Bactericidal Effect of Underwater Plasma Treatment on Waste Brine from Kimchi Production Process and the Evaluation of Reusability of Plasma-Treated Waste Brine in Salting Kimchi Cabbage
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
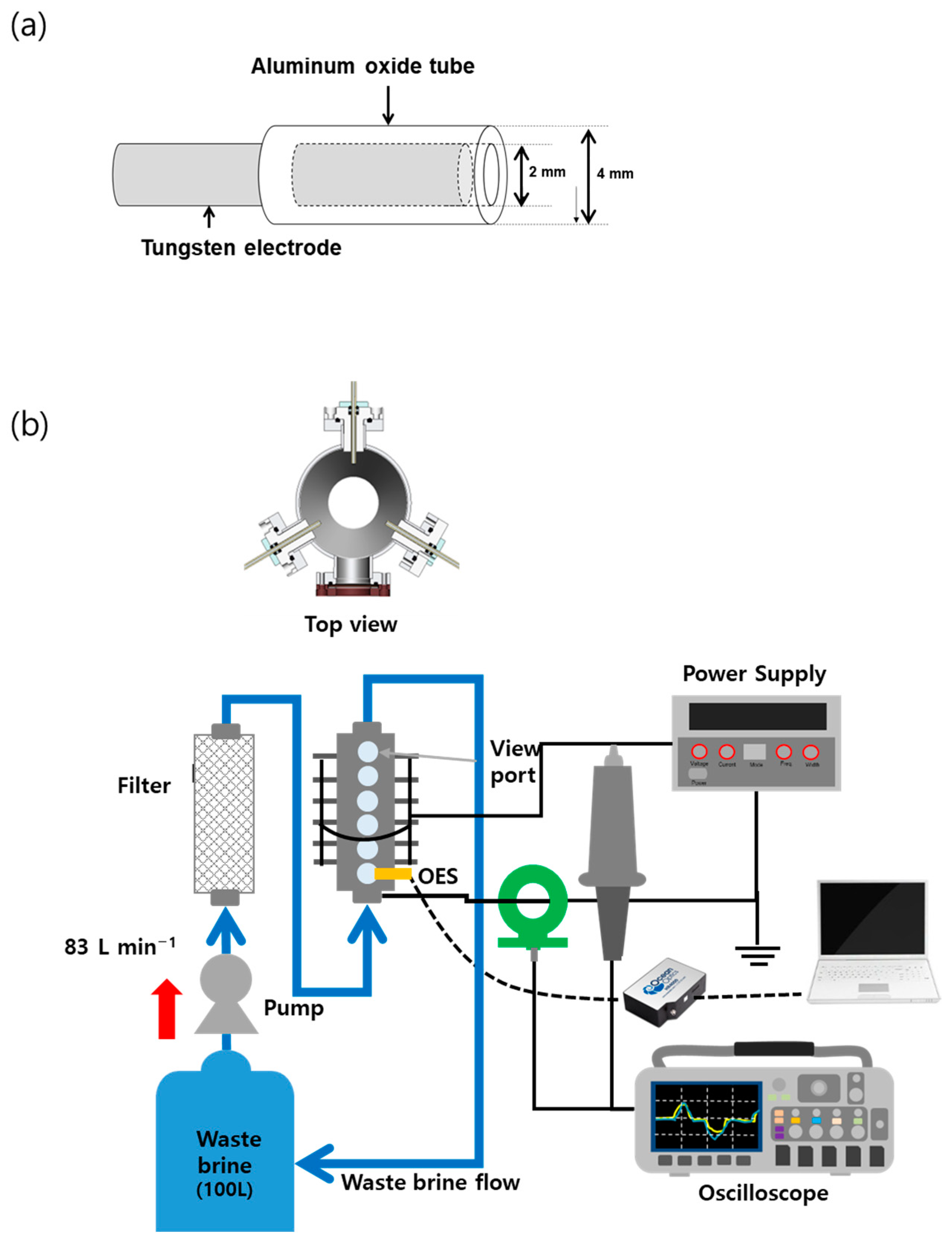
2.2. Plasma Device and Operation
2.3. Water Analysis
2.4. Preparation of Salted Kimchi Cabbage
2.5. Bacterial Sample Analysis
2.6. Modeling of Bacterial Inactivation
2.6.1. Log-Linear Model
2.6.2. Weibull Inactivation Model
2.7. Analysis Salinity, pH, Acidity, and Reducing Sugar Content
2.8. Statistical Analysis
3. Results
3.1. Characteristics of Waste Brine
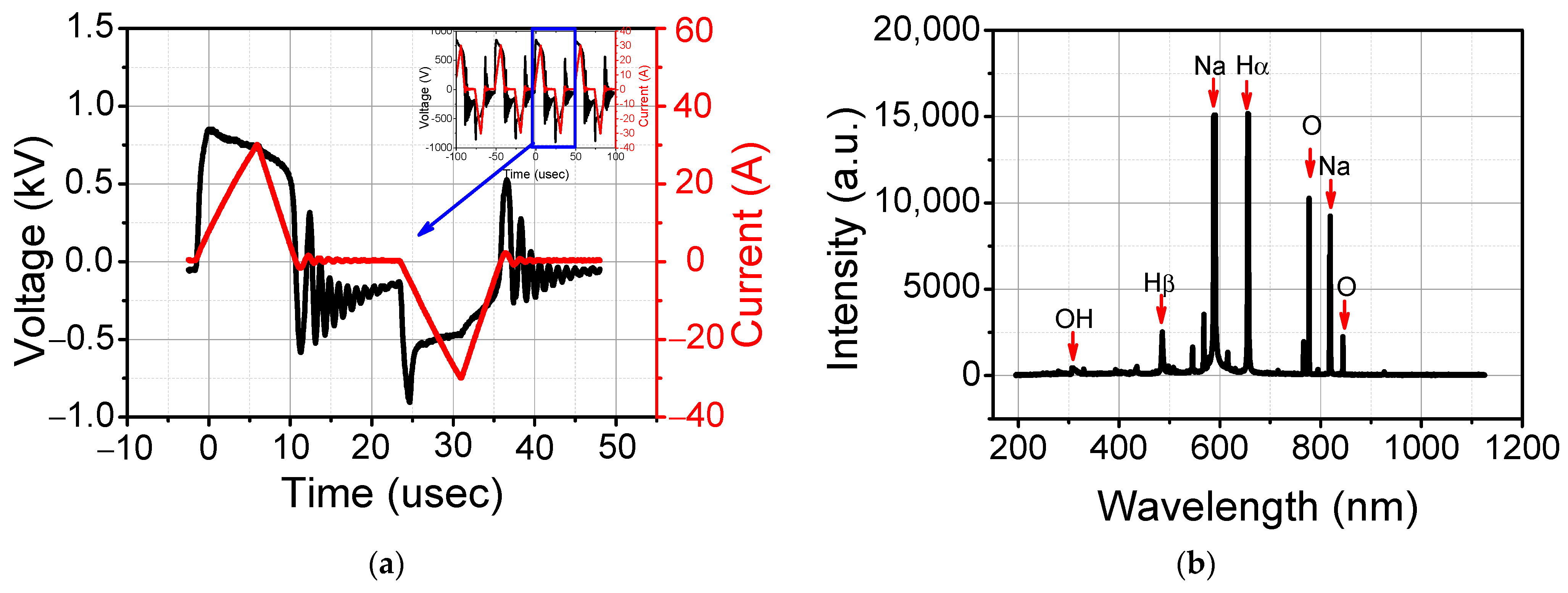
3.2. Plasma Characteristics
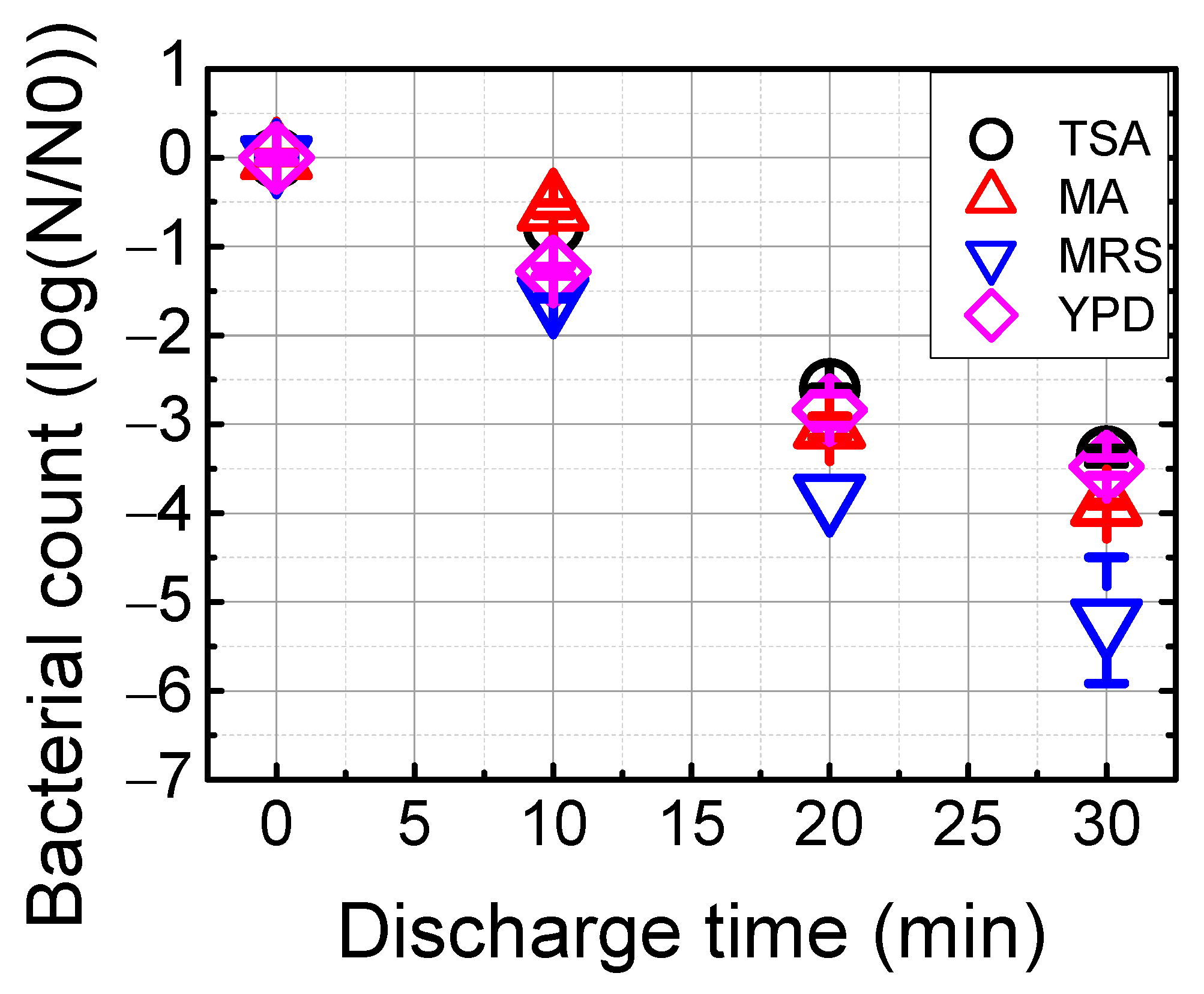
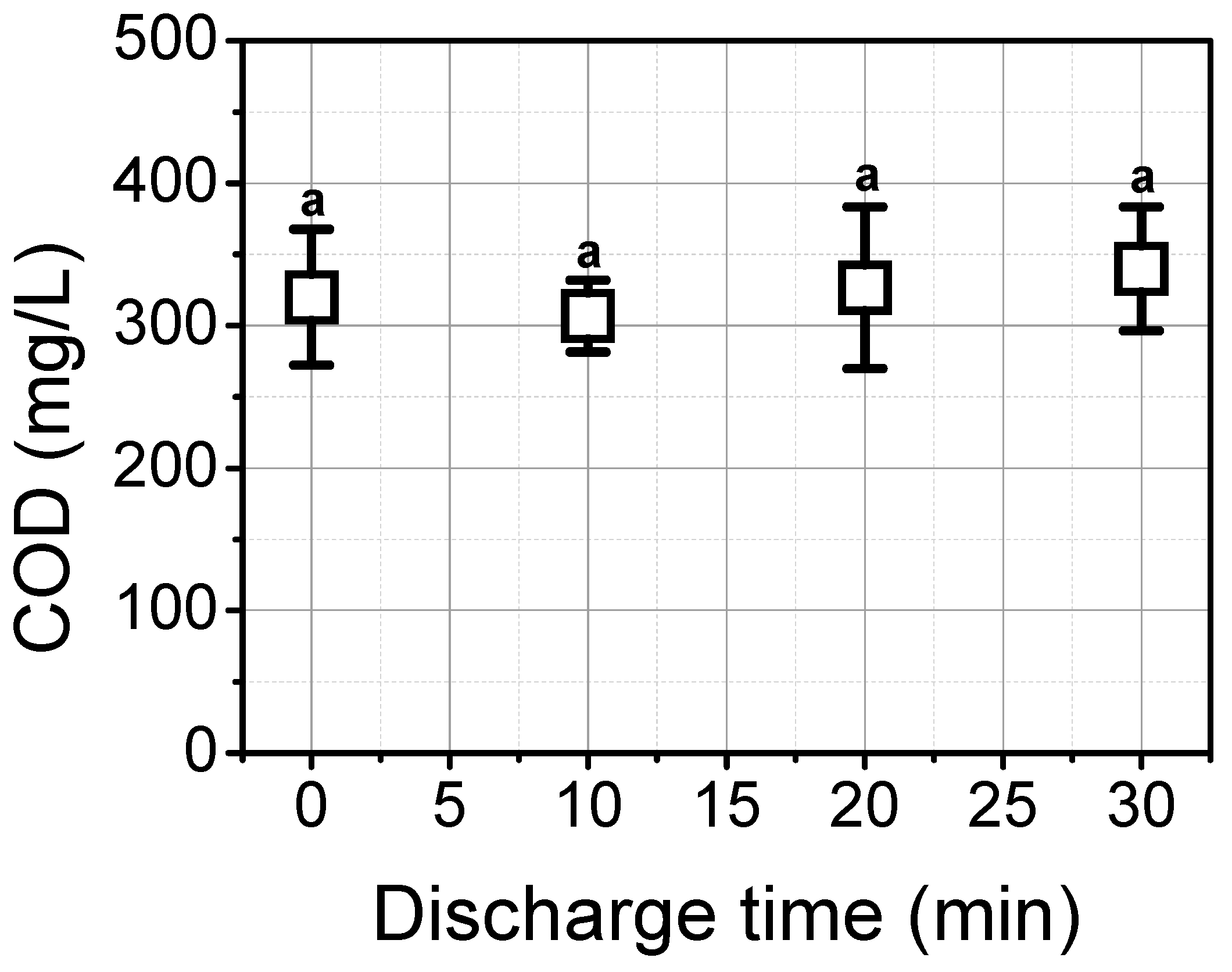
3.3. Reduction of Microorganisms and Organic Matter in Waste Brine with the Underwater Plasma
3.4. The Microbial Inactivation and Changes in Quality of Kimchi Cabbage Salted by PTWB
3.4.1. Microbial Inactivation of Kimchi Cabbage
3.4.2. Salinity, pH, Acidity, and Reducing Sugar Content of Kimchi Cabbage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shim, J.-Y.; Kim, D.-G.; Park, J.-T.; Kandpal, L.M.; Hong, S.; Cho, B.-K.; Lee, W.-H. Physicochemical Quality Changes in Chinese Cabbage with Storage Period and Temperature: A Review. J. Biosyst. Eng. 2016, 41, 373–388. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and Contamination Routes of Microbial Pathogens to Fresh Produce during Field Cultivation: A Review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, H.; Kim, S. Analysis of Components According to Different Collecting Time and Production Method in Sun-Dried Salt. Korean J. Food Preserv. 2013, 20, 791–797. [Google Scholar] [CrossRef]
- Inatsu, Y.; Bari, M.L.; Kawasaki, S.; Isshiki, K. Survival of Escherichia coli O157:H7, Salmonella enteritidis, Staphylococcus aureus, and Listeria monocytogenes in Kimchi. J. Food Prot. 2004, 67, 1497–1500. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Sung, J.-M. Hygienic Process of Recycling Salting Solution of Salted Chinese Cabbage. Bull. Food Technol. 2013, 26, 92–100. [Google Scholar]
- Lee, K.H. Effect of Ozone Treatment for Sanitation of Chinese Cabbage and Salted Chinese Cabbage. J. Korean Soc. Food Sci. Nutr. 2008, 37, 90–96. [Google Scholar] [CrossRef]
- Jung, J.Y.; Seo, E.; Jang, K.I.; Kim, T.J.; Han, N.S.; Yoon, H.S. Monitoring of Microbial Changes in Salted Cabbage (Jeolimbaechu) during Recycled Brining Operation. Food Sci. Biotechnol. 2011, 20, 223–227. [Google Scholar] [CrossRef]
- Han, G.J.; Choi, H.S.; Lee, S.M.; Lee, E.J.; Park, S.E.; Park, K.Y. Addition of Starters in Pasteurized Brined Baechu Cabbage Increased Kimchi Quality and Health Functionality. J. Korean Soc. Food Sci. Nutr. 2011, 40, 110–115. [Google Scholar] [CrossRef]
- Mazhar, M.A.; Khan, N.A.; Ahmed, S.; Khan, A.H.; Hussain, A.; Rahisuddin; Changani, F.; Yousefi, M.; Ahmadi, S.; Vambol, V. Chlorination Disinfection By-Products in Municipal Drinking Water—A Review. J. Clean. Prod. 2020, 273, 123159. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-Liquid Interactions: A Review and Roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Jiang, B.; Zheng, J.; Qiu, S.; Wu, M.; Zhang, Q.; Yan, Z.; Xue, Q. Review on Electrical Discharge Plasma Technology for Wastewater Remediation. Chem. Eng. J. 2014, 236, 348–368. [Google Scholar] [CrossRef]
- Xu, Z.; Lan, Y.; Ma, J.; Shen, J.; Han, W.; Hu, S.; Ye, C.; Xi, W.; Zhang, Y.; Yang, C.; et al. Applications of Atmospheric Pressure Plasma in Microbial Inactivation and Cancer Therapy: A Brief Review. Plasma Sci. Technol. 2020, 22, 103001. [Google Scholar] [CrossRef]
- Lim, J.; Byeon, Y.S.; Hong, E.J.; Ryu, S.; Kim, S.B. Effect of Post-Discharge Time of Plasma-Treated Water (PTW) on Microbial Inactivation and Quality of Fresh-Cut Potatoes. J. Food Process. Preserv. 2021, 45, 15387. [Google Scholar] [CrossRef]
- Zeghioud, H.; Nguyen-Tri, P.; Khezami, L.; Amrane, A.; Assadi, A.A. Review on Discharge Plasma for Water Treatment: Mechanism, Reactor Geometries, Active Species and Combined Processes. J. Water Process Eng. 2020, 38, 101664. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Choi, S.; Lyakhov, K.; Shaislamov, U.; Mongre, R.K.; Jeong, D.K.; Suresh, R.; Lee, H.J. High-Frequency Underwater Plasma Discharge Application in Antibacterial Activity. Plasma Phys. Reports 2017, 43, 381–392. [Google Scholar] [CrossRef]
- Fang, Y.; Hariu, D.; Yamamoto, T.; Komarov, S. Acoustic Cavitation Assisted Plasma for Wastewater Treatment: Degradation of Rhodamine B in Aqueous Solution. Ultrason. Sonochem. 2019, 52, 318–325. [Google Scholar] [CrossRef]
- Byeon, Y.S.; Hong, E.J.; Yoo, S.; Lho, T.; Yoon, S.-Y.; Kim, S.B.; Yoo, S.J.; Ryu, S. Ballast Water Treatment Test at Pilot-Scale Using an Underwater Capillary Discharge Device. Plasma Chem. Plasma Process. 2017, 37, 1405–1416. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hong, Y.C.; Lee, S.J.; Kim, J.H.; Lee, B.J. Underwater Capillary Discharge on the Penetrability of a Membrane. Surf. Coatings Technol. 2013, 228, S482–S485. [Google Scholar] [CrossRef]
- Hong, Y.C.; Park, H.J.; Lee, B.J.; Kang, W.S.; Uhm, H.S. Plasma Formation Using a Capillary Discharge in Water and Its Application to the Sterilization of E. coli. Phys. Plasmas 2010, 17, 053502. [Google Scholar] [CrossRef]
- Ryu, S.M.; Hong, E.J.; Seok, D.C.; Yoo, S.R.; Kim, Y.J.; Lho, T.; Lee, B.J. Characteristics of Discharged Sea Water Generated by Underwater Plasma System. Curr. Appl. Phys. 2011, 11, S87–S93. [Google Scholar] [CrossRef]
- Hong, Y.C.; Huh, J.Y.; Ma, S.H.; Kim, K.I. Inactivation of Microorganisms by Radical Droplets from Combination of Water Discharge and Electro-Spraying. J. Electrostat. 2018, 91, 56–60. [Google Scholar] [CrossRef]
- Lee, H.G.; Jeong, S.; Park, J.Y.; Yoo, S.R. Effect of Pasteurization on Delayed Kimchi Ripening and Regression Analysis for Shelf Life Estimation of Kimchi. Food Sci. Nutr. 2019, 7, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Song, K.B.; Choi, E.J.; Kim, H.K.; Park, H.W.; Chun, H.H. Combined Effects of High Hydrostatic Pressure Treatment and Red Ginseng Concentrate Supplementation on the Inactivation of Foodborne Pathogens and the Quality of Ready-to-Use Kimchi Sauce. LWT 2019, 114, 108410. [Google Scholar] [CrossRef]
- Bialka, K.L.; Demirci, A.; Puri, V.M. Modeling the Inactivation of Escherichia coli O157:H7 and Salmonella enterica on Raspberries and Strawberries Resulting from Exposure to Ozone or Pulsed UV-Light. J. Food Eng. 2008, 85, 444–449. [Google Scholar] [CrossRef]
- Lee, H.Y.; Haque, M.A.; Cho, K.M. Changes in Physicochemical Property and Lactic Acid Bacterial Community during Kimchi Fermentation at Different Temperatures. J. Appl. Biol. Chem. 2020, 63, 429–437. [Google Scholar] [CrossRef]
- Jung, H.; Lee, E.; Han, S.; Han, E. Evaluation of Brine Reuse on Salting of Chinese Using Electrochemical Process. J. Korean Soc. Water Wastewater 2014, 28, 541–548. [Google Scholar] [CrossRef]
- Lim, J.; Hong, E.J.; Kim, S.B.; Ryu, S. The Effect of Gap Distance between a Pin and Water Surface on the Inactivation of Escherichia Coli Using a Pin-to-Water Plasma. Int. J. Mol. Sci. 2022, 23, 5423. [Google Scholar] [CrossRef]
- Woloszko, J.; Stalder, K.R.; Brown, I.G. Plasma Characteristics of Repetitively-Pulsed Electrical Discharges in Saline Solutions Used for Surgical Procedures. IEEE Trans. Plasma Sci. 2002, 30, 1376–1383. [Google Scholar] [CrossRef]
- Němcová, L.; Nikiforov, A.; Leys, C.; Krcma, F. Chemical Efficiency of H2O2 Production and Decomposition of Organic Compounds under Action of DC Underwater Discharge in Gas Bubbles. IEEE Trans. Plasma Sci. 2011, 39, 865–870. [Google Scholar] [CrossRef]
- Van Kessel, J.; Strom, S.; Deason, H.; Van Moorlehem, E.; Berube, N.; Hauta, S.; Fernando, C.; Hill, J.; Fonstad, T.; Gerdts, V. Time and Temperature Requirements for Heat Inactivation of Pathogens to Be Applied to Swine Transport Trailers. J. Swine Health Prod. 2021, 29, 19–28. [Google Scholar] [CrossRef]
- Jaisan, C.; An, D.S.; Lee, D.S. Application of Physical Gas Absorbers in Manipulating the CO2 Pressure of Kimchi Package. J. Food Sci. 2018, 83, 3002–3008. [Google Scholar] [CrossRef] [PubMed]
- Bing, J.; Hu, C.; Zhang, L. Enhanced Mineralization of Pharmaceuticals by Surface Oxidation over Mesoporous γ-Ti-Al2O3 Suspension with Ozone. Appl. Catal. B Environ. 2017, 202, 118–126. [Google Scholar] [CrossRef]
- You, S.Y.; Yang, J.S.; Kim, S.H.; Hwang, I.M. Changes in the Physicochemical Quality Characteristics of Cabbage Kimchi with Respect to Storage Conditions. J. Food Qual. 2017, 2017, 9562981. [Google Scholar] [CrossRef]
- De Villiers, M.M.; Wurster, D.E.; Narsai, K. Stability of Lactic Acid and Glycolic Acid in Aqueous Systems Subjected to Acid Hydrolysis and Thermal Decomposition. J. Cosmet. Sci. 1997, 48, 165–174. [Google Scholar]
- Matsuoka, M.; Kamegawa, T.; Rakhmawaty, D.; Kitano, M.; Wada, K.; Anpo, M. Photocatalytic Decomposition of Lactic Acid in Water on a Photoelectrochemical Circuit System Consisting of a Rod-Type TiO2 Electrode and Silicon Solar Cell. Top. Catal. 2008, 47, 162–165. [Google Scholar] [CrossRef]
- Kozakova, Z.; Klimova, E.J.; Obradovic, B.M.; Dojcinovic, B.P.; Krcma, F.; Kuraica, M.M.; Olejnickova, Z.; Sykora, R.; Vavrova, M. Comparison of Liquid and Liquid-Gas Phase Plasma Reactors for Discoloration of Azo Dyes: Analysis of Degradation Products. Plasma Process. Polym. 2018, 15, 1700178. [Google Scholar] [CrossRef]
- Ucar, Y.; Ceylan, Z.; Durmus, M.; Tomar, O.; Cetinkaya, T. Application of Cold Plasma Technology in the Food Industry and Its Combination with Other Emerging Technologies. Trends Food Sci. Technol. 2021, 114, 355–371. [Google Scholar] [CrossRef]
- Kilonzo-Nthenge, A.; Liu, S.; Yannam, S.; Patras, A. Atmospheric Cold Plasma Inactivation of Salmonella and Escherichia coli on the Surface of Golden Delicious Apples. Front. Nutr. 2018, 5, 120. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Yuan, Y.; Fan, Y.; Guo, K.; Yue, T. Inactivation of Yeast in Apple Juice Using Gas-Phase Surface Discharge Plasma Treatment with a Spray Reactor. LWT 2018, 97, 530–536. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, J.H.; Lv, X.; Sun, D.W. Assessing the Inactivation Efficiency of Ar/O2 Plasma Treatment against Listeria monocytogenes Cells: Sublethal Injury and Inactivation Kinetics. LWT 2019, 111, 318–327. [Google Scholar] [CrossRef]
- Bai, Y.; Muhammad, A.I.; Hu, Y.; Koseki, S.; Liao, X.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Inactivation Kinetics of Bacillus cereus Spores by Plasma Activated Water (PAW). Food Res. Int. 2020, 131, 109041. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jung, J.; Cho, Y.; Lee, S.-J.; Kim, S.-H.; Park, K.; Kang, S. Quality Changes in Brined Baechu Cabbage Using Different Types of Polyethylene Film, and Salt Content during Storage. Korean J. Food Preserv. 2009, 16, 605–611. [Google Scholar]
- Ryu, J.P.; Yang, J.H.; Chung, Y.B.; Lee, S., II; Han, E.S. Quality Characteristics of Baechu-Kimchi Salted at High Salt Concentration for a Short Time. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1913–1919. [Google Scholar] [CrossRef]
- Lee, M.-H.; Lee, G.-D.; Son, K.; Yoon, S.-R.; Kim, J.S.; Kwon, J.-H. Changes in Organoleptic and Rheological Properties of Chinese Cabbage with Salting Condition. J. Korean Soc. Food Sci. Nutr. 2002, 31, 417–422. [Google Scholar] [CrossRef]
- Song, J.E.; Sun, K.M.; Han, J.S. Effects of the Salting of Chinese Cabbage on Taste and Fermentation of Kimchi. Korean J. Food Cook. Sci. 1995, 11, 226–232. [Google Scholar]
- Kim, D.-G.; Kim, B.-K.; Kim, N.-H. Effect of Reducing Sugar Content in Chinese Cabbage on Kimchi Fermentation. J. Korean Soc. Food Nutr. 1994, 23, 73–77. [Google Scholar]
- Park, S.S.; Sung, J.M.; Jeong, J.W.; Park, K.J.; Lim, J.H. Quality Changes of Salted Chinese Cabbages with Electrolyzed Water Washing and a Low Storage Temperature. J. Korean Soc. Food Sci. Nutr. 2013, 42, 615–620. [Google Scholar] [CrossRef]
- Song, H.; Cheon, S.; Yoo, S.; Chung, Y.B.; Seo, H. Changes in Quality Characteristics of Salted Kimchi Cabbage and Kimchi Paste during Storage. Korean J. Food Preserv. 2016, 23, 459–470. [Google Scholar] [CrossRef]

| pH | Conductivity (mS cm−1) | Salinity (%) | COD (mg L−1) |
|---|---|---|---|
| 5.71 ± 0.14 | 100.7 ± 8.71 | 7.00 ± 0.33 | 320 ± 95.4 |
| TSA (CFU mL−1) | MA (CFU mL−1) | MRS (CFU mL−1) | YPD (CFU mL−1) |
| 5.87 ± 0.01 | 6.09 ± 0.06 | 5.71 ± 0.02 | 5.55 ± 0.05 |
| Model | Agar | Parameter | R2 | |
|---|---|---|---|---|
| Log-linear | TSA | −0.115 | 0.983 | |
| MA | −0.131 | 0.962 | ||
| MRS | −0.177 | 0.996 | ||
| YPD | −0.124 | 0.989 | ||
| Weibull | TSA | 4.47 | 0.954 | |
| 1.098 | ||||
| MA | 4.886 | 0.921 | ||
| 1.238 | ||||
| MRS | 2.447 | 0.989 | ||
| 1.000 | ||||
| YPD | 2.314 | 0.977 | ||
| 0.825 | ||||
| After 0 Day | After 7 Days | |||||||
|---|---|---|---|---|---|---|---|---|
| TSA | MRS | MA | YPD | TSA | MRS | MA | YPD | |
| NMB | 5.89 ± 0.18 a | 5.88 ± 0.18 a | 5.82 ± 0.03 a | 5.94 ± 0.28 a | 5.34 ± 0.04 b | 4.30 ± 0.25 a | 4.53 ± 0.07 a | 4.62 ± 0.21 a |
| WB | 6.75 ± 0.04 b | 6.83 ± 0.01 b | 6.50 ± 0.04 b | 6.60 ± 0.06 b | 5.98 ± 0.02 c | 5.92 ± 0.05 b | 5.65 ± 0.03 b | 6.01 ± 0.04 b |
| PTWB | 6.07 ± 0.05 a | 6.12 ± 0.3 a | 5.89 ± 0.07 a | 5.65 ± 0.72 a | 4.87 ± 0.20 a | 4.65 ± 0.27 a | 4.59 ± 0.25 a | 4.45 ± 0.02 a |
| After 0 Day | After 7 Days | |||||||
|---|---|---|---|---|---|---|---|---|
| Salinity (%) | pH | Acidity (%) | RS (mg L−1) | Salinity (%) | pH | Acidity (%) | RS (mg L−1) | |
| NMB | 1.96 ± 0.027 b | 6.2 ± 0.01 a | 0.161 ± 0.0019 b | 414.95 ± 4.46 b | 1.62 ± 0.023 b | 6.0 ± 0.01 a | 0.17 ± 0.0021 a | 409.79 ± 3.63 b |
| WB | 1.69 ± 0.023 a | 6.2 ± 0.01 a | 0.144 ± 0.0014 a | 428.86 ± 2.16 c | 1.51 ± 0.014 a | 5.9 ± 0.03 a | 0.17 ± 0.0014 a | 418.21 ± 3.06 c |
| PTWB | 1.95 ± 0.047 b | 6.1 ± 0.03 a | 0.163 ± 0.0056 b | 398.00 ± 3.98 a | 1.77 ± 0.014 c | 5.9 ± 0.02 a | 0.17 ± 0.0036 a | 353.25 ± 1.36 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Hong, E.J.; Kim, S.B.; Lee, M.-A.; Ryu, S. Bactericidal Effect of Underwater Plasma Treatment on Waste Brine from Kimchi Production Process and the Evaluation of Reusability of Plasma-Treated Waste Brine in Salting Kimchi Cabbage. Foods 2023, 12, 728. https://doi.org/10.3390/foods12040728
Lim J, Hong EJ, Kim SB, Lee M-A, Ryu S. Bactericidal Effect of Underwater Plasma Treatment on Waste Brine from Kimchi Production Process and the Evaluation of Reusability of Plasma-Treated Waste Brine in Salting Kimchi Cabbage. Foods. 2023; 12(4):728. https://doi.org/10.3390/foods12040728
Chicago/Turabian StyleLim, Junghyun, Eun Jeong Hong, Seong Bong Kim, Mi-Ai Lee, and Seungmin Ryu. 2023. "Bactericidal Effect of Underwater Plasma Treatment on Waste Brine from Kimchi Production Process and the Evaluation of Reusability of Plasma-Treated Waste Brine in Salting Kimchi Cabbage" Foods 12, no. 4: 728. https://doi.org/10.3390/foods12040728
APA StyleLim, J., Hong, E. J., Kim, S. B., Lee, M.-A., & Ryu, S. (2023). Bactericidal Effect of Underwater Plasma Treatment on Waste Brine from Kimchi Production Process and the Evaluation of Reusability of Plasma-Treated Waste Brine in Salting Kimchi Cabbage. Foods, 12(4), 728. https://doi.org/10.3390/foods12040728








