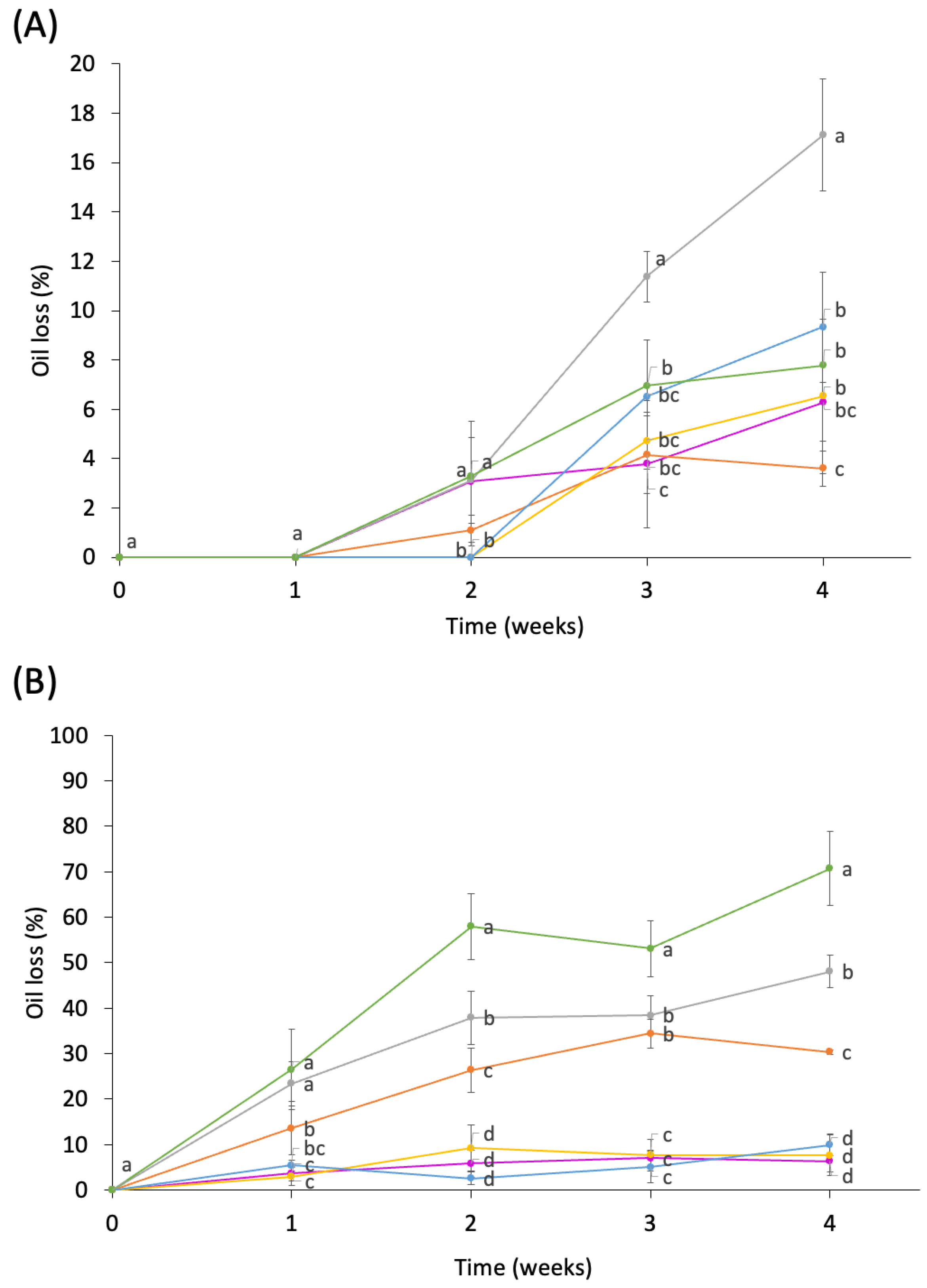
3.2. HIPE Physical Stability
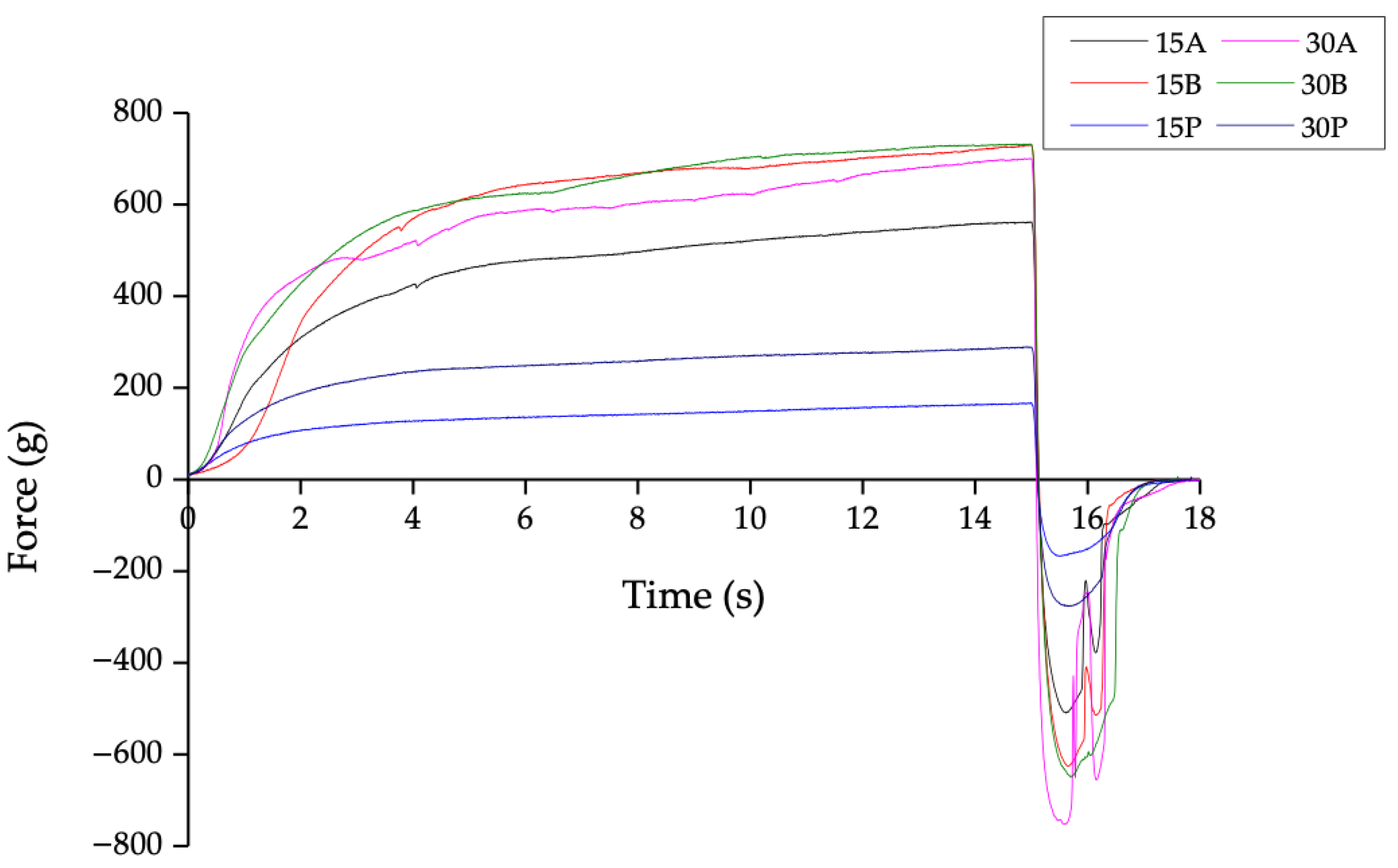
3.3. HIPE Textural Properties
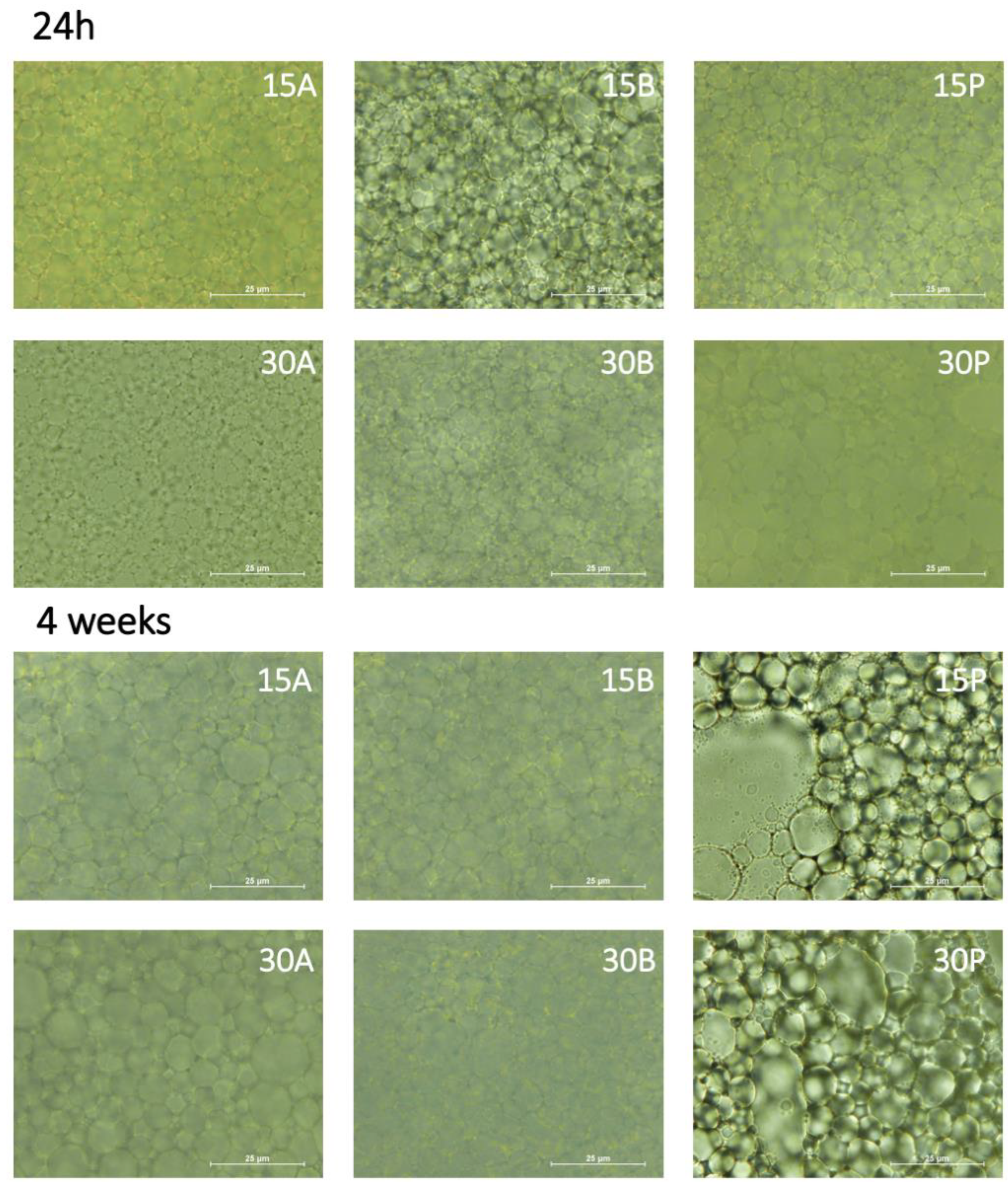
3.4. HIPE Microstructural Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making Sense of the “Clean Label” Trends: A Review of Consumer Food Choice Behavior and Discussion of Industry Implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef]
- Hadi, J.; Brightwell, G. Safety of Alternative Proteins: Technological, Environmental and Regulatory Aspects of Cultured Meat, Plant-Based Meat, Insect Protein and Single-Cell Protein. Foods 2021, 10, 1226. [Google Scholar] [CrossRef] [PubMed]
- Burger, T.G.; Zhang, Y. Recent Progress in the Utilization of Pea Protein as an Emulsifier for Food Applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Goldstein, N.; Reifen, R. The Potential of Legume-Derived Proteins in the Food Industry. Grain Oil Sci. Technol. 2022, 5, 167–178. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Adhikari, B. Maillard Reaction between Pea Protein Isolate and Maltodextrin via Wet-Heating Route for Emulsion Stabilisation. Futur. Foods 2022, 6, 100193. [Google Scholar] [CrossRef]
- Can Karaca, A.; Low, N.H.; Nickerson, M.T. Potential Use of Plant Proteins in the Microencapsulation of Lipophilic Materials in Foods. Trends Food Sci. Technol. 2015, 42, 5–12. [Google Scholar] [CrossRef]
- Cui, L.; Kimmel, J.; Zhou, L.; Chen, B.; Rao, J. Improving the Functionality of Pea Protein Isolate through Co-Spray Drying with Emulsifying Salt or Disaccharide. Food Hydrocoll. 2021, 113, 106534. [Google Scholar] [CrossRef]
- Chen, X.; Dai, Y.; Huang, Z.; Zhao, L.; Du, J.; Li, W.; Yu, D. Effect of Ultrasound on the Glycosylation Reaction of Pea Protein Isolate–Arabinose: Structure and Emulsifying Properties. Ultrason. Sonochem. 2022, 89, 106157. [Google Scholar] [CrossRef]
- Zha, F.; Rao, J.; Chen, B. Modification of Pulse Proteins for Improved Functionality and Flavor Profile: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3036–3060. [Google Scholar] [CrossRef]
- Liang, H.N.; Tang, C.H. PH-Dependent Emulsifying Properties of Pea [Pisum Sativum (L.)] Proteins. Food Hydrocoll. 2013, 33, 309–319. [Google Scholar] [CrossRef]
- Amine, C.; Dreher, J.; Helgason, T.; Tadros, T. Investigation of Emulsifying Properties and Emulsion Stability of Plant and Milk Proteins Using Interfacial Tension and Interfacial Elasticity. Food Hydrocoll. 2014, 39, 180–186. [Google Scholar] [CrossRef]
- Fernandez-Avila, C.; Arranz, E.; Guri, A.; Trujillo, A.J.; Corredig, M. Vegetable Protein Isolate-Stabilized Emulsions for Enhanced Delivery of Conjugated Linoleic Acid in Caco-2 Cells. Food Hydrocoll. 2016, 55, 144–154. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The Effect of Ultrasound Treatment on the Structural, Physical and Emulsifying Properties of Animal and Vegetable Proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef]
- Tamnak, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Muhammad, K. Physicochemical Properties, Rheological Behavior and Morphology of Pectin-Pea Protein Isolate Mixtures and Conjugates in Aqueous System and Oil in Water Emulsion. Food Hydrocoll. 2016, 56, 405–416. [Google Scholar] [CrossRef]
- Choe, U.; Lan, Y.; Chen, B.; Rao, J. Structural, Functional Properties of Phosphorylated Pea Protein Isolate by Simplified Co-Spray Drying Process. Food Chem. 2022, 393, 133441. [Google Scholar] [CrossRef]
- Qu, W.; Zhang, X.; Han, X.; Wang, Z.; He, R.; Ma, H. Structure and Functional Characteristics of Rapeseed Protein Isolate-Dextran Conjugates. Food Hydrocoll. 2018, 82, 329–337. [Google Scholar] [CrossRef]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. Pea Protein Isolate-Gum Arabic Maillard Conjugates Improves Physical and Oxidative Stability of Oil-in-Water Emulsions. Food Chem. 2019, 285, 130–138. [Google Scholar] [CrossRef]
- Guo, X.; Guo, X.; Meng, H.; Chen, X.; Zeng, Q.; Yu, S. Influences of Different Pectins on the Emulsifying Performance of Conjugates Formed between Pectin and Whey Protein Isolate. Int. J. Biol. Macromol. 2019, 123, 246–254. [Google Scholar] [CrossRef]
- Kutzli, I.; Weiss, J.; Gibis, M. Glycation of Plant Proteins via Maillard Reaction: Reaction Chemistry, Technofunctional Properties, and Potential Food Application. Foods 2021, 10, 376. [Google Scholar] [CrossRef]
- Shi, R.; Li, T.; Wang, C.; Yu, H.; Chen, W.; Wang, Y.; Qayum, A.; Jiang, Z.; Hou, J. Impacts of Preliminary Isolation and Enzymatic Treatment on Antioxidant Activities of Glycosylated Whey Protein Isolate with Inulin. J. Food Meas. Charact. 2020, 14, 3270–3279. [Google Scholar] [CrossRef]
- Ding, R.; Valicka, E.; Akhtar, M.; Ettelaie, R. Insignificant Impact of the Presence of Lactose Impurity on Formation and Colloid Stabilising Properties of Whey Protein–Maltodextrin Conjugates Prepared via Maillard Reactions. Food Struct. 2017, 12, 43–53. [Google Scholar] [CrossRef]
- Sedaghat Doost, A.; Nikbakht Nasrabadi, M.; Wu, J.; A’yun, Q.; Van der Meeren, P. Maillard Conjugation as an Approach to Improve Whey Proteins Functionality: A Review of Conventional and Novel Preparation Techniques. Trends Food Sci. Technol. 2019, 91, 1–11. [Google Scholar] [CrossRef]
- Pirestani, S.; Nasirpour, A.; Keramat, J.; Desobry, S. Preparation of Chemically Modified Canola Protein Isolate with Gum Arabic by Means of Maillard Reaction under Wet-Heating Conditions. Carbohydr. Polym. 2017, 155, 201–207. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Biochemistry, and Safety of Acrylamide. A Review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, B.; Tong, L.T.; Lu, C.; Li, S.; Sun, J.; Liu, L.; Wang, F. High Internal Phase Emulsions Stabilized Solely by Soy Protein Isolate. J. Food Eng. 2022, 318, 110905. [Google Scholar] [CrossRef]
- Zhao, Q.; Zaaboul, F.; Liu, Y.; Li, J. Recent Advances on Protein-Based Pickering High Internal Phase Emulsions (Pickering HIPEs): Fabrication, Characterization, and Applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1934–1968. [Google Scholar] [CrossRef]
- Wijaya, W.; Van der Meeren, P.; Wijaya, C.H.; Patel, A.R. High Internal Phase Emulsions Stabilized Solely by Whey Protein Isolate-Low Methoxyl Pectin Complexes: Effect of PH and Polymer Concentration. Food Funct. 2017, 8, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Erazo, E.M.; Bosqui, K.; Rabelo, R.S.; Kurozawa, L.E.; Hubinger, M.D. High Internal Phase Emulsions (HIPE) Using Pea Protein and Different Polysaccharides as Stabilizers. Food Hydrocoll. 2020, 105, 105775. [Google Scholar] [CrossRef]
- Wijaya, W.; Sun, Q.Q.; Vermeir, L.; Dewettinck, K.; Patel, A.R.; Van der Meeren, P. PH and Protein to Polysaccharide Ratio Control the Structural Properties and Viscoelastic Network of HIPE-Templated Biopolymeric Oleogels. Food Struct. 2019, 21, 100112. [Google Scholar] [CrossRef]
- Tirgarian, B.; Farmani, J.; Farahmandfar, R.; Milani, J.M.; Van Bockstaele, F. Ultra-Stable High Internal Phase Emulsions Stabilized by Protein-Anionic Polysaccharide Maillard Conjugates. Food Chem. 2022, 393, 133427. [Google Scholar] [CrossRef]
- Zhao, C.; Yin, H.; Yan, J.; Qi, B.; Liu, J. Structural and Physicochemical Properties of Soya Bean Protein Isolate/Maltodextrin Mixture and Glycosylation Conjugates. Int. J. Food Sci. Technol. 2020, 55, 3315–3326. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Qin, W.; Gu, J.; Zhang, H.; Duan, Y.; Ma, H. Structure and Functional Properties of Soy Protein Isolate-Lentinan Conjugates Obtained in Maillard Reaction by Slit Divergent Ultrasonic Assisted Wet Heating and the Stability of Oil-in-Water Emulsions. Food Chem. 2020, 331, 127374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, C.; Zhang, X.; Xia, S.; Jia, C.; Eric, K.; Abbas, S.; Feng, B.; Zhong, F. Effects of Maltodextrin Glycosylation Following Limited Enzymatic Hydrolysis on the Functional and Conformational Properties of Soybean Protein Isolate. Eur. Food Res. Technol. 2014, 238, 957–968. [Google Scholar] [CrossRef]
- Ye, Q.; Li, T.; Li, J.; Liu, L.; Dou, X.; Zhang, X. Development and Evaluation of Tea Polyphenols Loaded Water in Oil Emulsion with Zein as Stabilizer. J. Drug Deliv. Sci. Technol. 2020, 56, 101528. [Google Scholar] [CrossRef]
- Liu, H.; Xu, X.M.; Guo, S.D. Rheological, Texture and Sensory Properties of Low-Fat Mayonnaise with Different Fat Mimetics. LWT-Food Sci. Technol. 2007, 40, 946–954. [Google Scholar] [CrossRef]
- Zhang, Y.; Sharan, S.; Rinnan, Å.; Orlien, V. Survey on Methods for Investigating Protein Functionality and Related Molecular Characteristics. Foods 2021, 10, 2848. [Google Scholar] [CrossRef]
- Yin, S.W.; Tang, C.H.; Wen, Q.B.; Yang, X.Q.; Yuan, D.B. The Relationships between Physicochemical Properties and Conformational Features of Succinylated and Acetylated Kidney Bean (Phaseolus Vulgaris L.) Protein Isolates. Food Res. Int. 2010, 43, 730–738. [Google Scholar] [CrossRef]
- Paraman, I.; Hettiarachchy, N.S.; Schaefer, C. Glycosylation and Deamidation of Rice Endosperm Protein for Improved Solubility and Emulsifying Properties. Cereal Chem. 2007, 84, 593–599. [Google Scholar] [CrossRef]
- Zha, F.; Yang, Z.; Rao, J.; Chen, B. Conjugation of Pea Protein Isolate via Maillard-Driven Chemistry with Saccharide of Diverse Molecular Mass: Molecular Interactions Leading to Aggregation or Glycation. J. Agric. Food Chem. 2020, 68, 10157–10166. [Google Scholar] [CrossRef]
- O’Regan, J.; Mulvihill, D.M. Heat Stability and Freeze-Thaw Stability of Oil-in-Water Emulsions Stabilised by Sodium Caseinate-Maltodextrin Conjugates. Food Chem. 2010, 119, 182–190. [Google Scholar] [CrossRef]
- Chen, W.; Ma, X.; Wang, W.; Lv, R.; Guo, M.; Ding, T.; Ye, X.; Miao, S.; Liu, D. Preparation of Modified Whey Protein Isolate with Gum Acacia by Ultrasound Maillard Reaction. Food Hydrocoll. 2019, 95, 298–307. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. Influencing the Crystallization Behavior of Binary Mixtures of Stearyl Alcohol and Stearic Acid (SOSA) Using Ethylcellulose. Food Res. Int. 2017, 91, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Franzol, A.; Banin, T.M.; Brazil, T.R.; Rezende, M.C. Textural Analyses and Artificial Neural Network as Optimization Tools to Predict the Sensory Perception of Cosmetic Emulsions. J. Sens. Stud. 2022, 37, e12776. [Google Scholar] [CrossRef]
- César, F.C.S.; Campos, P.M.B.G. Influence of Vegetable Oils in the Rheology, Texture Profile and Sensory Properties of Cosmetic Formulations Based on Organogel. Int. J. Cosmet. Sci. 2020, 42, 494–500. [Google Scholar] [CrossRef]
- Fuhrmann, P.L.; Sala, G.; Stieger, M.; Scholten, E. Clustering of Oil Droplets in o/w Emulsions: Controlling Cluster Size and Interaction Strength. Food Res. Int. 2019, 122, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gao, H.; McClements, D.J.; Zhou, L.; Wu, J.; Zou, L. Stability, Rheology, and β-Carotene Bioaccessibility of High Internal Phase Emulsion Gels. Food Hydrocoll. 2019, 88, 210–217. [Google Scholar] [CrossRef]
- Mu, L.; Zhao, M.; Yang, B.; Zhao, H.; Cui, C.; Zhao, Q. Effect of Ultrasonic Treatment on the Graft Reaction between Soy Protein Isolate and Gum Acacia and on the Physicochemical Properties of Conjugates. J. Agric. Food Chem. 2010, 58, 4494–4499. [Google Scholar] [CrossRef] [PubMed]
- Foudazi, R.; Qavi, S.; Masalova, I.; Malkin, A.Y. Physical Chemistry of Highly Concentrated Emulsions. Adv. Colloid Interface Sci. 2015, 220, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Tcholakova, S.; Denkov, N.D.; Lips, A. Comparison of solid particles, globular proteins and surfactants as emulsifiers. Phys. Chem. Chem. Phys. 2008, 10, 1608–1627. [Google Scholar] [CrossRef]
| Textural Parameters | Sample | Storage Time | ||
|---|---|---|---|---|
| Fresh | 2 Weeks | 4 Weeks | ||
| Consistency (g·s) | 15A | 6442 ± 262 bA | 6019 ± 203 bB | 2324 ± 139 cC |
| 15B | 8781 ± 231 aA | 4888 ± 366 dB | 3411 ± 341 aB | |
| 15P | 2313 ± 287 dB | 4979 ± 312 cdA | 1445 ± 263 dC | |
| 30A | 8553 ± 335 aA | 6734 ± 126 aB | 2671 ± 276 bC | |
| 30B | 8713 ± 403 aA | 5334 ± 222 cB | 3098 ± 39 aC | |
| 30P | 3647 ± 251 cB | 4209 ± 272 eA | 2019 ± 77 cC | |
| Cohesiveness (g) | 15A | 479 ± 25 cA | 460 ± 50 bA | 240 ± 21 aB |
| 15B | 649 ± 29 bA | 428 ± 37 bcB | 258 ± 30 aC | |
| 15P | 171 ± 15 eB | 430 ± 2 bcA | 150 ± 40 bB | |
| 30A | 760 ± 25 aA | 565 ± 35 aB | 259 ± 27 aC | |
| 30B | 654 ± 11 bA | 460 ± 21 bB | 265 ± 19 aC | |
| 30P | 290 ± 26 dB | 406 ± 32 cA | 176 ± 12 bC | |
| Firmness (g) | 15A | 559 ± 28 bA | 442 ± 54 bB | 260 ± 39 aC |
| 15B | 734 ± 19 aA | 406 ± 20 bB | 264 ± 29 aC | |
| 15P | 174 ± 16 dB | 429 ± 22 bA | 125 ± 10 cC | |
| 30A | 716 ± 35 aA | 568 ± 31 aB | 260 ± 21 aC | |
| 30B | 739 ± 28 aA | 531 ± 34 aB | 275 ± 29 aC | |
| 30P | 299 ± 26 cB | 348 ± 24 cA | 171 ± 6 bC | |
| Viscosity index (g·s) | 15A | 514 ± 17 cB | 656 ± 36 aA | 264 ± 46 bcC |
| 15B | 642 ± 38 bA | 485 ± 108 bB | 165 ± 119 dC | |
| 15P | 225 ± 23 dB | 502 ± 19 bA | 191 ± 14 dB | |
| 30A | 807 ± 70 aA | 261 ± 18 cB | 308 ± 7 aB | |
| 30B | 672 ± 31 bA | 515 ± 23 bB | 291 ± 3 aC | |
| 30P | 234 ± 5 dA | 166 ± 30 dB | 223 ± 12 cdA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morell, P.; López-García, A.; Hernando, I.; Quiles, A. Improving Pea Protein Emulsifying Capacity by Glycosylation to Prepare High-Internal-Phase Emulsions. Foods 2023, 12, 870. https://doi.org/10.3390/foods12040870
Morell P, López-García A, Hernando I, Quiles A. Improving Pea Protein Emulsifying Capacity by Glycosylation to Prepare High-Internal-Phase Emulsions. Foods. 2023; 12(4):870. https://doi.org/10.3390/foods12040870
Chicago/Turabian StyleMorell, Pere, Adrián López-García, Isabel Hernando, and Amparo Quiles. 2023. "Improving Pea Protein Emulsifying Capacity by Glycosylation to Prepare High-Internal-Phase Emulsions" Foods 12, no. 4: 870. https://doi.org/10.3390/foods12040870
APA StyleMorell, P., López-García, A., Hernando, I., & Quiles, A. (2023). Improving Pea Protein Emulsifying Capacity by Glycosylation to Prepare High-Internal-Phase Emulsions. Foods, 12(4), 870. https://doi.org/10.3390/foods12040870










