Effects of Different Drying Methods on the Selenium Bioaccessibility and Antioxidant Activity of Cardamine violifolia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Drying Methods
2.2.1. Far-Infrared Drying (FIRD)
2.2.2. Vacuum Drying (VD)
2.2.3. Microwave Vacuum Drying (MVD)
2.2.4. Hot Air Drying (HD)
2.2.5. Freeze Drying (FD)
2.3. Determination of the Se Content
2.4. Determination of Se Species
2.5. In Vitro Gastrointestinal Simulated Digestion Test
2.6. Scanning Electron Microscopy (SEM)
2.7. Color Measurement
2.8. Extraction of Free Phenolics and Flavonoids from CVLs
2.9. Determination of the Total Polyphenol Content (TPC) and Total Flavonoid Content (TFC)
2.10. Antioxidant Activities
2.10.1. DPPH Radical Scavenging Capacity Assay
2.10.2. ABTS Radical Scavenging Capacity Assay
2.10.3. Ferric Reducing Capacity Assay
2.11. Statistical Analysis
3. Results and Discussion
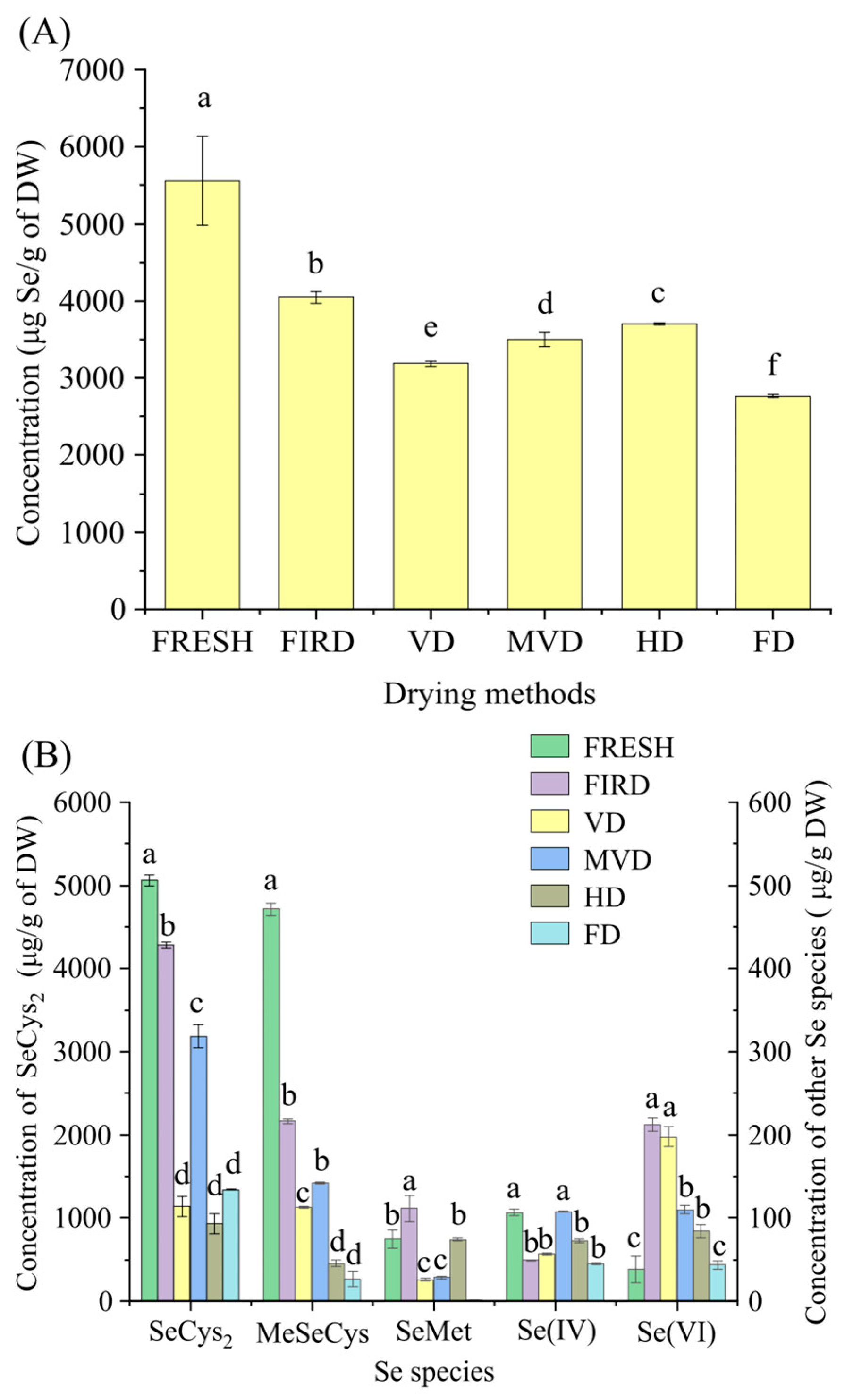
3.1. Effects of the Drying Method on the Se Content and Species of CVLs
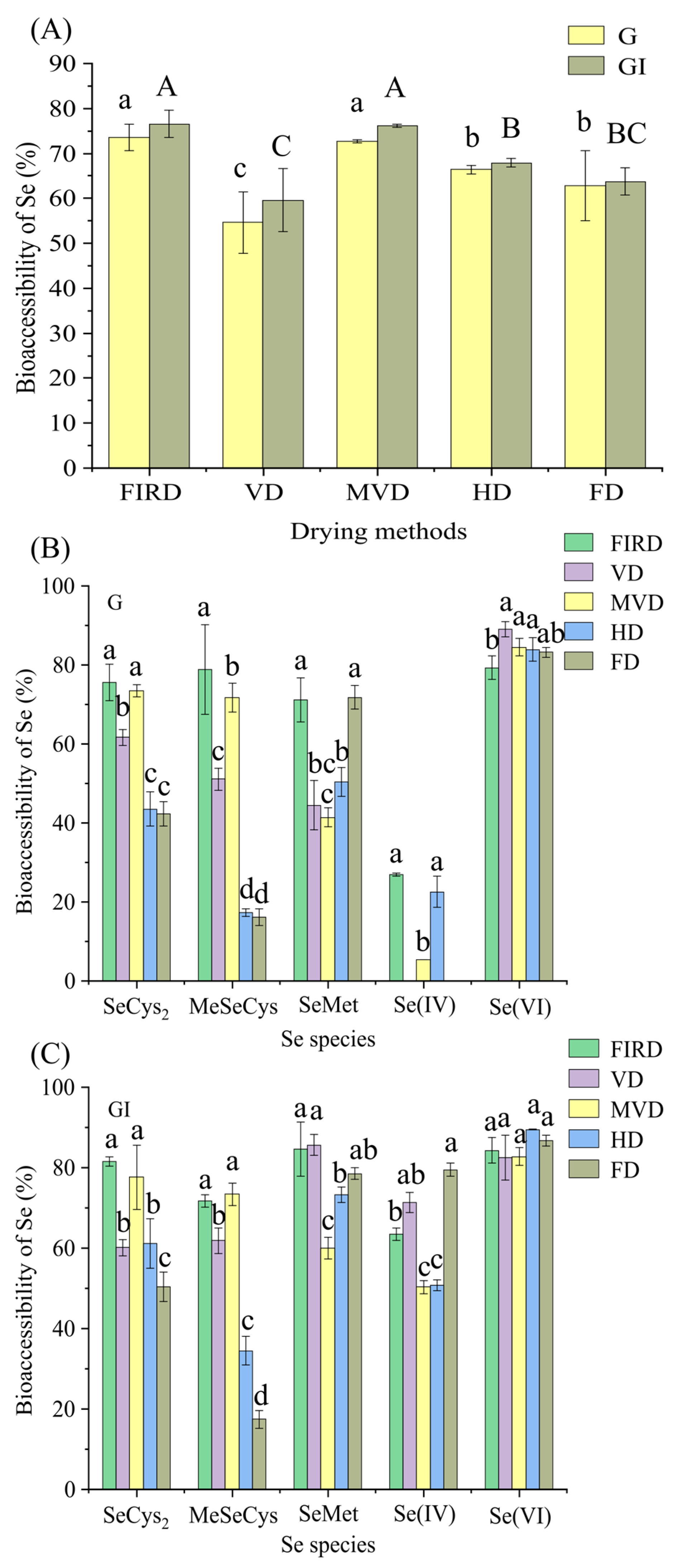
3.2. Effects of the Drying Method on Se Bioaccessibility
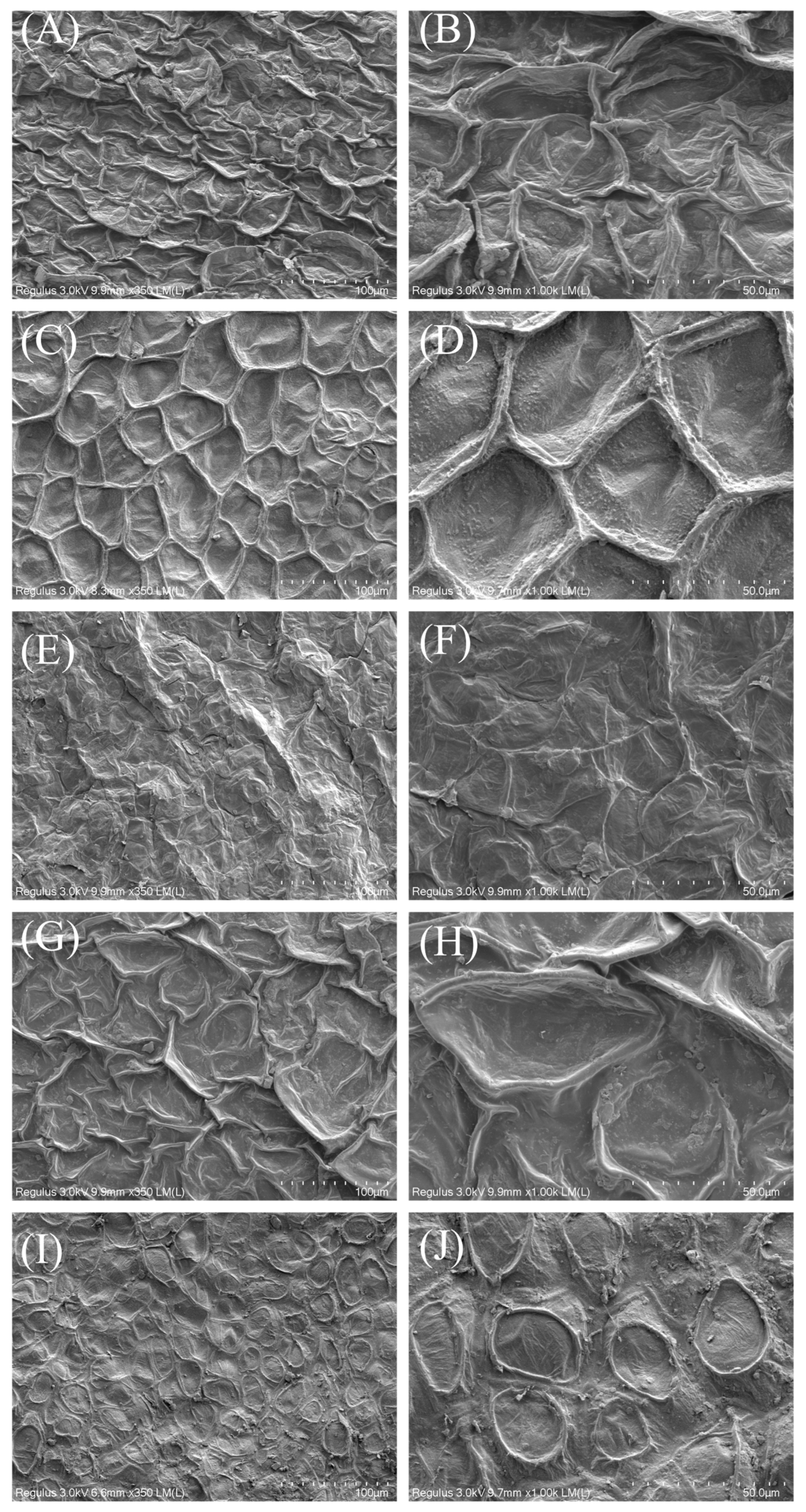
3.3. Morphology of Dried CVLs
3.4. Effect of Different Drying Methods on Color
3.5. Total Polyphenol Content (TPC) and Total Flavonoid Content (TFC) of Dried CVLs
3.6. Assessment of Antioxidant Activities
3.7. Correlation Matrix Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwarz, K.; Foltz, C.M. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- FairweatherTait, S.J. Bioavailability of selenium. Eur. J. Clin. Nutr. 1997, 51, S20–S23. [Google Scholar]
- Dong, Z.; Liu, Y.; Dong, G.; Wu, H. Effect of boiling and frying on the selenium content, speciation, and in vitro bioaccessibility of selenium-biofortified potato (Solanum tuberosum L.). Food Chem. 2021, 348, 129150. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Zurdo, D.; Gomez-Gomez, B.; Teresa Perez-Corona, M.; Madrid, Y. Impact of fish growing conditions and cooking methods on selenium species in swordfish and salmon fillets. J. Food Compost. Anal. 2019, 83, 103275. [Google Scholar] [CrossRef]
- Meiru, W.; Xin, C.; Meng, L.; Shen, R.; Yuan, L.; Jia, G.; Tian, Y. Effects of different exogenous selenium on Se accumulation, nutrition quality, elements uptake, and antioxidant response in the hyperaccumulation plant Cardamine violifolia. Ecotoxicol. Environ. Saf. 2020, 204, 111045. [Google Scholar] [CrossRef]
- Liu, R.; Yin, H.; Li, L.; Huang, K.; Li, H.; Zhao, J.; Mao, Z. First report of meloidogyne incognita on Cardamine Violifohia in China. Plant Dis. 2021, 106, 1998. [Google Scholar] [CrossRef]
- Sager, M. Selenium in agriculture, food, and nutrition. Pure Appl. Chem. 2006, 78, 111–133. [Google Scholar] [CrossRef]
- Kapolna, E.; Fodor, P. Bioavailability of selenium from selenium-enriched green onions (Allium fistulosum) and chives (Allium schoenoprasum) after ‘in vitro’ gastrointestinal digestion. Int. J. Food Sci. Nutr. 2007, 58, 282–296. [Google Scholar] [CrossRef]
- Zhou, F.; Peng, Q.; Wang, M.; Liu, N.; Quang Toan, D.; Zhai, H.; Liang, D. Influence of processing methods and exogenous selenium species on the content and in vitro bioaccessibility of selenium in Pleurotus eryngii. Food Chem. 2021, 338, 127661. [Google Scholar] [CrossRef]
- Ruby, M.V.; Schoof, R.; Brattin, W.; Goldade, M.; Post, G.; Harnois, M.; Chappell, W. Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environ. Sci. Technol. 1999, 33, 3697–3705. [Google Scholar] [CrossRef]
- Thiry, C.; Ruttens, A.; De Temmerman, L.; Schneider, Y.J.; Pussemier, L. Current knowledge in species-related bioavailability of selenium in food. Food Chem. 2012, 130, 767–784. [Google Scholar] [CrossRef]
- Moreda-Pineiro, J.; Moreda-Pineiro, A.; Bermejo-Barrera, P. In vivo and in vitro testing for selenium and selenium compounds bioavailability assessment in foodstuff. Crit. Rev. Food Sci. Nutr. 2017, 57, 805–833. [Google Scholar] [CrossRef] [PubMed]
- Khanam, A.; Platel, K. Influence of domestic processing on the bioaccessibility of selenium from selected food grains and composite meals. J. Food Sci. Tech. Mys. 2016, 53, 1634–1639. [Google Scholar] [CrossRef]
- Ye, M.; Li, J.; Yu, R.; Cong, X.; Huang, D.; Li, Y.; Zhu, S. Selenium Speciation in Selenium-Enriched Plant Foods. Food Anal. Methods 2022, 15, 1377–1389. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Brodkorb, A. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.I.; Mohamed, D.A.; Chwen, L.T.; Akit, H.; Samsudin, A.A. Effect of sodium selenite, selenium yeast, and bacterial enriched protein on chicken egg yolk color, antioxidant profiles, and oxidative stability. Foods 2021, 10, 871. [Google Scholar] [CrossRef]
- Wei, Q.; Yamada, R.; Araki, T.; Ogawa, Y. Changes in morphological and functional characteristics of tea leaves during Japanese green tea (Sencha) manufacturing process. Food Bioproc. Tech. 2022, 15, 82–91. [Google Scholar] [CrossRef]
- Cheak, T.E.; Ching, L.H.; Sze, P.O.; Chung, L.L.; Choon, H.T. Hybridization of freeze drying and impacts on drying kinetics and dried product quality of kedondong fruits. Dry. Technol. 2022, 40, 1–12. [Google Scholar] [CrossRef]
- Hamrouni-Sellami, I.; Rahali, F.Z.; Rebey, I.B.; Bourgou, S.; Limam, F.; Marzouk, B. Total Phenolics, Flavonoids, and Antioxidant Activity of Sage (Salvia officinalis L.) Plants as Affected by Different Drying Methods. Food Bioproc. Tech. 2013, 6, 806–817. [Google Scholar] [CrossRef]
- Mo, H.; Zhang, M.; Sun, J. Effect of drying process parameters on dehydrated cabbage enriched with selenium. Dry. Technol. 2006, 24, 1657–1663. [Google Scholar] [CrossRef]
- Perez, M.B.; Avila Maniero, M.; Londonio, A.; Smichowski, P.; Wuilloud, R.G. Effects of common cooking heat treatments on selenium content and speciation in garlic. J. Food Compost. Anal. 2018, 70, 54–62. [Google Scholar] [CrossRef]
- Ledesma-Escobar, C.A.; Priego-Capote, F.; Luque de Castro, M.D. Effect of sample pretreatment on the extraction of lemon (Citrus limon) components. Talanta 2016, 153, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Khanam, A.; Platel, K. Bioaccessibility of selenium, selenomethionine and selenocysteine from foods and influence of heat processing on the same. Food Chem. 2016, 194, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Aboud, S.A.; Altemimi, A.B.; Al-HiIphy, A.R.S.; Lee, Y.C.; Cacciola, F. A comprehensive review on infrared heating applications in food processing. Molecules 2019, 24, 4125. [Google Scholar] [CrossRef]
- Sun, G.X.; Van de Wiele, T.; Alava, P.; Tack, F.M.G.; Laing, G.D. Bioaccessibility of selenium from cooked rice as determined in a simulator of the human intestinal tract (SHIME). J. Sci. Food Agric. 2017, 97, 3540–3545. [Google Scholar] [CrossRef]
- Lavu, R.V.S.; Van De Wiele, T.; Pratti, V.L.; Tack, F.; Du Laing, G. Selenium bioaccessibility in stomach, small intestine and colon: Comparison between pure Se compounds, Se-enriched food crops and food supplements. Food Chem. 2016, 197, 382–387. [Google Scholar] [CrossRef]
- Khanam, A.; Platel, K. Bioavailability and bioactivity of selenium from wheat (Triticum aestivum), maize (Zea mays), and pearl millet (Pennisetum glaucum), in selenium-deficient rats. J. Agric. Food Chem. 2019, 67, 6366–6376. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Sentkowska, A. Selenium in plant foods: Speciation analysis, bioavailability, and factors affecting composition. Crit. Rev. Food Sci. Nutr. 2021, 61, 1340–1352. [Google Scholar] [CrossRef]
- Lu, X.; He, Z.; Lin, Z.; Zhu, Y.; Yuan, L.; Liu, Y.; Yin, X. Effects of Chinese cooking methods on the content and speciation of selenium in selenium bio-fortified cereals and soybeans. Nutrients 2018, 10, 317. [Google Scholar] [CrossRef]
- Shi, X.W.; Liu, Y.H.; Li, Z.Y.; Sun, X.; Li, X. Effects of radiation temperature on dehydration and moisture migration in banana slices during far-infrared radiation drying. J. Food Process. Preserv. 2020, 44, e14901. [Google Scholar] [CrossRef]
- Varley, M.C.; Neelakantan, S.; Clyne, T.W.; Dean, J.; Brooks, R.A.; Markaki, A.E. Cell structure, stiffness and permeability of freeze-dried collagen scaffolds in dry and hydrated states. Acta Biomater. 2016, 33, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Boateng, I.D.; Yang, X.M. Effect of different drying methods on product quality, bioactive and toxic components of Ginkgo biloba L. seed. J. Sci. Food Agric. 2021, 101, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
| Drying Methods | L* | a* | b* | H° | C* |
|---|---|---|---|---|---|
| FIRD | 55.88 ± 0.17 e | −4.50 ± 0.07 b | 19.31 ± 0.25 e | −76.88 ± 0.08 d | 19.91 ± 0.17 e |
| VD | 60.19 ± 0.21 b | −6.24 ± 0.06 d | 24.53 ± 0.35 a | −75.80 ± 0.28 c | 25.36 ± 0.30 a |
| MVD | 57.12 ± 0.38 d | −3.95 ± 0.04 a | 22.11 ± 0.37 bc | −79.91 ± 0.10 e | 22.60 ± 0.11 c |
| HD | 58.43 ± 0.19 c | −6.03 ± 0.06 c | 21.19 ± 0.20 d | −74.11 ± 0.12 b | 22.04 ± 0.21 d |
| FD | 62.42 ± 0.19 a | −7.26 ± 0.02 e | 21.87 ± 0.07 c | −71.60 ± 0.06 a | 23.05 ± 0.07 b |
| Drying Methods | TPC (mg GAE/100 g of DW) | TFC (mg RE/100 g of DW) | DPPH· IC50 (mg/mL) | ABTS+·IC50 (mg/mL) | TEAC (μ mol Trolox/g DW) | ||
|---|---|---|---|---|---|---|---|
| DPPH· | ABTS+· | FRA | |||||
| FIRD | 748.18 ± 1.92 c | 347.37 ± 6.23 b | 22.74 ± 3.98 c | 17.77 ± 1.67 b | 15.93 ± 1.32 a | 25.03 ± 0.52 a | 68.39 ± 2.38 a |
| VD | 990.67 ± 9.33 b | 320.10 ± 11.15 b | 17.19 ± 3.13 d | 12.06 ± 0.89 c | 17.75 ± 0.78 a | 26.20 ± 1.06 a | 69.01 ± 3.38 a |
| MVD | 550.81 ± 19.37 d | 419.09 ± 17.32 a | 36.88 ± 2.97 b | 23.07 ± 0.93 a | 11.82 ± 2.09 b | 22.78 ± 0.52 b | 47.26 ± 2.50 b |
| HD | 514.83 ± 17.32 d | 411.01 ± 12.45 a | 41.12 ± 2.19 a | 21.71 ± 0.49 ab | 11.21 ± 0.32 b | 24.23 ± 0.49 b | 42.14 ± 4.75 b |
| FD | 1134.72 ± 9.56 a | 418.08 ± 7.95 a | 19.62 ± 2.16 c | 11.39 ± 2.06 c | 16.58 ± 0.91 a | 25.76 ± 1.21 a | 76.20 ± 2.81 a |
| DPPH• Radical Scavenging Activity | ABTS+• Radical Scavenging Activity | Ferric Reducing Activity | |
|---|---|---|---|
| TPC | −0.9058 * | −0.9775 ** | 0.9235 * |
| TFC | 0.6437 | 0.4407 | −0.4553 |
| TSC | −0.9756 ** | −0.8862 * | 0.8843 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Li, Y.; Yu, R.; Huang, D.; Chen, S.; Zhu, S. Effects of Different Drying Methods on the Selenium Bioaccessibility and Antioxidant Activity of Cardamine violifolia. Foods 2023, 12, 758. https://doi.org/10.3390/foods12040758
Wang P, Li Y, Yu R, Huang D, Chen S, Zhu S. Effects of Different Drying Methods on the Selenium Bioaccessibility and Antioxidant Activity of Cardamine violifolia. Foods. 2023; 12(4):758. https://doi.org/10.3390/foods12040758
Chicago/Turabian StyleWang, Peiyu, Yue Li, Ruipeng Yu, Dejian Huang, Shangwei Chen, and Song Zhu. 2023. "Effects of Different Drying Methods on the Selenium Bioaccessibility and Antioxidant Activity of Cardamine violifolia" Foods 12, no. 4: 758. https://doi.org/10.3390/foods12040758
APA StyleWang, P., Li, Y., Yu, R., Huang, D., Chen, S., & Zhu, S. (2023). Effects of Different Drying Methods on the Selenium Bioaccessibility and Antioxidant Activity of Cardamine violifolia. Foods, 12(4), 758. https://doi.org/10.3390/foods12040758








