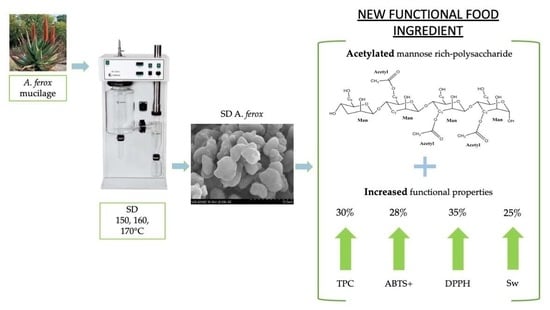
A New Functional Food Ingredient Obtained from Aloe ferox by Spray Drying
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Spray Drying (SD)
2.3. Scanning Electron Microscopy (SEM)
2.4. Color
2.5. Total Phenolic Content (TPC) and Antioxidant Capacity
2.6. Polysaccharide Analysis
2.6.1. Alcohol Insoluble Residues (AIRs) Preparation
2.6.2. Carbohydrate Analysis
2.7. FTIR and 1H NMR Analysis
2.8. Functional Properties
2.9. Statistical Analysis
3. Results and Discussion
3.1. SEM Observations
3.2. Color
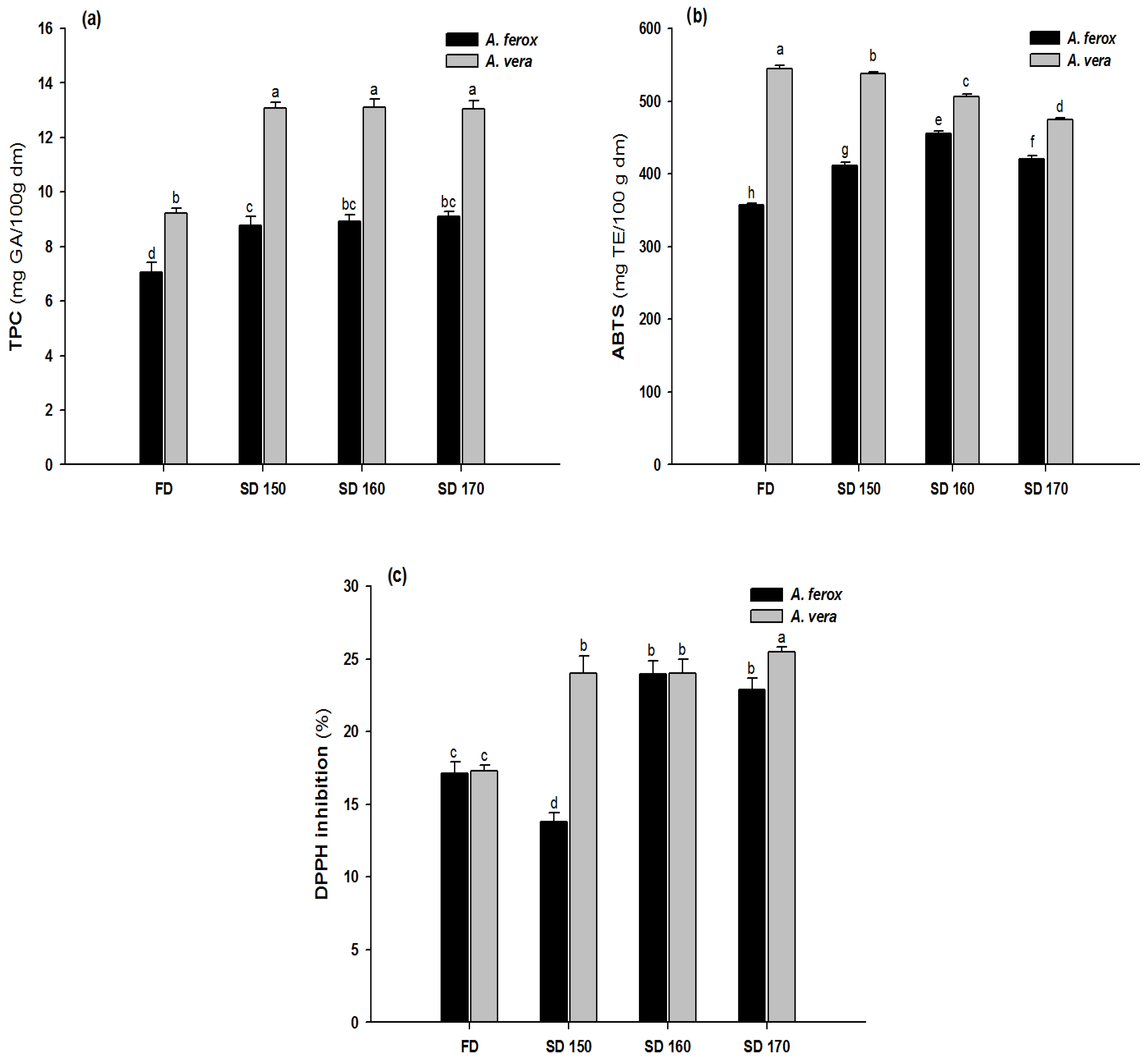
3.3. TPC and Antioxidant Capacity
3.4. Polysaccharide Composition
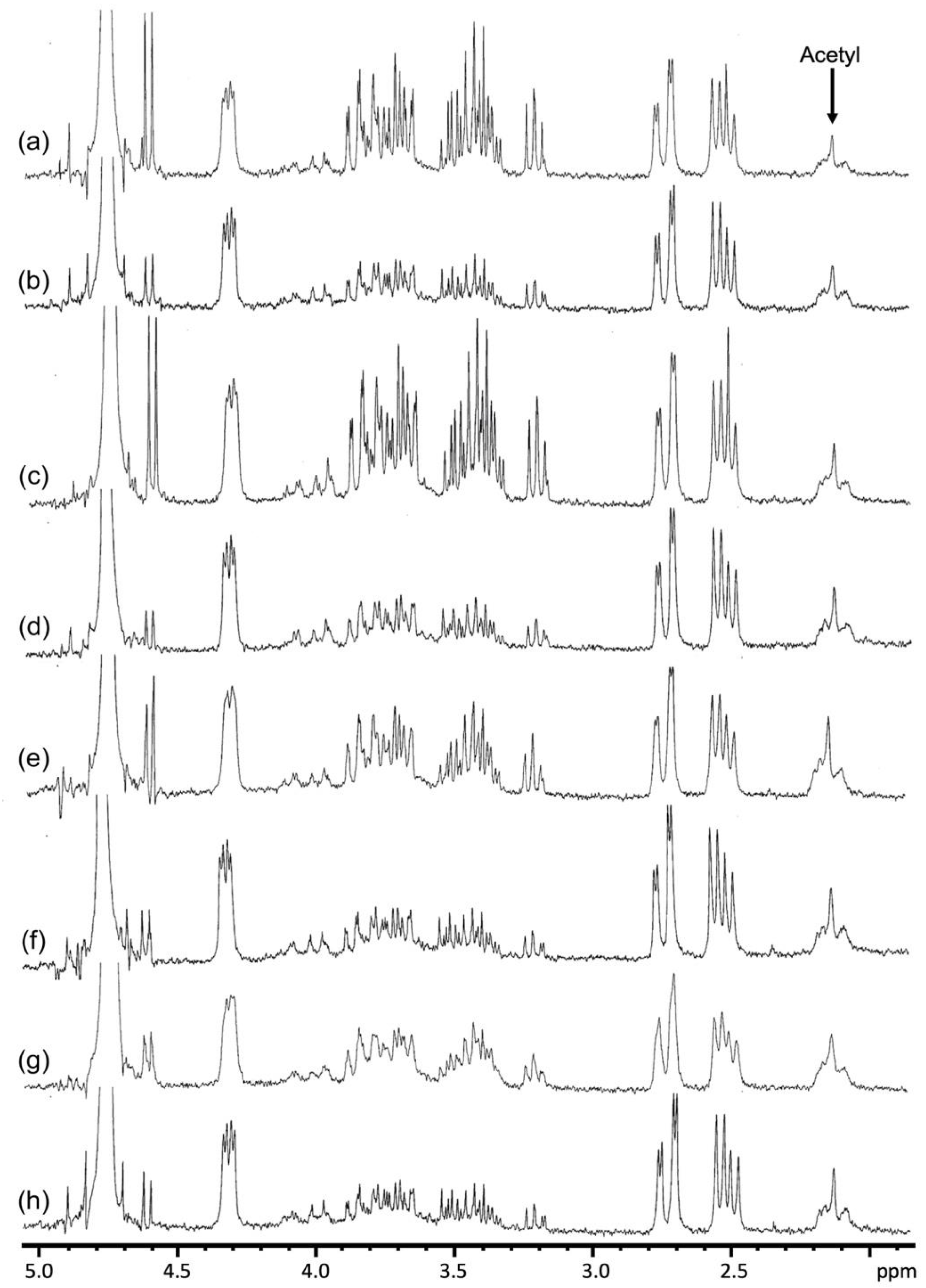
3.5. FTIR and 1H NMR Analysis of Aloe Samples
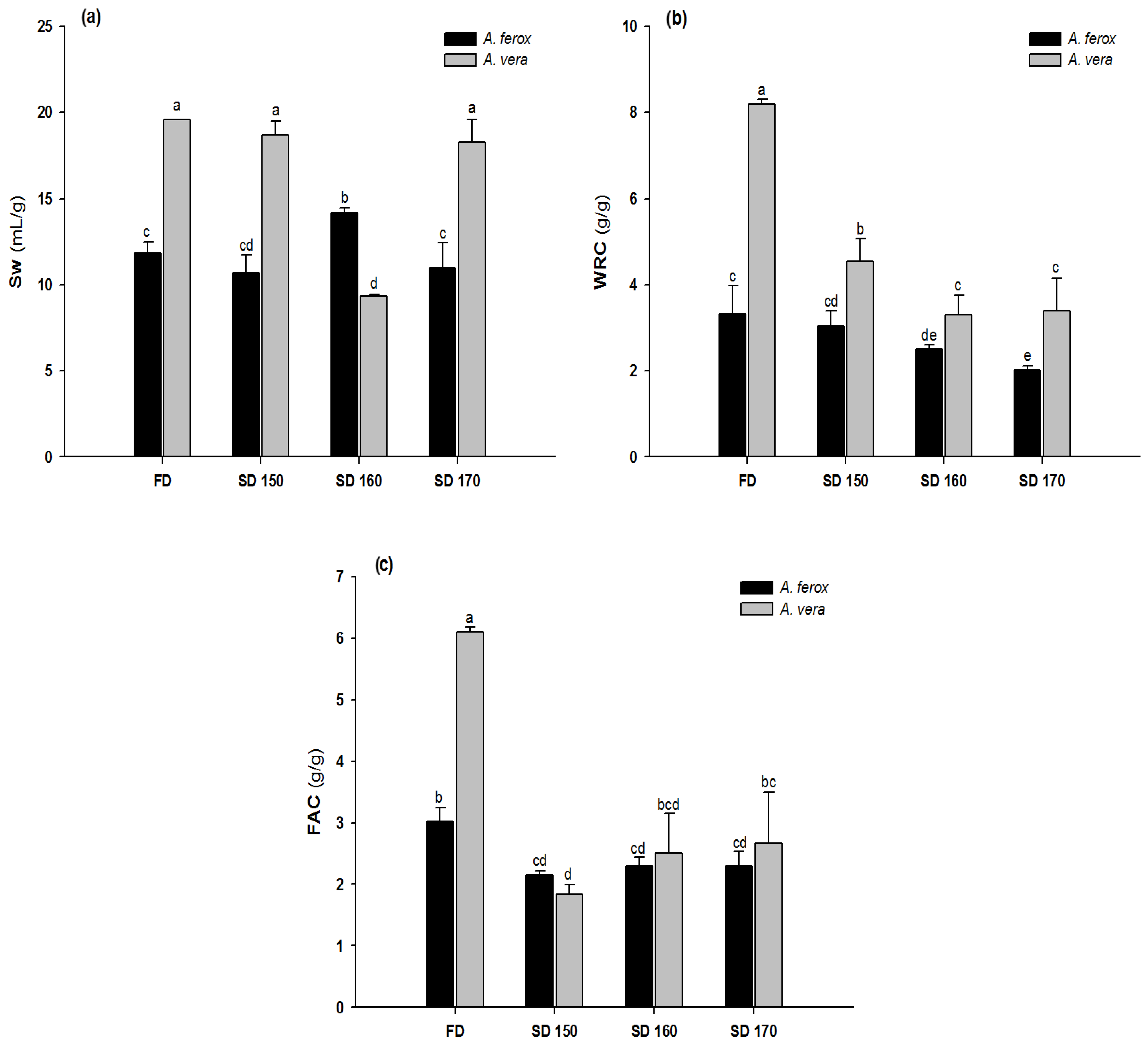
3.6. Functional Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avila-de la Rosa, G.; Alvarez-Ramirez, J.; Vernon-Carter, E.J.; Carrillo-Navas, H.; Pérez-Alonso, C. Viscoelasticity of chia (Salvia hispanica L.) seed mucilage dispersion in the vicinity of an oil-water interface. Food Hydrocoll. 2015, 49, 200–207. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Medina-Torres, L.; González-Laredo, R.F.; Rodríguez-González, V.M.; Eim, V.; Femenia, A. Influence of water deficit on the main polysaccharides and the rheological properties of Aloe vera (Aloe barbadensis miller) mucilage. Ind. Crops Prod. 2017, 109, 644–653. [Google Scholar] [CrossRef]
- Chokboribal, J.; Tachaboonyakiat, W.; Sangvanich, P.; Ruangpornvisuti, V.; Jettanacheawchankit, S.; Thunyakitpisal, P. Deacetylation affects the physical properties and bioactivity of acemannan, an extracted polysaccharide from Aloe vera. Carbohydr. Polym. 2015, 133, 556–566. [Google Scholar] [CrossRef]
- Femenia, A.; Sánchez, E.S.; Simal, S.; Rosselló, C. Compositional features of polysaccharides from Aloe vera (Aloe barbadensis miller) plant tissues. Carbohydr. Polym. 1999, 39, 109–117. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Rodríguez-González, V.M.; González-Laredo, R.F.; Eim, V.; González-Centeno, M.R.; Femenia, A. Effect of different drying procedures on the bioactive polysaccharide acemannan from Aloe vera (Aloe barbadensis miller). Carbohydr. Polym. 2017, 168, 327–336. [Google Scholar] [CrossRef]
- Rodríguez-González, V.M.; Femenia, A.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Candelas-Cadillo, M.G.; Ramírez-Baca, P.; Simal, S.; Rosselló, C. Effects of pasteurization on bioactive polysaccharide acemannan and cell wall polymers from Aloe barbadensis miller. Carbohydr. Polym. 2011, 86, 1675–1683. [Google Scholar] [CrossRef]
- Manna, S.; McAnalley, B.H. Determination of the position of the o-acetyl group in a β-(1→4)-mannan (acemannan) from Aloe barbardensis miller. Carbohydr. Res. 1993, 241, 317–319. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Núñez-Ramírez, D.M.; Calderas, F.; González-Laredo, R.F.; Minjares-Fuentes, R.; Valadez-García, M.A.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation of gallic acid by spray drying with Aloe vera mucilage (Aloe barbadensis miller) as wall material. Ind. Crops Prod. 2019, 138, 111461. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Núñez-Ramírez, D.M.; Calderas, F.; Bernad-Bernad, M.J.; Gracia-Mora, J.; Rodríguez-Ramírez, J.; González-Laredo, R.F.; Gallegos-Infante, J.A.; Manero, O. Curcumin encapsulation by spray drying using Aloe vera mucilage as encapsulating agent. J. Food Process Eng. 2019, 42, e12972. [Google Scholar] [CrossRef]
- Otalora, M.C.; Wilches-Torres, A.; Gomez Castano, J.A. Spray-drying microencapsulation of pink guava (Psidium guajava) carotenoids using mucilage from Opuntia ficus-indica cladodes and Aloe vera leaves as encapsulating materials. Polymers 2022, 14, 310. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Femenia, A.; Comas-Serra, F.; Rosselló, C.; Rodríguez-González, V.M.; González-Laredo, R.F.; Gallegos-Infante, J.A.; Medina-Torres, L. Effect of different drying procedures on physicochemical properties and flow behavior of Aloe vera (Aloe barbadensis miller) gel. LWT-Food Sci. Technol. 2016, 74, 378–386. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Role of acemannan o-acetyl group in murine radioprotection. Carbohydr. Polym. 2019, 207, 460–470. [Google Scholar] [CrossRef] [PubMed]
- FDA. Status of Certain Additional Over-the-Counterdrug Category ii and iii Active Ingredients; Final rule; Food and Drug Administration: Silver Spring, MD, USA, 2002; p. 31127. [Google Scholar]
- Chen, W.; Van Wyk, B.-E.; Vermaak, I.; Viljoen, A.M. Cape aloes—A review of the phytochemistry, pharmacology and commercialisation of Aloe ferox. Phytochem. Lett. 2012, 5, 1–12. [Google Scholar] [CrossRef]
- Mabusela, W.T.; Stephen, A.M.; Botha, M.C. Carbohydrate polymers from Aloe ferox leaves. Phytochemistry 1990, 29, 3555–3558. [Google Scholar] [CrossRef]
- O’Brien, C.; Van Wyk, B.E.; Van Heerden, F.R. Physical and chemical characteristics of Aloe ferox leaf gel. S. Afr. J. Bot. 2011, 77, 988–995. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Santiago-Adame, R.; Calderas, F.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Núñez-Ramírez, D.M.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation by spray drying of laurel infusions (Litsea glaucescens) with maltodextrin. Ind. Crops Prod. 2016, 90, 1–8. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.-L. Proanthocyanidin composition and antioxidant potential of the stem winemaking byproducts from 10 different grape varieties (Vitis vinifera l.). J. Agric. Food Chem. 2012, 60, 11850–11858. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Calderas, F.; Minjares, R.; Femenia, A.; Sánchez-Olivares, G.; Gónzalez-Laredo, F.R.; Santiago-Adame, R.; Ramirez-Nuñez, D.M.; Rodríguez-Ramírez, J.; Manero, O. Structure preservation of Aloe vera (Barbadensis miller) mucilage in a spray drying process. LWT-Food Sci. Technol. 2016, 66, 93–100. [Google Scholar] [CrossRef]
- Femenia, A.; Robertson, J.A.; Waldron, K.W.; Selvendran, R.R. Cauliflower (Brassica oleracea l), globe artichoke (Cynara scolymus) and chicory witloof (Cichorium intybus) processing by-products as sources of dietary fibre. J. Sci. Food Agric. 1998, 77, 511–518. [Google Scholar] [CrossRef]
- Alvarado-Morales, G.; Minjares-Fuentes, R.; Contreras-Esquivel, J.C.; Montañez, J.; Meza-Velázquez, J.A.; Femenia, A. Application of thermosonication for Aloe vera (Aloe barbadensis miller) juice processing: Impact on the functional properties and the main bioactive polysaccharides. Ultrason. Sonochem. 2019, 56, 125–133. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- León-Martínez, F.M.; Méndez-Lagunas, L.L.; Rodríguez-Ramírez, J. Spray drying of nopal mucilage (opuntia ficus-indica): Effects on powder properties and characterization. Carbohydr. Polym. 2010, 81, 864–870. [Google Scholar] [CrossRef]
- Santhalakshmy, S.; Don Bosco, S.J.; Francis, S.; Sabeena, M. Effect of inlet temperature on physicochemical properties of spray-dried jamun fruit juice powder. Powder Technol. 2015, 274, 37–43. [Google Scholar] [CrossRef]
- Tan, S.P.; Kha, T.C.; Parks, S.E.; Stathopoulos, C.E.; Roach, P.D. Effects of the spray-drying temperatures on the physiochemical properties of an encapsulated bitter melon aqueous extract powder. Powder Technol. 2015, 281, 65–75. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Galanakis, C.M.; Brnčić, M.; Grimi, N.; Boussetta, N.; Mota, M.J.; Saraiva, J.A.; Patras, A.; Tiwari, B.; Barba, F.J. Fruit juice sonication: Implications on food safety and physicochemical and nutritional properties. Food Res. Int. 2015, 77 Pt 4, 743–752. [Google Scholar] [CrossRef]
- Eshun, K.; He, Q. Aloe vera: A valuable ingredient for the food, pharmaceutical and cosmetic industries—A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Vanga, S.K.; Raghavan, V. High-intensity ultrasound processing of kiwifruit juice: Effects on the microstructure, pectin, carbohydrates and rheological properties. Food Chem. 2020, 313, 126121. [Google Scholar] [CrossRef]
- Lu, W.; Yang, X.; Shen, J.; Li, Z.; Tan, S.; Liu, W.; Cheng, Z. Choosing the appropriate wall materials for spray-drying microencapsulation of natural bioactive ingredients: Taking phenolic compounds as examples. Powder Technol. 2021, 394, 562–574. [Google Scholar] [CrossRef]
- Li, H.; Xie, L.; Ma, Y.; Zhang, M.; Zhao, Y.; Zhao, X. Effects of drying methods on drying characteristics, physicochemical properties and antioxidant capacity of okra. LWT 2019, 101, 630–638. [Google Scholar] [CrossRef]
- Breaud, C.; Lallemand, L.; Mares, G.; Mabrouki, F.; Bertolotti, M.; Simmler, C.; Greff, S.; Mauduit, M.; Herbette, G.; Garayev, E.; et al. Lc-ms based phytochemical profiling towards the identification of antioxidant markers in some endemic aloe species from mascarene islands. Antioxidants 2023, 12, 50. [Google Scholar] [CrossRef]
- Medina-Torres, L.; García-Cruz, E.E.; Calderas, F.; González Laredo, R.F.; Sánchez-Olivares, G.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E.; Rodríguez-Ramírez, J. Microencapsulation by spray drying of gallic acid with nopal mucilage (Opuntia ficus indica). LWT-Food Sci. Technol. 2013, 50, 642–650. [Google Scholar] [CrossRef]
- Otálora, M.C.; Carriazo, J.G.; Osorio, C.; Nazareno, M.A. Encapsulation of cactus (opuntia megacantha) betaxanthins by ionic gelation and spray drying: A comparative study. Food Res. Int. 2018, 111, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Antigo, J.L.D.; Stafussa, A.P.; de Cassia Bergamasco, R.; Madrona, G.S. Chia seed mucilage as a potential encapsulating agent of a natural food dye. J. Food Eng. 2020, 285, 110101. [Google Scholar] [CrossRef]
- Minjares-Fuentes, J.R.; Femenia, A. Effect of processing on the bioactive polysaccharides and phenolic compounds from Aloe vera (Aloe barbadensis miller). In Dietary Fiber Functionality in Food and Nutraceuticals; Hosseinian, F., Oomah, B.D., Campos-Vega, R., Eds.; Wiley Blackwell: Oxford, UK, 2017; pp. 263–287. [Google Scholar]
- Minjares-Fuentes, R.; Femenia, A.; Comas-Serra, F.; Rodríguez-González, V.M. Compositional and structural features of the main bioactive polysaccharides present in the Aloe vera plant. J. AOAC Int. 2018, 101, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- McConaughy, S.D.; Stroud, P.A.; Boudreaux, B.; Hester, R.D.; McCormick, C.L. Structural characterization and solution properties of a galacturonate polysaccharide derived from Aloe vera capable of in situ gelation. Biomacromolecules 2008, 9, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Gentilini, R.; Bozzini, S.; Munarin, F.; Petrini, P.; Visai, L.; Tanzi, M.C. Pectins from Aloe vera: Extraction and production of gels for regenerative medicine. J. Appl. Polym. Sci. 2014, 131, 39760. [Google Scholar] [CrossRef]
- Bozzi, A.; Perrin, C.; Austin, S.; Arce Vera, F. Quality and authenticity of commercial Aloe vera gel powders. Food Chem. 2007, 103, 22–30. [Google Scholar] [CrossRef]
- Diehl, B.; Teichmuller, E.E. Aloe vera, quality inspection and identification. Agro Food Ind. Hi-Tech 1998, 9, 14–16. [Google Scholar]
- Nejatzadeh-Barandozi, F.; Enferadi, S. Ft-ir study of the polysaccharides isolated from the skin juice, gel juice, and flower of Aloe vera tissues affected by fertilizer treatment. Org. Med. Chem. Lett. 2012, 2, 33. [Google Scholar] [CrossRef]
- Reynolds, T.; Dweck, A.C. Aloe vera leaf gel: A review update. J. Ethnopharmacol. 1999, 68, 3–37. [Google Scholar] [CrossRef]
- Sharma, K.; Mittal, A.; Chauhan, N. Aloe vera as penetration enhancer. Int. J. Drug Dev. Res. 2015, 7, 31–43. [Google Scholar]
- Campestrini, L.H.; Silveira, J.L.M.; Duarte, M.E.R.; Koop, H.S.; Noseda, M.D. Nmr and rheological study of aloe barbadensis partially acetylated glucomannan. Carbohydr. Polym. 2013, 94, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tiku, A.B. Immunomodulatory potential of acemannan (polysaccharide from Aloe vera) against radiation induced mortality in swiss albino mice. Food Agric. Immunol. 2016, 27, 72–86. [Google Scholar] [CrossRef]
- Tong, X.; Lao, C.; Li, D.; Du, J.; Chen, J.; Xu, W.; Li, L.; Ye, H.; Guo, X.; Li, J. An acetylated mannan isolated from Aloe vera induce colorectal cancer cells apoptosis via mitochondrial pathway. Carbohydr. Polym. 2022, 291, 119464. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, V.M.; Femenia, A.; Minjares-Fuentes, R.; Gonzalez-Laredo, R.F. Functional properties of pasteurized samples of Aloe barbadensis miller: Optimization using response surface methodology. LWT-Food Sci. Technol. 2012, 47, 225–232. [Google Scholar] [CrossRef]
- Moyano, G.; Sáyago-Ayerdi, S.G.; Largo, C.; Caz, V.; Santamaria, M.; Tabernero, M. Potential use of dietary fibre from Hibiscus sabdariffa and Agave tequilana in obesity management. J. Funct. Food 2016, 21, 1–9. [Google Scholar] [CrossRef]
- Mackie, A.; Rigby, N.; Harvey, P.; Bajka, B. Increasing dietary oat fibre decreases the permeability of intestinal mucus. J. Funct. Food 2016, 26, 418–427. [Google Scholar] [CrossRef]
- Ma, M.; Mu, T. Anti-diabetic effects of soluble and insoluble dietary fibre from deoiled cumin in low-dose streptozotocin and high glucose-fat diet-induced type 2 diabetic rats. J. Funct. Food 2016, 25, 186–196. [Google Scholar] [CrossRef]
- Femenia, A.; García-Pascual, P.; Simal, S.; Rosselló, C. Effects of heat treatment and dehydration on bioactive polysaccharide acemannan and cell wall polymers from Aloe barbadensis miller. Carbohydr. Polym. 2003, 51, 397–405. [Google Scholar] [CrossRef]



| L | a* | b* | C | H° | ΔE | ||
|---|---|---|---|---|---|---|---|
| FD | |||||||
| A. ferox | 74.1 ± 0.0 e | −0.6 ± 0.0 e | 18.1 ± 0.0 abc | 18.1 ± 0.0 abc | 91.9 ± 0.9 a | - | |
| A. vera | 87.4 ± 0.0 a | −0.8 ± 0.0 e | 17.2 ± 0.0 bc | 17.2 ± 0.0 bc | 92.7 ± 1.1 a | - | |
| SD 150 | |||||||
| A. ferox | 85.7 ± 2.3 ab | 1.4 ± 0.0 d | 17.0 ± 2.7 bc | 17.1 ± 2.7 bc | 85.3 ± 0.8 b | 11.7 ± 1.8 a | |
| A. vera | 83.1 ± 1.2 bc | 1.8 ± 0.3 cd | 16.0 ± 0.7 c | 16.1 ± 0.7 c | 83.4 ± 1.0 bc | 5.8 ± 0.9 cd | |
| SD 160 | |||||||
| A. ferox | 83.4 ± 1.9 bc | 2.4 ± 0.8 bc | 18.9 ± 1.1 ab | 19.1 ± 1.2 ab | 82.7 ± 1.8 cd | 9.8 ± 0.5 b | |
| A. vera | 81.9 ± 2.0 c | 2.5 ± 0.5 bc | 16.5 ± 0.7 c | 16.4 ± 0.7 c | 81.4 ± 1.4 cd | 7.0 ± 0.9 c | |
| SD 170 | |||||||
| A. ferox | 77.9 ± 3.4 d | 3.1 ± 0.9 b | 19.3 ± 2.1 ab | 19.6 ± 2.2 a | 81.0 ± 1.6 d | 5.4 ± 0.4 d | |
| A. vera | 77.6 ± 0.8 d | 4.2 ± 0.3 a | 19.7 ± 0.5 a | 20.2 ± 0.6 a | 77.9 ± 0.7 e | 11.3 ± 0.3 a | |
| Sample | Carbohydrate Composition (mol%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rha | Fuc | Ara | Xyl | Man | Gal | Glc | UA | ||
| FD | |||||||||
| A. ferox | 2.0 ± 0.0 | 0.6 ± 0.1 | 2.1 ± 0.0 | 1.2 ± 0.0 | 59.5 ± 5.1 | 3.7 ± 0.1 | 20.8 ± 0.4 | 10.0 ± 0.4 | |
| A. vera | 1.2 ± 0.0 | 0.2 ± 0.0 | 2.0 ± 0.0 | 0.8 ± 0.1 | 69.7 ± 3.6 | 2.9 ± 0.0 | 14.8 ± 0.3 | 8.4 ± 0.2 | |
| SD 150 | |||||||||
| A. ferox | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.2 ± 0.0 | 0.0 ± 0.0 | 76.3 ± 2.6 | 3.1 ± 0.0 | 7.8 ± 0.3 | 10.6 ± 0.3 | |
| A. vera | 1.2 ± 0.0 | 0.4 ± 0.0 | 1.7 ± 0.1 | 0.6 ± 0.0 | 76.8 ± 4.5 | 2.7 ± 0.0 | 8.7 ± 0.3 | 7.9 ± 0.0 | |
| SD 160 | |||||||||
| A. ferox | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.4 ± 0.0 | 0.0 ± 0.0 | 72.7 ± 2.3 | 1.5 ± 0.0 | 7.5 ± 0.0 | 16.9 ± 0.4 | |
| A. vera | 0.5 ± 0.0 | 0.0 ± 0.0 | 1.9 ± 0.0 | 0.0 ± 0.0 | 76.4 ± 0.3 | 2.5 ± 0.1 | 9.4 ± 0.0 | 9.2 ± 0.1 | |
| SD 170 | |||||||||
| A. ferox | 0.4 ± 0.0 | 0.0 ± 0.0 | 1.5 ± 0.0 | 0.6 ± 0.0 | 74.7 ± 5.3 | 2.3 ± 0.1 | 8.0 ± 0.3 | 12.5 ± 0.2 | |
| A. vera | 1.1 ± 0.0 | 0.0 ± 0.0 | 1.7 ± 0.0 | 0.0 ± 0.0 | 74.8 ± 3.7 | 3.1 ± 0.0 | 10.3 ± 0.2 | 9.0 ± 0.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comas-Serra, F.; Martínez-García, J.J.; Pérez-Alba, A.; Sáenz-Esqueda, M.d.l.Á.; Candelas-Cadillo, M.G.; Femenia, A.; Minjares-Fuentes, R. A New Functional Food Ingredient Obtained from Aloe ferox by Spray Drying. Foods 2023, 12, 850. https://doi.org/10.3390/foods12040850
Comas-Serra F, Martínez-García JJ, Pérez-Alba A, Sáenz-Esqueda MdlÁ, Candelas-Cadillo MG, Femenia A, Minjares-Fuentes R. A New Functional Food Ingredient Obtained from Aloe ferox by Spray Drying. Foods. 2023; 12(4):850. https://doi.org/10.3390/foods12040850
Chicago/Turabian StyleComas-Serra, Francesca, Juan José Martínez-García, Alma Pérez-Alba, María de los Ángeles Sáenz-Esqueda, María Guadalupe Candelas-Cadillo, Antoni Femenia, and Rafael Minjares-Fuentes. 2023. "A New Functional Food Ingredient Obtained from Aloe ferox by Spray Drying" Foods 12, no. 4: 850. https://doi.org/10.3390/foods12040850
APA StyleComas-Serra, F., Martínez-García, J. J., Pérez-Alba, A., Sáenz-Esqueda, M. d. l. Á., Candelas-Cadillo, M. G., Femenia, A., & Minjares-Fuentes, R. (2023). A New Functional Food Ingredient Obtained from Aloe ferox by Spray Drying. Foods, 12(4), 850. https://doi.org/10.3390/foods12040850