Abstract
The aerial parts of Agastache rugosa are used as a food material and traditional medicine in Asia. A 50% ethanol extract exhibited potent xanthine oxidase (XO) inhibitory activity (IC50 = 32.4 µg/mL). To investigate the major components responsible for this effect, seven known compounds were identified from A. rugosa; among these, salvianolic acid B (2) was isolated from this plant for the first time. Moreover, acacetin (7) exhibited the most potent inhibitory activity with an IC50 value of 0.58 µM, lower than that of allopurinol (IC50 = 4.2 µM), which is commonly used as a XO inhibitor. Comparative activity screening revealed that the C6-bonded monosaccharides (3) or sugars substituted with acetyl or malonyl groups (4–6) are critical for XO inhibition when converted to aglycone (7). The most potent inhibitor (7) in the A. rugosa extract (ARE) exhibited mixed-type inhibition kinetics and reversible inhibition toward XO. Furthermore, the hydrolysis of ARE almost converted to an inhibitor (7), which displayed the highest efficacy; UPLC-qTof MS revealed an increased content, up to five times more compared with that before treatment. This study will contribute to the enhancement in the industrial value of ARE hydrolysates as a functional ingredient and natural drug toward the management of hyperuricemia and treatment of gout.
1. Introduction
The main causes of hyperuricemia are the over-production of uric acid, insufficient excretion in the urine, or a combination of both [1,2]. When blood levels of uric acid are high, uric acid enters the joints and crystallizes, causing joint inflammation; the inflammation causes swelling and pain in the joints, leading to gout and gouty arthritis which are excruciatingly painful [3,4]. Xanthine oxidase (XO, EC 1.17.3.2) is an important enzyme in the purine catabolic pathway in the body; it is present in significant concentrations in the gastrointestinal tract. XO oxidizes hypoxanthine into xanthine followed by xanthine conversion to uric acid [5]. Thus, XO inhibitors that block uric acid synthesis can play an important role in preventing hyperuricemia. The administration of allopurinol, a well-known representative XO inhibitor, is one of the clinical treatment methods for hyperuricemia and chronic gout [6]. However, despite the excellent efficacy of allopurinol, side effects such as nausea, diarrhea, or drowsiness may occur in general. In particular, in cases of renal dysfunction, it can be fatal; hypersensitivity syndromes such as gastrointestinal disorder, skin rash, or fever may also occur when diuretics are administered [7]. Therefore, the development of potential XO inhibitors from natural materials that increase effectiveness, reduce side effects, and guarantee safety is highly desirable. One such natural resource is Agastache rugosa (Fisch. and C.A.Mey.) Kuntze.
A. rugosa is a perennial plant belonging to the Lamiales order Lamiaceae family; it is known to form a colony and has high growth power even in barren mountain fields [8]. Geographically, it is mainly distributed in Northeast Asia, China, Japan, and Korea, where the leaves are used as spices and the flowers as tea. Moreover, in traditional medicine, the aerial part is used as an important source of medicinal resources [9,10]. It has been reported that the main use of A. rugosa is for treating vomiting, fever, diarrhea, anxiety, halitosis, nausea, and headaches [11,12]. Previous studies have revealed that the A. rugosa extract (ARE) displays an anti-osteoporotic effect on estrogen-deficiency-induced osteoporosis in animal models and its mechanism of inhibition on osteoclast differentiation has been elucidated [13]. There is also pharmacological evidence that ARE exhibits anti-inflammatory, anti-tumor, and anti-atherogenic effects [14,15]. These effects may be due to the proliferation of active compounds in ARE.
ARE has been reported to contain various types of functional metabolites including phenolic acids, triterpenes, essential oils, and flavonoids [16,17]. Many studies have reported that components isolated from A. rugosa contribute to bioactive effects; however, no detailed research regarding potential XO inhibitors has been performed. In particular, among the flavonoids, apigenin is known to be a strong XO inhibitor, and structurally very similar components of A. rugosa have been reported in the literature [18]. Thus, this study intends to confirm the XO inhibitory effect.
Therefore, to support the use of safe and highly potent natural inhibitors for the prevention, symptom relief, and treatment of hyperuricemia, we isolated the key constituents responsible for the XO inhibitory effect and investigated the mechanism of inhibition by kinetic mode analysis. In addition, for industrial use purposes, ARE was converted to a material that maximized the active ingredients through hydrolysis.
2. Materials and Methods
2.1. Plant Materials and Sample Preparation
The aerial part of A. rugosa is called Kwakhayang and was purchased from the oriental herbal market (Omniherb, Daegu, Republic of Korea) (http://www.omniherb.com/, accessed on 1 September 2022) that only handles herbs certified by the Korean Pharmacopoeia. A sample was ground to a powder state to ensure uniform extraction and stored at −80 °C in a cryogenic freezer until further experiment. A total of 5 different solvents for extraction were used, ethanol (EtOH), 70% EtOH in water (EtOH/H2O (7/3, v/v)), 50% EtOH, 30% EtOH, and distilled water (H2O). To prepare the various extracts for the activity-guided fractionation, 2 g of crushed aerial parts were immersed in 40 mL of each solvent and were extracted ultrasonically for 1 h at 25 °C. For hydrolysis, 2 mg of 50% ARE was treated with 1 mL of 50 mM phosphate buffer (pH 6.8) with 0.2 units of β-glucosidase. The reaction mixture was incubated at 37 °C for 2 h to completely hydrolyze the glucose moiety with the β form [19]. The samples were centrifuged at 4000× g for 5 min, filtered through a 0.22 μm PTFE membrane filter, and used for liquid chromatography analyses and enzymatic inhibition evaluations. For quantitative analyses, calibration curves were plotted with high linearity (r2 > 0.998) for 6 different concentrations (100 μg, 50 μg, 10 μg, 5 μg, 1 μg, and 0.1 μg/mL). HPLC-grade solvents such as water, acetonitrile (ACN), and ethanol were purchased from J. T. Baker (Phillipsburg, Pennsylvania, USA). β-Glucosidase from almonds, XO from bovine milk, xanthine, allopurinol, apigenin, sodium pyrophosphate, dimethyl sulfoxide (DMSO), and formic acid (FA) of MS-grade were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Instruments
The 1H and 13C nuclear magnetic resonance (NMR) spectra were obtained on a Bruker AM 400, using DMSO-d6 and CD3OD with TMS (Andover, MA, USA) as the internal standard. Electrospray ionization (ESI) high-resolution (HR) mass spectrometry (MS) spectra were analyzed on a quadrupole time-of-flight mass spectrometer (qTof PremierTM, Waters Corp., Milford, MA, USA). The ultra-performance liquid chromatography (UPLC) system equipped with a UV detector, binary gradient module, and an auto sampler was from Waters Corp. (Milford, MA, USA). Separation and purification of single compounds were performed using a medium-pressure liquid chromatography (MPLC) and recycling HPLC (YMC Co., Ltd., Kyoto, Japan) with a reversed-phase (RP) cartridge and preparative RP column (250 mm × 20 mm, i.d. 5 μm). All the enzymatic assay experiments were performed on a multi-mode microplate reader, SpectraMax M2 series (Molecular device, San Jose, CA, USA).
2.3. Extraction, Fractionation, and Isolation of A. rugosa
The air-dried aerial part (600 g) of A. rugosa was ground using a blender (SHMFP-40000; HANIL Co., Ltd., Seoul, South Korea) and extracted twice with 50% EtOH (12 L) for 4 days each at room temperature (25 °C). The filtered and combined extract was evaporated, resulting in 67.8 g (yield 11.3%) of crude extract. A portion of the sample (50 g) of this extract was subjected to column chromatography (CC) on a Diaion HP20SS (12 × 50 cm, 1.2 kg) eluted with MeOH/H2O mixed solvents (10:90 (2 L), 30:70 (2 L), 50:50 (4 L), 70:30 (4 L), 90:10 (2 L), and 100:0 (2 L)), to provide seven fractions (A–G). Fraction B (3.2 g) was fractionated on an C18 column cartridge using MPLC with a linear gradient of 35–50% MeOH/H2O at an 18 mL/min flow rate to afford 5 fractions (B1–B4). The B3 subfraction, enriched with 1 and 2, was purified by preparative HPLC using 50% MeOH as mobile phase and afforded 1 (124 mg) and 2 (2.7 mg). Fractions C–E (8.9 g) enriched with 3–6 were fractionated via RP-MPLC with a linear gradient of 45–60% MeOH/H2O to give fractions C1–C9. The C3 subfraction, enriched with 3 and 4, was further purified by recycling preparative HPLC using 55% MeOH in H2O and afforded 3 (184 mg) and 4 (92 mg). Subsequent separation of subfractions (C5–C7), enriched with 5 and 6, were combined and further purified by recycling preparative HPLC mode using 40% ACN in H2O as the mobile phase to afford 5 (5.2 mg) and 6 (7.4 mg). Fraction F (0.7 g) enriched with 7 on Sephadex LH-20 with 60% ACN as the eluent yielded 7 (16.4 mg). All isolated compounds (1–7) were identified based on the following physicochemical and spectroscopic data [16,20].
Rosmarinic acid (1). White powder; λmax (MeOH) 200, 329 nm; HR-ESI-MS, m/z 359.0777 [M–H]− (calcd. for C18H15O8 359.0767). 1H NMR and 13C NMR data, see Supplementary Materials.
Salvianolic acid B (2). Amorphous white powder; [α]20D +94.0 (c 0.7, CH3OH); λmax (MeOH) 254, 287, 329 nm; HR-ESI-MS, m/z 717.1453 [M–H]− (calcd. for C36H30O16 717.1456). 1H NMR (500 MHz, CD3OD) δH 3.10-2.95 (4H, m, H-7″α, 7″β, 7‴α, 7‴β), 4.40 (1H, d, J = 4.4 Hz, H-3), 5.11–5.20 (2H, m, H-8″, 8‴), 5.83 (1H, d, J = 4.4 Hz, H-2), 6.29 (1H, d, J = 16.0 Hz, H-11‴), 6.46 (1H, dd, J = 2.0, 8.0 Hz, H-6″), 6.57-6.65 (8H, m, H-6‴, 6′, 5′, 5‴, 2″, 2′, 2‴, 5″), 6.76 (1H, d, J = 8.4 Hz, H-6), 7.17 (1H, d, J = 16.0 Hz, H-5), 7.68 (1H, d, J = 16.0 Hz, H-12‴). 13C NMR (125 MHz, CD3OD) δC 172.4 (C-9″), 170.1 (C-10″), 168.1 (C-9‴), 166.9 (C-10‴), 148.9 (C-8), 146.5 (C-7), 146.4 (C-3‴, 4‴), 145.9 (C-3′, 4′), 145.1 (C-3″,4″), 133.7 (C-1′), 129.2 (C-1″), 128.7 (C-1‴), 126.2 (C-9), 124.7 (C-4), 144.1 (C-12‴), 121.9 (C-5, 6″, 6‴), 118.3 (C-6, 6′), 117.5 (C-2″, 2‴), 116.3 (C-5′, 5″, 5‴, 11‴), 113.2 (C-2′), 88.8 (C-2), 74.4 (C-8″), 74.4 (C-8″), 57.2 (C-3), 37.8 (C-7‴), and 37.2 (C-7″).
Tilianin (3). White-yellowish powder; λmax (MeOH) 267, 332 nm; HR-ESI-MS, m/z 447.1286 [M+H]+ (calcd. for C22H23O10 447.1291). 1H NMR and 13C NMR data, see Supplementary Materials.
Acacetin 7-O-(6-O-malonyl)-β-D-glucoside (4). Yellowish amorphous powder; λmax (MeOH) 267, 332 nm; HR-ESI-MS, m/z 533.1293 [M+H]+ (calcd. for C25H25O13 533.1295). 1H NMR and 13C NMR data, see Supplementary Materials.
Acacetin 7-O-(2″-O-acetyl)-β-D-glucoside (5). Yellowish crystalline powder; λmax (MeOH) 267, 332 nm; HR-ESI-MS, m/z 489.1394 [M+H]+ (calcd. for C24H25O11 489.1397). 1H NMR and 13C NMR data, see Supplementary Materials.
Acacetin 7-O-(2″-O-acetyl-6″-O-malonyl)-β-D-glucoside (6). Pale-brown amorphous powder; λmax (MeOH) 267, 332 nm; HR-ESI-MS, m/z 575.1399 [M+H]+ (calcd. for C27H27O14 575.1401). 1H NMR and 13C NMR data, see Supplementary Materials.
Acacetin (7). White-yellowish powder; λmax (MeOH) 267, 332 nm; HR-ESI-MS, m/z 285.0755 [M+H]+ (calcd. for C16H13O5 285.0763). 1H NMR and 13C NMR data, see Supplementary Materials.
2.4. UPLC-qTof Mass Spectrometry Analysis
The identification of the main component peaks was performed by a UPLC-coupled PDA detector. An amount of 2.0 μL of each sample was injected into a BEH C18 column (i.d., 1.7 μm, 100 × 2.1 mm) at a flow rate of 0.4 mL (per min) and eluted using a chromatographic gradient of 2 mobile phases (A: H2O containing 0.1% FA; B: ACN containing 0.1% FA). The linear gradient elution was optimized as follows: 0 min, 10% B; 0–1 min, 10% B; 1–8 min, 10–40% B; 8–11 min, 40–90% B; 11–11.3 min, 90–100% B; 11.3–13.3 min, 100% B; 13.3–13.4 min, 100–10% B; and 13.4–15 min, return to initial elution condition of 10% B. The qTof MS was operated in two different ion modes (negative and positive) under the following conditions: cone voltage 30 V, capillary voltage 2.5 kV, source temperature 110 °C, and desolvation temperature 400 °C. A sprayer with a calibration (lock MS) standard solution of leucine-enkephalin ([M–H]− m/z 554.2615, [M+H]+ m/z 556.2771) was used. The UV chromatograms were obtained using Empower 2, and full scan MS data and MS2 spectra were collected using the MassLynx software.
2.5. XO Inhibitory Activity and XO Kinetic Assay
The bovine milk XO activity was assayed following the previously published procedures [21,22]. Allopurinol and apigenin were used as the positive controls for the enzyme assay protocol. The compounds (1–7) for evaluation of inhibitory effects were dissolved in DMSO at 4 mM and further diluted at various concentrations. First, 125 µL of 100 mM sodium pyrophosphate buffer (HCl, pH 7.5), 30 µL of 0.1 units XO enzyme, 5 µL of test samples, and 40 µL of the substrate (0.5 mM xanthine) were mixed. The reaction mixture (200 µL) in a 300 µL well plate was incubated at 37 °C and the UV absorbance at 295 nm was screened every 1 min for 15 min. The inhibition mechanism and constants (Km, Vmax, and Ki) of a binding site for the inhibitor to a free enzyme (KI) or an enzyme–substrate complex (KIS) were determined using the Lineweaver–Burk and Dixon plots [23].
1/V = Km/Vmax (1 + [I]/KI) 1/S + 1/Vmax
Slope = Km/KI Vmax [I] + Km/Vmax
Intercept = 1/KIS Vmax [I] + 1/Vmax
2.6. Statistical Analysis
All measurements of the XO enzyme activities and individual compound contents were repeated in triplicate and the data were expressed as mean ± SD (standard deviations) using SigmaPlot version 10.0 (Systat Software Inc., San Jose, CA, USA).
3. Results and Discussion
3.1. Bioassay-Guided Isolation and Identification of Compounds
The ARE was prepared using five different polar solvents (EtOH, 70% EtOH, 50% EtOH, 30% EtOH, and H2O) and tested for their XO inhibitory activity. The degree of in vitro enzyme inhibition was measured spectrophotometrically by determining the uric acid formation in absorbance at 295 nm, using xanthine (C5H4N4O2) as the substrate. All extracts investigated, except the H2O extract, showed more than 50% inhibitory activity against XO at 125 μg/mL (Table 1).

Table 1.
XO Inhibitory effects of different solvent extracts and isolated compounds 1–7 in aerial parts of A. rugosa.
In particular, the 50% EtOH solvent (IC50 = 32.4 μg/mL) extracted the maximum XO inhibitory substances. In fact, the 50% ARE exhibited inhibitory effects statistically similar to those of the 70% ARE (IC50 = 31.9 μg/mL). The 50% EtOH extract was selected considering its industrial use parameters, such as the unit cost of EtOH, ease of powdering, and especially the point with the highest yield (11.3%). The high potency of the 50% EtOH extract via activity-guided fractionation encouraged us to identify compounds responsible for its XO inhibitory effects.
As a result of separation and purification through repeated CC, seven XO inhibitory phenolic compounds (1 and 2) and flavonoids (3–7) were isolated from 50% ARE (Figure 1). The isolated compounds 1–7 were identified as rosmarinic acid (1), salvianolic acid B (2), tilianin (3), acacetin 7-O-(6″-O-malonyl)-β-D-glucopyranoside (4), acacetin 7-O-(2″-O-acetyl)-β-D-glucopyranoside (5), acacetin 7-O-(2″-O-acetyl-6″-O-malonyl)-β-D-glucopyranoside (6), and acacetin (7) by comparing our spectroscopic analysis data (Supplementary Materials, Figures S1–S12 and Table S1) with previously reported data [16,20]. Interestingly, the trace compound salvianolic acid B (2) was first identified in the ARE. The structure of 2 was elucidated using established spectroscopic analysis data of NMR and ESI-HR-MS. Among the isolated compounds, 7 exhibited the highest XO inhibitory effect with a molecular formula of C16H12O5 as established by the [M+H]+ ion at m/z 285.0755 (calcd. 285.0763) in the ESI-HR-MS analysis using qTof MS. The 13C-NMR data enabled carbons corresponding to seven C–C double bonds and one carbonyl group to be identified, thus accounting for 8 of the 11 degrees of unsaturation. The extra three degrees of unsaturation were ascribed to three aromatic rings. In the 1H-NMR spectrum, the presence of seven aromatic protons indicated a trisubstituted typical flavonoid skeleton. Four proton values for ring B protons appeared in the form of two sets of doublets (J = 8.8 Hz) at δ 8.02 (2H, H-2′, H-6′) and 7.10 (2H, H-3′, H-5′) with their corresponding carbons at 128.3 and 114.6 ppm, respectively. Thus, 7 was identified as acacetin.
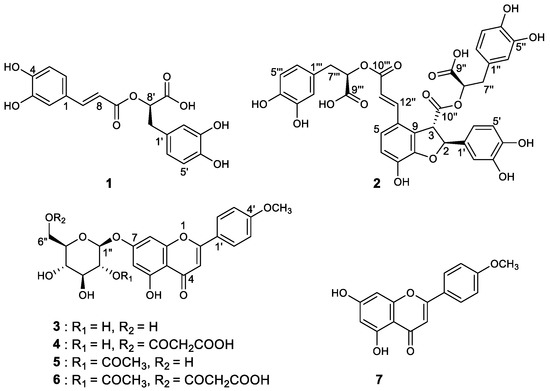
Figure 1.
Structures of compounds 1–7 isolated from A. rugosa.
A. rugosa is a well-known aromatic plant; most studies have focused on its volatile components including essential oils such as estragole, limonene, menthone, and pulegone. Until recently, Hong et al. reported that the essential oil components of A. rugosa help improving human concentration and freshness of the human brain [24]. Furthermore, the non-volatile polar components of A. rugosa are important, including acacetin (7) and their derivatives because they induce a very strong effect on the XO inhibitory activity according to our study.
3.2. XO Inhibitory Activity of Identified Compounds
All isolated compounds 1–7 were evaluated for their inhibitory effects on XO activity and their inhibitory mechanism was derived through kinetic analysis. Phenolic acid derivatives (1 and 2), flavone glycosides (3, 4, 5, and 6), and flavone aglycone (7) had a concentration-dependent effect (Figure 2A). Nile et al. reported that phenolic acid derivatives containing caffeic acid, ferulic acid, and sinapic acid are effective against XO [25]. Lin et al. also reported flavonoids such as apigenin, luteoline, kaempferol, and quercetin had potent XO inhibitory effects [18]. However, the XO inhibitory effect showed that phenolic acids and flavonoid compounds were inactive (IC50: >25 μM) except for acacetin (7), a flavonoid aglycone (Table 1).
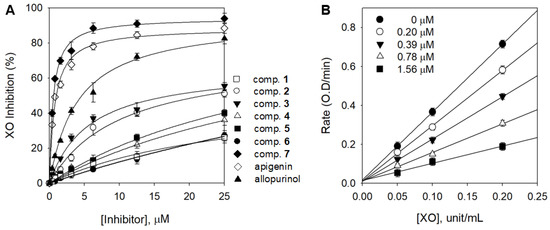
Figure 2.
(A) Inhibitory effects of compounds (1–7) on the activity of XO based on the oxidation of xanthine to uric acid. (B) Reversible relationship of the catalytic activity of XO between enzyme concentrations in compound 7 at different concentrations.
Compound 7 exhibited considerably higher inhibitory activity (75.5%) at the same concentration of 5 μM compared with apigenin (67.2%) and allopurinol (51.9%), which were used as positive controls. Acacetin (7) (IC50 = 0.58 µM), which is more effective than apigenin (IC50 = 0.87 µM) and is known to have the highest inhibitory activity among flavonoids, was revealed for the first time. It appears that inhibition was higher when (7) has a methoxy group instead of a hydroxyl group at the 4′-position. These results are in agreement with those reported by Liu, in which some flavonoids have higher inhibitory activity when they are substituted with methoxy groups [26]. Acacetin glycosides (3–6) showed significantly lower inhibitory activities, between 26.4 and >100 µM, compared with aglycone 7. This difference may be due to the presence of the monosaccharide glucoside and the additional malonyl, acetyl, or acetyl–malonyl on the sugar moiety. Nguyen et al. used luteolin and luteolin 7-O-glucoside and revealed that a glycoside is more than 10 times weaker than its aglycone [27]. Biochemically, the XO inhibition is associated with the hydrogen bonding between the phenolic hydroxyl functionalities of the substrates and the abundant amino groups in the peptide chain of the enzyme. Therefore, the glycosyl substitution of the A-ring C6 of acacetin (3–6) more effectively prevents the binding to the XO active site than aglycone 7 [28]. As shown in Figure 2B, to confirm the reversible inhibition caused by (7), a plot of the various inhibitor concentrations (0 μM, 0.20 μM, 0.39 μM, 0.78 μM, and 1.56 μM) versus its activity at different enzyme concentrations (0 unit/mL, 0.05 unit/mL, 0.1 unit/mL, and 0.2 unit/mL) was analyzed.
The kinetic behaviors of the oxidation of xanthine catalyzed by XO at different concentrations of inhibitor were analyzed using both Lineweaver–Burk (Figure 3A) and Dixon plots (Figure 3B). Figure 3A shows the mixed-type inhibitor, indicating that Vmax was decreased, whereas Km increased with the increasing concentration of 7 from 0 to 1.56 μM. The two possible inhibition mechanisms were investigated to reveal which one gives this kinetic profile. The mechanisms of mixed inhibition can be investigated through the changes of the substrate with inhibitor followed by measurements of the residual enzyme–substrate complex. Parameters of free enzyme (KI) and the enzyme–substrate complex (KIS) can be fitted to Equations (2) and (3) [29]. The secondary plots of the straight lines of the slope and intercept showed that KI (0.57 μM) was much less than KIS (1.14 μM). It is thus reasonable to conclude that 7 is allowed as mixed type I when the inhibitor prefers the free enzyme over the substrate–enzyme complex. The values of KIS were approximately two times greater, suggesting a weaker binding of 7 to the XO-xanthine intermediate. This indicates that the inhibition mechanism is competitive and predominated over noncompetitive. The Ki value of 7 was determined to be 0.61 μM by the Dixon plots (Figure 3B). Further analysis of the progress of the inhibition was undertaken by preincubating the enzyme with 7, prior to the addition of the substrate. The enzyme lost no more than 10% of its activity across the whole assay due to intrinsic decay (control). When the enzyme was preincubated with an inhibitor (0.2 μM) for a period of time, a sustained decrease in activity was observed, demonstrating that it has a time-dependent inhibitory behavior (Figure 3C).
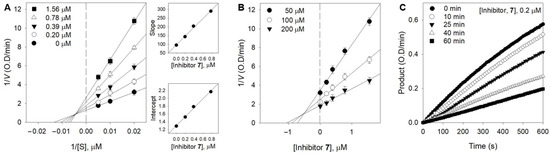
Figure 3.
Enzymatic kinetics of XO inhibition by 7. (A) Lineweaver–Burk plots of 7. (Inset) The secondary plots of the slope and the intercept of the straight lines versus the concentration of 3 throughout Equations (1)–(3) are shown. (B) Dixon plots of 7. The graphical symbols are substrate concentrations (50 µM, ●; 100 µM, ○; 200 µM, ▼). (C) Inhibition as a function of preincubation time for 7 at 0.2 µM.
3.3. UPLC-qTof Mass Spectrometry Profiles
To increase the industrial applicability of the ARE, the extraction conditions were optimized and the content of potent XO inhibitor (7), which had the highest inhibitory activity, was analyzed using UPLC-qTof MS. The extracts for quantitative analysis were repeatedly sonicated for 30 min at 25 °C using 5 different solvents (EtOH, 70% EtOH, 50% EtOH, 30% EtOH, and H2O) and the most abundant peaks (1–7) were identified in 50% ARE. As shown in Table 1, the 50% ARE showing the highest inhibitory activity on XO showed the highest yield of 11.3% compared with the other extracts; the content of each peak was also the most abundant. The contents of 7 isolated compounds in the different ARE were analyzed using a UPLC at 265 nm, the maximum UV wavelength of flavonoid derivatives. All detected peaks showed molecular ions with masses consistent with those of our isolated compounds (1, m/z 359.0777 [M–H]−; 2, m/z 717.1453 [M–H]−; 3, m/z 447.1286 [M+H]+; 4, m/z 533.1293 [M+H]+; 5, m/z 489.1394 [M+H]+; 6, m/z 575.1399 [M+H]+; 7, m/z 285.0755 [M+H]+). The standard curve demonstrated a highly validated correlation coefficient (r2 > 0.998) and was linear and reproducible. As a result of the quantitative analysis, it was confirmed that the contents of 1–7 in the extracted 50% EtOH were 4.05 mg/g, 0.06 mg/g, 9.04 mg/g, 5.12 mg/g, 0.37 mg/g, 0.45 mg/g, and 1.96 mg/g per dry sample, respectively. Table 2 shows the retention time (tR), UV absorption maxima (λmax), and MS spectral data, including molecular ion and elemental composition of the peaks (1–7) [16,20]. A PDA chromatogram of 50% ARE (Figure 4A) shows that acacetin (7), which has the highest inhibitory activity on XO, is accumulated as a monosaccharide glycoside (3) or mostly substituted with units of malonyl-glc (4), acetyl-glc (5), or acetyl-malonyl-glc (6). Thus, to hydrolyze glycoside compounds (3–6) to aglycone 7 (Figure 4B), 50% ARE was treated with β-glucosidase at 37 °C for 2 h. Gratifyingly, the hydrolyzed 50% ARE exhibited 5-fold higher XO inhibition than the control ARE with IC50 = 6.2 µg/mL and IC50 = 32.4 µg/mL, respectively. Furthermore, it was almost converted to inhibitor 7 (10.2 mg/g per dry sample) and the content increased by more than 5 times compared with that before treatment (1.96 mg/g per dry sample). According to Kim et al., the acacetin (7) content increased to 3 mg/g per dry sample level when ARE was treated with lactic acid bacteria [30]. However, such an increase in the 7 content caused by bacterial treatment indicates that the selective hydrolysis of only the glucose moiety with the β form or its complete conversion to an aglycone is difficult. In addition, regarding the industrial use of the hydrolysis extract of 50% ARE, more research is needed involving acid hydrolysis in addition to treatment with enzymes or bacteria because acid hydrolysis is more competitive in terms of manufacturing cost.

Table 2.
Spectroscopic characteristics and contents (mg/g) of the seven investigated compounds from the aerial parts of A. rugosa.
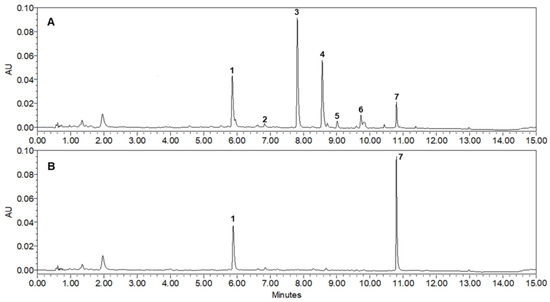
Figure 4.
UPLC-PDA chromatograms of 50% EtOH extract from the aerial parts of A. rugosa (265 nm): (A) Control of 50% ARE; (B) hydrolysis of 50% ARE with 0.2 units of β-glucosidase. The order of peaks (1–7) is: 1. rosmarinic acid, 2. salvianolic acid B, 3. tilianin, 4. acacetin 7-O-(6″-O-malonyl)-β-D-glucopyranoside, 5. acacetin 7-O-(2″-O-acetyl)-β-D-glucopyranoside, 6. acacetin 7-O-(2″-O-acetyl-6″-O-malonyl)-β-D-glucopyranoside, and 7. acacetin.
4. Conclusions
The ARE exhibited a significant inhibitory effect on XO in vitro. Importantly, it was revealed for the first time that the most potent inhibitor was acacetin (7) with a low micromolar inhibition (IC50 = 0.58 μM) against XO. The evaluation of the detailed kinetic analysis using double-reciprocal plots revealed that 7 exhibited a mixed-type inhibition (Ki = 0.61 μM). The index/active ingredient information was provided, and rapid analysis methods for standardization and quality control of the ARE were conducted. In addition, this work presents the possibility of developing a material that maximizes the active ingredient through hydrolysis; we expect that the ARE can be industrially used as a natural drug for the management of hyperuricemia and treatment of gout.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12030573/s1, Figures S1−S4. 1H-NMR spectra of 1–7. Figures S5−S8. 13C-NMR spectra of 1–7. Figures S9−S12. Identification of 1–7 by UPLC-qTOf MS. Table S1. 1H and 13C-NMR assign of compounds (1–7).
Author Contributions
Conceptualization, H.W.R.; Methodology, H.J.Y.; Data curation, H.W.R.; Writing—original draft, H.J.Y.; Supervision, D.-S.K.; Project administration, D.-S.K. This study was performed based on the design of H.W.R. and D.-S.K., the corresponding authors. All authors approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2022R1C1C1009298).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gibson, T. Hyperuricemia, gout and the kidney. Curr. Opin. Rheumatol. 2012, 24, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Bitik, B.; Öztürk, M.A. An old disease with new insights: Update on diagnosis and treatment of gout. Eur. J. Rheumatol. 2014, 1, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zheng, A.; Xu, P.; Wang, J.; Xue, T.; Dai, S.; Pan, S.; Guo, Y.; Xie, X.; Li, L.; et al. High-protein diet induces hyperuricemia in a new animal model for studying human gout. Int. J. Mol. Sci. 2020, 21, 2147. [Google Scholar]
- Martillo, M.A.; Nazzal, L.; Crittenden, D.B. The crystallization of monosodium urate. Curr. Rheumatol. Rep. 2014, 16, 400. [Google Scholar] [CrossRef]
- Fukunari, A.; Okamoto, K.; Nishino, T.B.; Eger, T.; Pai, E.F.; Kamezawa, M.; Yamada, I.; Kato, N. Y-700 [1-[3-Cyano-4-(2,2-dimethylpropoxy)phenyl]-1H-pyrazole-4-carboxylic acid]: A potent xanthine oxidoreductase inhibitor with hepatic excretion. J. Pharmacol. Exp. Ther. 2004, 311, 519–528. [Google Scholar] [CrossRef]
- Huo, L.-N.; Wang, W.; Zhang, C.-Y.; Shi, H.-B.; Liu, Y.; Liu, X.H.; Guo, B.-H.; Zhao, D.-M.; Gao, H. Bioassy-guided isolation and identification of xanthine oxidase inhibitory constituents from the leaves of Perilla frutescens. Molecules 2015, 20, 17848–17859. [Google Scholar] [CrossRef]
- Orhan, I.E.; Deniz, F.S.S. Natural products and extracts as xantine oxidase inhibitors—A hope for gout disease? Curr. Pharm. Des. 2021, 27, 143–158. [Google Scholar] [CrossRef]
- Anand, S.; Pang, E.; Livanos, G.; Mantri, N. Characterization of Physico-Chemical Properties and Antioxidant Capacities of Bioactive Honey Produced from Australian Grown Agastache rugosa and its Correlation with Colour and Poly-Phenol Content. Molecules 2018, 23, 108. [Google Scholar] [CrossRef]
- Hong, S.; Cha, K.H.; Kwon, D.Y.; Son, Y.J.; Kim, S.M.; Choi, J.H.; Yoo, G.; Nho, C.W. Agastache rugosa ethanol extract suppresses bone loss via induction of osteoblast differentiation with alteration of gut microbiota. Phytomedicine 2021, 84, 153517. [Google Scholar] [CrossRef]
- Cao, P.; Xie, P.; Wang, X.; Wang, J.; Wei, J.; Kang, W.Y. Chemical constituents and coagulation activity of Agastache rugosa. BMC Complement. Altern. Med. 2017, 17, 93. [Google Scholar] [CrossRef]
- Oh, Y.R.; Lim, H.W.; Huang, Y.H.; Kwon, H.S.; Jin, C.D.; Kim, K.H.; Lim, C.J. Attenuating properties of Agastache rugosa leaf extract against ultraviolet-B-induced photoaging via up-regulating glutathione and superoxide dismutase in a human keratinocyte cell line. J. Photochem. Photobiol. B Biol. 2016, 163, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.; Gu, M.J.; Han, J.; Cho, W.; Ma, J.Y. Agastache rugosa Kuntze extract, containing the active component rosmarinic acid, prevents atherosclerosis through up-regulation of the cyclin-dependent kinase inhibitors p21WAF1/CIP1 and p27KIP1. J. Funct. Foods 2017, 30, 30–38. [Google Scholar] [CrossRef]
- Jang, S.A.; Hwang, Y.H.; Kim, T.; Yang, H.; Lee, J.; Seo, Y.H.; Park, J.I.; Ha, H. Water extract of Agastache rugosa prevents ovariectomy-induced bone loss by inhibiting osteoclastogenesis. Foods 2020, 9, 1181. [Google Scholar] [CrossRef]
- Lee, Y.; Lim, H.W.; Ryu, I.W.; Huang, Y.H.; Park, M.; Chi, Y.M.; Lim, C.J. Anti-inflammatory, barrier-protective, and antiwrinkle properties of Agastache rugosa Kuntze in human epidermal keratinocytes. BioMed Res. Int. 2020, 2020, 1759067. [Google Scholar] [CrossRef] [PubMed]
- Shin, S. Essential oil compounds from Agastache rugosa as antifungal agents against Trichophyton species. Arch. Pharm. Res. 2004, 27, 295–299. [Google Scholar] [CrossRef]
- An, J.H.; Yuk, H.J.; Kim, D.Y.; Nho, C.W.; Lee, D.H.; Ryu, H.W.; Oh, S.R. Evaluation of phytochemicals in Agastache rugosa (Fisch. & C.A.Mey.) Kuntze at different growth stages by UPLC-QTof-MS. Ind. Crops Prod. 2018, 112, 608–616. [Google Scholar]
- Yeo, H.J.; Park, C.H.; Park, Y.E.; Hyeon, H.; Kim, J.K.; Lee, S.Y.; Park, S.U. Metabolic profiling and antioxidant activity during flower development in Agastache rugose. Physiol. Mol. Biol. Plants 2021, 27, 445–455. [Google Scholar] [CrossRef]
- Lin, C.M.; Chen, C.S.; Chen, C.T.; Liang, Y.C.; Lin, J.K. Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem. Biophys. Res. Commun. 2002, 294, 167–172. [Google Scholar] [CrossRef]
- Yuk, H.J.; Song, Y.H.; Curtis-Long, M.J.; Kim, D.W.; Woo, S.G.; Lee, Y.B.; Uddin, Z.; Kim, C.Y.; Park, K.H. Ethylene induced a high accumulation of dietary isoflavones and expression of isoflavonoid biosynthetic genes in soybean (Glycine max) leaves. J. Agric. Food Chem. 2016, 64, 7315–7324. [Google Scholar] [CrossRef]
- Ai, C.B.; Li, L.N. Stereostructure of salvianolic acid B and isolation of salvianolic acid C from Salvia miltiorrhiza. J. Nat. Prod. 1988, 51, 145–149. [Google Scholar] [CrossRef]
- Yuk, H.J.; Lee, Y.S.; Ryu, H.W.; Kim, S.H.; Kim, D.S. Effects of Toona sinensis leaf extract and its chemical constituents on xanthine oxidase activity and serum uric acid levels in potassium oxonate-induced hyperuricemic rats. Molecules 2018, 23, 3254. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.J.; Kim, J.W.; Sung, Y.Y.; Kim, D.S. Phloroglucinol derivatives from Dryopteris crassirhizoma as potent xanthine oxidase inhibitors. Molecules 2021, 26, 122. [Google Scholar] [CrossRef]
- Kim, J.Y.; Wang, Y.; Li, Z.P.; Baiseitova, A.; Ban, Y.J.; Park, K.H. Xanthine oxidase inhibition and anti-LDL oxidation by prenylated isoflavones from Flemingia philippinensis root. Molecules 2020, 25, 3074. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Jang, H.; Bo, S.; Kim, M.; Deepa, P.; Park, J.; Sowndhararajan, K.; Kim, S. Changes in human electroencephalographic activity in response to Agastache rugosa essential oil exposure. Behav. Sci. 2022, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Keum, Y.S.; Nile, A.S.; Kwon, Y.D.; Kim, D.H. Potential cow milk xanthine oxidase inhibitory and antioxidant activity of selected phenolic acid derivatives. J. Biochem. Mol. Toxicol. 2018, 32, e22005. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, L.; Ren, L.; Xie, Y. Advances in structures required of polyphenols for xanthine oxidase inhibition. Food Front. 2020, 1, 152–167. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Awale, S.; Tezuka, Y.; Ueda, J.Y.; Tran, Q.L.; Kadota, S. Xanthine oxidase inhibitors from the flowers of Chrysanthemum sinense. Planta Medica 2006, 72, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Fernandes, E.; Roleira, F. Progress towards the discovery of xanthine oxidase inhibitors. Curr. Med. Chem. 2002, 9, 195–217. [Google Scholar] [CrossRef]
- Seo, W.D.; Kim, J.W.; Ryu, H.W.; Kim, J.H.; Han, S.I.; Ra, J.E.; Seo, K.H.; Jang, K.C.; Lee, J.H. Identification and characterisation of coumarins from the roots of Angelica dahurica and their inhibitory effects against cholinesterase. J. Funct. Foods 2013, 5, 1421–1431. [Google Scholar] [CrossRef]
- Kim, N.Y.; Kwon, H.S.; Lee, H.Y. Effect of inhibition on tyrosinase and melanogenesis of Agastache rugosa Kuntze by lactic acid bacteria fermentation. J. Cosmet. Dermatol. 2016, 16, 407–415. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).