Vacuum-Assisted Block Freeze Concentration Studies in Cheese Whey and Its Potential in Lactose Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
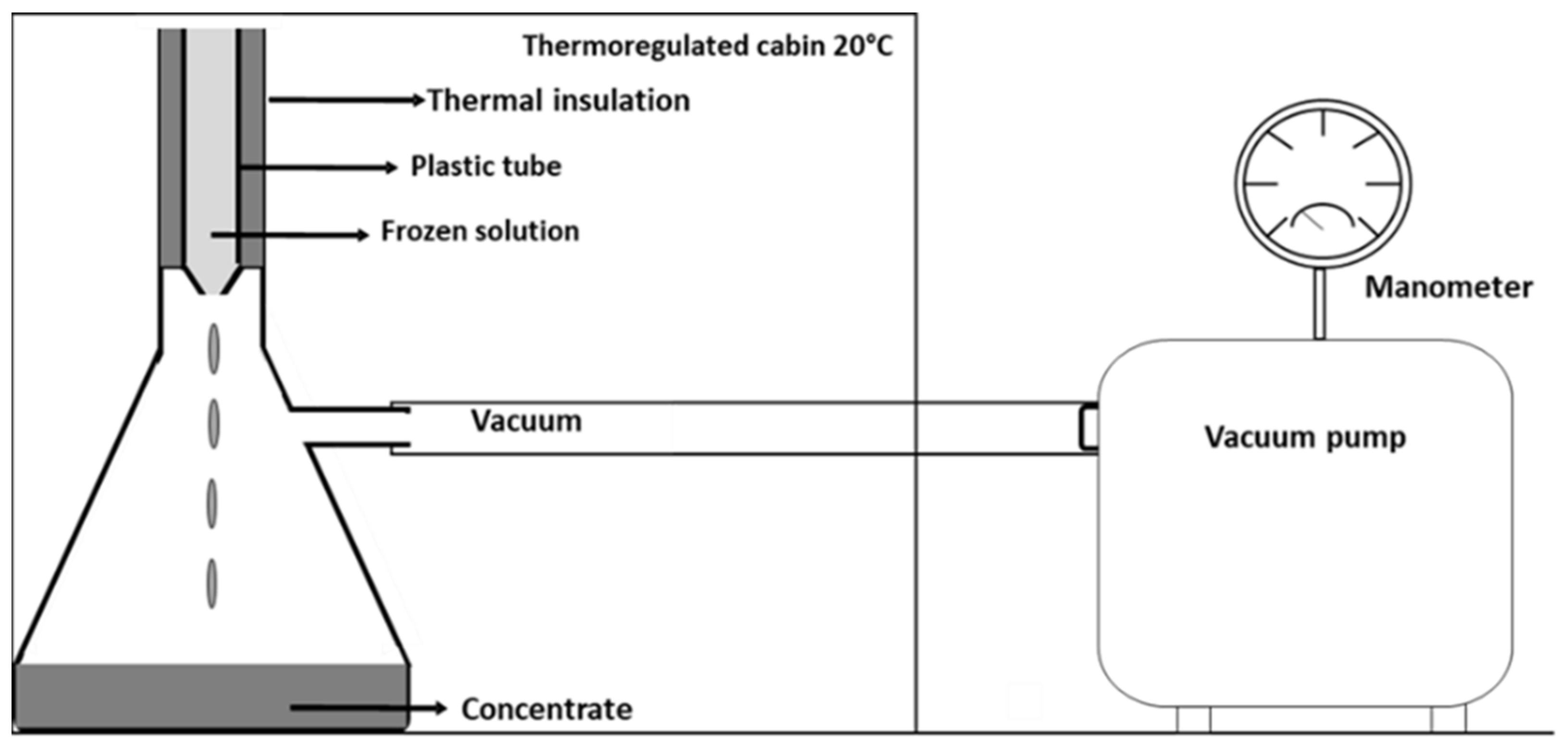
2.2. Freezing and Vacuum Suction Procedure
2.3. Experimental Design
2.3.1. Effect of Factors on Response Variables
2.3.2. Vacuum-Assisted BFC Tests on Three Types of Cheese Whey
2.4. Lyophilization Process
2.5. Response Variables
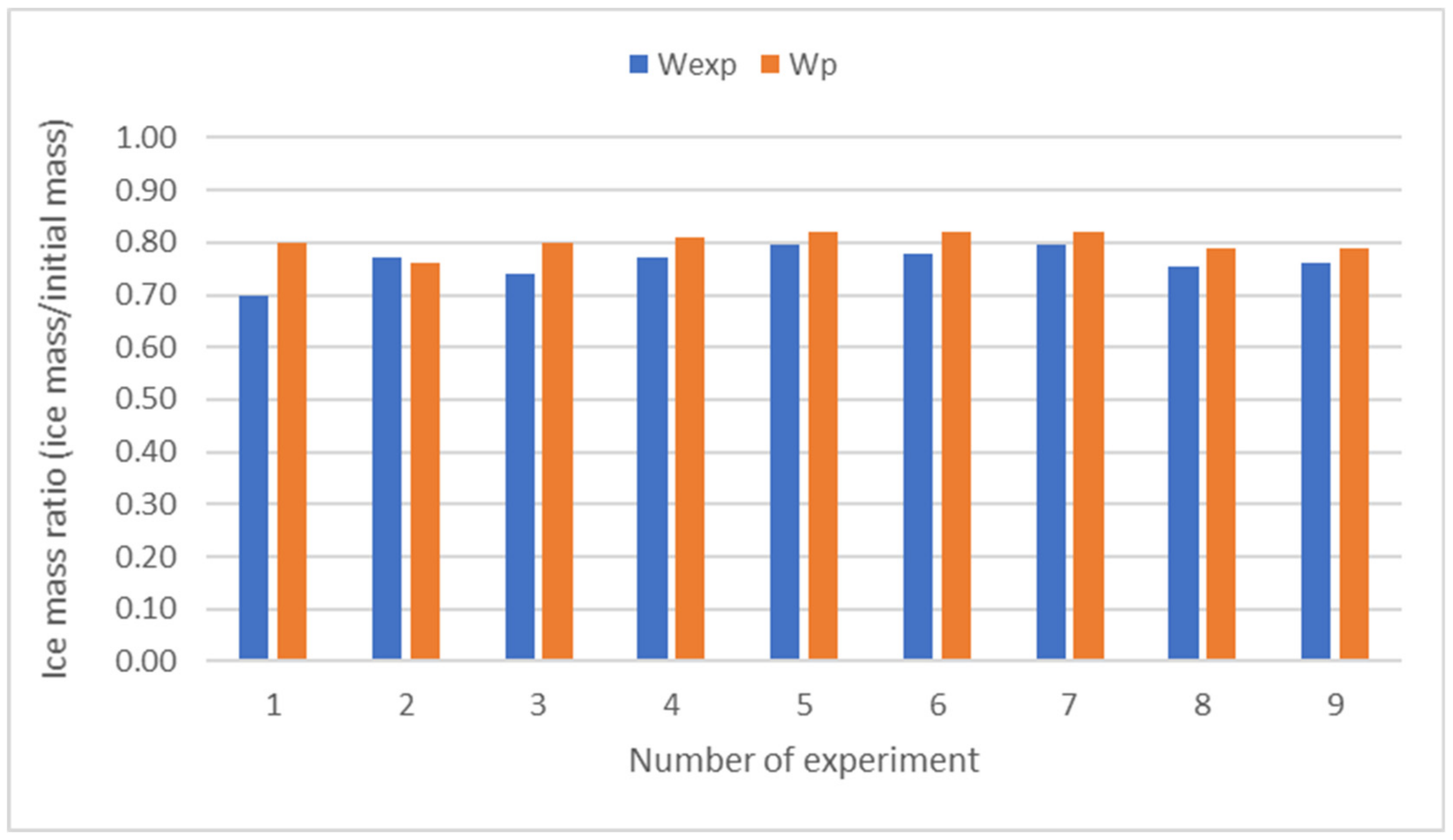
2.6. Validation of Results
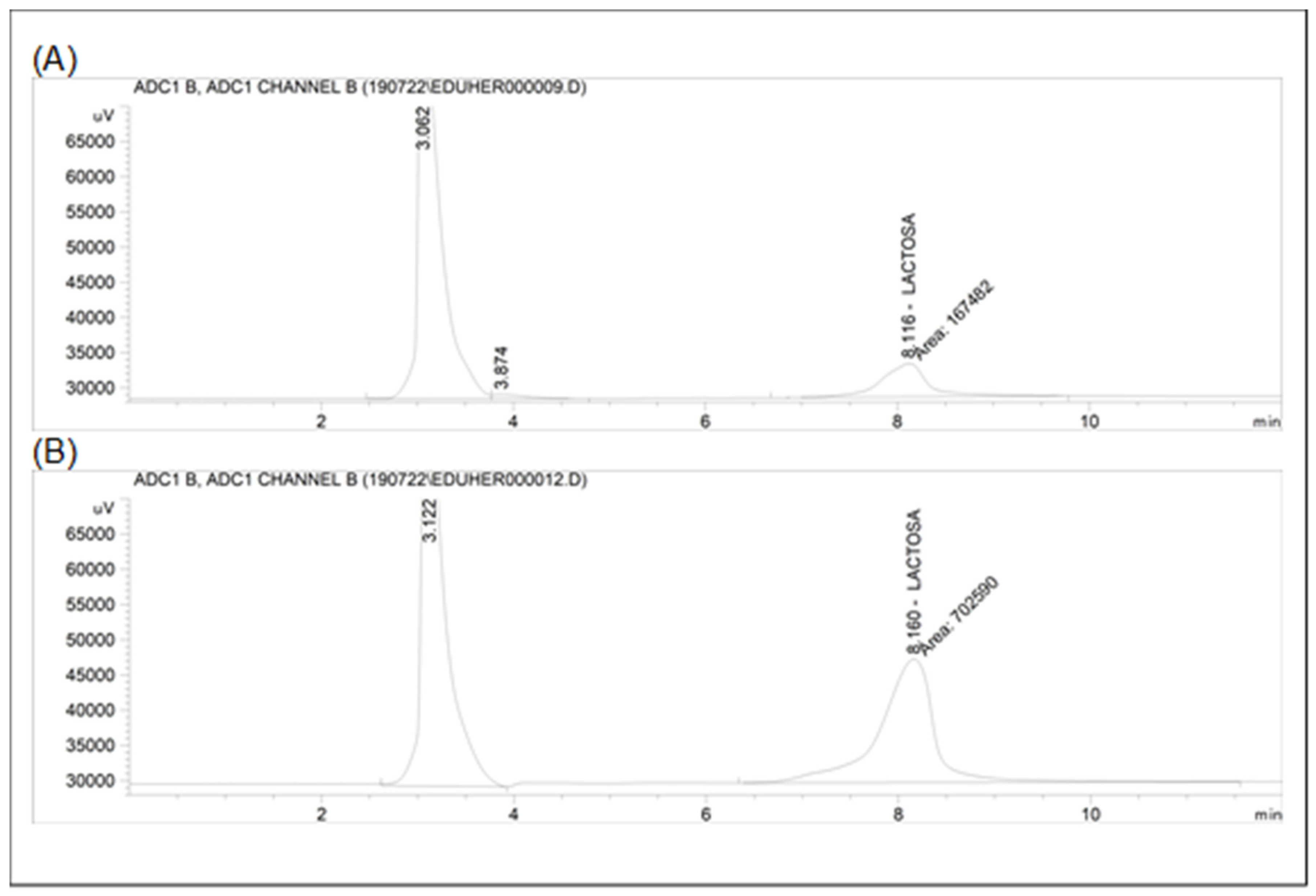
2.7. Lactose Concentration
2.8. Statistical Analysis
3. Results
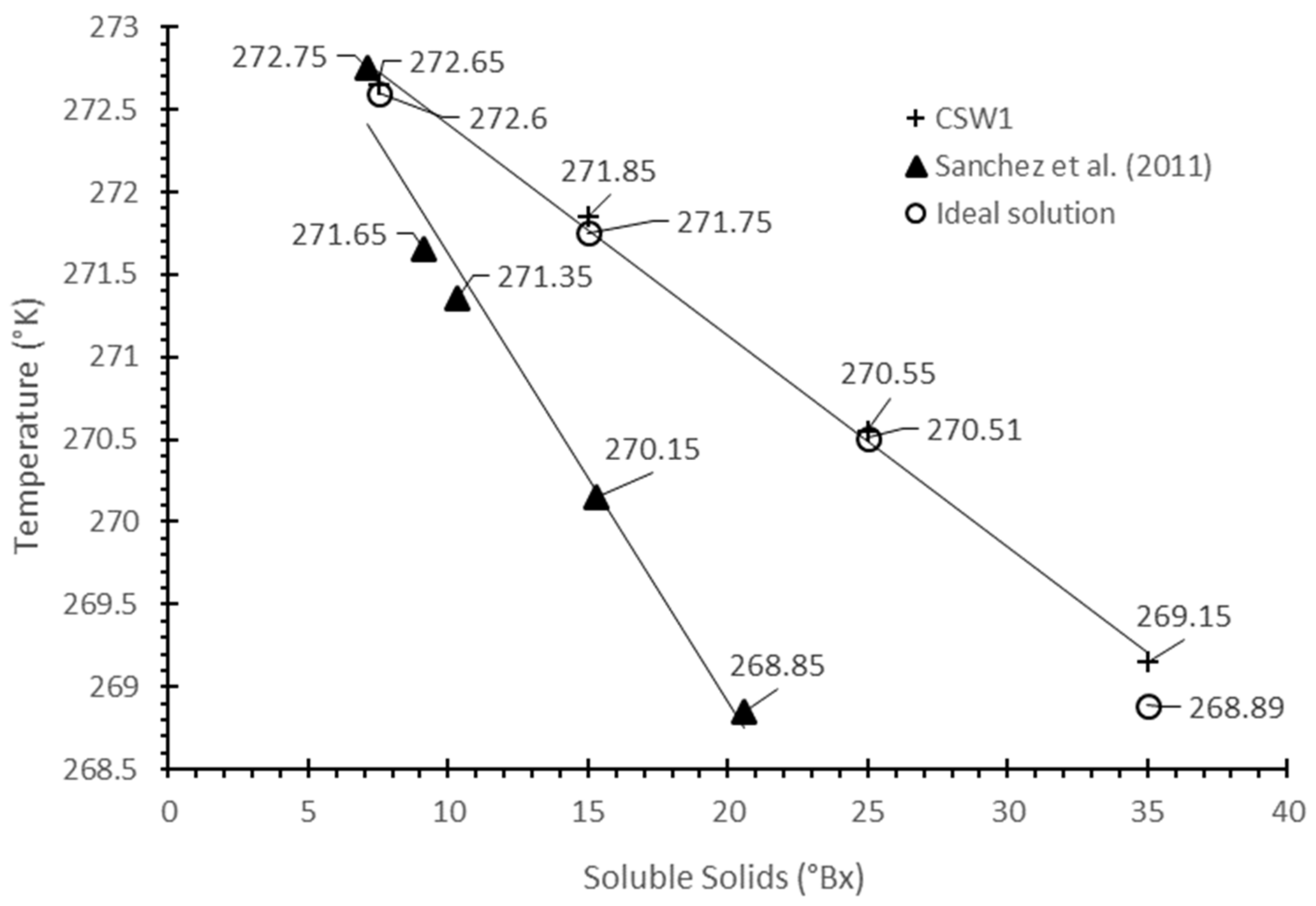
3.1. Freezing Point Depression
3.2. Solute Yield and Concentration Index
3.3. Vacuum-Assisted BFC Tests on Three Types of Whey
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belén, F.; Raventós, M.; Hernández, E. Management of cheese whey by film freeze concentration. Environ. Eng. Manag. J. 2018, 17, 1373–1383. [Google Scholar] [CrossRef]
- Aider, M.; de Halleux, D.; Akbache, A. Whey cryoconcentration and impact on its composition. J. Food Eng. 2007, 82, 92–102. [Google Scholar] [CrossRef]
- Aider, M.; de Halleux, D.; Melnikova, I. Skim milk whey cryoconcentration and impact on the composition of the concentrated and ice fractions. Food Bioproc. Tech. 2009, 2, 80–88. [Google Scholar] [CrossRef]
- Raventós, M.; Hernández, E.; Auleda, J.; Ibarz, A. Concentration of aqueous sugar solutions in multi-plate cryoconcentrator. J. Food Eng. 2007, 79, 577–585. [Google Scholar] [CrossRef]
- Aider, M.; de Halleux, D. Production of concentrated cherry and apricot juices by cryoconcentration technology. LWT-Food Sci. Technol. 2008, 41, 1768–1775. [Google Scholar] [CrossRef]
- Petzold, G.; Orellana, P.; Moreno, J.; Cerda, E.; Parra, P. Vacuum-assisted block freeze concentration applied to wine. Innov. Food Sci. Emerg. Technol. 2016, 36, 330–335. [Google Scholar] [CrossRef]
- Hsieh, H.C. Desalinating process. US Patent 2008, 7, 467–526. [Google Scholar]
- Petzold, G.; Niranjan, K.; Aguilera, J.M. Vacuum-assisted freeze concentration of sucrose solutions. J. Food Eng. 2013, 115, 357–361. [Google Scholar] [CrossRef]
- Pardo, J.M.; Sánchez, R. Block freeze concentration intensification by means of vacuum and microwave pulses. Eng. Compet. 2015, 17, 143–151. [Google Scholar]
- Moreno, F.L.; Raventós, M.; Hernández, E.; Ruiz, Y. Block freeze-concentration of coffee extract: Effect of freezing and thawing stages on solute recovery and bioactive compounds. J. Food Eng. 2014, 120, 158–166. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Petzold, G.; Torres, N.; Aguilera, M. Elaboration of orange juice concentrate by vacuum-assisted block freeze concentration. J. Food Process. Preserv. 2017, 2017, e13438. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Petzold, G.; Pierre, L.; Pensaben, J.M. Protection of polyphenols in blueberry juice by vacuum-assisted block freeze concentration. Food Chem. Toxicol. 2017, 109, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Russel, A.; Chen, X.; Robertson, L. Ice fouling on a subcooled metal surface examined by thermo-response and electrical conductivity. J. Food Eng. 2003, 59, 421–429. [Google Scholar] [CrossRef]
- Sung-Hee, P.; Jee-Yeon, K.; Geun-Pyo, H.; Hae-Soo, K.; Sang-Gi, M. Effect of ice recrystallization on freeze concentration of milk solutes in a lab-scale unit. Food Sci. Biotechnol. 2006, 15, 196–201. [Google Scholar]
- Aider, M.; de Halleux, D.; Melnikova, I. Gravitational and microwave-assisted thawing during milk whey cryoconcentration. J. Food Eng. 2008, 88, 373–380. [Google Scholar] [CrossRef]
- Aider, M.; de Halleux, M.; Melnikova, I. Skim acidic milk whey cryoconcentration and assessment of its functional properties: Impact of processing conditions. Innov. Food Sci. Emerg. Technol. 2009, 10, 334–341. [Google Scholar] [CrossRef]
- Habib, B.; Farid, M. Freeze concentration of milk and saline solutions in a liquid-solid fluidized bed. part I. experimental. Chem. Eng. Process. Process Intensif. 2007, 46, 1400–1411. [Google Scholar] [CrossRef]
- Chen, P.; Chen, X.D. A generalized correlation of solute inclusion in ice formed from aqueous solutions and food liquids on sub-cooled surface. Can. J. Chem. Eng. 2000, 78, 312–319. [Google Scholar] [CrossRef]
- Sánchez, J.; Hernández, E.; Auleda, J.M.; Raventós, M. Freeze concentration of whey in a falling-film based pilot plant: Process and characterization. J. Food Eng. 2011, 103, 147–155. [Google Scholar] [CrossRef]
- Mollea, C.; Marmo, L.; Bosco, F. Valorisation of cheese whey, a by-product from the dairy industry. In Food Industry, 1st ed.; Muzzalupo, I., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Zandona, E.; Blažić, M.; Režek, A. Whey utilization: Sustainable uses and environmental approach. Food Technol. Biotechnol. 2021, 59, 147–161. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yana, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Prestes, A.A.; Helm, C.V.; Esmerino, E.A.; Silva, R.; da Cruz, A.G.; Prudencio, E.S. Freeze concentration techniques as alternative methods to thermal processing in dairy manufacturing: A review. J. Food Sci. 2022, 87, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.L.; Quintanilla-Carvajal, M.X.; Sotelo, L.I.; Osorio, C.; Raventós, M.; Hernández, E.; Ruiz, Y. Volatile compounds, sensory quality and ice morphology in falling-film and block freeze concentration of coffee extract. J. Food Eng. 2015, 164, 64–71. [Google Scholar] [CrossRef]
- Khajehei, F.; Niakousari, M.; Eskandari, M.H.; Sarshar, M. Production of pomegranate juice concentrate by complete block cryoconcentration process. J. Food Process Eng. 2015, 38, 488–498. [Google Scholar] [CrossRef]
- Nakagawa, K.; Maebashi, S.; Maeda, K. Freeze-thawing as a path to concentrate aqueous solution. Sep. Purif. Technol. 2010, 73, 403–408. [Google Scholar] [CrossRef]
- Guerra-Valle, M.; Lillo-Perez, S.; Petzold, G.; Orellana-Palma, P. Effect of freeze crystallization on quality properties of two endemic Patagonian berries juices: Murta (Ugni molinae) and arrayan (Luma apiculata). Foods 2021, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Schuster-Wolff-Bühring, R.; Michael, R.; Hinrichs, J. A new liquid chromatography method for the simultaneous and sensitive quantification of lactose and lactulose in milk. Dairy Sci. Technol. 2011, 91, 27–37. [Google Scholar] [CrossRef]
- Raventós, M.; Hernández, E.; Auleda, J.M. Freeze concentration applications in fruit processing. In Advances in Fruit Processing Technologies, 1st ed.; Rodrigues, S., Narciso-Fernandes, F.A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 263–286. [Google Scholar]
- Bakshi, A.S.; Johnson, R.M. Calorimetric studies on whey freeze concentration. J. Food Sci. 1983, 48, 1279–1283. [Google Scholar] [CrossRef]
- Muñoz, I.D.B.; Rubio, A.; Blanco, M.; Raventós, M.; Hernández, E.; Prudêncio, E.S. Progressive freeze concentration of skimmed milk in an agitated vessel: Effect of the coolant temperature and stirring rate on process performance. Food Sci. Technol. Int. 2019, 25, 150–159. [Google Scholar] [CrossRef]
- Gulfo, R.; Auleda, J.M.; Moreno, F.L.; Ruiz, Y.; Hernández, E.; Raventós, M. Multi-plate freeze concentration: Recovery of solutes occluded in the ice and determination of thawing time. Food Sci. Technol. Int. 2013, 20, 405–419. [Google Scholar] [CrossRef]
- Yee, P.L.; Wakisaka, M.; Shirai, Y.; Hassan, M.A. Effects of single food components on freeze concentration by freezing and thawing technique. Japan J. Food Eng. 2003, 4, 77–82. [Google Scholar] [CrossRef]
- Pham, Q.T. Advances in food freezing/thawing/freeze concentration modelling and techniques. Japan J. Food Eng. 2008, 9, 21–32. [Google Scholar] [CrossRef]
- Petzold, G.; Orellana, P.; Moreno, J.; Cuevas, C. Process parameters of vacuum-assisted freeze concentration. Chem. Eng. Trans. 2017, 57, 1789–1794. [Google Scholar] [CrossRef]
- Lamkaddam, I.U.; Vega, E.; Colón, J.; Ponsá, S.; Llenas, L.; Mora, M. Progressive freeze concentration of cheese whey for protein and lactose recovery. Int. Dairy J. 2023, 139, 105572. [Google Scholar] [CrossRef]
- Korotkiy, I.A.; Korotkaya, E.V.; Neverov, E.N.; Plotnikov, I.B.; Efremov, D.A. Separatory freezing and cryoconcentration of milk and whey. IOP Conf. Ser. Earth Environ. Sci. 2021, 852, 012052. [Google Scholar] [CrossRef]
- Kawasaki, K.; Matsuda, A.; Kadota, H. Freeze concentration of equal molarity solutions with ultrasonic irradiation under constant freezing rate: Effect of solute. Chem. Eng. Res. Des. 2006, 84, 107–112. [Google Scholar] [CrossRef]
- Lewicki, P.P. Raoult’s law based food water sorption isotherm. J. Food Eng. 2000, 43, 31–40. [Google Scholar] [CrossRef]
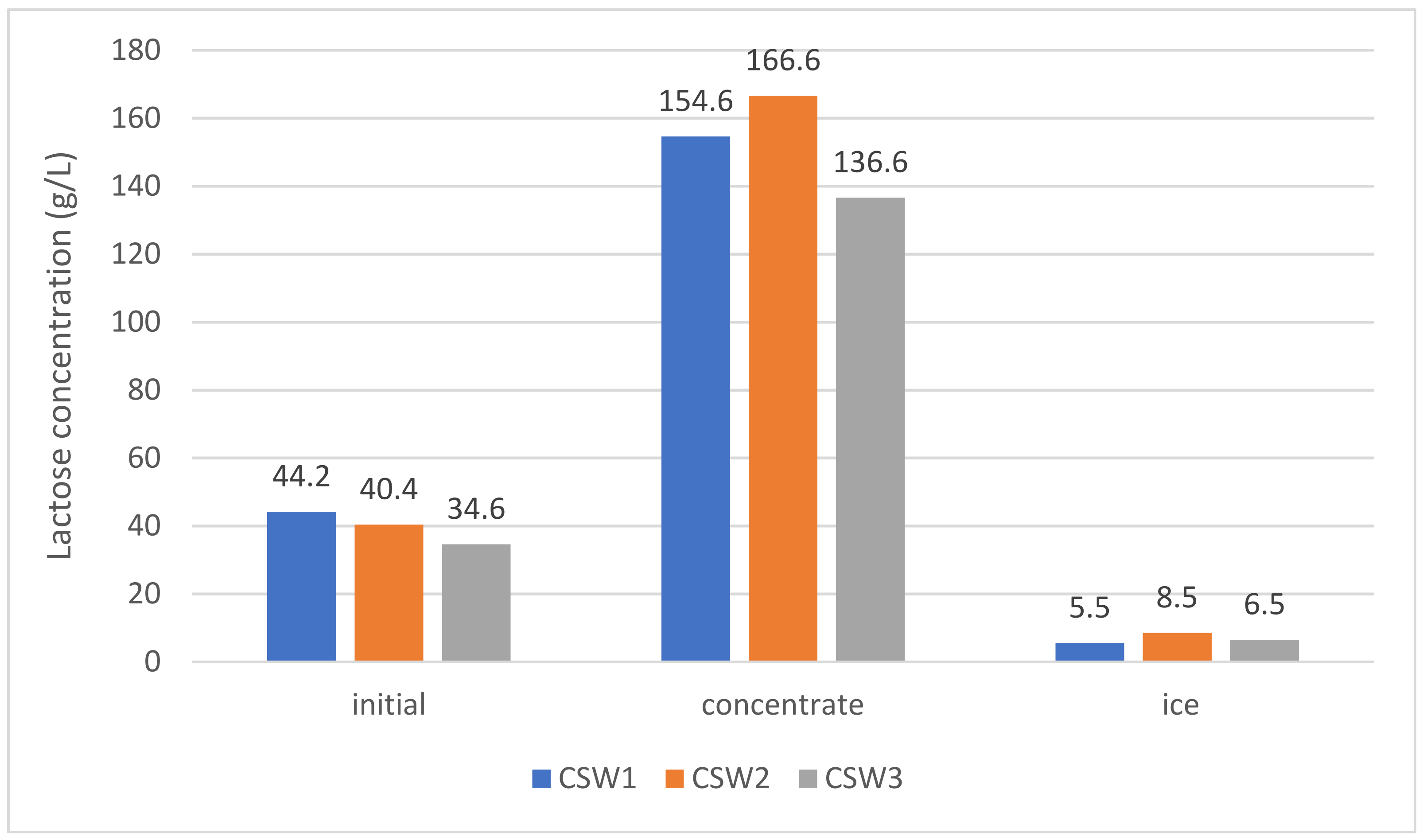
| C0 (°Bx) | t (min) | V (kPa) | |
|---|---|---|---|
| Minimum | 7.5 | 20 | 10 |
| Maximum | 19 | 60 | 70 |
| V (kPa) | C0 (°Bx) | t (min) | Y (%) | CI |
|---|---|---|---|---|
| 10 | 7.5 | 20 | 28.4 ± 4.4 b | 5.3 ± 0.4 a |
| 10 | 7.5 | 60 | 65.5 ± 3.8 a | 3.4 ± 0.2 b |
| 70 | 7.5 | 20 | 9.4 ± 4.8 cde | 4.5 ± 0.2 a |
| 70 | 7.5 | 60 | 36.3 ± 10 b | 3.2 ± 0.7 b |
| 10 | 19 | 20 | 7.4 ± 3.9 de | 2.6 ± 0.1 bc |
| 10 | 19 | 60 | 24.3 ± 2.0 bc | 1.7 ± 0.1 cd |
| 70 | 19 | 20 | 0.26 ± 0.1 e | 1.2 ± 0.3 d |
| 70 | 19 | 60 | 21.9 ± 1.0 bcd | 1.5 ± 0.2 cd |
| Y | CI | |||
|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | |
| Vacuum (V) | 39.08 | <0.000 * | 14.57 | 0.002 * |
| Concentration (C0) | 86.03 | <0.000 * | 197.47 | <0.000 * |
| Time (t) | 123.44 | <0.000 * | 32.34 | <0.000 * |
| V•C0 | 17.68 | 0.001 * | 0.53 | 0.479 |
| V•t | 0.34 | 0.566 | 7.11 | 0.017 * |
| C0•t | 7.57 | 0.014 * | 16.79 | 0.001 * |
| V•C0•t | 2.60 | 0.127 | 0.57 | 0.461 |
| Whey Type | C0 (°Bx) | CI | Y (%) |
|---|---|---|---|
| CSW1 | 6.6 | 3.5 ± 0.1 | 73 ± 1 |
| CSW2 | 5.7 | 3.8 ± 0.2 | 69 ± 1 |
| CSW3 | 6.4 | 3.8 ± 0.2 | 71 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, N.; Quinteros, G.; Blanco, M.; Samsuri, S.; Amran, N.A.; Orellana-Palma, P.; Schwinden, E.; Hernández, E. Vacuum-Assisted Block Freeze Concentration Studies in Cheese Whey and Its Potential in Lactose Recovery. Foods 2023, 12, 836. https://doi.org/10.3390/foods12040836
Gil N, Quinteros G, Blanco M, Samsuri S, Amran NA, Orellana-Palma P, Schwinden E, Hernández E. Vacuum-Assisted Block Freeze Concentration Studies in Cheese Whey and Its Potential in Lactose Recovery. Foods. 2023; 12(4):836. https://doi.org/10.3390/foods12040836
Chicago/Turabian StyleGil, Noelia, Gisela Quinteros, Monica Blanco, Shafirah Samsuri, Nurul Aini Amran, Patrico Orellana-Palma, Elane Schwinden, and Eduardo Hernández. 2023. "Vacuum-Assisted Block Freeze Concentration Studies in Cheese Whey and Its Potential in Lactose Recovery" Foods 12, no. 4: 836. https://doi.org/10.3390/foods12040836
APA StyleGil, N., Quinteros, G., Blanco, M., Samsuri, S., Amran, N. A., Orellana-Palma, P., Schwinden, E., & Hernández, E. (2023). Vacuum-Assisted Block Freeze Concentration Studies in Cheese Whey and Its Potential in Lactose Recovery. Foods, 12(4), 836. https://doi.org/10.3390/foods12040836









