Bifidobacterium animalis A12 and Lactobacillus salivarius M18-6 Alleviate Alcohol Injury by keap1-Nrf2 Pathway and Thioredoxin System
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbiology Experiment
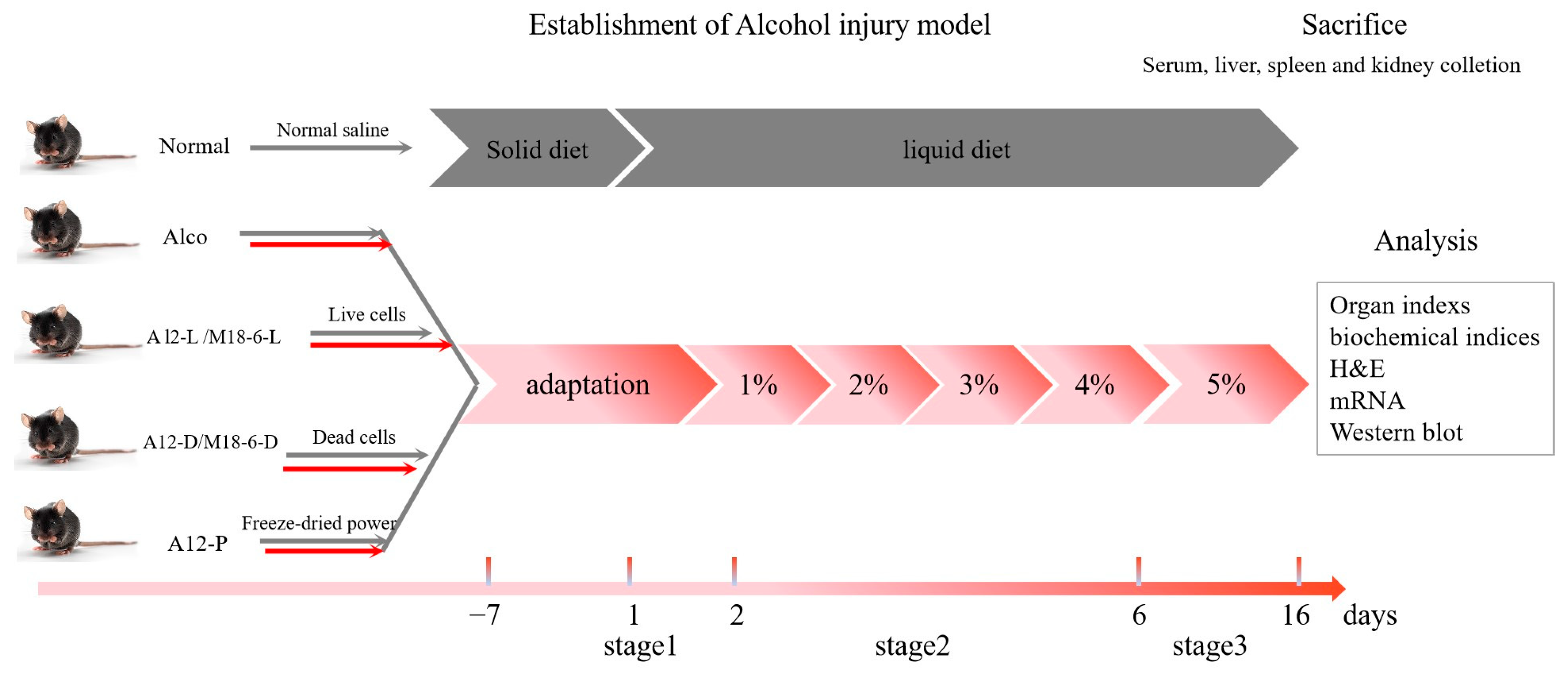
2.2. Animal Experimental Design
2.3. Serum Biochemistry Determination
2.4. Liver Biochemistry Determination
2.5. Gene Expression Analysis
2.6. Western Blot
2.7. Haematoxylin and Eosin (H&E) Staining of Mouse Liver
2.8. GC-MS Detection of Short-Chain Fatty Acids in Mouse Faeces
2.9. Statistical Analysis
3. Results
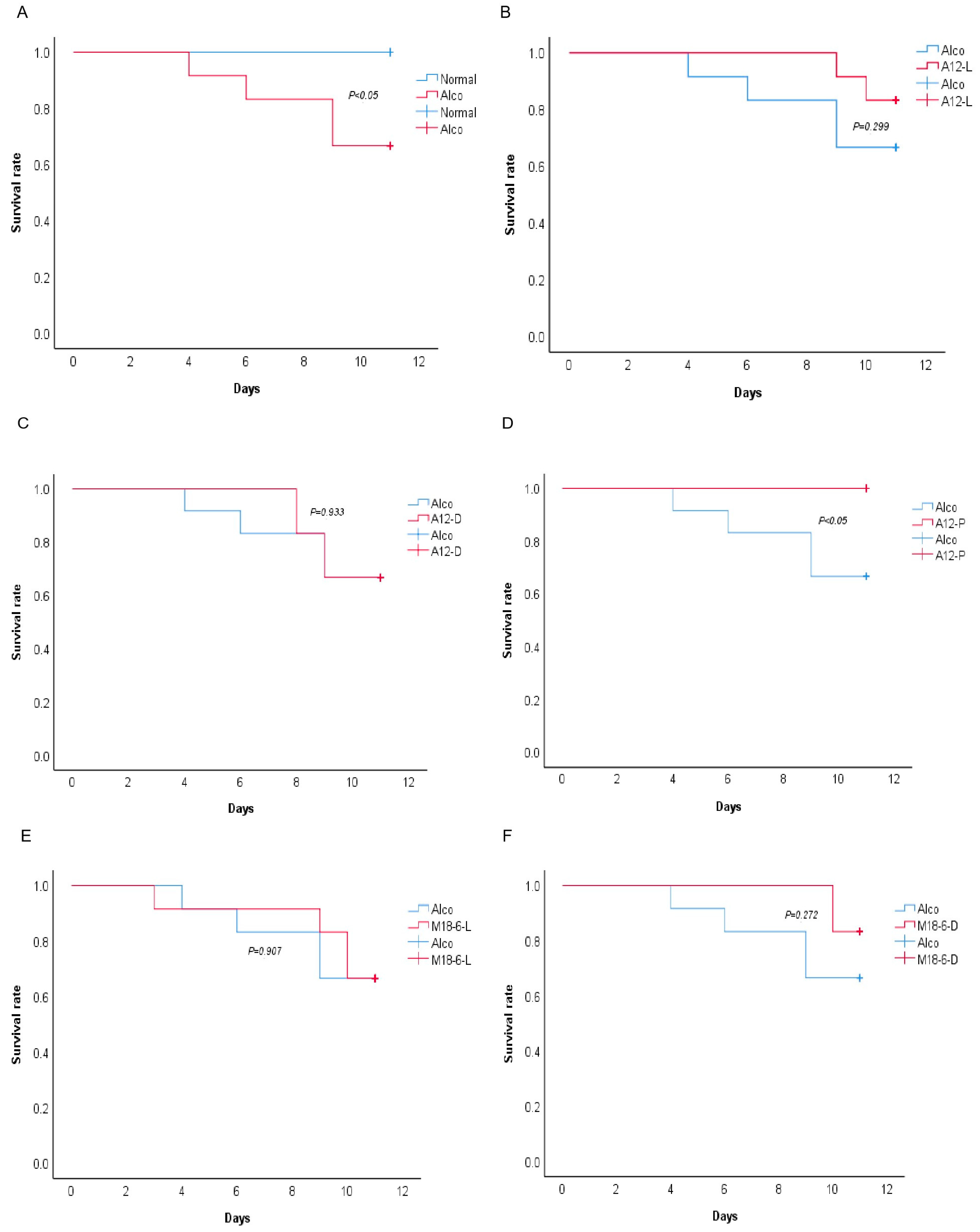
3.1. Effect of B. Animal A12 and L. salivarius M18-6 on Morphology and Survival Rate of Mice with Alcohol Injury
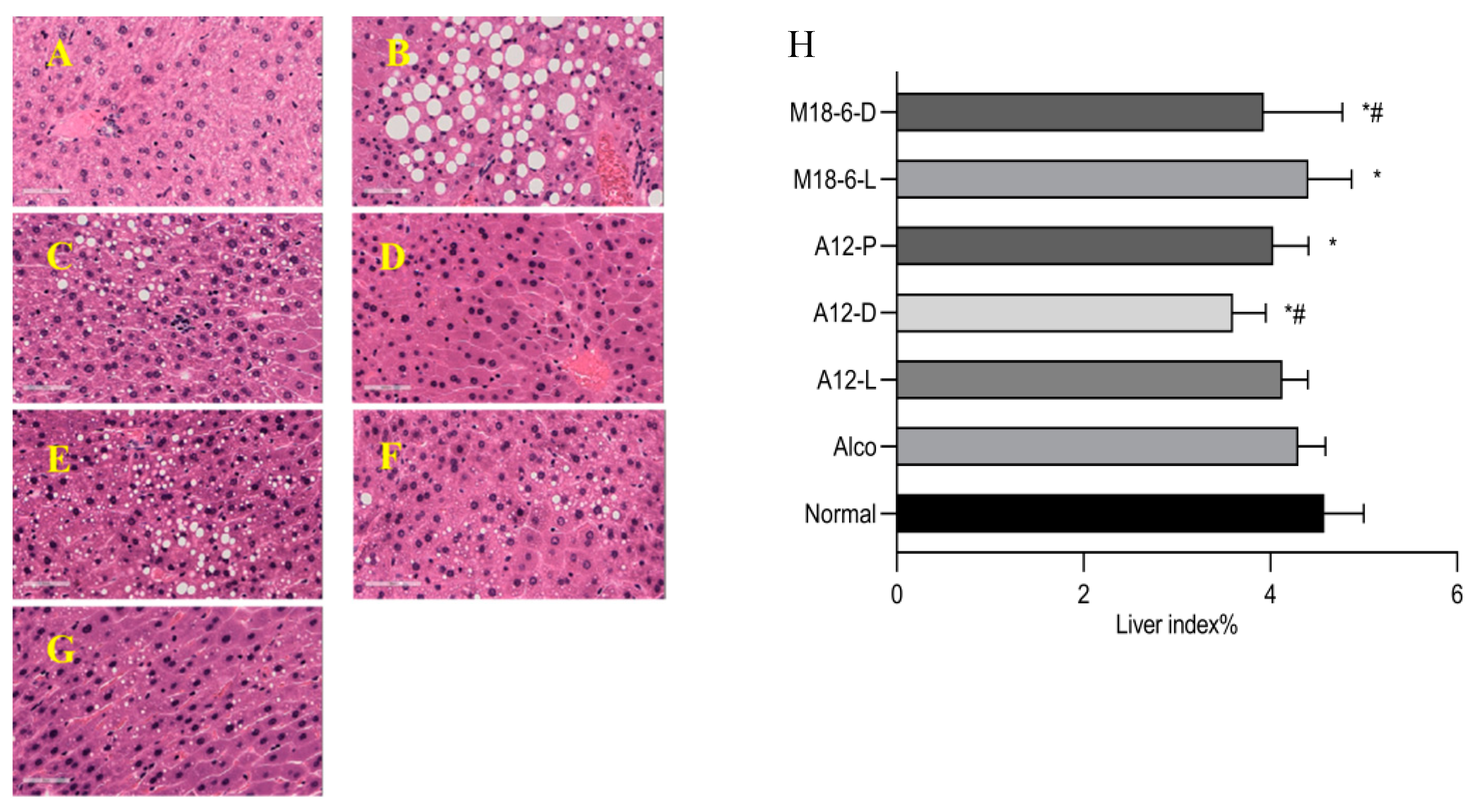
3.2. Effect of B. animalis A12 and L. salivarius M18-6 on Liver Histopathology in Mice with Alcohol Injury
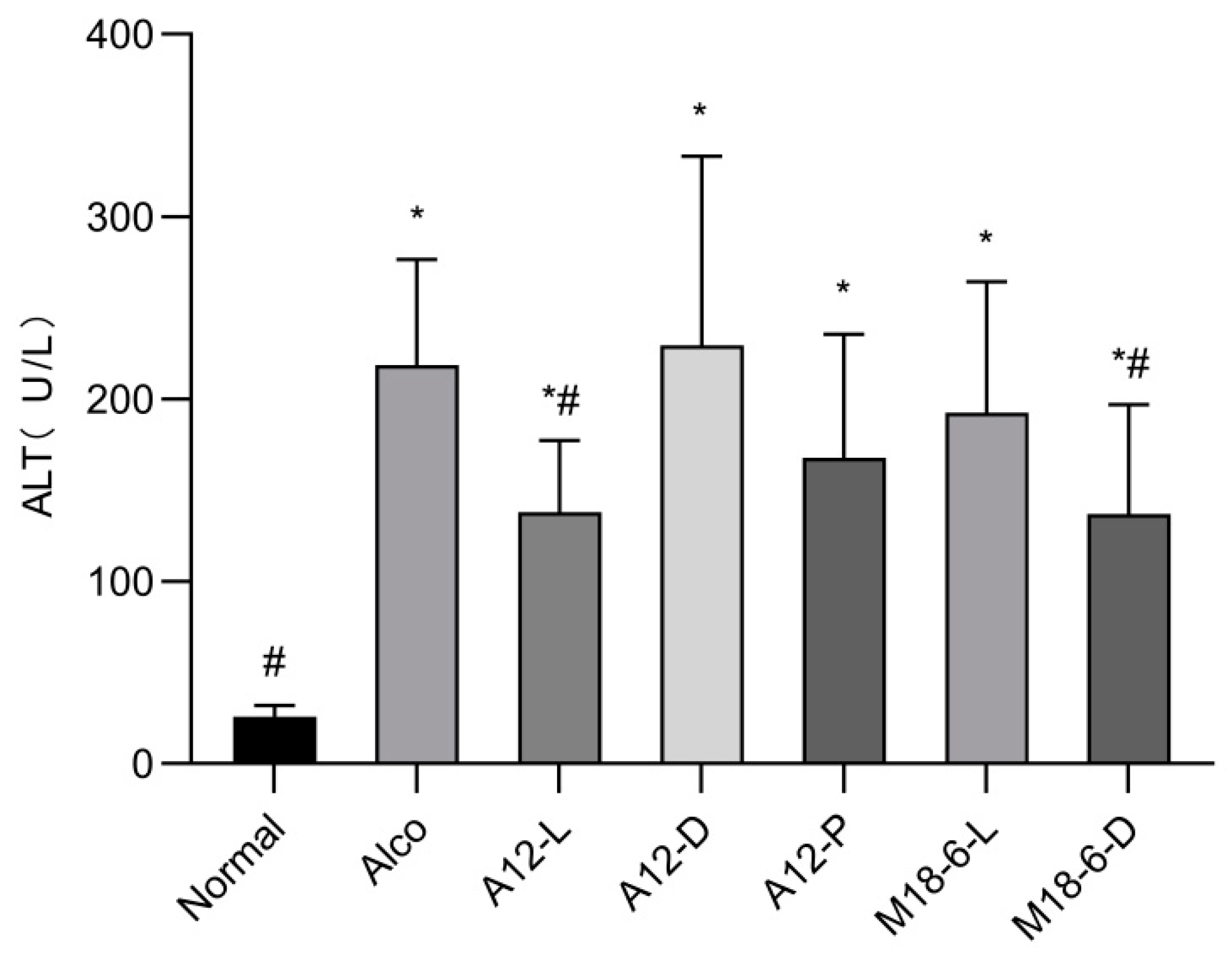
3.3. Effect of B. animalis A12 and L. salivarius M18-6 on Serum ALT in Mice with Alcohol Injury
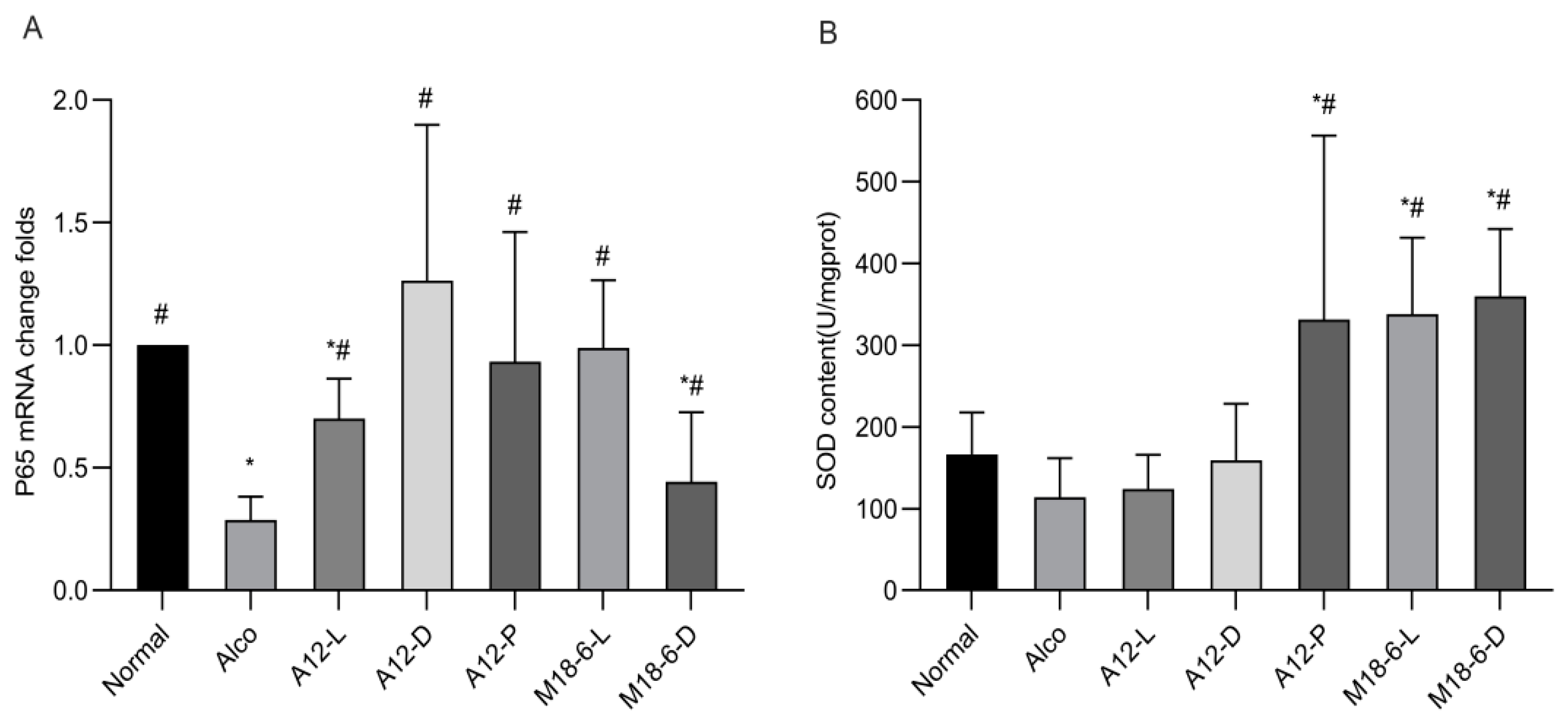
3.4. Effect of B. animalis A12 and L. salivarius M18-6 on Immune Level and Liver Oxidative Status in Mice
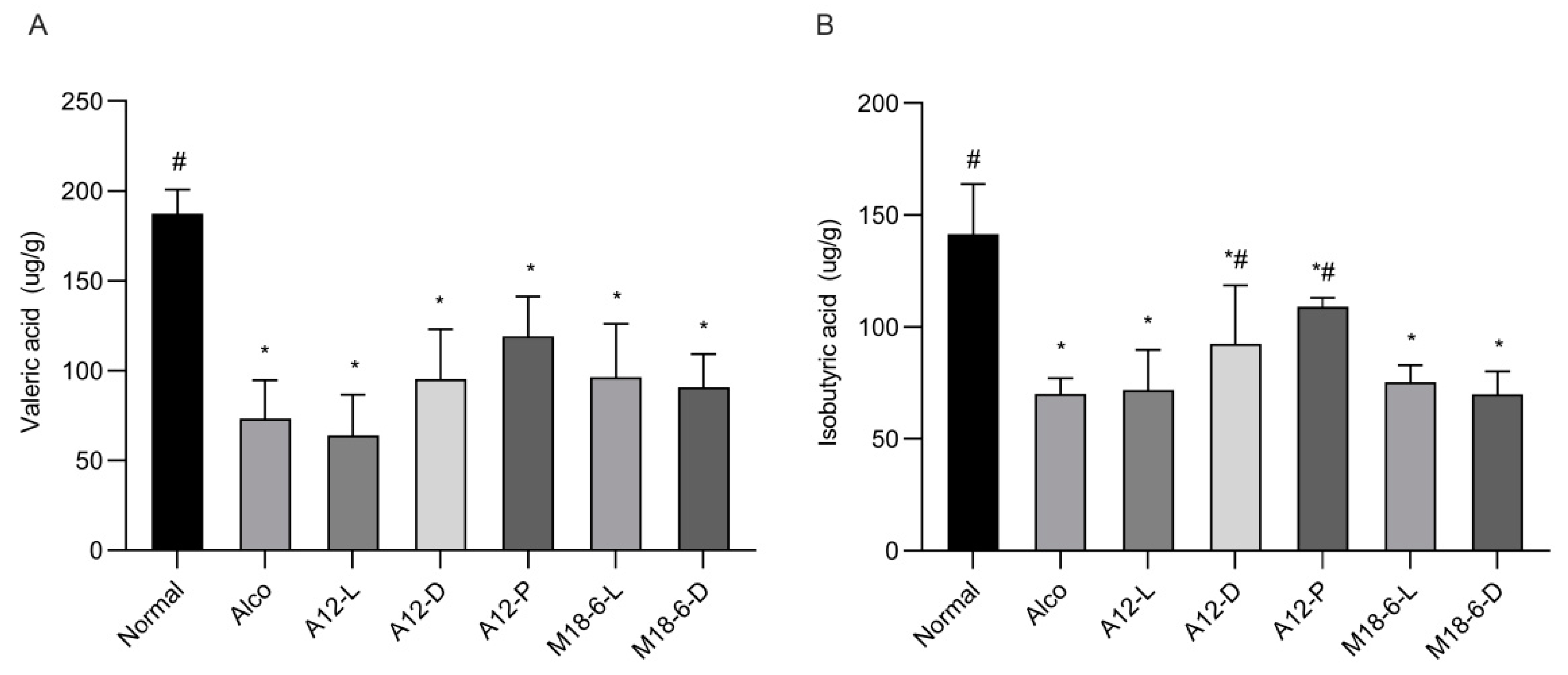
3.5. Effect of B. animalis A12 and L. salivarius M18-6 on Short Chain Fatty Acid Content
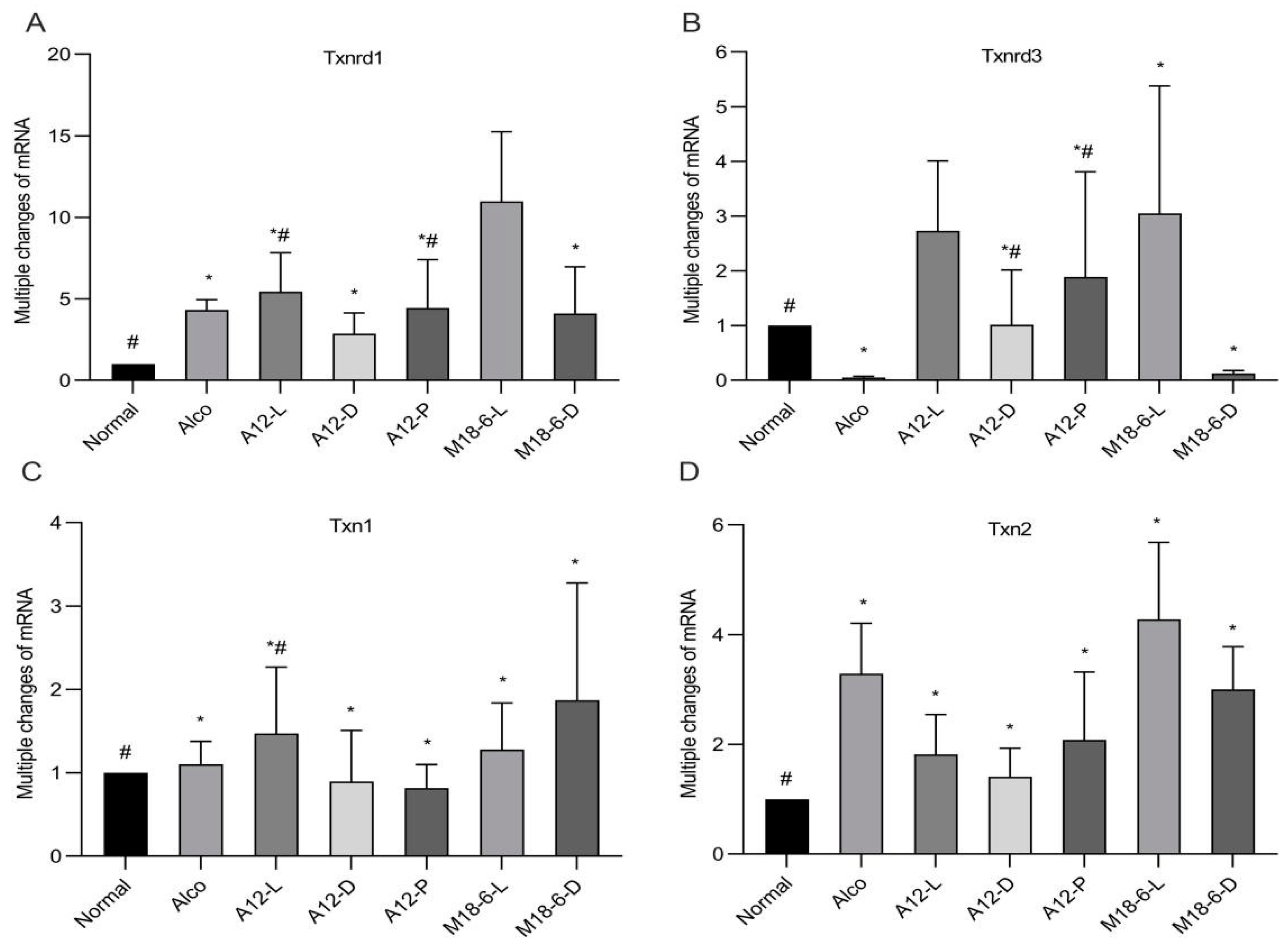
3.6. Effect of B. animalis A12 and L. salivarius M18-6 on Gene Expression of Thioredoxin System in Mice
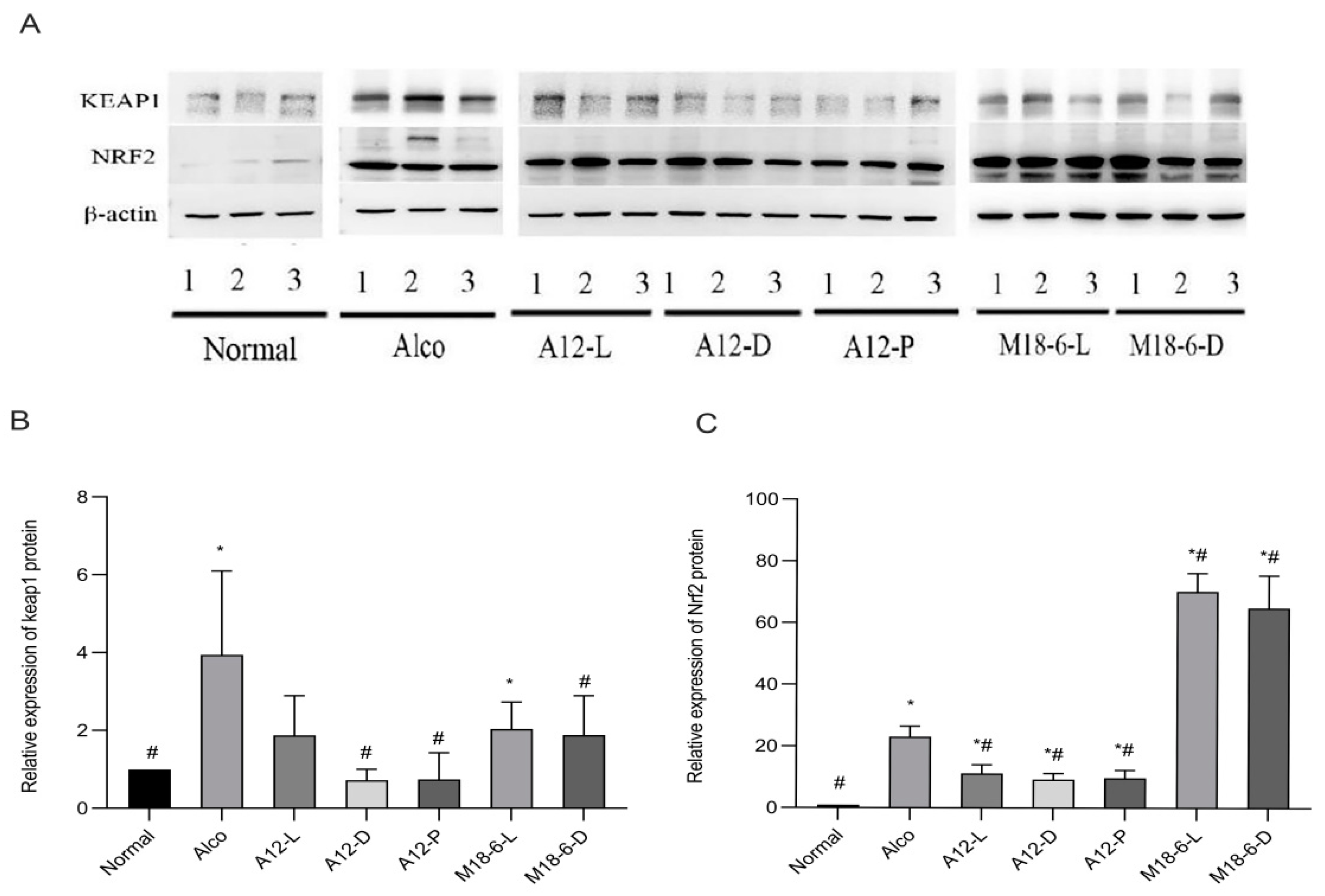
3.7. Effect of B. animalis A12 and L. salivarius M18-6 on Immune Proteins in Mice with Alcohol Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine transaminase |
| SCFAS | Short chain fatty acids |
| SOD | Superoxide dismutase |
| Txnrd | Thioredoxin reductase |
| Txn | Thioredoxin |
| Keap1 | Kelch-1ike ECH- associated protein l |
| Nrf2 | Nuclear factor erythroid2-related factor 2 |
| NF-kB | Nuclear factor-kappa B |
References
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- George, A.K.; Behera, J.; Kelly, K.E.; Zhai, Y.; Tyagi, N. Hydrogen sulfide, endoplasmic reticulum stress and alcohol mediated neurotoxicity. Brain Res. Bull. 2017, 130, 251–256. [Google Scholar] [CrossRef]
- Bienia, A.; Sodolski, W.; Luchowska, E. The effect of chronic alcohol abuse on gastric and duodenal mucosa. Ann. Univ. Mariae Curie-Sklodowska Sect. D Med. 2002, 57, 570–582. [Google Scholar]
- Koh, P.O.; Kim, M.O. Ethanol exposure decreases cell proliferation and increases apoptosis in rat testes. J. Vet. Med. Sci. 2006, 68, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Mcclain, C.; Hill, D.; Schmidt, J.; Diehl, A. Cytokines and alcoholic liver disease. Semin. Liver Dis. 1993, 13, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhang, J.; Yao, J.H.; Zheng, Y.; Song, J.; Wang, M. Research progress in mechanism of oxidative stress in alcoholic liver disease. Chin. Pharmacol. Bull. 2017, 33, 1353–1356. [Google Scholar]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Fuenzalida, C.; Dufeu, M.S.; Poniachik, J.; Roblero, J.P.; Valenzuela, P.L.; Beltrán, C.J. Probiotics-Based Treatment as an Integral Approach for Alcohol Use Disorder in Alcoholic Liver Disease. Front. Pharmacol. 2021, 12, 729950. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Cho, D.; Huang, E.; Ju, Y.S.; Holzapfel, W.H. Amelioration of alcohol induced gastric ulcers through the administration of lactobacillus plantarum apsulloc 331261 isolated from green tea. Front. Microbiol. 2020, 11, 420. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Lin, S.W.; Chen, Y.L.; Chen, C.C. Effect of probiotics Lactobacillus paracasei GKS6, L. plantarum GKM3, and L. rhamnosus GKLC1 on alleviating alcohol-induced alcoholic liver disease in a mouse model. Nutr. Res. Pract. 2020, 14, 299–308. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Souza, L.K.M.; Araújo, T.S.L.; Araújo, S.; Nogueira, K.M.; Sousa, F.B.M.; Medeiros, J.V.R. Lactobacillus reuteri dsm 17938 protects against gastric damage induced by ethanol administration in mice: Role of trpv1/substance p axis. Nutrients 2019, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.K.; Kang, G.D.; Kim, W.K.; Han, M.J.; Kim, D.H. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 simultaneously alleviate ethanol-induced gastritis and hepatic injury in mice. J. Funct. Foods 2017, 38, 389–398. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.A.; Kang, S.A.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef]
- Panebianco, C.; Eddine, F.B.N.; Forlani, G.; Palmieri, G.; Tatangelo, L.; Villani, A.; Xu, L.; Accolla, R.; Pazienza, V. Probiotic Bifidobacterium lactis, anti-oxidant vitamin E/C and anti-inflammatory dha attenuate lung inflammation due to pm2.5 exposure in mice. Benef. Microbes 2018, 10, 69–75. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, H.; Bai, L.; Lv, Y.; Li, R. Protective effects of probiotics on acute alcohol-induced liver injury in mice through alcohol metabolizing enzymes activation and hepatic tnf-α response reduction. J. Funct. Foods 2019, 59, 234–241. [Google Scholar] [CrossRef]
- Shukla, P.K.; Meena, A.S.; Manda, B.; Gomes-Solecki, M.; Dietrich, P.; Dragatsis, I.; Rao, R. Lactobacillus plantarum prevents and mitigates alcohol-induced disruption of colonic epithelial tight junctions, endotoxemia, and liver damage by an EGF receptor-dependent mechanism. FASEB J. 2018, 32, 6274–6292. [Google Scholar] [CrossRef]
- Yang, L.C.; Lin, S.W.; Li, I.C.; Chen, Y.P.; Tzu, S.Y.; Chou, W.; Lin, W.H. Lactobacillus plantarum GKM3 and Lactobacillus paracasei GKS6 Supplementation Ameliorates Bone Loss in Ovariectomized Mice by Promoting Osteoblast Differentiation and Inhibiting Osteoclast Formation. Nutrients 2020, 12, 1914. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, I.O.; Tan, P.L.; Eor, J.Y.; Kim, S.H. Protective effect of lactobacillus fermentum la12 in an alcohol-induced rat model of alcoholic steatohepatitis. Korean J. Food Sci. Anim. Resour. 2017, 37, 931–939. [Google Scholar]
- Gu, Z.; Wu, Y.; Wang, Y.; Sun, H.; Wang, Y. Lactobacillus rhamnosus granules dose-dependently balance intestinal microbiome disorders and ameliorate chronic alcohol-induced liver injury. J. Med. Food 2019, 23, 114–124. [Google Scholar] [CrossRef]
- Bertola, A.; Mathews, S.; Ki, S.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Xu, M.; Hua, W.; Bertola, A.; Gao, B. Animals models of gastrointestinal and liver diseases. animal models of alcohol-induced liver disease: Pathophysiology, translational relevance, and challenges. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G819–G823. [Google Scholar] [CrossRef] [PubMed]
- Maricic, I.; Sheng, H.; Marrero, I.; Seki, E.; Kisseleva, T.; Chaturvedi, S.; Kumar, V. Inhibition of type I natural killer T cells by retinoids or following sulfatide-mediated activation of type II natural killer T cells attenuates alcoholic liver disease in mice. Hepatology 2015, 61, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, J.; Zhang, H.; Xie, Y.; Jin, J. Bifidobacterium from breastfed infant faeces prevent high-fat-diet-induced glucose tolerance impairment, mediated by the modulation of glucose intake and the incretin hormone secretion axis. J. Sci. Food Agric. 2020, 100, 3308–3318. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, H.; Jia, Y.; Xie, Y.; Liu, H.; Jin, J. Evaluation of antioxidative function of Lactobacillus salivarius M18-6 in vitro and its antioxidant mechanisms. Food Ferment. Ind. 2021, 47, 132–137. [Google Scholar]
- Duan, C.C.; Zhao, Y.J.; Huang, C.; Zhao, Z.; Gao, L.; Niu, C.H.; Li, S.Y. Hepatoprotective effects of Lactobacillus plantarum C88 on LPS/D-GalN–induced acute liver injury in mice. J. Funct. Foods 2018, 43, 146–153. [Google Scholar] [CrossRef]
- Palmer, M. Dr. Textbook of Melissa Palmer’s guide to hepatitis and liver disease. Libr. J. 2004, 125. [Google Scholar]
- Kirpich, I.A.; Solovieva, N.V.; Leikhter, S.N.; Shidakova, N.A.; Lebedeva, O.V.; Sidorov, P.I.; Cave, M. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: A pilot study. Alcohol 2008, 42, 675–682. [Google Scholar] [CrossRef]
- Xing, H.C.; Li, L.J.; Xu, K.J.; Shen, T.; Chen, Y.B.; Sheng, J.F.; Zheng, S.S. Protective role of supplement with foreign Bifidobacterium and Lactobacillus in experimental hepatic ischemia-reperfusion injury. J. Gastroenterol. Hepatol. 2006, 21, 647–656. [Google Scholar] [CrossRef]
- Zhang, N.; Tian, Y.; Wang, Y.; Fan, Y.; Zhang, Y.; Xing, X.; Wang, Y. Ameliorative effect of Lactobacillus plantarum Lp2 against cyclophosphamide-induced liver injury in mice. Food Chem. Toxicol. 2022, 169, 113433. [Google Scholar] [CrossRef]
- Dong, J.; Ping, L.; Xie, Q.; Liu, D.; Zhao, L.; Evivie, S.; Huo, G. Lactobacillus plantarum KLDS1.0386 with antioxidant capacity ameliorates the lipopolysaccharide-induced acute liver injury in mice by NF-κB and Nrf2 pathway. Food Biosci. 2022, 47, 101589. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.Z.; Zhang, R.N.; He, C.X.; Chen, G.Y.; Fan, J.G. Sodium butyrateattenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Tilg, H. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef]
- Smith, P.; Howitt, M.; Panikov, N.; Michaud, M.; Gallini, C.; Bohlooly, Y.; Garrett, W. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Peran, L.; Sierra, S.; Comalada, M.; Lara-Villoslada, F.; Bailón, E.; Nieto, A.; Gálvez, J. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br. J. Nutr. 2007, 97, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F.; Luo, H.; Liu, A.; Jiang, Y. Protective effect of butyrate against ethanol-induced gastric ulcers in mice by promoting the anti-inflammatory, anti-oxidant and mucosal defense mechanisms. Int. Immunopharmacol. 2016, 30, 179–187. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Shi, Y.; Su, W.; Chen, J.; Zhang, Z.; Wang, G.; Wang, F. Short Chain Fatty Acid Acetate Protects against Ethanol-Induced Acute Gastric Mucosal Lesion in Mice. Biol. Pharm. Bull. 2017, 40, 1439–1446. [Google Scholar] [CrossRef]
- Santos, C.V.D.; Rey, P. Plant thioredoxins are key actors in the oxidative stress response. Trends Plant Sci. 2006, 11, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Whayne, T.F.J.; Parinandi, N.; Maulik, N. Thioredoxins in cardiovascular disease. Can. J. Physiol. Pharmacol. 2015, 93, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Yodoi, J. Extracellular thioredoxin: A therapeutic tool to combat inflammation. Cytokine Growth Factor Rev. 2013, 24, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Pyo, M.C.; Yang, S.Y.; Chun, S.H.; Oh, N.S.; Lee, K.W. Protective effects of maillard reaction products of whey protein concentrate against oxidative stress through an nrf2-dependent pathway in hepg2 cells. Biol. Pharm. Bull. 2016, 39, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Mo, Z. Keap1-Nrf2 signaling pathway in angiogenesis and vascular diseases. J. Tissue Eng. Regen. Med. 2020, 14, 869–883. [Google Scholar] [CrossRef]
- Taguchi, K.; Yamamoto, M. The keap1-nrf2 system as a molecular target of cancer treatment. Cancers 2020, 13, 46. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. Nrf2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Renaud, C.O.; Ziros, P.G.; Chartoumpekis, D.V.; Bongiovanni, M.; Sykiotis, G.P. Keap1/nrf2 signaling: A new player in thyroid pathophysiology and thyroid cancer. Front. Endocrinol. 2019, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuźniak, V.; Paluszczak, J.; Baer-Dubowska, W. The Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatment. Pharmacol. Rep. 2017, 69, 393–402. [Google Scholar] [CrossRef]
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol. Genom. 2018, 50, 77–97. [Google Scholar] [CrossRef]
- Saeedi, B.J.; Liu, K.H.; Hunter-Chang, S.; Camacho, M.C.; Eboka, R.U.; Owens, J.A.; Neish, A.S. Gut Resident Lactobacilli Activate Hepatic Nrf2 and Protect Against Oxidative Liver Injury. Cell Metab. 2020, 31, 956–968. [Google Scholar] [CrossRef]
| Gene | Oligonucleotide (5′–3′) | Oligonucleotide (3′–5′) | Product(bp) |
|---|---|---|---|
| Keap1 | TCGAAGGCATCCACCCTAAG | CTCGAACCACGCTGTCAATCT | 135 |
| Nrf2 | TAGATGACCATGAGTCGCTTGC | GCCAAACTTGCTCCATGTCC | 153 |
| P65 | ACTGCCGGGATGGCTACTAT | TCTGGATTCGCTGGCTAATGG | 126 |
| Txn1 | GTGGTGTGGACCTTGCAAAA | CTGGCAGTCATCCACTCCA | 100 |
| Txnrd1 | GGGTCCTATGACTTCGACCTG | AGTCGGTGTGACAAAATCCAAG | 117 |
| Txn2 | TTCCCTCACCTCTAAGACCCT | CCTGGACGTTAAAGGTCGTCA | 122 |
| Txnrd3 | GCACGCGGGTTAAGGAACT | TGGGCACCGTTTTCTGGTTAC | 129 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ma, J.; Jing, N.; Zhang, H.; Xie, Y.; Liu, H.; Shan, X.; Ren, J.; Jin, J. Bifidobacterium animalis A12 and Lactobacillus salivarius M18-6 Alleviate Alcohol Injury by keap1-Nrf2 Pathway and Thioredoxin System. Foods 2023, 12, 439. https://doi.org/10.3390/foods12030439
Zhang Y, Ma J, Jing N, Zhang H, Xie Y, Liu H, Shan X, Ren J, Jin J. Bifidobacterium animalis A12 and Lactobacillus salivarius M18-6 Alleviate Alcohol Injury by keap1-Nrf2 Pathway and Thioredoxin System. Foods. 2023; 12(3):439. https://doi.org/10.3390/foods12030439
Chicago/Turabian StyleZhang, Yan, Jingsheng Ma, Nanqing Jing, Hongxing Zhang, Yuanhong Xie, Hui Liu, Xiangfen Shan, Jianhua Ren, and Junhua Jin. 2023. "Bifidobacterium animalis A12 and Lactobacillus salivarius M18-6 Alleviate Alcohol Injury by keap1-Nrf2 Pathway and Thioredoxin System" Foods 12, no. 3: 439. https://doi.org/10.3390/foods12030439
APA StyleZhang, Y., Ma, J., Jing, N., Zhang, H., Xie, Y., Liu, H., Shan, X., Ren, J., & Jin, J. (2023). Bifidobacterium animalis A12 and Lactobacillus salivarius M18-6 Alleviate Alcohol Injury by keap1-Nrf2 Pathway and Thioredoxin System. Foods, 12(3), 439. https://doi.org/10.3390/foods12030439





