Volatile Compounds in Green and Roasted Arabica Specialty Coffee: Discrimination of Origins, Post-Harvesting Processes, and Roasting Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Roasting
2.3. Analysis of Volatile Compounds
2.4. Statistical Analysis
3. Results
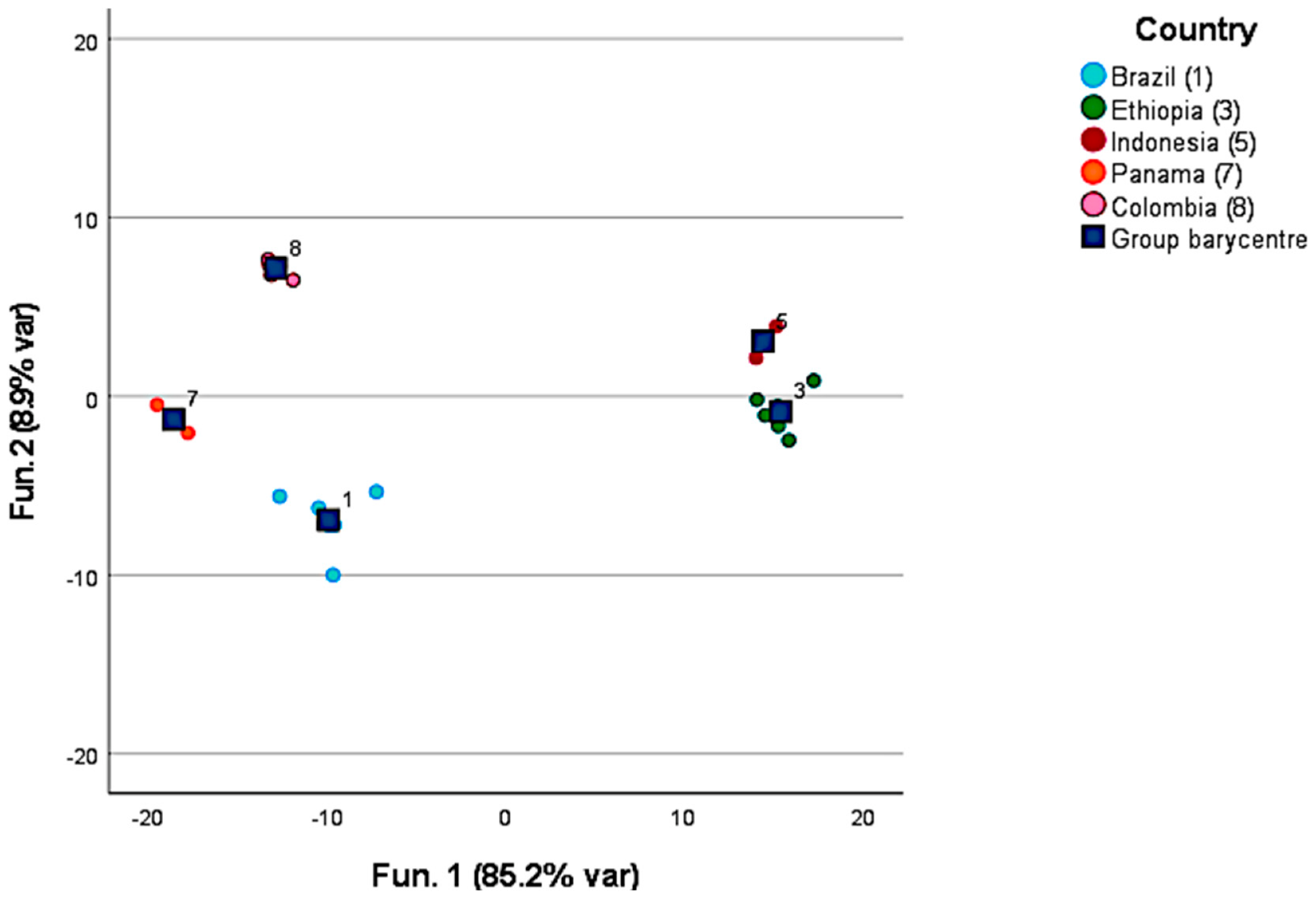
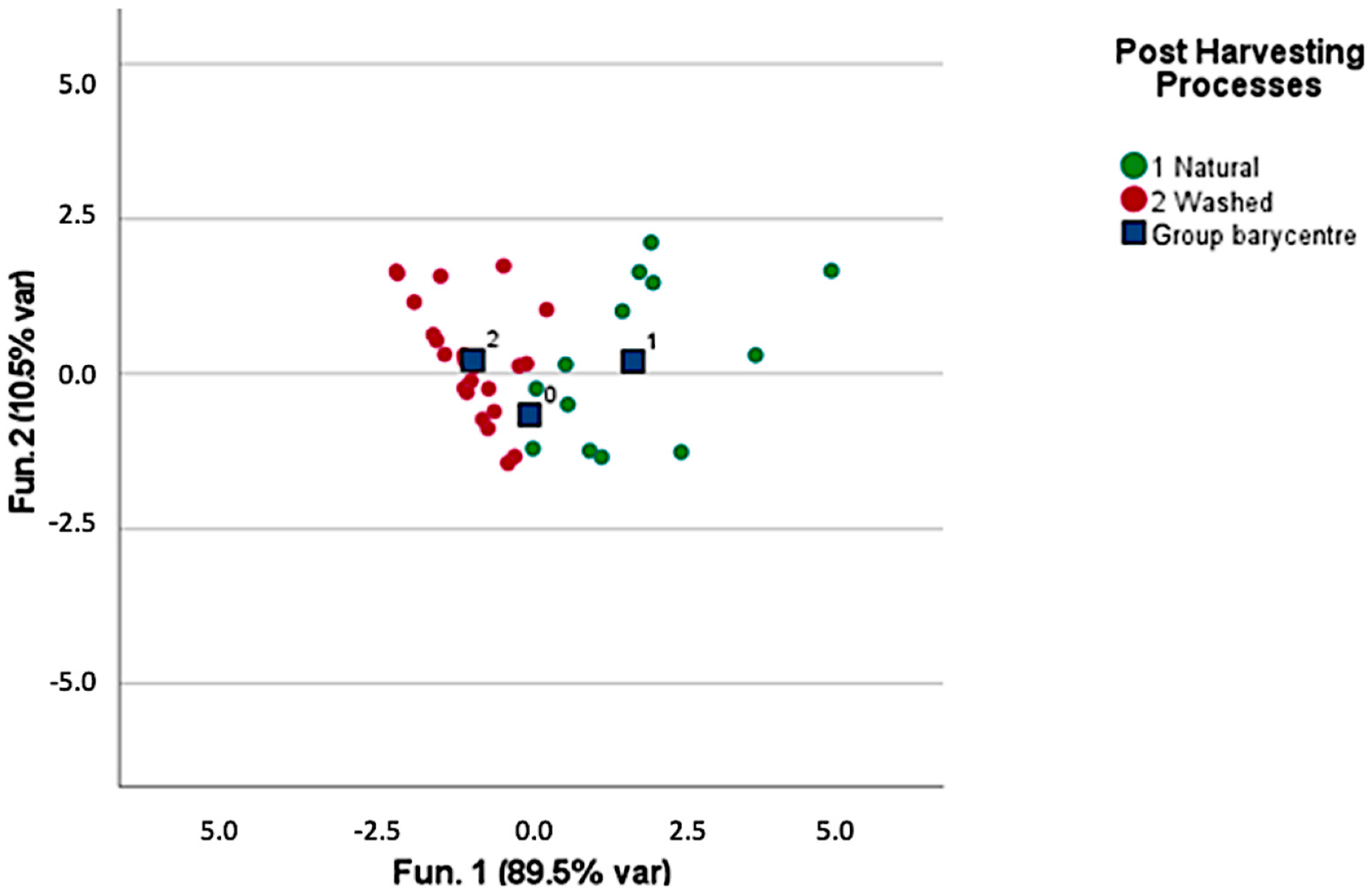
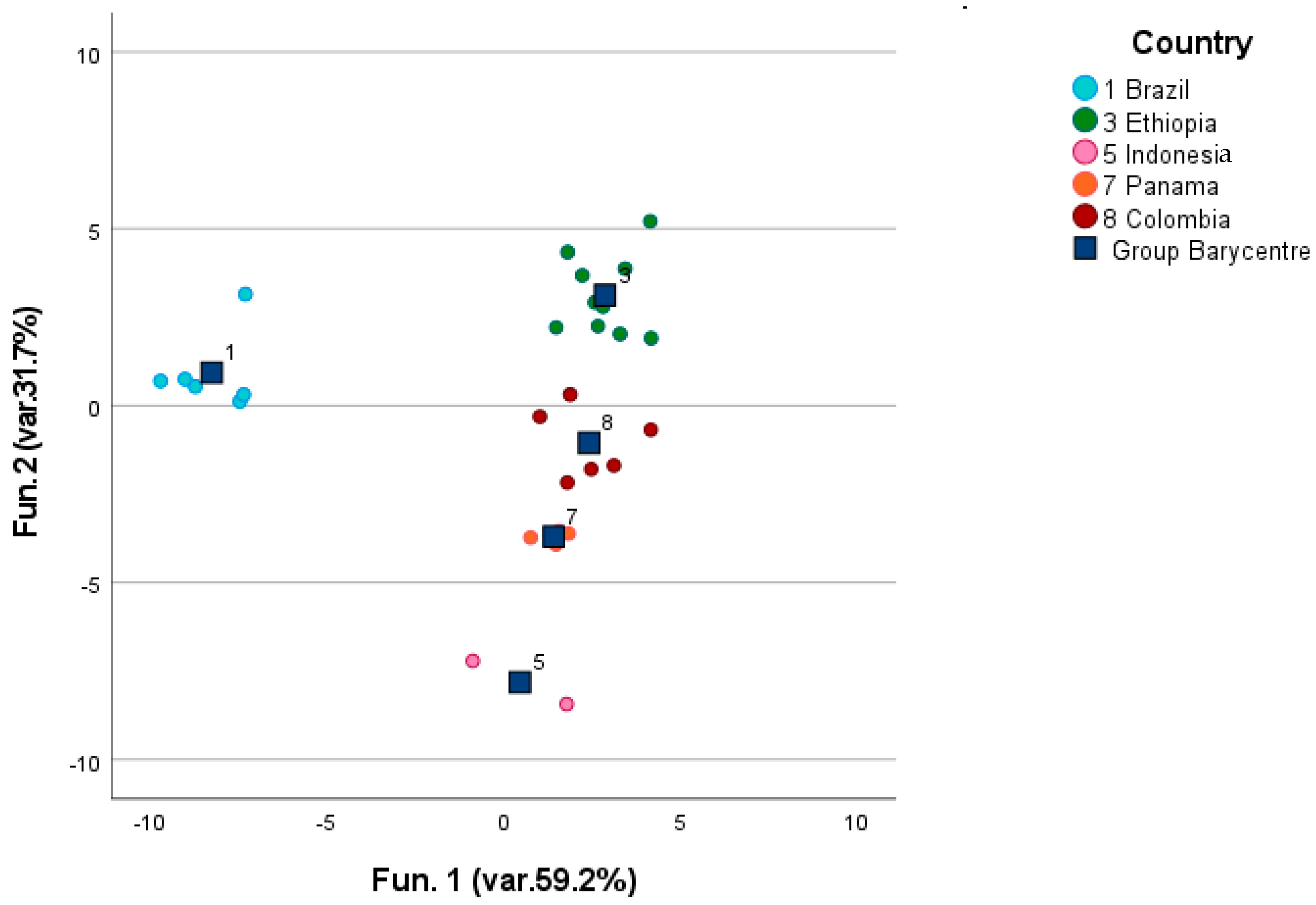
3.1. Volatile Compounds in Green Coffee
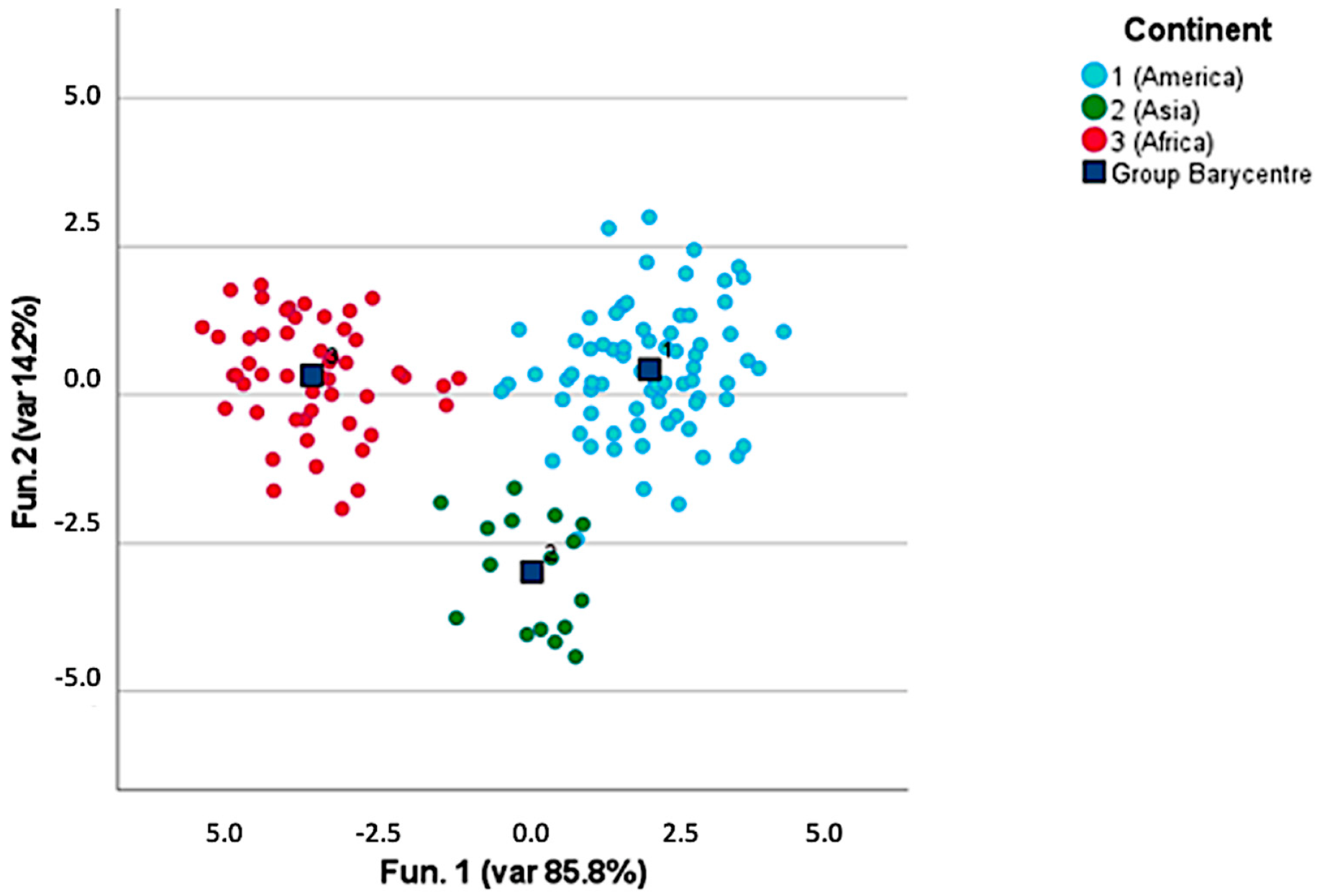
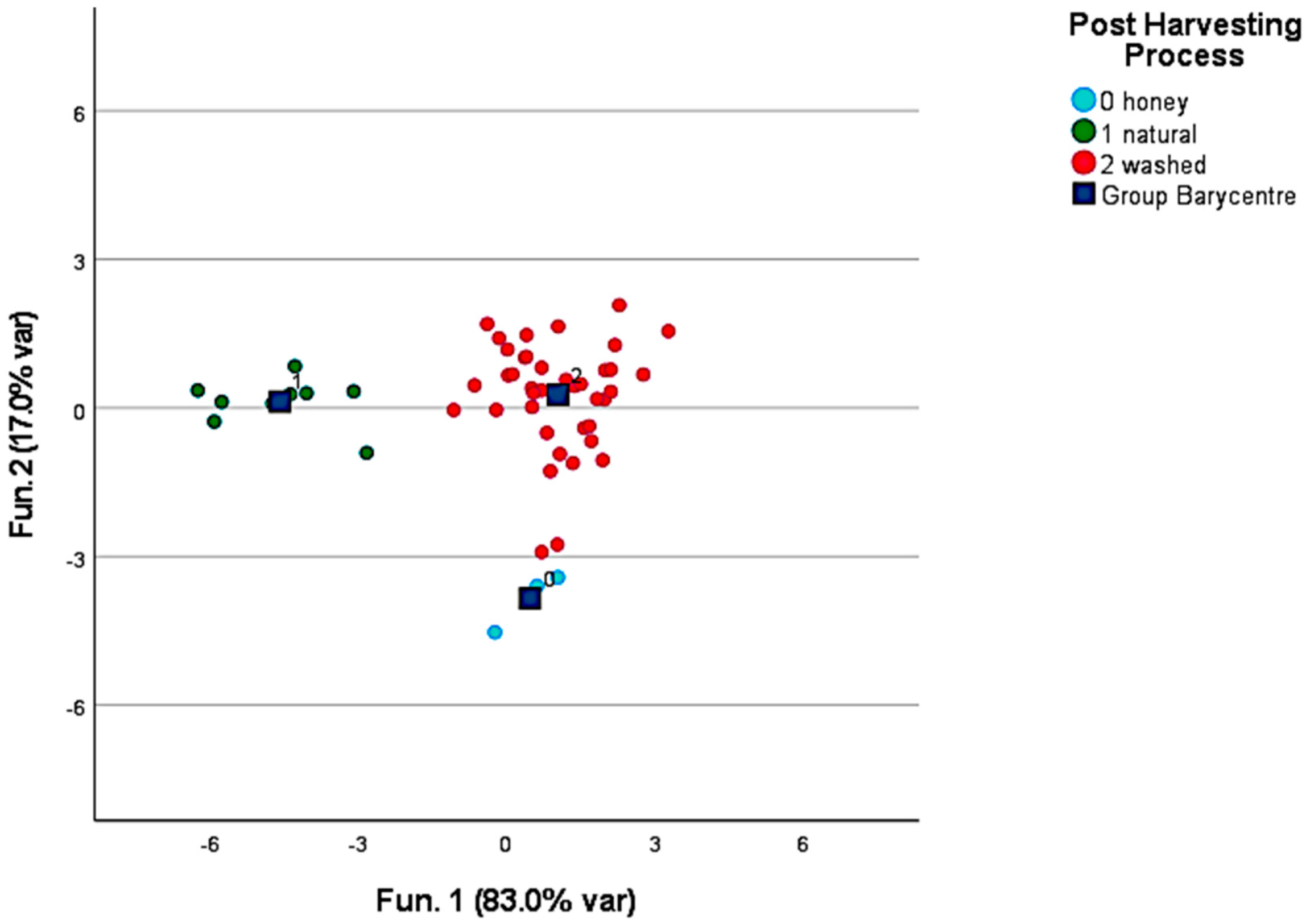
3.2. Volatile Compounds in Roasted Coffee
4. Discussion
4.1. Green Coffee Origin and Discrimination and Characterization Based on the Post-Harvesting Processes
4.2. Discrimination and Characterization of the Origin, Post-Harvesting Processes, and Roasting Level of Roasted Coffee
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flament, I.; Bessière-Thomas, Y. Coffee Flavor Chemistry; Wiley: New York, NY, USA, 2002; 410p. [Google Scholar]
- Abdelwareth, A.; Zayed, A.; Farag, M.A. Chemometrics-Based Aroma Profiling for Revealing Origin, Roasting Indices, and Brewing Method in Coffee Seeds and Its Commercial Blends in the Middle East. Food Chem. 2021, 349, 129162. [Google Scholar] [CrossRef] [PubMed]
- Credence Research Specialty Coffee Market Size, Share, Growth and Forecast to 2026. Available online: https://www.credenceresearch.com/report/specialty-coffee-market (accessed on 30 June 2021).
- Specialty Coffee Association What Is Specialty Coffee? Available online: https://sca.coffee/research/what-is-specialty-coffee (accessed on 30 June 2021).
- Specialty Coffee Association. Building an Understanding Based on Attributes towards a Deeinition of Specialty Coffee: An SCA White Paper; Specialty Coffee Association: Santa Ana, CA, USA, 2021. [Google Scholar]
- Cantergiani, E.; Brevard, H.; Krebs, Y.; Feria-Morales, A.; Amadò, R.; Yeretzian, C. Characterisation of the Aroma of Green Mexican Coffee and Identification of Mouldy/Earthy Defect. Eur. Food Res. Technol. 2001, 212, 648–657. [Google Scholar] [CrossRef]
- Toci, A.T.; Farah, A. Volatile Fingerprint of Brazilian Defective Coffee Seeds: Corroboration of Potential Marker Compounds and Identification of New Low Quality Indicators. Food Chem. 2014, 153, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liu, C.; Liu, X.; Degn, T.K.; Munchow, M.; Fisk, I. Determination of Volatile Marker Compounds of Common Coffee Roast Defects. Food Chem. 2016, 211, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Liberto, E.; Ruosi, M.R.; Cordero, C.; Rubiolo, P.; Bicchi, C.; Sgorbini, B. Non-Separative Headspace Solid Phase Microextraction—Mass Spectrometry Profile as a Marker To Monitor Coffee Roasting Degree. J. Agric. Food Chem. 2013, 61, 1652–1660. [Google Scholar] [CrossRef]
- De Toledo, P.R.A.B.; de Melo, M.M.R.; Pezza, H.R.; Toci, A.T.; Pezza, L.; Silva, C.M. Discriminant Analysis for Unveiling the Origin of Roasted Coffee Samples: A Tool for Quality Control of Coffee Related Products. Food Control 2017, 73, 164–174. [Google Scholar] [CrossRef]
- Zakidou, P.; Plati, F.; Matsakidou, A.; Varka, E.-M.; Blekas, G.; Paraskevopoulou, A.; Mildner-Szkudlarzp, S.; Majcher, M. Molecules Single Origin Coffee Aroma: From Optimized Flavor Protocols and Coffee Customization to Instrumental Volatile Characterization and Chemometrics. Molecules 2021, 26, 4609. [Google Scholar] [CrossRef]
- Avelino, J.; Barboza, B.; Araya, J.C.; Fonseca, C.; Davrieux, F.; Guyot, B.; Cilas, C. Effects of Slope Exposure, Altitude and Yield on Coffee Quality in Two Altitude Terroirs of Costa Rica, Orosi and Santa María de Dota. J. Sci. Food Agric. 2005, 85, 1869–1876. [Google Scholar] [CrossRef]
- Bertrand, B.; Boulanger, R.; Dussert, S.; Ribeyre, F.; Berthiot, L.; Descroix, F.; Joët, T. Climatic Factors Directly Impact the Volatile Organic Compound Fingerprint in Green Arabica Coffee Bean as Well as Coffee Beverage Quality. Food Chem. 2012, 135, 2575–2583. [Google Scholar] [CrossRef]
- Gonzalez-Rios, O.; Suarez-Quiroz, M.L.; Boulanger, R.; Barel, M.; Guyot, B.; Guiraud, J.P.; Schorr-Galindo, S. Impact of “Ecological” Post-Harvest Processing on the Volatile Fraction of Coffee Beans: I. Green Coffee. J. Food Compos. Anal. 2007, 20, 289–296. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. Microbiological and Chemical Characteristics of Wet Coffee Fermentation Inoculated With Hansinaspora Uvarum and Pichia Kudriavzevii and Their Impact on Coffee Sensory Quality. Front. Microbiol. 2021, 12, 713969. [Google Scholar] [CrossRef]
- Panighel, A.; Flamini, R. Applications of Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry (SPME-GC/MS) in the Study of Grape and Wine Volatile Compounds. Molecules 2014, 19, 21291–21309. [Google Scholar] [CrossRef]
- Pott, D.M.; Vallarino, J.G.; Osorio, S. Profiling Volatile Compounds in Blackcurrant Fruit Using Headspace Solid-Phase Microextraction Coupled to Gas Chromatography-Mass Spectrometry. J. Vis. Exp. 2021, 2021, e62421. [Google Scholar] [CrossRef]
- Toci, A.T.; Farah, A. Volatile Compounds as Potential Defective Coffee Beans’ Markers. Food Chem. 2008, 108, 1133–1141. [Google Scholar] [CrossRef]
- Vezzulli, F.; Triachini, S.; Mulazzi, A.; Lambri, M.; Bertuzzi, T. Acrylamide: Impact of Precursors Concentration, Origin, Post-Harvesting Process and Roasting Level in High-Quality Arabica and Robusta Coffee. Int. J. Food Sci. Technol. 2022, 57, 7468–7476. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of Single Bean Coffee Volatile Compounds of Arabica and Robusta Roasted Coffees Analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef]
- Liardon, R.; Ott, U.; Daget, N. Analysis of Coffee Headspace Profiles by Multivariate Statistics. In Analysis of Volatiles; De Gruyter: Berlin, Germany, 1984. [Google Scholar] [CrossRef]
- Semmelroch, P.; Grosch, W. Analysis of Roasted Coffee Powders and Brews by Gas Chromatography-Olfactometry of Headspace Samples. LWT-Food Sci. Technol. 1995, 28, 310–313. [Google Scholar] [CrossRef]
- Semmelroch, P.; Grosch, W. Studies on Character Impact Odorants of Coffee Brews. J. Agric. Food Chem. 1996, 44, 537–543. [Google Scholar] [CrossRef]
- Bhumiratana, N.; Adhikari, K.; Chambers, E. Evolution of Sensory Aroma Attributes from Coffee Beans to Brewed Coffee. LWT-Food Sci. Technol. 2011, 44, 2185–2192. [Google Scholar] [CrossRef]
- Sanz, C.; Ansorena, D.; Bello, J.; Cid, C. Optimizing Headspace Temperature and Time Sampling for Identification of Volatile Compounds in Ground Roasted Arabica Coffee. J. Agric. Food Chem. 2001, 49, 1364–1369. [Google Scholar] [CrossRef]
- Buffo, R.A.; Cardelli-Freire, C. Coffee FLavour: An Overview. Flavour Fragr. J. 2004, 19, 99–104. [Google Scholar] [CrossRef]
- Mayer, F.; Czerny, M.; Grosch, W. Sensory Study of the Character Impact Aroma Compounds of a Coffee Beverage. Eur. Food Res. Technol. 2000, 211, 272–276. [Google Scholar] [CrossRef]
- Moon, J.K.; Shibamoto, T. Role of Roasting Conditions in the Profile of Volatile Flavor Chemicals Formed from Coffee Beans. J. Agric. Food Chem. 2009, 57, 5823–5831. [Google Scholar] [CrossRef] [PubMed]
- Guyot, B.; Gueule, D.; Manez, J.-C.; Perriot, J.; Giron, J.; Villain, L. Influence de l’altitude et de l’ombrage Sur La Qualité Des Cafés Arabica. Plant. Rech. Dev. 1996, 3, 272–283. [Google Scholar]
- Bertrand, B.; Vaast, P.; Alpizar, E.; Etienne, H.; Davrieux, F.; Charmetant, P. Comparison of Bean Biochemical Composition and Beverage Quality of Arabica Hybrids Involving Sudanese-Ethiopian Origins with Traditional Varieties at Various Elevations in Central America. Tree Physiol. 2006, 26, 1239–1248. [Google Scholar] [CrossRef]
- Decazy, F.; Avelino, J.; Guyot, B.; Perriot, J.J.; Pineda, C.; Cilas, C. Quality of Different Honduran Coffees in Relation to Several Environments. J. Food Sci. 2003, 68, 2356–2361. [Google Scholar] [CrossRef]
- Tsegay, G.; Redi-Abshiro, M.; Chandravanshi, B.S.; Ele, E.; Mohammed, A.M.; Mamo, H. Volatile Profile of Green Coffee Beans from Coffea arabica L. Plants Grown at Different Altitudes in Ethiopia. Bull. Chem. Soc. Ethiop. 2019, 33, 401–413. [Google Scholar] [CrossRef]
- Marin, K.; Požrl, T.; Zlatić, E.; Plestenjak, A. A New Aroma Index to Determine the Aroma Quality of Roasted and Ground Coffee during Storage. Food Technol. Biotechnol. 2008, 46, 442–447. [Google Scholar]
- De Maria, C.A.B.; Moreira, R.F.A.; Trugo, L.C. Componentes Voláteis Do Café Torrado. Parte I: Compostos Heterocíclicos. Quim. Nova 1999, 22, 209–217. [Google Scholar] [CrossRef]



| Alcohols | LRI | Linear Alkanes | LRI |
|---|---|---|---|
| Ethanol | 565 | Trimethyl-octane | 904 |
| 3-Methylbutanol | 742 | n-Decan | 1000 |
| 2,3-Butanediol | 804 | 2,6-Dimethyloctane | 1016 |
| 2-Heptanol | 873 | Tetramethyl-octane | 1068 |
| Octanol | 1007 | 3-Methylnonane | 1064 |
| Phenylethyl alcohol | 1126 | Tetramethyl-heptane | 1026 |
| Acids | LRI | Undecane | 1100 |
| Acetic acid | 595 | 3-Methylundecane | 1176 |
| 3-Metyl butanoic acid (isovaleric acid) | 856 | Dodecane | 1200 |
| 2-Methyl butanoic acid | 863 | 3-Methyl-tridecane | 1374 |
| 3-Methyl-but-2-en-oic acid | 884 | Tetradecane | 1400 |
| Pentanoic acid (valeric acid) | 894 | Pentadecane | 1500 |
| 3-Methyl-pentanoic acid | 903 | Terpenes | LRI |
| Esters | LRI | β-Pinene | 898 |
| Methyl ester isovaleric acid | 809 | Farnesane (2,6,10-trimethyl-dodecane) | 1241 |
| 2-Methyl-ethyl ester butanoic acid | 840 | α-Pinene | 930 |
| 3-Methyl-ethylestere butanoic acid (ethyl ester isovaleric acid) | 843 | Carene | 1010 |
| Propanoic acid methyl hexyl ester | 1058 | D-limonene | 1023 |
| Aldehydes | LRI | α-Linalool | 1104 |
| Hexanal | 798 | Pyrazines e pyridines | LRI |
| Benzaldehyde | 907 | Methylpyrazine | 832 |
| Nonanal | 1110 | 3-Methoxypyrazine-2-isobutyle | 1181 |
| Ketones | LRI | Furans | LRI |
| 1-Methoxy-2-propanone | 582 | 2-Pentylfuran | 985 |
| Cyclopentanone-2-sec-butyl | 1221 | Furfuryl-alcohol | 831 |
| 6,10,14-Trimethyl-2-pentadecanone | 1752 | Contaminants | LRI |
| Diphenyl-propane | 1654 | Ethylbenzene | 848 |
| Alkaloids | LRI | Tributyl-phosphate | 1602 |
| Caffeine | 1772 | Phthalic acid dibutyl-ester | 1764 |
| Diethyl phthalate | 1558 |
| Furans and Derivatives | LRI | Pyrazole | LRI |
|---|---|---|---|
| Dihydro 2-methyl-3-furanone | 820 | Pyrazole-3,4-pyrimidine | 1227 |
| Furfural | 842 | Pyridines | LRI |
| Furfuryl alcohol | 831 | Pyridine | 776 |
| Furfuryl formate | 890 | 1-Acetyl-1,4-dihydropyridine | 990 |
| 3-Furanone-2,5-dimethyl-2-((hydroxy-1-acetyl) ethyl) | 930 | Pyrazines | LRI |
| 5-Methylfurfural | 942 | Methylpyrazine | 832 |
| Furfuryl acetate | 955 | 4,6-Dimethylpyrimidine | 906 |
| 1-Propanone-2-furanyl | 968 | 2-Ethyl-6-methylpyrazine | 959 |
| 2,2-Bifuran | 1023 | 2-Ethyl-5-methylpyrazine | 962 |
| Alanine N ethyl furfuryl ester | 1072 | 2-Methyl-6-vinyl pyrazine | 982 |
| Furan, 2,2′-methylenebis | 1117 | 2-Acetyl pyrazine | 998 |
| 4-Hydroxy-2,5-dimethyl-3(2H)-furanone | 1128 | 3-Ethyl-2,5-dimethyl pyrazine | 1112 |
| 5-Methyl-2,2-dimethylene furan | 1217 | 1-(6-Methyl-2-pyrazinyl) ethenone | 1163 |
| Furfuryl methyl disulfide | 1258 | 2-Acetyl-3-methyl pyrazine | 1155 |
| Furfuryl-3-methylbutanoate | 1262 | 5-Methyl-6,7-dihydro-5H-cyclopenta pyrazine | 1181 |
| 3-Phenylfuran | 1272 | 3,5-Diethyl-2-methylpyrazine | 1197 |
| 6-(5-Methyl-furan-2-yl)-hexan-2-one | 1291 | 2-Methyl-5(1-propenyl) pyrazine | 1242 |
| 2,2′-Difurylmethane | 1130 | 3,5-Dimethyl-2-acetyl pyrazine | 1265 |
| Furfuryl methylamine | 1401 | 6-Methyl-2-isoamyl pyrazine | 1294 |
| Ketones | LRI | Acids | LRI |
| 2,3-Pentanedione | 590 | Isovaleric acid | 856 |
| 3-Methyl-1,2-cyclopentanedione | 1013 | 2-Methylene-4-hydroxy butyric acid | 1028 |
| 2,4-Dimethyl-1,3-cyclopentanedione | 1050 | Pyrroles | LRI |
| Methyl acetyl acetone | 1143 | 2-Formyl-1-methylpyrrole | 965 |
| 3-Ethyl-2-hydroxy-cyclopentene-1-one | 1135 | Pyrrole-2-carboxyaldehyde | 1007 |
| Terpenes | LRI | Pyrrole-2-acetonitrile-1-methyl | 1150 |
| Linalool | 1104 | Alcohols | LRI |
| Damascenone (2,6,6-trimethylcyclohexa-1,3-diene) | 1379 | Maltol | 1157 |
| 4-Ethyl-2-methoxy phenol | 1320 | ||
| 2-Methoxy-4-vinyl phenol | 1353 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vezzulli, F.; Lambri, M.; Bertuzzi, T. Volatile Compounds in Green and Roasted Arabica Specialty Coffee: Discrimination of Origins, Post-Harvesting Processes, and Roasting Level. Foods 2023, 12, 489. https://doi.org/10.3390/foods12030489
Vezzulli F, Lambri M, Bertuzzi T. Volatile Compounds in Green and Roasted Arabica Specialty Coffee: Discrimination of Origins, Post-Harvesting Processes, and Roasting Level. Foods. 2023; 12(3):489. https://doi.org/10.3390/foods12030489
Chicago/Turabian StyleVezzulli, Fosca, Milena Lambri, and Terenzio Bertuzzi. 2023. "Volatile Compounds in Green and Roasted Arabica Specialty Coffee: Discrimination of Origins, Post-Harvesting Processes, and Roasting Level" Foods 12, no. 3: 489. https://doi.org/10.3390/foods12030489
APA StyleVezzulli, F., Lambri, M., & Bertuzzi, T. (2023). Volatile Compounds in Green and Roasted Arabica Specialty Coffee: Discrimination of Origins, Post-Harvesting Processes, and Roasting Level. Foods, 12(3), 489. https://doi.org/10.3390/foods12030489








