Levels and Health Risk Assessment of Polycyclic Aromatic Hydrocarbons in Vegetable Oils and Frying Oils by Using the Margin of Exposure (MOE) and the Incremental Lifetime Cancer Risk (ILCR) Approach in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemicals and Reagents
2.3. Sample Analysis
2.4. Apparatus Conditions
2.5. Method Validation
2.6. Statistical Analysis
2.7. Consumption Data
2.8. Exposure Assessment
2.9. Risk Characterization
3. Results and Discussion
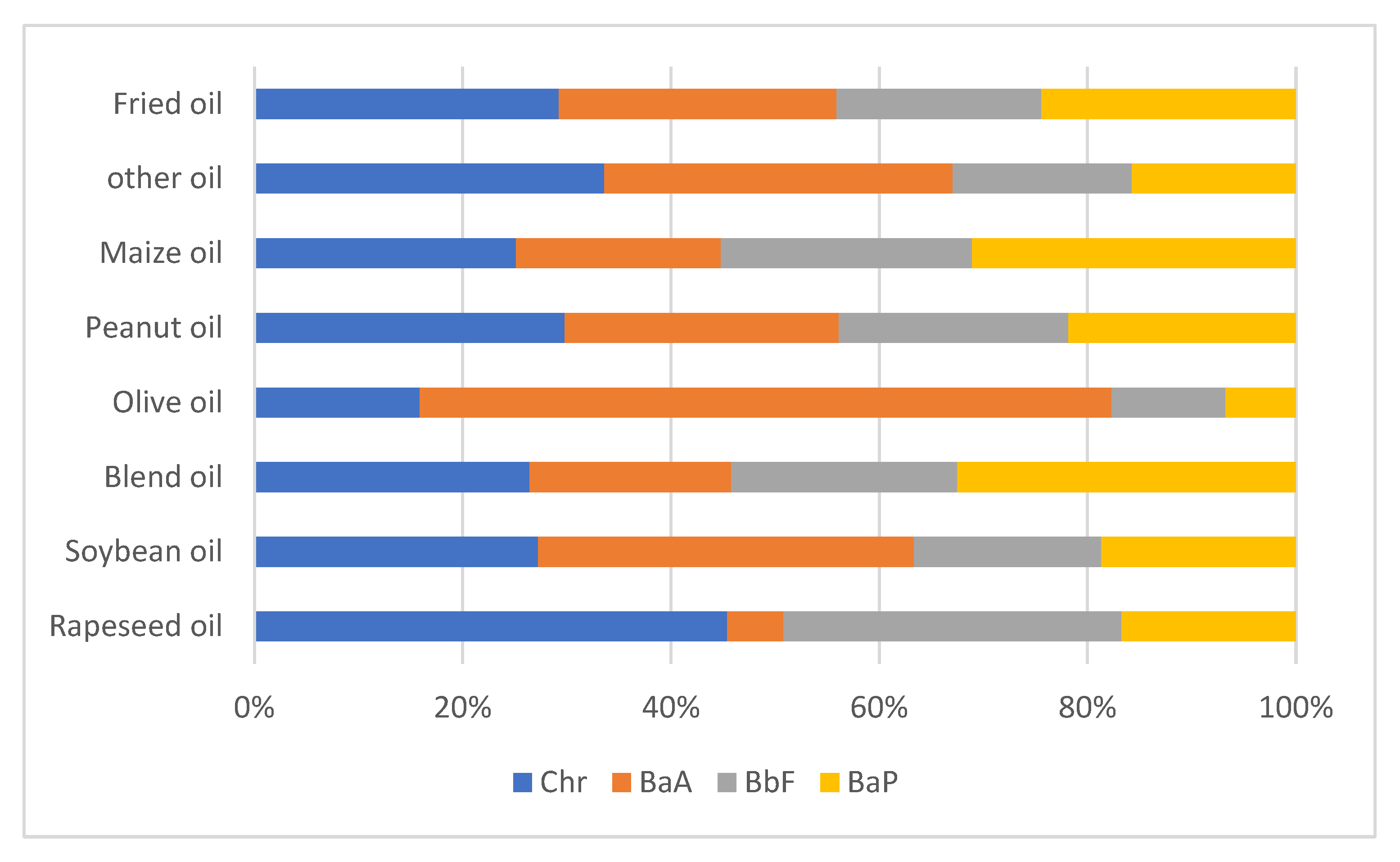
3.1. Levels of PAHs in Vegetable Oils
3.2. Levels of PAHs in Frying Oils (Vegetable Oils Used for Frying)
3.3. Estimated Daily Intake
3.4. Risk Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qiu, Y.Y.; Gong, Y.X.; Ni, H.G. Contribution of soil erosion to PAHs in surface water in china. Sci. Total Environ. 2019, 686, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Bai, W.; Nagae, M.; Takao, Y. Seasonal fluctuation of polycyclic aromatic hydrocarbons and aerosol genotoxicity in long-range transported air mass observed at the western end of Japan. Int. J. Environ. Res. 2020, 17, 1210. [Google Scholar] [CrossRef]
- Ihunwo, O.C.; Shahabinia, A.R.; Udo, K.S.; Bonnail, E.; Onyema, M.O.; Dibofori-Orji, A.N.; Mmom, P.C. Distribution of polycyclic aromatic hydrocarbons in Woji Creek, in the Niger Delta. Environ. Res. Commun. 2019, 1, 125001. [Google Scholar] [CrossRef]
- Walid, B.A.; Ali, A.; Takoua, M. Polycyclic aromatic hydrocarbons in mullet (Chelon auratus) from two lagoons of great ecological and economic importance in Tunisia: Levels, sources and human health risk implications. J. Sea Res. 2023, 192, 102325. [Google Scholar]
- Zainal, P.N.S.; Alang Ahmad, S.A.; Abdul Aziz, S.F.N.; Rosly, N.Z. Polycyclic Aromatic Hydrocarbons: Occurrence, Electroanalysis, Challenges, and Future Outlooks. Crit. Rev. Anal. Chem. 2022, 52, 878–896. [Google Scholar] [CrossRef]
- Cao, W.; Geng, S.; Zou, J.; Wang, Y.; Guo, Y.; Zhu, Y.; Dou, J. Post relocation of industrial sites for decades: Ascertain sources and human risk assessment of soil polycyclic aromatic hydrocarbons-sciencedirect. Ecotoxicol. Environ. Saf. 2020, 198, 110646. [Google Scholar] [CrossRef] [PubMed]
- Diekmann, A.; Giese, U.; Schaumann, I. Polycyclic aromatic hydrocarbons in consumer goods made from recycled rubber material: A review. Chemosphere 2019, 220, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, J.; Cheng, L.; Deng, Y.; Li, Y. The associations between prenatal exposure to polycyclic aromatic hydrocarbon metabolites, umbilical cord blood mitochondrial DNA copy number, and children’s neurobehavioral development. Environ. Pollut. 2020, 265, 114594. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, L.; Yu, Y.; Tan, L. Distribution, source, risk and phytoremediation of polycyclic aromatic hydrocarbons (PAHs) in typical urban landscape waters recharged by reclaimed water. J. Environ. Manag. 2023, 330, 117214. [Google Scholar] [CrossRef]
- Miao, Y.; Kong, X.; Li, C. Distribution, sources, and toxicity assessment of polycyclic aromatic hydrocarbons in surface soils of a heavy industrial city, Liuzhou, China. Environ. Monit. Assess. 2018, 190, 164. [Google Scholar] [CrossRef]
- Peng, Y.; He, S.-Y.; Wang, F.-H.; Zheng, H.-B.; Meng, Z. Determination of polycyclic aromatic hydrocarbons in edible oil by magnetic solid phase extraction based on a mesoporous molybdenum disulfide/graphite prior to gas chromatography-mass spectrometry. Microchem. J. 2022, 183, 108146. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Bansal, V.; Kim, K.H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Purcaro, G.; Navas, J.A.; Guardiola, F.; Conte, L.S.; Moret, S. Polycyclic aromatic hydrocarbons in frying oils and snacks. J. Food Prot. 2006, 69, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Das, D.N.; Bhutia, S.K. Inevitable dietary exposure of benzo[a]pyrene: Carcinogenic risk assessment an emerging issues and concerns. Curr. Opin. Food Sci. 2018, 24, 16–25. [Google Scholar] [CrossRef]
- European Food Safety Agency. Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on polycyclic aromatic. Hydrocarbons in food. EFSA J. 2008, 724, 1–114. [Google Scholar]
- IARC. Monographs on the evaluation of carcinogenic risks to humans. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 93, 9–38. [Google Scholar]
- Famiyeh, L.; Chen, K.; Xu, J.; Sun, Y.; He, J. A review on analysis methods, source identification, and cancer risk evaluation of atmospheric polycyclic aromatic hydrocarbons. Sci. Total Environ. 2021, 789, 147741. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Zhang, H.; Niu, Y.; Fu, Y.; Nie, J.; Yang, A.; Zhao, J.; Yang, J. Mediation effect of AhR expression between polycyclic aromatic hydrocarbons exposure and oxidative DNA damage among Chinese occupational workers. Environ. Pollut. 2018, 243, 972–977. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, Y.; Cui, X. Urinary polycyclic aromatic hydrocarbon metabolites and adult asthma: A case-control study. Sci. Rep. 2018, 8, 7658. [Google Scholar] [CrossRef]
- EUR-Lex. EC/835/2011. COMMISSION REGULATION (EU) No 835/2011 of 19 August 2011. Amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, L215, 4–8. [Google Scholar]
- GB2762-2012; National Food Safety Standard of China. Standards for Limits of Contaminants in Food. Standards Press of China: Beijing, China, 2012. (In Chinese)
- Mojtaba, Y.; Ghazal, S.; Nasim, K.; Vahid, G.M.; Yadolah, F.; Hedayat, H. Polycyclic aromatic hydrocarbons (PAHs) content of edible vegetable oils in Iran: A risk assessment study. Food Chem. Toxicol. 2018, 118, 480–489. [Google Scholar]
- An, K.J.; Liu, Y.L.; Liu, H.L. Relationship between total polar components and polycyclic aromatic hydrocarbons in fried edible oil. Food Addit. Contam. Part A 2017, 34, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.H.; Chen, S.; Chen, C.J.; Huang, C.W.; Chen, B.H. Evaluation of analysis of polycyclic aromatic hydrocarbons by the quechers method and gas chromatography–mass spectrometry and their formation in poultry meat as affected by marinating and frying. J. Agric. Food Chem. 2012, 60, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Y.; Sun, L.J.; Ma, L.G. Investigation of benzo(a) pyrene contamination in edible vegetable oil sold in Henan Province from 2017 to 2019. J. Hyg. Res. 2020, 49, 759–794. [Google Scholar]
- Wu, R.-N. Contamination characteristics and health risk assessment of polycyclic aromatic hydrocarbons in edible vegetable oil in Beijing. Chin. J. Oil Crop Sci. 2016, 38, 843–849. [Google Scholar]
- Guan, R.; Chen, Y.; Yong-Bo, L.I.; Zuo, B.; Shen, N.M. Investigation on benzo(a)pyrene pollution status of edible vegetable oil in Xi’an from 2011 to 2013. Chin. J. Health Lab. Technol. 2014, 24, 2413–2414. [Google Scholar]
- Yang, X.Q.; Cao, X.L.; Liu, S.H. Investigation on benzo(a) pyrene residue in edible vegetable oil of jinan during 2011–2013. Chin. J. Health Lab. Technol. 2014, 24, 1646–1647. [Google Scholar]
- Galeone, C.; Talamini, R.; Levi, F.; Pelucchi, C.; Negri, E.; Giacosa, A.; Montella, M.; Franceschi, S.; La Vecchia, C. Fried foods, olive oil and colorectal cancer. Ann. Oncol. 2007, 18, 36–39. [Google Scholar] [CrossRef]
- Jánská, M.; Tomaniová, M.; Hajslová, J.; Kocourek, V. Optimization of the procedure for the determination of polycyclic aromatic hydrocarbons and their derivatives in fish tissue: Estimation of measurements uncertainty. Food Addit. Contam. 2006, 23, 309–325. [Google Scholar] [CrossRef]
- Dadar, M.; Adel, M.; Nasrollahzadeh Saravi, H.; Fakhri, Y. Trace element concentration and its risk assessment in common kilka (Clupeonella cultriventris caspia Bordin, 1904) from southern basin of Caspian Sea. Toxin Rev. 2017, 36, 222–227. [Google Scholar] [CrossRef]
- USEPA. Guidelines for exposure assessment. Fed. Regist. 1992, 57, 22888–22938. [Google Scholar]
- Li, G.; Wu, S.; Wang, L.; Akoh, C.C. Concentration, dietary exposure and health risk estimation of polycyclic aromatic hydrocarbons (PAHs) in youtiao, a Chinese traditional fried food. Food Control 2016, 59, 328–336. [Google Scholar] [CrossRef]
- Xu, L.R.; Wu, G.C.; Zhang, Y.R.; Wang, Q.J.; Zhao, C.W.; Zhang, H.; Jin, Q.Z.; Wang, X.G. Evaluation of glycerol core aldehydes formation in edible oils under restaurant deep frying. Food Res. Int. 2020, 137, 109696. [Google Scholar] [CrossRef]
- Tfouni, S.; Padovani, G.R.; Reis, R.M.; Furlani, R.; Camargo, M. Incidence of polycyclic aromatic hydrocarbons in vegetable oil blends. Food Control 2014, 46, 539–543. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Zhu, Y.; Shi, L.K.; Guo, Y.; Wei, L.; Zhang, H.; Wang, X.G.; Jin, Q.Z. Physicochemical properties and health risk assessment of polycyclic aromatic hydrocarbons of flavor rapeseed oils in China. J. Sci. Food Agric. 2020, 100, 3351–3359. [Google Scholar] [CrossRef] [PubMed]
- Alomirah, H.; Al-Zenki, S.; Al-Hooti, S.; Zaghloul, S.; Sawaya, W.; Ahmed, N.; Kannan, K. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control 2020, 22, 2028–2035. [Google Scholar] [CrossRef]
- Drabova, L.; Tomaniova, M.; Kalachova, K.; Kocourek, V.; Hajslova, J.; Pulkrabova, J. Application of solid phase extraction and two-dimensional gas chromatography coupled with time-of-flight mass spectrometry for fast analysis of polycyclic aromatic hydrocarbons in vegetable oils. Food Control 2013, 33, 489–497. [Google Scholar] [CrossRef]
- Lee, J.G.; Suh, J.H.; Yoon, H.J. Occurrence and risk characterization of polycyclic aromatic hydrocarbons of edible oils by the margin of exposure (MOE) approach. Appl. Biol. Chem. 2019, 62, 51. [Google Scholar] [CrossRef]
- Jiang, D.; Xin, C.; Li, W.; Chen, J.; Li, F.; Chu, Z.; Shao, L. Quantitative analysis and health risk assessment of polycyclic aromatic hydrocarbons in edible vegetable oils marketed in Shandong of China. Food Chem. Toxicol. 2015, 83, 61–67. [Google Scholar] [CrossRef]
- Wang, Y.C.; Qiao, M.; Liu, Y.X.; Arp, H.P.; Zhu, Y.G. Comparison of polycyclic aromatic hydrocarbon uptake pathways and risk assessment of vegetables from waste-water irrigated areas in northern China. J. Environ. Monit. 2011, 13, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Xia, B.; Dai, X. Residues of persistent organic pollutants in frequently-consumed vegetables and assessment of human health risk based on consumption of vegetables in Huizhou, South China. Chemosphere 2013, 93, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, R.; Bu, Q.; Cai, Q.; Cui, J.Z. Comparison of PAH content, potential risk in vegetation, and bare soil near Daqing oil well and evaluating the effects of soil properties on PAHs. Environ. Sci. Pollut. Res. 2019, 26, 25071–25083. [Google Scholar] [CrossRef] [PubMed]
- Al Nasir, F.; Batarseh, M.I. Agricultural reuse of reclaimed water and uptake of organic compounds: Pilot study at mutah university wastewater treatment plant, Jordan. Chemosphere 2008, 72, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Suh, J.H.; Yoon, H.J. The effects of extracting procedures on occurrence of polycyclic aromatic hydrocarbons in edible oils. Food Sci. Biotechnol. 2020, 29, 1181–1186. [Google Scholar] [CrossRef]
- Kiralan, S.S.; Tekin, A. Reducing polycyclic aromatic hydrocarbons (PAHs) in olive pomace oil using short-path molecular distillation. Food Addit. Contam. Part A 2020, 37, 401–407. [Google Scholar] [CrossRef]
- Hao, X.W.; Yin, Y.; Feng, S.J.; Du, X.; Yu, J.Y.; Yao, Z.L. Characteristics of polycyclic aromatic hydrocarbons in food oils in Beijing catering services. Environ. Sci. Pollut. Res. 2016, 23, 24932–24942. [Google Scholar] [CrossRef]
- Jung, M.Y.; Bock, J.Y.; Baik, S.O.; Lee, J.H.; Lee, T.K. Effects of roasting on pyrazine contents and oxidative stability of red pepper seed oil prior to its extraction. J. Agric. Food Chem. 1999, 47, 1700–1704. [Google Scholar] [CrossRef]
- Yang, C.Y.; Mandal, P.K.; Han, K.H.; Fukushima, M.; Choi, K.; Kim, C.J. Capsaicin and tocopherol in red pepper seed oil enhances the thermal oxidative stability during frying. J. Food Sci. Technol. 2010, 47, 162–165. [Google Scholar] [CrossRef]
- Saito, E.; Tanaka, N.; Miyazaki, A.; Tsuzaki, M. Concentration and particle size distribution of polycyclic aromatic hydrocarbons formed by thermal cooking. Food Chem. 2014, 153, 285–291. [Google Scholar] [CrossRef]
- Antoniolli, P.R.; Vicente, E.; Tfouni, S.A.V.; Rojo Camargo, M.C. Polycyclic aromatic hydrocarbons in Brazilian commercial soybean oils and dietary exposure. Food Addit. Contam. Part B Surveill. 2011, 2, 152–159. [Google Scholar]
- Kang, B.; Lee, B.-M.; Shin, H.-S. Determination of Polycyclic Aromatic Hydrocarbon (PAH) Content and Risk Assessment from Edible Oils in Korea. J. Toxicol. Environ. Health Part A Curr. Issues 2014, 77, 1359–1371. [Google Scholar] [CrossRef]
- Barzegar, G.; Rezaei Kalantary, R.; Bashiry, M.; Jaafarzadeh, N.; Ghanbari, F.; Shakerinejad, G.; Khatebasreh, M.; Sabaghan, M. Measurement of polycyclic aromatic hydrocarbons in edible oils and potential health risk to consumers using Monte Carlo simulation, southwest Iran. Environ. Sci. Pollut. Res. Int. 2023, 30, 5126–5136. [Google Scholar] [CrossRef] [PubMed]
- Shariatifar, N.; Dadgar, M.; Fakhri, Y.; Shahsavari, S.; Moazzen, M.; Ahmadloo, M.; Kiani, A.; Aeenehvand, S.; Nazmara, S.; Khanegah, A.M. Levels of polycyclic aromatic hydrocarbons in milk and milk powder samples and their likely risk assessment in Iranian population. J. Food Compos. Anal. 2020, 85, 103331. [Google Scholar] [CrossRef]

| PAHs | Time Window (min) | λ Excitation (nm) | λ Emission (nm) |
|---|---|---|---|
| Nap, Ace, Flo | 0 | 270 | 324 |
| Phe, Ant | 11.8 | 248 | 375 |
| Flt | 14.0 | 280 | 462 |
| Pyr BaA Chr | 14.9 | 270 | 446 |
| BbF | 19.0 | 256 | 446 |
| BkF BaP DBahA BghiP | 20.9 | 292 | 410 |
| IcdP | 24.7 | 290 | 355 |
| Age | No. | Rapeseed Oil | Soybean Oil | Blend Oil | Olive Oil | Peanut Oil | Maize Oil | Other Oil | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | P95 | Mean | P95 | Mean | P95 | Mean | P95 | Mean | P95 | Mean | P95 | Mean | P95 | Mean | P95 | ||
| 2–6 (male) | 75 | 19.15 | 57.83 | 2.94 | 31.80 | 0.00 | 0.00 | 0.00 | 0.00 | 2.70 | 25.20 | 1.81 | 14.46 | 4.99 | 32.28 | 31.60 | 65.64 |
| 2–6 (female) | 65 | 14.12 | 59.91 | 3.29 | 29.20 | 0.11 | 0.00 | 0.00 | 0.00 | 6.72 | 40.76 | 1.83 | 4.00 | 4.43 | 28.14 | 30.49 | 75.88 |
| 7–12 (male) | 479 | 14.81 | 60.22 | 3.19 | 29.27 | 0.12 | 0.00 | 0.00 | 0.00 | 5.04 | 38.16 | 0.29 | 0.00 | 9.22 | 43.12 | 32.66 | 71.57 |
| 7–12 (female) | 472 | 14.89 | 60.95 | 3.83 | 33.82 | 0.11 | 0.00 | 0.00 | 0.00 | 3.70 | 31.45 | 1.04 | 0.00 | 8.64 | 39.78 | 32.22 | 70.05 |
| 13–17 (male) | 224 | 16.75 | 64.75 | 4.48 | 35.85 | 0.00 | 0.00 | 0.00 | 0.00 | 3.34 | 28.92 | 0.61 | 0.00 | 11.74 | 59.17 | 36.92 | 81.76 |
| 13–17 (female) | 219 | 16.70 | 61.77 | 3.40 | 29.26 | 0.00 | 0.00 | 0.00 | 0.00 | 2.57 | 24.49 | 1.40 | 0.00 | 8.91 | 44.33 | 32.99 | 69.01 |
| >18 (male) | 5760 | 14.24 | 66.80 | 4.48 | 36.83 | 0.27 | 0.00 | 0.22 | 0.00 | 6.80 | 47.51 | 1.59 | 0.00 | 10.46 | 56.87 | 38.06 | 82.86 |
| >18 (female) | 6607 | 13.95 | 66.62 | 4.46 | 36.33 | 0.28 | 0.00 | 0.21 | 0.00 | 6.91 | 47.58 | 1.64 | 0.00 | 10.54 | 56.44 | 38.00 | 82.50 |
| Gender | Preschoolers (2–6) | School-Agers (7–12) | Youths (13–17) | Adults (>18) |
|---|---|---|---|---|
| female | 23.02 ± 6.23 | 32.52 ± 10.49 | 50.40 ± 13.21 | 60.88 ± 11.12 |
| male | 22.06 ± 4.35 | 31.52 ± 9.50 | 50.33 ± 12.15 | 61.64 ± 11.17 |
| Parameter | BaP | Chr | BaA | BbF | BkF | DBahA | BghiP | IcdP | Pyr | Flt | Ant | Phe | Fluo | Ace | Nap | PAH4 | PAH8 | PAH15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| vegetable oil | Incidence > LOD(%) | 52 | 60.8 | 46.6 | 53.4 | 41.9 | 34.5 | 45.3 | 22.3 | 83.1 | 70.9 | 59.5 | 92.6 | 76.4 | 50 | 87.2 | / | / | / |
| Mean | 2.16 | 3.37 | 2.63 | 2.34 | 1.18 | 0.91 | 1.36 | 0.7 | 9.43 | 16.78 | 4.62 | 31.84 | 13.25 | 20.18 | 61.08 | 10.49 | 14.63 | 171.81 | |
| Media | 0.4 | 0.98 | 0.15 | 0.46 | 0.15 | 0.15 | 0.15 | 0.15 | 4.96 | 2.63 | 0.98 | 14.05 | 6.34 | 0.35 | 20.45 | 3.94 | 6.895 | 122.67 | |
| P90 | 6.18 | 7.6 | 5.68 | 5.14 | 3.35 | 2.8 | 3.84 | 1.65 | 25.3 | 40.52 | 10.05 | 73.61 | 23.78 | 50.6 | 167.5 | 28.54 | 37.47 | 347.42 | |
| MIN | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.6 | 1.2 | 2.25 | |
| MAX | 25.5 | 45.1 | 60.4 | 52.6 | 15.4 | 9.47 | 22.8 | 10.5 | 104 | 394 | 121 | 488 | 190 | 293 | 1072 | 123.5 | 134.75 | 1670.9 | |
| frying oil | Incidence > LOD(%) | 64.6 | 70.8 | 64.6 | 52.1 | 58.3 | 50 | 47.9 | 39.6 | 68.8 | 85.4 | 75 | 93.8 | 89.6 | 68.8 | 91.7 | / | / | / |
| Mean | 3.59 | 4.3 | 3.92 | 2.9 | 1.68 | 0.86 | 1.6 | 0.88 | 62.32 | 21.77 | 7.18 | 42.52 | 22.37 | 11.74 | 122.45 | 14.72 | 19.74 | 310.1 | |
| Media | 0.5 | 1.2 | 0.8 | 0.61 | 0.48 | 0.22 | 0.5 | 0.25 | 1.82 | 3.19 | 3.2 | 18.3 | 9.2 | 3.5 | 14 | 5.01 | 8.43 | 121.74 | |
| P90 | 8.37 | 14.26 | 7.96 | 6.18 | 4.35 | 1.91 | 3.68 | 2.13 | 33.43 | 34.85 | 17.34 | 66.38 | 43.78 | 17.86 | 443.7 | 33.39 | 45.08 | 652.71 | |
| MIN | 0.05 | 0.1 | 0.1 | 0.1 | 0.05 | 0.1 | 0.1 | 0.1 | 0.15 | 0.15 | 0.1 | 0.25 | 0.05 | 0.15 | 0.15 | 0.4 | 0.7 | 3.75 | |
| MAX | 61 | 42.2 | 74.4 | 32.1 | 12.2 | 6.89 | 11.4 | 6 | 1299 | 336 | 79.6 | 565 | 267 | 121 | 716 | 143 | 152.21 | 3285.06 |
| Oil Tyle | No. Samples | BaP | PAH4 | PAH8 | PAH15 | TEQBap | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | ||
| Rapeseed oil | 20 | 2.40 | <RL-25.5 | 14.32 | <RL-118.85 | 17.98 | <RL-134.7 | 141.54 | <RL-607.45 | 4.01 | 0.37–33.59 |
| Soybean oil | 28 | 1.55 | <RL-10.3 | 8.28 | <RL-47.46 | 11.97 | <RL-66.11 | 103.82 | 7.99–360.42 | 3.38 | 0.37–21.2 |
| Blend oil | 23 | 1.94 | <RL-21.1 | 5.98 | <RL-30.2 | 8.80 | <RL-38.4 | 213.82 | <RL-1314.05 | 3.41 | 0.37–22.85 |
| Olive oil | 7 | 0.39 | <RL-1.1 | 5.83 | <RL-32.37 | 9.16 | <RL-34.91 | 154.49 | 24.67–287.35 | 2.01 | 00.47–4.18 |
| Peanut oil | 21 | 3.31 | <RL-17.1 | 15.18 | <RL-88.32 | 20.41 | <RL-103.36 | 278.72 | 24.96–1022.01 | 5.43 | 0.39–20.63 |
| Maize oil | 20 | 2.21 | <RL-11.9 | 7.12 | <RL-27.88 | 11.31 | 1.44–40.02 | 143.00 | 9.95–762.25 | 4.11 | 0.38–14.46 |
| Other oil | 20 | 2.36 | <RL-22.1 | 15.01 | 1.44–123.5 | 20.91 | 2.41–134.75 | 171.59 | 5.88–1670.9 | 4.58 | 0.38–32.05 |
| Frying oil | 48 | 3.37 | <RL-24.4 | 10.9 | 0.3–129.50 | 18.30 | 0.70–152.21 | 246.81 | 3.75–2160.21 | 4.53 | 0.37–29.64 |
| Age | BaP | PAH2 | PAH4 | PAH8 | PAH15 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | P95 | Mean | P95 | Mean | P95 | Mean | P95 | Mean | P95 | |
| 2–6 (male) | 0.244 | 0.539 | 0.249 | 0.549 | 0.307 | 0.669 | 0.407 | 0.867 | 0.426 | 0.907 |
| 2–6 (female) | 0.236 | 0.631 | 0.240 | 0.647 | 0.296 | 0.795 | 0.391 | 1.041 | 0.411 | 1.090 |
| 7–12 (male) | 0.217 | 0.496 | 0.222 | 0.503 | 0.277 | 0.625 | 0.366 | 0.816 | 0.384 | 0.854 |
| 7–12 (female) | 0.202 | 0.443 | 0.207 | 0.454 | 0.361 | 0.787 | 0.345 | 0.751 | 0.361 | 0.787 |
| 13–17 (male) | 0.124 | 0.295 | 0.127 | 0.301 | 0.159 | 0.385 | 0.212 | 0.509 | 0.222 | 0.540 |
| 13–17 (female) | 0.110 | 0.249 | 0.113 | 0.256 | 0.141 | 0.308 | 0.188 | 0.401 | 0.197 | 0.425 |
| >18 (male) | 1.135 | 2.544 | 1.159 | 2.603 | 1.445 | 3.249 | 1.931 | 4.247 | 2.026 | 4.466 |
| >18 (female) | 1.149 | 2.575 | 1.172 | 2.630 | 1.463 | 3.278 | 1.954 | 4.294 | 2.051 | 4.505 |
| Age | BaP | PAH2 | PAH4 | PAH8 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | P95 | Mean | P95 | Mean | P95 | Mean | P95 | |
| 2–6 (male) | 287,331 | 129,796 | 682,822 | 309,666 | 1,106,062 | 507,993 | 1,202,838 | 565,324 |
| 2–6 (female) | 297,110 | 110,945 | 707,412 | 262,873 | 1,147,686 | 427,482 | 1,251,968 | 470,923 |
| 7–12 (male) | 322,775 | 141,188 | 767,165 | 338,271 | 1,228,542 | 544,341 | 1,337,984 | 600,647 |
| 7–12 (female) | 345,760 | 158,029 | 821,743 | 374,764 | 1,317,361 | 600,400 | 1,422,205 | 652,464 |
| 13–17 (male) | 565,447 | 237,560 | 1,343,280 | 564,872 | 2,142,262 | 882,870 | 2,308,208 | 962,884 |
| 13–17 (female) | 633,619 | 280,790 | 1,504,933 | 665,110 | 2,413,834 | 1,105,143 | 2,604,790 | 1,220,536 |
| >18 (male) | 61,658 | 27,516 | 146,729 | 65,305 | 235,263 | 104,649 | 253,796 | 115,378 |
| >18 (female) | 60,922 | 27,190 | 144,991 | 64,646 | 232,470 | 103,737 | 250,733 | 114,122 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Wu, P.; Zhou, P.; Luo, P. Levels and Health Risk Assessment of Polycyclic Aromatic Hydrocarbons in Vegetable Oils and Frying Oils by Using the Margin of Exposure (MOE) and the Incremental Lifetime Cancer Risk (ILCR) Approach in China. Foods 2023, 12, 811. https://doi.org/10.3390/foods12040811
Liu Q, Wu P, Zhou P, Luo P. Levels and Health Risk Assessment of Polycyclic Aromatic Hydrocarbons in Vegetable Oils and Frying Oils by Using the Margin of Exposure (MOE) and the Incremental Lifetime Cancer Risk (ILCR) Approach in China. Foods. 2023; 12(4):811. https://doi.org/10.3390/foods12040811
Chicago/Turabian StyleLiu, Qing, Pinggu Wu, Pingping Zhou, and Pengjie Luo. 2023. "Levels and Health Risk Assessment of Polycyclic Aromatic Hydrocarbons in Vegetable Oils and Frying Oils by Using the Margin of Exposure (MOE) and the Incremental Lifetime Cancer Risk (ILCR) Approach in China" Foods 12, no. 4: 811. https://doi.org/10.3390/foods12040811
APA StyleLiu, Q., Wu, P., Zhou, P., & Luo, P. (2023). Levels and Health Risk Assessment of Polycyclic Aromatic Hydrocarbons in Vegetable Oils and Frying Oils by Using the Margin of Exposure (MOE) and the Incremental Lifetime Cancer Risk (ILCR) Approach in China. Foods, 12(4), 811. https://doi.org/10.3390/foods12040811





