Feeding Aquilaria sinensis Leaves Modulates Lipid Metabolism and Improves the Meat Quality of Goats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Nutritional Ingredients Analysis of A. sinensis Leaves
2.3. Bioactive Compounds Analysis of A. sinensis Leaves
2.4. Serum Biochemistry Analysis of Goats
2.5. UPLC-MS/MS Metabolomics Analysis of Serum and Muscle Samples
2.6. Statistical Analysis
3. Results
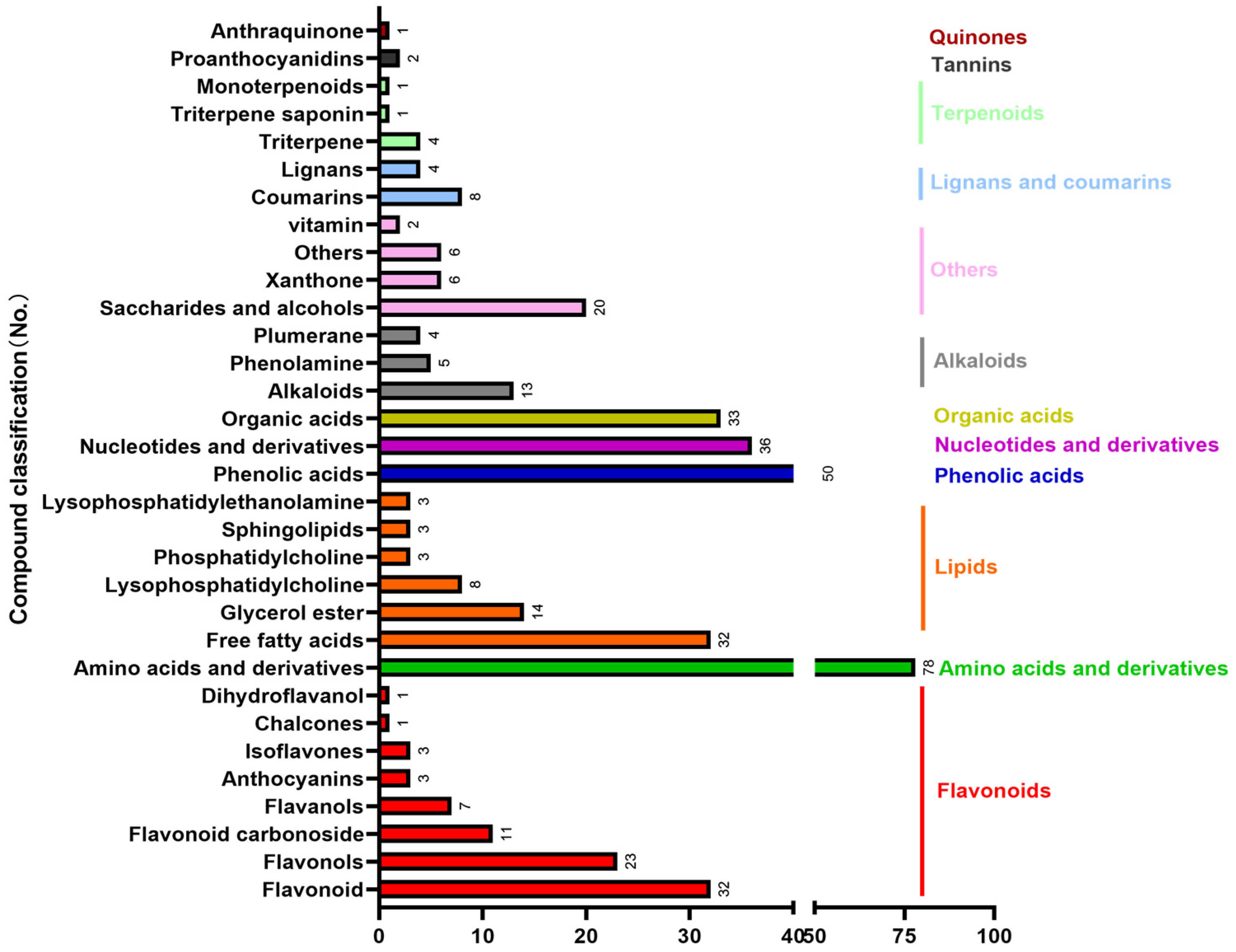
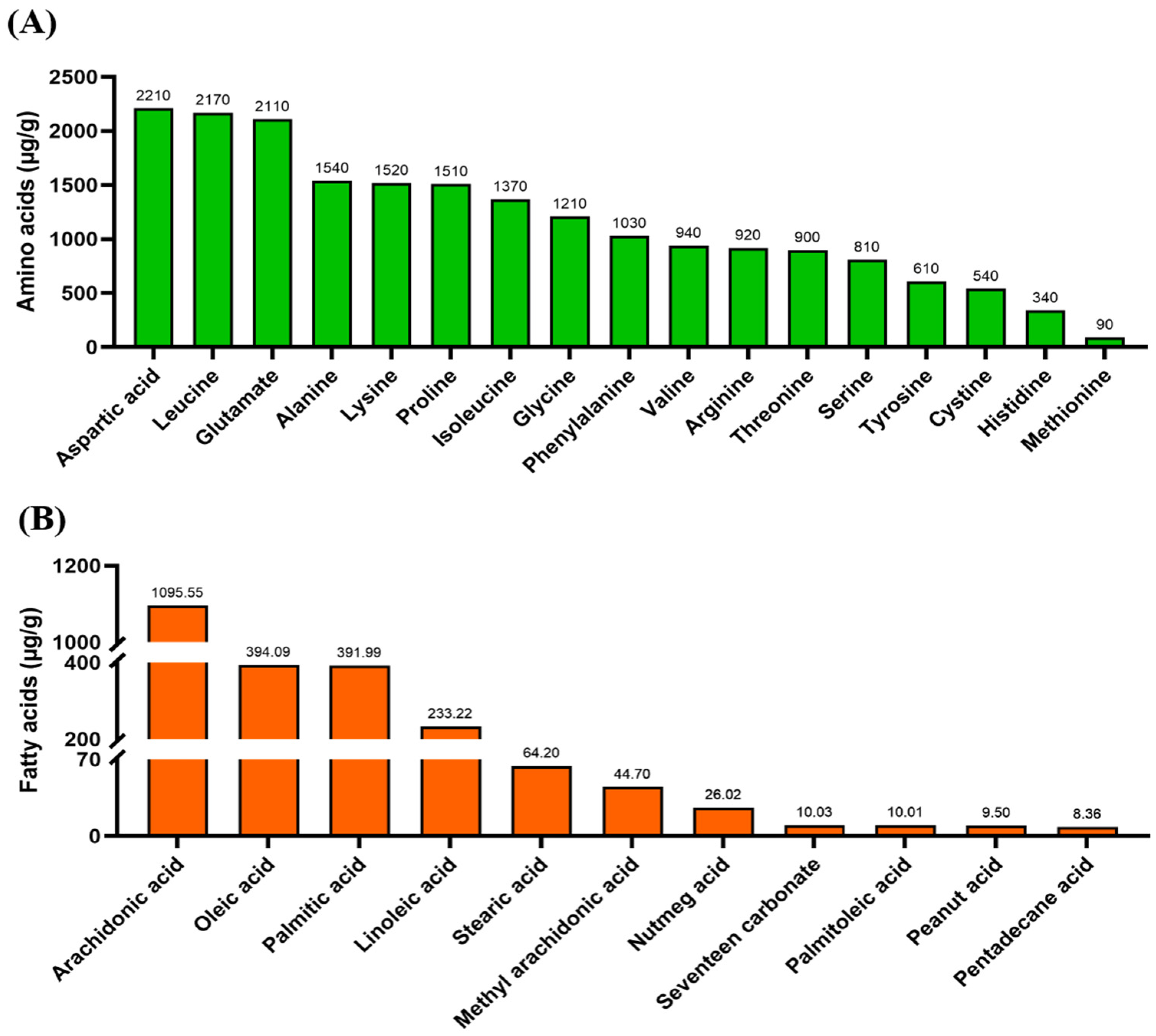
3.1. Nutritional Ingredients and Bioactive Compounds of A. sinensis Leaves
3.2. Effects of Feeding A. sinensis Leaves on Growth Performance and Serum Biochemical Indexes of Goats
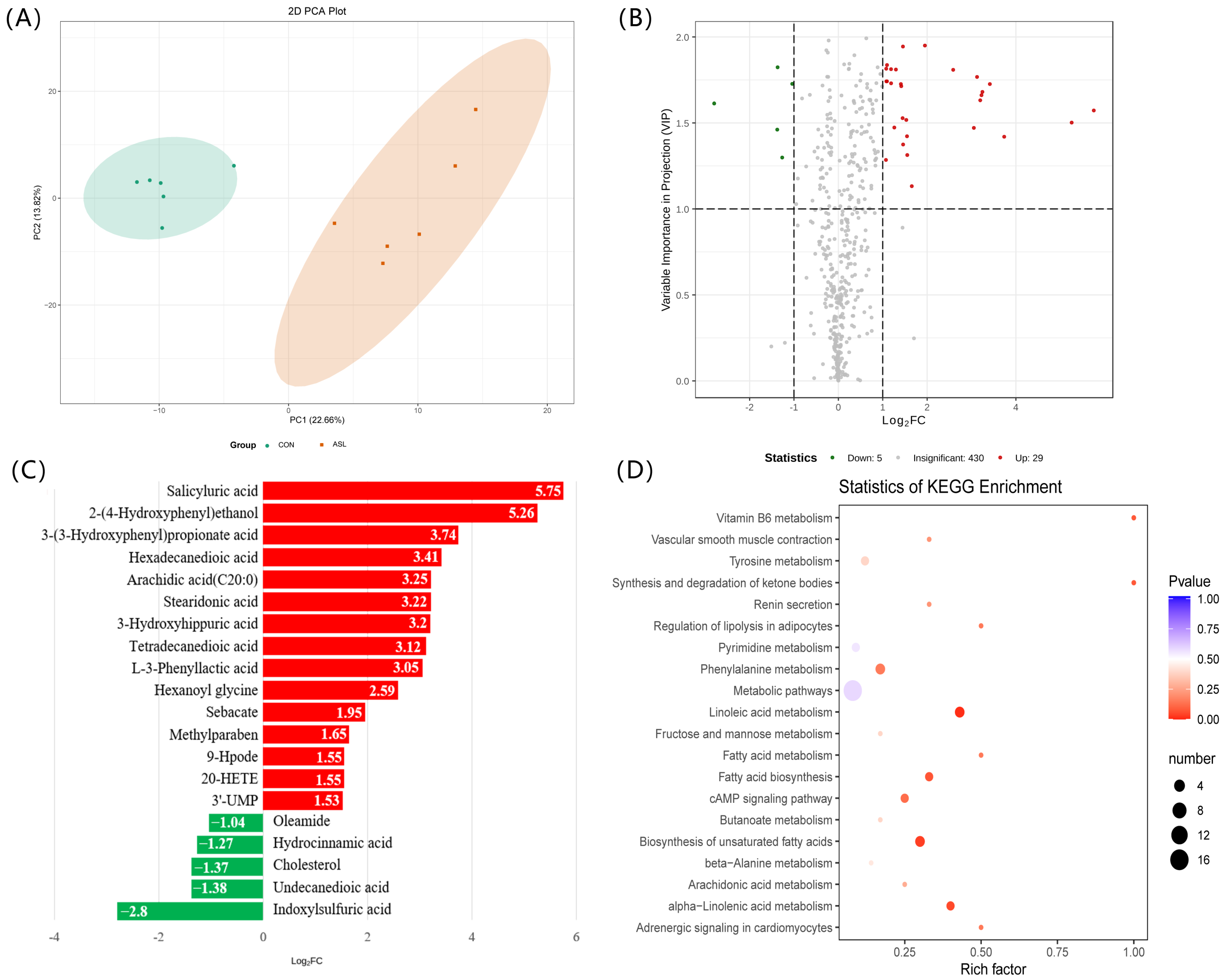
3.3. Changes of the Metabolites and the Corresponding Pathways of Serum and Muscle in Goats
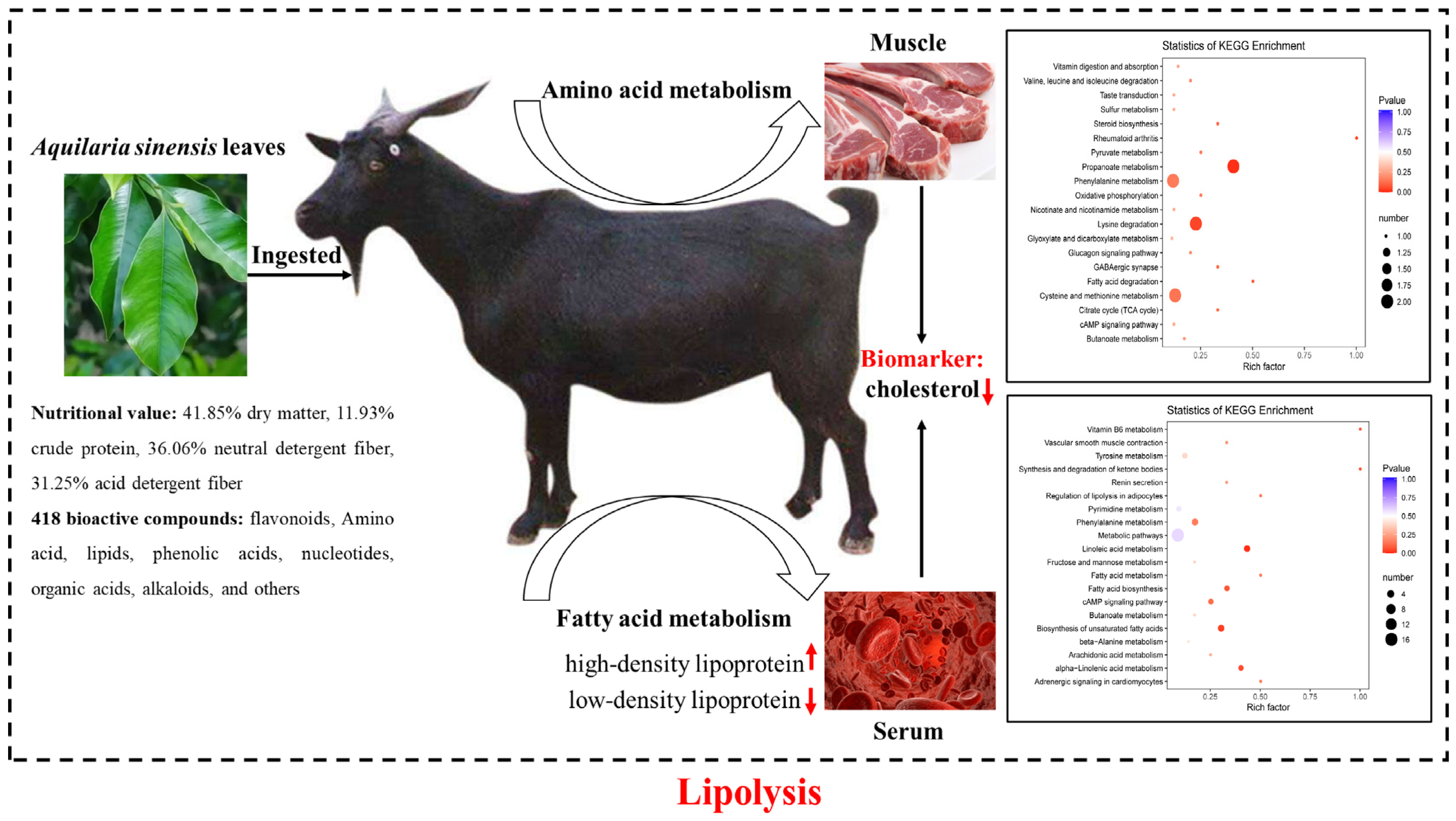
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, G.; Liu, C.; Sun, W. Pollination and seed dispersal of Aquilaria sinensis (Lour.) Gilg (Thymelaeaceae): An economic plant species with extremely small populations in China. Plant Divers. 2016, 38, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.-W.; Zhao, J.-P.; Liu, Y.-B.; Qiu, Y.-X.; Xie, Q.-L.; Li, M.-J.; Khan, I.A.; Wang, W. Advance in Studies on Chemical Constituents, Pharmacology and Quality Control of Aquilaria sinensis. Digit. Chin. Med. 2018, 1, 316–330. [Google Scholar] [CrossRef]
- Hsiao, S.-W.; Wu, Y.-C.; Mei, H.-C.; Chen, Y.-H.; Hsiao, G.; Lee, C.-K. Constituents of Aquilaria sinensis Leaves Upregulate the Expression of Matrix Metalloproteases 2 and 9. Molecules 2021, 26, 2537. [Google Scholar] [CrossRef] [PubMed]
- Wongwad, E.; Pingyod, C.; Saesong, T.; Waranuch, N.; Wisuitiprot, W.; Sritularak, B.; Temkitthawon, P.; Ingkaninan, K. Assessment of the bioactive components, antioxidant, antiglycation and anti-inflammatory properties of Aquilaria crassna Pierre ex Lecomte leaves. Ind. Crop. Prod. 2019, 138, 111448. [Google Scholar] [CrossRef]
- Adam, A.Z.; Lee, S.Y.; Mohamed, R. Pharmacological properties of agarwood tea derived from Aquilaria (Thymelaeaceae) leaves: An emerging contemporary herbal drink. J. Herb. Med. 2017, 10, 37–44. [Google Scholar] [CrossRef]
- Sanon, H.O.; Kaboré-Zoungrana, C.; Ledin, I. Growth and carcass characteristics of male Sahelian goats fed leaves or pods of Pterocarpus lucens or Acacia senegal. Livest. Sci. 2008, 117, 192–202. [Google Scholar] [CrossRef]
- Oni, A.O.; Arigbede, O.M.; Oni, O.O.; Onwuka, C.F.I.; Anele, U.Y.; Oduguwa, B.O.; Yusuf, K.O. Effects of feeding different levels of dried cassava leaves (Manihot esculenta, Crantz) based concentrates with Panicum maximum basal on the performance of growing West African Dwarf goats. Livest. Sci. 2010, 129, 24–30. [Google Scholar] [CrossRef]
- Idamokoro, E.M.; Masika, P.J.; Muchenje, V. Vachellia karroo leaf meal: A promising non-conventional feed resource for improving goat production in low-input farming systems of Southern Africa. Afr. J. Range Forage Sci. 2016, 33, 141–153. [Google Scholar] [CrossRef]
- Ghavipanje, N.; Nasri, M.H.F.; Farhangfar, H.; Modaresi, J. In Situ, In Vitro and In Vivo nutritive value assessment of Barberry leaf as a roughage for goat feeding. Small Rumin. Res. 2016, 141, 94–98. [Google Scholar] [CrossRef]
- Babiker, E.E.; Juhaimi, F.A.L.; Ghafoor, K.; Abdoun, K.A. Comparative study on feeding value of Moringa leaves as a partial replacement for alfalfa hay in ewes and goats. Livest. Sci. 2017, 195, 21–26. [Google Scholar] [CrossRef]
- Fernández, C.; Pérez-Baena, I.; Marti, J.V.; Palomares, J.L.; Jorro-Ripoll, J.; Segarra, J.V. Use of orange leaves as a replacement for alfalfa in energy and nitrogen partitioning, methane emissions and milk performance of murciano-granadina goats. Anim. Feed Sci. Technol. 2019, 247, 103–111. [Google Scholar] [CrossRef]
- García, E.M.; López, A.; Zimerman, M.; Hernández, O.; Arroquy, J.I.; Nazareno, M.A. Enhanced oxidative stability of meat by including tannin-rich leaves of woody plants in goat diet. Asian-Australas. J. Anim. Sci. 2019, 32, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Kausar, T.; Kausar, M.A.; Khan, S.; Haque, S.; Azad, Z.R.A.A. Optimum Additive Composition to Minimize Fat in Functional Goat Meat Nuggets: A Healthy Red Meat Functional Food. Processes 2021, 9, 475. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Sahfi, M.E.; Elnesr, S.S.; Alqaisi, O.; El-Kassas, S.; Al-wajeeh, A.S.; Taha, A.E.; Abd E-Hack, M.E. Phytochemical characteristics of Paulownia trees wastes and its use as unconventional feedstuff in animal feed. Anim. Biotechnol. 2022, 33, 586–593. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Zhan, H.; Xiong, Y.; Wang, Z.; Dong, W.; Zhou, Q.; Xie, S.; Li, X.; Zhao, S.; Ma, Y. Integrative analysis of transcriptomic and metabolomic profiles reveal the complex molecular regulatory network of meat quality in Enshi black pigs. Meat Sci. 2022, 183, 108642. [Google Scholar] [CrossRef]
- Zhang, N.; Xue, S.; Song, J.; Zhou, X.; Zhou, D.; Liu, X.; Hong, Z.; Xu, D. Effects of various artificial agarwood-induction techniques on the metabolome of Aquilaria sinensis. BMC Plant Biol. 2021, 21, 591. [Google Scholar] [CrossRef]
- Alam, J.; Mujahid, M.; Badruddeen; Jahan, Y.; Bagga, P.; Rahman, M.A. Hepatoprotective potential of ethanolic extract of Aquilaria agallocha leaves against paracetamol induced hepatotoxicity in SD rats. J. Tradit. Complement. Med. 2017, 7, 9–13. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.-Q.; Wang, H.; Mei, W.-L.; Dai, H.-F. Natural products in agarwood and Aquilaria plants: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 528–565. [Google Scholar] [CrossRef]
- Cappai, M.G.; Liesegang, A.; Dimauro, C.; Mossa, F.; Pinna, W. Circulating electrolytes in the bloodstream of transition Sarda goats make the difference in body fluid distribution between single vs. twin gestation. Res. Vet. Sci. 2019, 123, 84–90. [Google Scholar] [CrossRef]
- Chae, H.-S.; Dale, O.; Mir, T.M.; Avula, B.; Zhao, J.; Khan, I.A.; Khan, S.I. A Multitarget Approach to Evaluate the Efficacy of Aquilaria sinensis Flower Extract against Metabolic Syndrome. Molecules 2022, 27, 629. [Google Scholar] [CrossRef] [PubMed]
- Peet, D.J.; Janowski, B.A.; Mangelsdorf, D.J. The LXRs: A new class of oxysterol receptors. Curr. Opin. Genet. Dev. 1998, 8, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.-T.; Liu, S.-Y.; Kuang, Y.-J.; Jiang, Z.-M.; Lin, Y.; Xie, Z.-S.; Liu, E.H. Network pharmacology analysis and experimental validation to explore the mechanism of sea buckthorn flavonoids on hyperlipidemia. J. Ethnopharmacol. 2021, 264, 113380. [Google Scholar] [CrossRef]
- Yang, M.H.; Ali, Z.; Khan, S.I.; Khan, I.A. Characterization of chemical constituents from Thymelaea hirsuta with PPARα/γ modulation activity. Planta Med. 2014, 80, PD124. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Lee, J.W.; Mun, H.S.; Yang, C.J. Effects of supplementation with green tea by-products on growth performance, meat quality, blood metabolites and immune cell proliferation in goats. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Rajion, M.A.; Goh, Y.M. Effects of Oils Rich in Linoleic and α-Linolenic Acids on Fatty Acid Profile and Gene Expression in Goat Meat. Nutrients 2014, 6, 3913–3928. [Google Scholar] [CrossRef] [PubMed]
- Subi, S.; Lee, S.J.; Shiwani, S.; Singh, N.K. Differential characterization of myogenic satellite cells with linolenic and retinoic acid in the presence of thiazolidinediones from prepubertal Korean black goats. Asian-Australas. J. Anim. Sci. 2018, 31, 439–448. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, Z.; Tu, J.; Wang, Z.; Gao, X.; Deng, K.; El-Samahy, M.A.; You, P.; Fan, Y.; Wang, F. γ-Linolenic Acid Prevents Lipid Metabolism Disorder in Palmitic Acid-Treated Alpha Mouse Liver-12 Cells by Balancing Autophagy and Apoptosis via the LKB1-AMPK-mTOR Pathway. J. Agric. Food Chem. 2021, 69, 8257–8267. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Li, Y.; Shi, B.; Guo, X.; Zhao, Y.; Yan, S. Flaxseed Oil and Heated Flaxseed Supplements Have Different Effects on Lipid Deposition and Ileal Microbiota in Albas Cashmere Goats. Animals 2021, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.Y.; Sim, G.Y.; Ahn, J.-H. Synthesis of 4-Hydroxybenzoic Acid Derivatives in Escherichia coli. J. Agric. Food Chem. 2020, 68, 9743–9749. [Google Scholar] [CrossRef]
- Ramalingam, V.; Song, Z.; Hwang, I. The potential role of secondary metabolites in modulating the flavor and taste of the meat. Food Res. Int. 2019, 122, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Subbaraj, A.K.; Kim, Y.H.B.; Fraser, K.; Farouk, M.M. A hydrophilic interaction liquid chromatography–mass spectrometry (HILIC–MS) based metabolomics study on colour stability of ovine meat. Meat Sci. 2016, 117, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, Y.-Q.; Yuan, Y.-X.; Xu, P.-W.; Zhang, C.; Li, F.; Wang, L.-N.; Yin, C.; Zhang, L.; Cai, X.-C.; et al. Succinate induces skeletal muscle fiber remodeling via SUCNR1 signaling. EMBO Rep. 2019, 20, e47892. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Zamudio, G.D.; Silva, L.H.P.; Vieira, N.M.; Vilela, R.S.R.; Assis, D.E.F.; Assis, G.J.F.; Estrada, M.M.; Rodrigues, R.T.S.; Duarte, M.S.; Chizzotti, M.L. Effect of short-term dietary protein restriction before slaughter on meat quality and skeletal muscle metabolomic profile in culled ewes. Livest. Sci. 2022, 261, 104956. [Google Scholar] [CrossRef]
- Milićević, D.; Vranić, D.; Mašić, Z.; Parunović, N.; Trbović, D.; Nedeljković-Trailović, J.; Petrović, Z. The role of total fats, saturated/unsaturated fatty acids and cholesterol content in chicken meat as cardiovascular risk factors. Lipids Health Dis. 2014, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D.; Srichaikul, K.K.; Jenkins, D.J.A. Cardiovascular Harm From Egg Yolk and Meat: More Than Just Cholesterol and Saturated Fat. J. Am. Heart. Assoc. 2021, 10, e017066. [Google Scholar] [CrossRef] [PubMed]
- Werdi Pratiwi, N.M.; Murray, P.J.; Taylor, D.G. Total cholesterol concentrations of the muscles in castrated Boer goats. Small Rumin. Res. 2006, 64, 77–81. [Google Scholar] [CrossRef]

| Item | Diets | |
|---|---|---|
| CON | ASL | |
| Ingredients (%) | ||
| Corn straw silage | 48.8 | 33.8 |
| A. sinensis leaves | 20.0 | |
| Peanut vine | 15.5 | 10.5 |
| Corn | 15.2 | 15.5 |
| Soybean meal | 13.8 | 13.5 |
| Wheat bran | 4.1 | 4.1 |
| Salt | 0.5 | 0.5 |
| Dicalcium phosphate | 1.1 | 1.1 |
| Limestone | 0.5 | 0.5 |
| Premix 1 | 0.5 | 0.5 |
| Nutrient composition (%) | ||
| Crude protein | 10.98 | 11.05 |
| Neutral detergent fiber | 41.15 | 38.50 |
| Acid detergent fiber | 25.64 | 24.82 |
| Calcium | 0.64 | 0.64 |
| Phosphorus | 0.41 | 0.41 |
| Digestible energy (MJ/kg) | 10.76 | 10.35 |
| Item | Contents (%) |
|---|---|
| Dry matter | 41.85 |
| Organic matter | 37.10 |
| Crude protein | 11.93 |
| Crude fat | 3.46 |
| Neutral detergent fiber | 36.06 |
| Acid detergent fiber | 31.25 |
| Item | Diets | SEM | p Value | |
|---|---|---|---|---|
| CON | ASL | |||
| Growth performance | ||||
| Initial weight (kg) | 16.4 | 17.0 | 1.13 | 0.32 |
| Final weight (kg) | 21.3 | 21.9 | 1.33 | 0.45 |
| Average daily intake (g/d) | 697.8 | 705.4 | 60.8 | 0.78 |
| Average daily gain (g/d) | 53.5 | 53.7 | 6.51 | 0.92 |
| Serum biochemical indexes | ||||
| Total protein (g/L) | 68.9 | 61.4 | 3.39 | 0.92 |
| Albumin (g/L) | 24.4 | 24.8 | 1.08 | 0.14 |
| Glutamic pyruvic transaminase (U/L) | 11.2 | 13.6 | 0.78 | 0.51 |
| Glutamic oxyacetic transaminase (U/L) | 64.5 | 83.9 | 9.87 | 0.57 |
| Glucose (mmol/L) | 5.85 | 3.62 | 0.17 | 0.23 |
| Blood urea nitrogen (mmol/L) | 8.68 | 8.78 | 0.79 | 0.48 |
| Uric acid (µmol/L) | 25.2 | 16.3 | 1.25 | 0.06 |
| Cholesterol (mmol/L) | 2.11 | 1.49 | 0.12 | 0.01 |
| Triglyceride (mmol/L) | 0.34 | 0.39 | 0.07 | 0.40 |
| High-density lipoprotein (mmol/L) | 1.42 | 1.82 | 0.11 | 0.01 |
| Low-density lipoprotein (mmol/L) | 0.78 | 0.45 | 0.04 | 0.09 |
| Compounds | Classification | VIP | Fold Change | Log2FC |
|---|---|---|---|---|
| Indoxylsulfuric acid | Organic acid and its derivatives | 1.612864 | 0.143783 | −2.798038 |
| Hydrocinnamic acid | Organic acid and its derivatives | 1.298589 | 0.41591 | −1.265657 |
| Undecanedioic acid | Fatty acyls | 1.460975 | 0.384958 | −1.377228 |
| Cholesterol | Lipids | 1.823414 | 0.387121 | −1.369145 |
| Oleamide | Lipids fatty acids | 1.727046 | 0.486523 | −1.039421 |
| Salicyluric acid | Benzene and substituted derivatives | 1.572335 | 53.98397 | 5.754459 |
| 2-(4-Hydroxyphenyl)ethanol | Benzene and substituted derivatives | 1.501871 | 38.21139 | 5.255931 |
| 4,4′-Methylenedianiline | Benzene and substituted derivatives | 1.374475 | 2.748404 | 1.458594 |
| 3-(3-Hydroxyphenyl)Propionate acid | Organic acid and its derivatives | 1.41958 | 13.32459 | 3.736019 |
| 3-Hydroxyhippuric acid | Organic acid and its derivatives | 1.631329 | 9.162949 | 3.195812 |
| L-3-Phenyllactic acid | Organic acid and its derivatives | 1.471008 | 8.295528 | 3.052334 |
| Sebacate | Organic acid and its derivatives | 1.949887 | 3.862228 | 1.949434 |
| N-Oleoyl glycine | Organic acid and its derivatives | 1.944144 | 2.741058 | 1.454733 |
| Imidazoleacetic acid | Organic acid and its derivatives | 1.527683 | 2.726868 | 1.447245 |
| Malonicacid | Organic acid and its derivatives | 1.741892 | 2.135244 | 1.094401 |
| 3-Hydroxybutyrate | Organic acid and its derivatives | 1.741623 | 2.115696 | 1.081132 |
| Hexadecanedioic acid | Lipids fatty acids | 1.72588 | 10.64918 | 3.412671 |
| Arachidic Acid(C20:0) | Lipids fatty acids | 1.680261 | 9.482547 | 3.245275 |
| Stearidonic acid | Lipids fatty acids | 1.661258 | 9.344554 | 3.224126 |
| Tetradecanedioic acid | Lipids fatty acids | 1.767233 | 8.718257 | 3.12404 |
| α-Linolenic acid(C18:3N3) | Lipids fatty acids | 1.713567 | 2.66958 | 1.416613 |
| γ-Linolenic acid(C18:3N6) | Lipids fatty acids | 1.725919 | 2.653937 | 1.408134 |
| Palmitoleic acid(C16:1) | Lipids fatty acids | 1.810366 | 2.455625 | 1.29609 |
| 8,15-Dihete | Lipids fatty acids | 1.730287 | 2.278096 | 1.187828 |
| Hexanoyl glycine | Amino acid metabolomics | 1.808671 | 6.006694 | 2.586571 |
| N-Acetylneuraminic acid | Amino acid metabolomics | 1.473051 | 2.395256 | 1.26018 |
| Methylparaben | Benzoic acid and its derivatives | 1.132065 | 3.146752 | 1.653863 |
| 9-Hpode | Lipids | 1.313142 | 2.93127 | 1.551526 |
| 20-HETE [20-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid] | Oxidized lipid | 1.422309 | 2.920729 | 1.546329 |
| 9,10-DiHOME [(±)9,10-dihydroxy-12Z-octadecenoic acid] | Oxidized lipid | 1.28463 | 2.101768 | 1.071603 |
| 3′-UMP | Nucleotide metabolomics | 1.517275 | 2.888509 | 1.530325 |
| 4-Pyridoxic acid | Pyridine and pyridine derivatives | 1.812439 | 2.275612 | 1.186255 |
| Epinephrine | Hormones | 1.836576 | 2.13752 | 1.095938 |
| L-Rhamnose | Carbohydrate metabolomics | 1.815123 | 2.111195 | 1.07806 |
| Compounds | Classification | VIP | Fold Change | Log2FC |
|---|---|---|---|---|
| Quinine | Alkaloid | 1.117945 | 0.005174 | −7.59449 |
| (±)5-HETE [(±)5-hydroxy−6E,8Z,11Z,14Z-eicosatetraenoic acid] | Oxidized lipid | 1.45057 | 0.08001 | −3.643675 |
| Sebacate | Organic acid and its derivatives | 1.199688 | 0.207823 | −2.266575 |
| Enterodiol | Phenols and its derivatives | 1.690837 | 0.239295 | −2.063138 |
| 5-amino-1-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]imidazole-4-carboxamide | Nucleotide metabolomics | 1.591578 | 0.345404 | −1.533644 |
| Cholesterol | Lipids | 1.112062 | 0.381854 | −1.388905 |
| Cortisol 21-acetate | Lipids | 1.233468 | 0.39921 | −1.324779 |
| 12-Hete | Lipids | 1.159916 | 0.485659 | −1.041984 |
| Marmesin | Carbohydrate metabolomics | 1.639964 | 0.408265 | −1.292422 |
| (±)12-HETE [(±)12-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid] | Oxidized lipid | 1.381799 | 0.433116 | −1.207174 |
| 6-β-hydroxytestosterone | Hormones | 1.017614 | 0.47224 | −1.082408 |
| 3-Indolebutyric acid | Indole and its derivatives | 1.031324 | 0.488706 | −1.032961 |
| Salicyluric acid | Benzene and substituted derivatives | 1.852642 | 68.24099 | 6.092567 |
| Diphenyl ether | Benzene and substituted derivatives | 1.190736 | 2.148731 | 1.103485 |
| L-Cystathionine | Amino acid metabolomics | 2.034695 | 7.419325 | 2.891288 |
| 3-Hydroxyhippuric acid | Organic acid and its derivatives | 1.030264 | 6.878891 | 2.782176 |
| Vanillic acid | Organic acid and its derivatives | 1.674148 | 3.891708 | 1.960404 |
| Methylmalonic acid | Organic acid and its derivatives | 1.391963 | 2.752149 | 1.460559 |
| Aminomalonic acid | Organic acid and its derivatives | 1.477349 | 2.632414 | 1.396387 |
| Glutaric acid | Organic acid and its derivatives | 2.310351 | 2.204869 | 1.140693 |
| 3,4,5-Trimethoxycinnamic acid | Organic acid and its derivatives | 1.179124 | 2.199951 | 1.137471 |
| Methylparaben | Benzoic acid and its derivatives | 1.033092 | 6.023043 | 2.590493 |
| γ-glutamyl-leucine | Amino acid metabolomics | 1.750045 | 2.192277 | 1.13243 |
| Hexanoyl glycine | Amino acid metabolomics | 1.785677 | 3.271286 | 1.709858 |
| N-Isovaleroylglycine | Amino acid metabolomics | 1.558525 | 3.061615 | 1.614293 |
| Succinic acid | Amino acid metabolomics | 1.496548 | 2.774461 | 1.472208 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, L.; Wang, G.; Tong, X.; Yang, H.; Sun, H.; Zhang, Z.; Xu, B.; Li, D.; Zhang, S.; Li, G. Feeding Aquilaria sinensis Leaves Modulates Lipid Metabolism and Improves the Meat Quality of Goats. Foods 2023, 12, 560. https://doi.org/10.3390/foods12030560
Min L, Wang G, Tong X, Yang H, Sun H, Zhang Z, Xu B, Li D, Zhang S, Li G. Feeding Aquilaria sinensis Leaves Modulates Lipid Metabolism and Improves the Meat Quality of Goats. Foods. 2023; 12(3):560. https://doi.org/10.3390/foods12030560
Chicago/Turabian StyleMin, Li, Gang Wang, Xiong Tong, Huaigu Yang, Hao Sun, Zhifei Zhang, Bin Xu, Dagang Li, Sheng Zhang, and Guanghong Li. 2023. "Feeding Aquilaria sinensis Leaves Modulates Lipid Metabolism and Improves the Meat Quality of Goats" Foods 12, no. 3: 560. https://doi.org/10.3390/foods12030560
APA StyleMin, L., Wang, G., Tong, X., Yang, H., Sun, H., Zhang, Z., Xu, B., Li, D., Zhang, S., & Li, G. (2023). Feeding Aquilaria sinensis Leaves Modulates Lipid Metabolism and Improves the Meat Quality of Goats. Foods, 12(3), 560. https://doi.org/10.3390/foods12030560









