Multispecies Bacterial Biofilms and Their Evaluation Using Bioreactors
Abstract
:1. Introduction
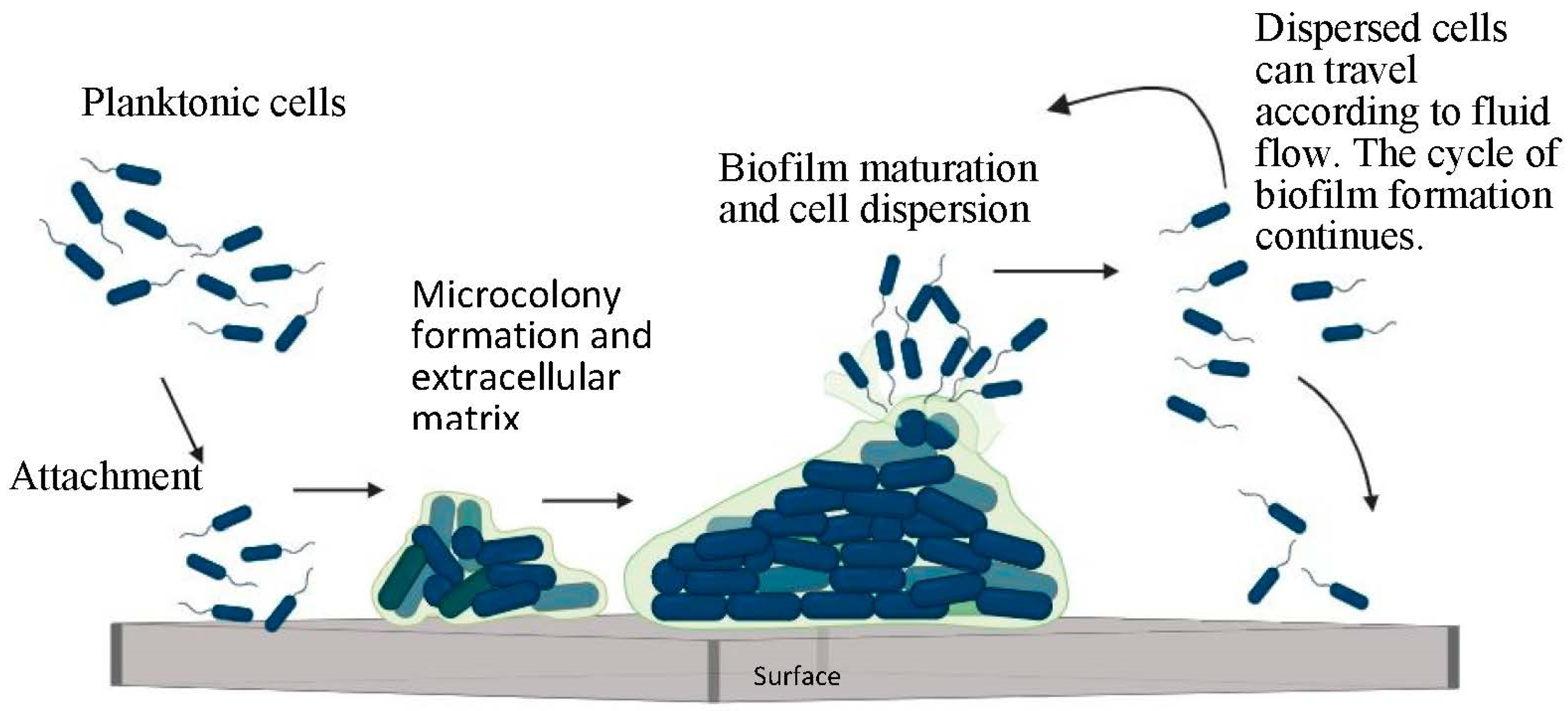
1.1. Stages of Biofilm Formation
1.2. Social Dynamics: Cooperative and Competitive Interactions in Biofilm Consortia
1.3. Influence of Fluid Dynamics on Biofilm Formation
1.4. Influence of Surface Material on Biofilm Formation
2. Types of Bioreactors
| Classification | Bioreactors | Microorganisms | Study | References |
|---|---|---|---|---|
| Operation mode | Batch process | Lactobacillus helvetics | Biomass assessed for antimicrobial and probiotic properties | [75] |
| Bacillus sp., Lysinibacillus sp., Kerstesia sp. | Wastewater treatment evaluation with decolorization/removal of Amaranth dye | [76] | ||
| Fed-batch process | Lactobacillus casei | Evaluation of use of plastic-composite supports in fermentation; periodical spike to maintain ~8% glucose in a reactor | [77] | |
| Bacillus subtilis natto | Biofilm formation in glycerol and glucose-based media; bioreactor cycle every 12 h for Vitamin K extraction | [78] | ||
| Continuous flow process | Cronobacter, Listeria monocytogenes, Salmonella and S. aureus | Multispecies biofilm formation in CDC reactor under turbulent flows to mimic dairy processing | [79] | |
| Streptomyces sp. | Streptomyces biofilms used for the removal of insecticides on polyurethane foam pieces | [80] | ||
| Static bioreactors | Lab equipment | Poultry slaughterhouse wastewater isolates (Comamonas sp.) | Bioflocculants were produced by optimizing conditions inside conical flask bioreactors using Comamonas sp. bacterial biofilms | [81] |
| Enterobacter cloacae, Klebsiella oxytoca, Serratia odorifera, and Saccharomyces cerevisiae Salmonella isolates from swine Salmonella isolates of produce and poultry origin | Biofilm formation on moving bed media for the removal of mercury from wastewater Efficacy of natural antimicrobials in biofilm removal Comparative evaluation of bacterial sources in the context of biofilm formation | [82,83,84] | ||
| Scaffolds | S. aureus, E. coli, and P. aeruginosa | Biofilm formation in clinical and food industries using Ɛ-caprolactone scaffold and curcumin nanofibers | [85] | |
| Lactiplantibacillus plantarum | Biofilm formation on electrospun ethyl cellulose nanofiber scaffolds to improve self-resistance of probiotics during production | [86] | ||
| Microfluidic devices | Enterococcus faecalis, S. aureus, Klebsiella pneumoniae and P. aeruginosa | Evaluation of 3D-printed polylactic acid surfaces to construct a microfluidic device and its suitability in biofilm formation studies | [87] | |
| P. aeruginosa | Biofilm formation in microfluidic channels under different oxygen availability conditions | [88] | ||
| Dynamic bioreactors | Stir-tank | Shigha-toxigenic E. coli, L. monocytogenes | Efficacy of peptides used in removal of pathogenic biofilms | [89] |
| Xylaria karyophthora, Clostridium aceticum, S. aureus | Inhibition of Candida albicans and Staphylococcus aureus biofilms using cytochalasins from Xylaria karyophthora | [90] | ||
| Drip flow | S. aureus and P. aeruginosa | Mixed-species biofilm formation for evaluation of an anti-biofilm treatment | [91] | |
| T. reesei and T. harzianum | Adhesion of fungal biofilms on Viton rubber, stainless steel, PTFE, silicone rubber and glass | [92] | ||
| Fluidized bed biofilm | Nitrospira, Nitrobacter | Carbonaceous oxidization and nitrification of wastewater with biofilm | [93] | |
| Comamonas, Thiobacillus, Pseudomonas, Thauera, Nitrospira | Multispecies biofilms used for the removal of chemical oxygen demand and ammonia nitrogen | [94] | ||
| Modified Robbins Device | Staphylococcus epidermidis | Adhesion of S. epidermides to glass, siliconized glass, plasma-conditioned glass, titanium, stainless-steel, and Teflon | [95] | |
| Candida albicans and S. aureus | Evaluation of disinfectants used for biofilm removal on oral medical devices | [96] | ||
| Flow chamber | Multiple oral commensal and pathogenic bacteria | Oral multispecies biofilm evaluation used in BHI/vitamin K medium | [97] | |
| E. coli | Biofilm formation on oral implant materials: glass and implant steel | [98] | ||
| Rotating disk type | Blakeslea trispora | B. trispora biofilms for carotene production in fermentation system | [99] | |
| Shewanella colwelliana | Effects of surfaces on S. colvelliana biofilms and in melanin production | [100] |
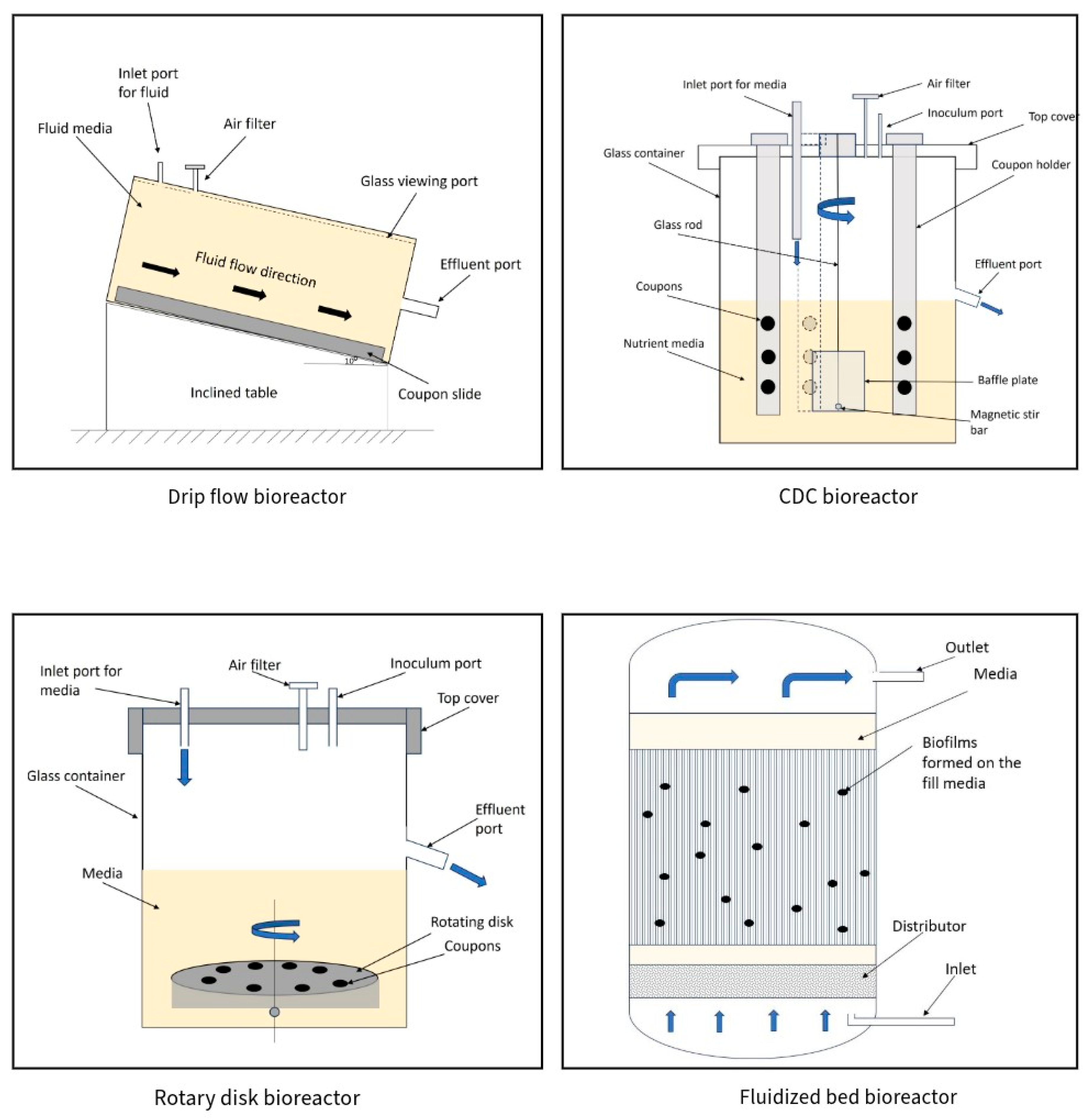
2.1. Classification Based on Bioreactor Operation Mode:
2.1.1. Batch Process Reactor
2.1.2. Fed-Batch Process Bioreactor
2.1.3. Continuous Flow Process
2.2. Classification Based on Working Principles
2.3. Classification Based on Scale:
3. Sanitary Design in Food Processing
Surface Coating to Prevent Biofilm Formation
4. Conclusions
5. Disclaimer
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Achinas, S.; Charalampogiannis, N.; Euverink, G.J.W. A Brief Recap of Microbial Adhesion and Biofilms. Appl. Sci. 2019, 9, 2801. [Google Scholar] [CrossRef]
- Cappitelli, F.; Polo, A.; Villa, F. Biofilm Formation in Food Processing Environments Is Still Poorly Understood and Controlled. Food Eng. Rev. 2014, 6, 29–42. [Google Scholar] [CrossRef]
- Lewandowski, Z.; Beyenal, H. Fundamentals of Biofilm Research; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-46655-960-8. [Google Scholar]
- Bai, X.; Nakatsu, C.H.; Bhunia, A.K. Bacterial Biofilms and Their Implications in Pathogenesis and Food Safety. Foods 2021, 10, 2117. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial Biofilms: From the Natural Environment to Infectious Diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, R. Biofilms: Microbial Cities of Scientific Significance. J. Microbiol. Exp. 2014, 1, 14. [Google Scholar] [CrossRef]
- Pönisch, W.; Eckenrode, K.B.; Alzurqa, K.; Nasrollahi, H.; Weber, C.; Zaburdaev, V.; Biais, N. Pili Mediated Intercellular Forces Shape Heterogeneous Bacterial Microcolonies Prior to Multicellular Differentiation. Sci. Rep. 2018, 8, 16567. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Tian, X. Quorum Sensing and Bacterial Social Interactions in Biofilms. Sensors 2012, 12, 2519–2538. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The Biofilm Life Cycle: Expanding the Conceptual Model of Biofilm Formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.; Banin, E. Multi-Species Biofilms: Living with Friendly Neighbors. FEMS Microbiol. Rev. 2012, 36, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cheng, S.; Li, C.; Sun, Y.; Huang, H. Shear Stress Affects Biofilm Structure and Consequently Current Generation of Bioanode in Microbial Electrochemical Systems (MESs). Front. Microbiol. 2019, 10, 398. [Google Scholar] [CrossRef]
- Lee, K.W.K.; Periasamy, S.; Mukherjee, M.; Xie, C.; Kjelleberg, S.; Rice, S.A. Biofilm Development and Enhanced Stress Resistance of a Model, Mixed-Species Community Biofilm. ISME J. 2014, 8, 894–907. [Google Scholar] [CrossRef]
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Resistance of Bacterial Biofilms to Disinfectants: A Review. Biofouling 2011, 27, 1017–1032. [Google Scholar] [CrossRef]
- Mitri, S.; Xavier, J.B.; Foster, K.R. Social Evolution in Multispecies Biofilms. Proc. Natl. Acad. Sci. USA 2011, 108, 10839–10846. [Google Scholar] [CrossRef]
- Rendueles, O.; Ghigo, J.-M. Mechanisms of Competition in Biofilm Communities. Microbiol. Spectr. 2015, 3, 319–342. [Google Scholar] [CrossRef]
- Chu, W.; Zere, T.R.; Weber, M.M.; Wood, T.K.; Whiteley, M.; Hidalgo-Romano, B.; Valenzuela, E.; McLean, R.J.C. Indole Production Promotes Escherichia coli Mixed-Culture Growth with Pseudomonas Aeruginosa by Inhibiting Quorum Signaling. Appl. Environ. Microbiol. 2012, 78, 411–419. [Google Scholar] [CrossRef]
- Govaert, M.; Smet, C.; Graeffe, A.; Walsh, J.L.; Van Impe, J.F.M. Inactivation of L. monocytogenes and S. typhimurium Biofilms by Means of an Air-Based Cold Atmospheric Plasma (CAP) System. Foods 2020, 9, 157. [Google Scholar] [CrossRef]
- Alonso, V.P.P.; Harada, A.M.M.; Kabuki, D.Y. Competitive and/or Cooperative Interactions of Listeria Monocytogenes with Bacillus cereus in Dual-Species Biofilm Formation. Front. Microbiol. 2020, 11, 177. [Google Scholar] [CrossRef]
- Maes, S.; De Reu, K.; Van Weyenberg, S.; Lories, B.; Heyndrickx, M.; Steenackers, H. Pseudomonas Putida as a Potential Biocontrol Agent against Salmonella Java Biofilm Formation in the Drinking Water System of Broiler Houses. BMC Microbiol. 2020, 20, 373. [Google Scholar] [CrossRef] [PubMed]
- Wucher, B.R.; Winans, J.B.; Elsayed, M.; Kadouri, D.E.; Nadell, C.D. Breakdown of Clonal Cooperative Architecture in Multispecies Biofilms and the Spatial Ecology of Predation. Proc. Natl. Acad. Sci. USA 2023, 120, e2212650120. [Google Scholar] [CrossRef] [PubMed]
- Camus, L.; Briaud, P.; Vandenesch, F.; Moreau, K. How Bacterial Adaptation to Cystic Fibrosis Environment Shapes Interactions Between Pseudomonas Aeruginosa and Staphylococcus Aureus. Front. Microbiol. 2021, 12, 617784. [Google Scholar] [CrossRef] [PubMed]
- Miryala, S.; Nair, V.G.; Chandramohan, S.; Srinandan, C.S. Matrix Inhibition by Salmonella Excludes Uropathogenic E. Coli from Biofilm. FEMS Microbiol. Ecol. 2021, 97, fiaa214. [Google Scholar] [CrossRef]
- Cavalcanti, I.M.G.; Nobbs, A.H.; Ricomini-Filho, A.P.; Jenkinson, H.F.; Del Bel Cury, A.A. Interkingdom Cooperation between Candida Albicans, Streptococcus Oralis and Actinomyces Oris Modulates Early Biofilm Development on Denture Material. Pathog. Dis. 2016, 74, ftw002. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Jin, X.; Hong, S.H. Probiotic Escherichia coli Inhibits Biofilm Formation of Pathogenic E. coli via Extracellular Activity of DegP. Sci. Rep. 2018, 8, 4939. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; Cargo, R.; Rupp, C.J.; Wilson, S.; Klapper, I. Biofilm Material Properties as Related to Shear-Induced Deformation and Detachment Phenomena. J. Ind. Microbiol. Biotechnol. 2002, 29, 361–367. [Google Scholar] [CrossRef]
- Moreira, J.; Teodósio, J.S.; Simões, M.; Melo, L.F.; Mergulhão, F.J. Biofilm Formation under Turbulent Conditions: External Mass Transfer versus Shear Stress. In Proceedings of the 10th International Conference on Heat Exchanger Fouling and Cleaning, Budapest, Hungary, 9–14 June 2013. [Google Scholar]
- Tsagkari, E.; Sloan, W.T. Turbulence Accelerates the Growth of Drinking Water Biofilms. Bioprocess Biosyst. Eng. 2018, 41, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.O.; Kuehn, M.; Wuertz, S.; Neu, T.; Melo, L.F. Effect of Flow Regime on the Architecture of a Pseudomonas Fluorescens Biofilm. Biotechnol. Bioeng. 2002, 78, 164–171. [Google Scholar] [CrossRef]
- Fernandes, S.; Gomes, I.B.; Simões, L.C.; Simões, M. Overview on the Hydrodynamic Conditions Found in Industrial Systems and Its Impact in (Bio)Fouling Formation. Chem. Eng. J. 2021, 418, 129348. [Google Scholar] [CrossRef]
- Reynolds, O. XXIX. An Experimental Investigation of the Circumstances Which Determine Whether the Motion of Water Shall Be Direct or Sinuous, and of the Law of Resistance in Parallel Channels. Philos. Trans. R. Soc. Lond. 1883, 174, 935–982. [Google Scholar]
- Fysun, O.; Kern, H.; Wilke, B.; Langowski, H.-C. Evaluation of Factors Influencing Dairy Biofilm Formation in Filling Hoses of Food-Processing Equipment. Food Bioprod. Process. Trans. Inst. Chem. Eng. Part C 2019, 113, 39–48. [Google Scholar] [CrossRef]
- Freiberger, F.; Budde, J.; Ateş, E.; Schlüter, M.; Pörtner, R.; Möller, J. New Insights from Locally Resolved Hydrodynamics in Stirred Cell Culture Reactors. Processes 2022, 10, 107. [Google Scholar] [CrossRef]
- Goeres, D.M.; Pedersen, S.; Warwood, B.; Walker, D.K.; Parker, A.E.; Mettler, M.; Sturman, P. Design and Fabrication of Biofilm Reactors. In Recent Trends in Biofilm Science and Technology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–88. ISBN 978-0-12819-497-3. [Google Scholar]
- Stoodley, P.; Warwood, B.K. Use of Flow Cells and Annular Reactors to Study Biofilms. In Biofilms in Industry, Medicine and Environmental Biotechnology; Lens, P., O’Flaherty, V., Moran, A., Stoodley, P., Mahony, T., Eds.; IWA Publishing: London, UK, 2003; pp. 197–213. ISBN 978-1-84339-019-0. [Google Scholar]
- Teodósio, J.S.; Simões, M.; Melo, L.F.; Mergulhão, F.J. Flow Cell Hydrodynamics and Their Effects on E. coli Biofilm Formation under Different Nutrient Conditions and Turbulent Flow. Biofouling 2011, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Oder, M.; Arlič, M.; Bohinc, K.; Fink, R. Escherichia coli Biofilm Formation and Dispersion under Hydrodynamic Conditions on Metal Surfaces. Int. J. Environ. Health Res. 2018, 28, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.; Mergulhão, F.; Melo, L.; Simões, M. The Effect of Shear Stress on the Formation and Removal of Bacillus cereus Biofilms. Food Bioprod. Process. 2015, 93, 242–248. [Google Scholar] [CrossRef]
- Vieira, M.J. The Role of Hydrodynamic Stress on the Phenotypic Characteristics of Single and Binary Biofilms of Pseudomonas Fluorescens. Water Sci. Technol. 2007, 55, 437–445. [Google Scholar]
- Abld, M.; Xuereb, C.; Bertrand, J. Modeling of the 3D Hydrodynamics of 2-blade Impellers in Stirred Tanks Filled with a Highly Viscous Fluid. Can. J. Chem. Eng. 1994, 72, 184–193. [Google Scholar] [CrossRef]
- Ismadi, M.-Z.; Hourigan, K.; Fouras, A. Experimental Characterisation of Fluid Mechanics in a Spinner Flask Bioreactor. Processes 2014, 2, 753–772. [Google Scholar] [CrossRef]
- Kadic, E.; Heindel, T.J. Hydrodynamic Considerations in Bioreactor Selection and Design. In Proceedings of the ASME 2010 3rd Joint US-European Fluids Engineering Summer Meeting, Montreal, QC, Canada, 1–5 August 2010; Volume 1, pp. 2149–2159. [Google Scholar]
- Csapai, A.; Toc, D.A.; Popa, F.; Tosa, N.; Pascalau, V.; Costache, C.; Botan, A.; Popa, C.O. 3D Printed Microfluidic Bioreactors Used for the Preferential Growth of Bacterial Biofilms through Dielectrophoresis. Micromachines 2022, 13, 1377. [Google Scholar] [CrossRef]
- Recupido, F.; Toscano, G.; Tatè, R.; Petala, M.; Caserta, S.; Karapantsios, T.D.; Guido, S. The Role of Flow in Bacterial Biofilm Morphology and Wetting Properties. Colloids Surf. B Biointerfaces 2020, 192, 111047. [Google Scholar] [CrossRef]
- Liduino, V.S.; Payão Filho, J.C.; Cravo-Laureau, C.; Lutterbach, M.T.; Camporese Sérvulo, E.F. Comparison of Flow Regimes on Biocorrosion of Steel Pipe Weldments: Fluid Characterization and Pitting Analysis. Int. Biodeterior. Biodegrad. 2019, 144, 104750. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, H.; Zheng, G.; Dong, F.; Liu, C. Dynamic Changes in Biofilm Structures under Dynamic Flow Conditions. Appl. Environ. Microbiol. 2022, 88, e01072-22. [Google Scholar] [CrossRef]
- Chang, J.; He, X.; Bai, X.; Yuan, C. The Impact of Hydrodynamic Shear Force on Adhesion Morphology and Biofilm Conformation of Bacillus sp. Ocean Eng. 2020, 197, 106860. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, M.; Zhang, H.; Yang, S.; Song, K.-Y.; Yin, R.; Zhang, W. Effects of Hydrophilicity, Adhesion Work, and Fluid Flow on Biofilm Formation of PDMS in Microfluidic Systems. ACS Appl. Bio Mater. 2020, 3, 8386–8394. [Google Scholar] [CrossRef] [PubMed]
- Sherman, E.; Bayles, K.; Moormeier, D.; Endres, J.; Wei, T. Observations of Shear Stress Effects on Staphylococcus Aureus Biofilm Formation. mSphere 2019, 4, 10–128. [Google Scholar] [CrossRef]
- Gomes, I.B.; Lemos, M.; Fernandes, S.; Borges, A.; Simões, L.C.; Simões, M. The Effects of Chemical and Mechanical Stresses on Bacillus cereus and Pseudomonas Fluorescens Single- and Dual-Species Biofilm Removal. Microorganisms 2021, 9, 1174. [Google Scholar] [CrossRef]
- Fabbri, S.; Li, J.; Howlin, R.P.; Rmaile, A.; Gottenbos, B.; De Jager, M.; Starke, E.M.; Aspiras, M.; Ward, M.T.; Cogan, N.G.; et al. Fluid-Driven Interfacial Instabilities and Turbulence in Bacterial Biofilms. Environ. Microbiol. 2017, 19, 4417–4431. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chand, D.V.; Chandra, J.; Anderson, J.M.; Ghannoum, M.A. Shear Stress Modulates the Thickness and Architecture of Candida Albicans Biofilms in a Phase-Dependent Manner. Mycoses 2009, 52, 440–446. [Google Scholar] [CrossRef]
- Chen, M.J.; Zhang, Z.; Bott, T.R. Effects of Operating Conditions on the Adhesive Strength of Pseudomonas Fluorescens Biofilms in Tubes. Colloids Surf. B Biointerfaces 2005, 43, 61–71. [Google Scholar] [CrossRef]
- Arnold, J.W.; Bailey, G.W. Surface Finishes on Stainless Steel Reduce Bacterial Attachment and Early Biofilm Formation: Scanning Electron and Atomic Force Microscopy Study. Poult. Sci. 2000, 79, 1839–1845. [Google Scholar] [CrossRef]
- Medilanski, E.; Kaufmann, K.; Wick, L.Y.; Wanner, O.; Harms, H. Influence of the Surface Topography of Stainless Steel on Bacterial Adhesion. Biofouling 2002, 18, 193–203. [Google Scholar] [CrossRef]
- Rivas, L.; Fegan, N.; Dykes, G.A. Attachment of Shiga Toxigenic Escherichia coli to Stainless Steel. Int. J. Food Microbiol. 2007, 115, 89–94. [Google Scholar] [CrossRef]
- De-la-Pinta, I.; Cobos, M.; Ibarretxe, J.; Montoya, E.; Eraso, E.; Guraya, T.; Quindós, G. Effect of Biomaterials Hydrophobicity and Roughness on Biofilm Development. J. Mater. Sci. Mater. Med. 2019, 30, 77. [Google Scholar] [CrossRef]
- Goulter-Thorsen, R.M.; Taran, E.; Gentle, I.R.; Gobius, K.S.; Dykes, G.A. Surface Roughness of Stainless Steel Influences Attachment and Detachment of Escherichia coli O157. J. Food Prot. 2011, 74, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Mafu, A.A.; Plumety, C.; Deschênes, L.; Goulet, J. Adhesion of Pathogenic Bacteria to Food Contact Surfaces: Influence of pH of Culture. Int. J. Microbiol. 2011, 2011, 972494. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Catchmark, J.M.; Demirci, A. Enhanced Production of Bacterial Cellulose by Using a Biofilm Reactor and Its Material Property Analysis. J. Biol. Eng. 2009, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Roveto, P.M.; Benavidez, A.; Schuler, A.J. Effects of Methyl, Ester, and Amine Surface Groups on Microbial Activity and Communities in Nitrifying Biofilms. ACS Appl. Bio Mater. 2022, 5, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Vongkampang, T.; Sreenivas, K.; Grey, C.; van Niel, E.W.J. Immobilization Techniques Improve Volumetric Hydrogen Productivity of Caldicellulosiruptor Species in a Modified Continuous Stirred Tank Reactor. Biotechnol. Biofuels Bioprod. 2023, 16, 25. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Hrycauk, S.; Holman, D.B.; Ells, T.C. Microbial Dynamics in Mixed-Culture Biofilms of Salmonella Typhimurium and Escherichia coli O157:H7 and Bacteria Surviving Sanitation of Conveyor Belts of Meat Processing Plants. Microorganisms 2023, 11, 421. [Google Scholar] [CrossRef]
- Stewart, P.S. Biophysics of Biofilm Infection. Pathog. Dis. 2014, 70, 212–218. [Google Scholar] [CrossRef]
- Paul, E.; Ochoa, J.C.; Pechaud, Y.; Liu, Y.; Liné, A. Effect of Shear Stress and Growth Conditions on Detachment and Physical Properties of Biofilms. Water Res. 2012, 46, 5499–5508. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Dertli, E.; Mayer, M.J.; Narbad, A. Impact of the Exopolysaccharide Layer on Biofilms, Adhesion and Resistance to Stress in Lactobacillus Johnsonii FI9785. BMC Microbiol. 2015, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Rodrigues, J.B.; de Souza, N.T.; Scarano, J.O.A.; de Sousa, J.M.; Lira, M.C.; de Figueiredo, R.C.B.Q.; de Souza, E.L.; Magnani, M. Efficacy of Using Oregano Essential Oil and Carvacrol to Remove Young and Mature Staphylococcus Aureus Biofilms on Food-Contact Surfaces of Stainless Steel. LWT 2018, 93, 293–299. [Google Scholar] [CrossRef]
- Faille, C.; Jullien, C.; Fontaine, F.; Bellon-Fontaine, M.-N.; Slomianny, C.; Benezech, T. Adhesion of Bacillus Spores and Escherichia coli Cells to Inert Surfaces. Can. J. Microbiol. 2002, 48, 728–738. [Google Scholar] [CrossRef]
- Bryers, J. Understanding and Controlling Detrimental Bioreactor Biofilms. Trends Biotechnol. 1991, 9, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, M.; Yang, L.; Jiang, Y.; Jiang, W.; Yu, Z.; Zhang, W.; Xin, F.; Jiang, M. Spatial Niche Construction of a Consortium-Based Consolidated Bioprocessing System. Green Chem. 2022, 24, 7941–7950. [Google Scholar] [CrossRef]
- Leiknes, T.; Ødegaard, H. The Development of a Biofilm Membrane Bioreactor. Desalination 2007, 202, 135–143. [Google Scholar] [CrossRef]
- An, S.-Q.; Hull, R.; Metris, A.; Barrett, P.; Webb, J.S.; Stoodley, P. An in Vitro Biofilm Model System to Facilitate Study of Microbial Communities of the Human Oral Cavity. Lett. Appl. Microbiol. 2022, 74, 302–310. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, H.; Xiong, J.; Li, Y.; Luo, W. Enhanced Iturin a Production in a Two-Compartment Biofilm Reactor by Bacillus Velezensis ND. Front. Bioeng. Biotechnol. 2023, 11, 1102786. [Google Scholar] [CrossRef]
- Tsagkari, E.; Connelly, S.; Liu, Z.; McBride, A.; Sloan, W.T. The Role of Shear Dynamics in Biofilm Formation. NPJ Biofilms Microbiomes 2022, 8, 33. [Google Scholar] [CrossRef]
- Denkova-Kostova, R.; Goranov, B.; Tomova, T.; Yanakieva, V.; Blazheva, D.; Denkova, Z.; Kostov, G. Investigation of Probiotic Properties of Lactobacillus Helveticus 2/20 Isolated from Rose Blossom of Rosa Damascena Mill; EDP Sciences: Paris, France, 2023; Volume 58. [Google Scholar]
- Anjaneya, O.; Shrishailnath, S.; Guruprasad, K.; Nayak, A.S.; Mashetty, S.; Karegoudar, T. Decolourization of Amaranth Dye by Bacterial Biofilm in Batch and Continuous Packed Bed Bioreactor. Int. Biodeterior. Biodegrad. 2013, 79, 64–72. [Google Scholar] [CrossRef]
- Velázquez, A.; Pometto Iii, A.L.; Ho, K.-L.G.; Demirci, A. Evaluation of Plastic-Composite Supports in Repeated Fed-Batch Biofilm Lactic Acid Fermentation by Lactobacillus Casei. Appl. Microbiol. Biotechnol. 2001, 55, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Mahdinia, E.; Demirci, A.; Berenjian, A. Implementation of Fed-Batch Strategies for Vitamin K (Menaquinone-7) Production by Bacillus Subtilis Natto in Biofilm Reactors. Appl. Microbiol. Biotechnol. 2018, 102, 9147–9157. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.; Killington, A.; Fouhy, K.; Loh, M.; Malakar, P. The CDC Biofilm Bioreactor Is a Suitable Method to Grow Biofilms, and Test Their Sanitiser Susceptibilities, in the Dairy Context. Int. Dairy J. 2022, 126, 105264. [Google Scholar] [CrossRef]
- Briceño, G.; Levio, M.; González, M.E.; Saez, J.M.; Palma, G.; Schalchli, H.; Diez, M.C. Performance of a Continuous Stirred Tank Bioreactor Employing an Immobilized Actinobacteria Mixed Culture for the Removal of Organophosphorus Pesticides. 3 Biotech 2020, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Dlangamandla, C.; Dyantyi, S.A.; Mpentshu, Y.P.; Ntwampe, S.K.O.; Basitere, M. Optimisation of Bioflocculant Production by a Biofilm Forming Microorganism from Poultry Slaughterhouse Wastewater for Use in Poultry Wastewater Treatment. Water Sci. Technol. 2016, 73, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Radojević, I.; Jakovljević, V.; Grujić, S.; Ostojić, A.; Ćirković, K. Biofilm Formation by Selected Microbial Strains Isolated from Wastewater and Their Consortia: Mercury Resistance and Removal Potential. Res. Microbiol. 2023, 104092. [Google Scholar] [CrossRef] [PubMed]
- Keelara, S.; Thakur, S.; Patel, J. Biofilm Formation by Environmental Isolates of Salmonella and Their Sensitivity to Natural Antimicrobials. Foodborne Pathog. Dis. 2016, 13, 509–516. [Google Scholar] [CrossRef]
- Patel, J.; Singh, M.; Macarisin, D.; Sharma, M.; Shelton, D. Differences in Biofilm Formation of Produce and Poultry Salmonella Enterica Isolates and Their Persistence on Spinach Plants. Food Microbiol. 2013, 36, 388–394. [Google Scholar] [CrossRef]
- Pompa-Monroy, D.A.; Figueroa-Marchant, P.G.; Dastager, S.G.; Thorat, M.N.; Iglesias, A.L.; Miranda-Soto, V.; Pérez-González, G.L.; Villarreal-Gómez, L.J. Bacterial Biofilm Formation Using Pcl/Curcumin Electrospun Fibers and Its Potential Use for Biotechnological Applications. Materials 2020, 13, 5556. [Google Scholar] [CrossRef]
- Shi, J.; Li, S.-F.; Feng, K.; Han, S.-Y.; Hu, T.-G.; Wu, H. Improving the Viability of Probiotics under Harsh Conditions by the Formation of Biofilm on Electrospun Nanofiber Mat. Foods 2022, 11, 1203. [Google Scholar] [CrossRef]
- Toc, D.A.; Csapai, A.; Popa, F.; Popa, C.; Pascalau, V.; Tosa, N.; Botan, A.; Mihaila, R.M.; Costache, C.A.; Colosi, I.A. Easy and Affordable: A New Method for the Studying of Bacterial Biofilm Formation. Cells 2022, 11, 4119. [Google Scholar] [CrossRef]
- Skolimowski, M.; Weiss Nielsen, M.; Emnéus, J.; Molin, S.; Taboryski, R.; Sternberg, C.; Dufva, M.; Geschke, O. Microfluidic Dissolved Oxygen Gradient Generator Biochip as a Useful Tool in Bacterial Biofilm Studies. Lab Chip 2010, 10, 2162–2169. [Google Scholar] [CrossRef]
- Yin, H.-B.; Boomer, A.; Chen, C.-H.; Patel, J. Antibiofilm Efficacy of Peptide 1018 against Listeria Monocytogenes and Shiga Toxigenic Escherichia coli on Equipment Surfaces. J. Food Prot. 2019, 82, 1837–1843. [Google Scholar] [CrossRef]
- Lambert, C.; Shao, L.; Zeng, H.; Surup, F.; Saetang, P.; Aime, M.C.; Husbands, D.R.; Rottner, K.; Stradal, T.E.; Stadler, M. Cytochalasans Produced by Xylaria Karyophthora and Their Biological Activities. Mycologia 2023, 115, 277–287. [Google Scholar] [CrossRef]
- Regulski, M.; Myntti, M.F.; James, G.A. Anti-Biofilm Efficacy of Commonly Used Wound Care Products in In Vitro Settings. Antibiotics 2023, 12, 536. [Google Scholar] [CrossRef]
- Bajoul Kakahi, F.; Ly, S.; Tarayre, C.; Deschaume, O.; Bartic, C.; Wagner, P.; Compère, P.; Derdelinckx, G.; Blecker, C.; Delvigne, F. Modulation of Fungal Biofilm Physiology and Secondary Product Formation Based on Physico-Chemical Surface Properties. Bioprocess Biosyst. Eng. 2019, 42, 1935–1946. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, D.; Xu, Z.; Li, A.; Gao, H.; Hou, D. Enhanced Aerobic Granulation, Stabilization, and Nitrification in a Continuous-Flow Bioreactor by Inoculating Biofilms. Appl. Microbiol. Biotechnol. 2014, 98, 5737–5745. [Google Scholar] [CrossRef]
- Yuan, K.; Li, S.; Zhong, F. Treatment of Coking Wastewater in Biofilm-Based Bioaugmentation Process: Biofilm Formation and Microbial Community Analysis. J. Hazard. Mater. 2020, 400, 123117. [Google Scholar] [CrossRef]
- Linton, C.J.; Sherriff, A.; Millar, M.R. Use of a Modified Robbins Device to Directly Compare the Adhesion of Staphylococcus Epidermidis RP62A to Surfaces. J. Appl. Microbiol. 1999, 86, 194–202. [Google Scholar] [CrossRef]
- Coenye, T.; De Prijck, K.; De Wever, B.; Nelis, H.J. Use of the Modified Robbins Device to Study the in Vitro Biofilm Removal Efficacy of NitrAdineTM, a Novel Disinfecting Formula for the Maintenance of Oral Medical Devices. J. Appl. Microbiol. 2008, 105, 733–740. [Google Scholar] [CrossRef]
- Kommerein, N.; Doll, K.; Stumpp, N.S.; Stiesch, M. Development and Characterization of an Oral Multispecies Biofilm Implant Flow Chamber Model. PLoS ONE 2018, 13, e0196967. [Google Scholar] [CrossRef]
- Menzel, F.; Conradi, B.; Rodenacker, K.; Gorbushina, A.A.; Schwibbert, K. Flow Chamber System for the Statistical Evaluation of Bacterial Colonization on Materials. Materials 2016, 9, 770. [Google Scholar] [CrossRef]
- Roukas, T. Modified Rotary Biofilm Reactor: A New Tool for Enhanced Carotene Productivity by Blakeslea Trispora. J. Clean. Prod. 2018, 174, 1114–1121. [Google Scholar] [CrossRef]
- Mitra, S.; Gachhui, R.; Mukherjee, J. Enhanced Biofilm Formation and Melanin Synthesis by the Oyster Settlement-Promoting Shewanella Colwelliana Is Related to Hydrophobic Surface and Simulated Intertidal Environment. Biofouling 2015, 31, 283–296. [Google Scholar] [CrossRef]
- Bodean, M.F.; Regaldo, L.; Mayora, G.; Mora, C.; Giri, F.; Gervasio, S.; Popielarz, A.; Repetti, M.; Licursi, M. Effects of Herbicides and Fertilization on Biofilms of Pampean Lotic Systems: A Microcosm Study; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Mitra, S.; Murthy, G.S. Bioreactor Control Systems in the Biopharmaceutical Industry: A Critical Perspective. Syst. Microbiol. Biomanuf. 2022, 2, 91–112. [Google Scholar] [CrossRef]
- Yamuna Rani, K.; Ramachandra Rao, V.S. Control of Fermenters—A Review. Bioprocess Eng. 1999, 21, 77–88. [Google Scholar] [CrossRef]
- Germec, M.; Yatmaz, E.; Karahalil, E.; Turhan, İ. Effect of Different Fermentation Strategies on β-Mannanase Production in Fed-Batch Bioreactor System. 3 Biotech 2017, 7, 77. [Google Scholar] [CrossRef]
- Trappetti, C.; Oggioni, M.R. Chapter 13—Biofilm Formation Under In Vitro Conditions. In Streptococcus Pneumoniae; Brown, J., Hammerschmidt, S., Orihuela, C., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 245–255. ISBN 978-0-12410-530-0. [Google Scholar]
- Fisher, J.T.; Gurney, T.O.; Mason, B.M.; Fisher, J.K.; Kelly, W.J. Mixing and Oxygen Transfer Characteristics of a Microplate Bioreactor with Surface-Attached Microposts. Biotechnol. J. 2021, 16, e2000257. [Google Scholar] [CrossRef]
- Winterbottom, J.M.; King, M. Reactor Design for Chemical Engineers; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-35141-966-6. [Google Scholar]
- Singh, J.; Kaushik, N.; Biswas, S. Bioreactors–Technology & Design Analysis. Scitech J. 2014, 1, 28–36. [Google Scholar]
- Buckingham-Meyer, K.; Goeres, D.M.; Hamilton, M.A. Comparative Evaluation of Biofilm Disinfectant Efficacy Tests. J. Microbiol. Methods 2007, 70, 236–244. [Google Scholar] [CrossRef]
- Manner, S.; Goeres, D.M.; Skogman, M.; Vuorela, P.; Fallarero, A. Prevention of Staphylococcus Aureus Biofilm Formation by Antibiotics in 96-Microtiter Well Plates and Drip Flow Reactors: Critical Factors Influencing Outcomes. Sci. Rep. 2017, 7, 43854. [Google Scholar] [CrossRef]
- Yuan, L.; Sadiq, F.A.; Wang, N.; Yang, Z.; He, G. Recent Advances in Understanding the Control of Disinfectant-Resistant Biofilms by Hurdle Technology in the Food Industry. Crit. Rev. Food Sci. Nutr. 2021, 61, 3876–3891. [Google Scholar] [CrossRef]
- Bourdichon, F.; Betts, R.; Dufour, C.; Fanning, S.; Farber, J.; McClure, P.; Stavropoulou, D.A.; Wemmenhove, E.; Zwietering, M.H.; Winkler, A. Processing Environment Monitoring in Low Moisture Food Production Facilities: Are We Looking for the Right Microorganisms? Int. J. Food Microbiol. 2021, 356, 109351. [Google Scholar] [CrossRef]
- Soon, J.M.; Brazier, A.K.M.; Wallace, C.A. Determining Common Contributory Factors in Food Safety Incidents—A Review of Global Outbreaks and Recalls 2008–2018. Trends Food Sci. Technol. 2020, 97, 76–87. [Google Scholar] [CrossRef]
- Moerman, F.; Kastelein, J.; Rugh, T. Hygienic Design of Food Processing Equipment. In Food Safety Management; Elsevier: Amsterdam, The Netherlands, 2023; pp. 623–678. ISBN 978-0-12820-013-1. [Google Scholar]
- DeFlorio, W.; Liu, S.; White, A.R.; Taylor, T.M.; Cisneros-Zevallos, L.; Min, Y.; Scholar, E.M.A. Recent Developments in Antimicrobial and Antifouling Coatings to Reduce or Prevent Contamination and Cross-Contamination of Food Contact Surfaces by Bacteria. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3093–3134. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.A.; Boegli, L.; James, G.; Velasquillo, C.; Sánchez-Sánchez, R.; Martínez-Martínez, R.-E.; Martínez-Castañón, G.A.; Martinez-Gutierrez, F. Silver Nanoparticles with Antimicrobial Activities against Streptococcus Mutans and Their Cytotoxic Effect. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 360–366. [Google Scholar] [CrossRef]
- Ciolacu, L.; Zand, E.; Negrau, C.; Jaeger, H. Bacterial Attachment and Biofilm Formation on Antimicrobial Sealants and Stainless Steel Surfaces. Foods 2022, 11, 3096. [Google Scholar] [CrossRef]
- Hili, P.; Evans, C.S.; Veness, R.G. Antimicrobial Action of Essential Oils: The Effect of Dimethylsulphoxide on the Activity of Cinnamon Oil. Lett. Appl. Microbiol. 1997, 24, 269–275. [Google Scholar] [CrossRef]
- Raut, R.R.; Sawant, A.; Jamge, B. Antimicrobial Activity of Azadirachta Indica (Neem) against Pathogenic Microorganisms. J. Acad. Ind. Res. 2014, 3, 327–329. [Google Scholar]
- Lamarra, J.; Calienni, M.N.; Rivero, S.; Pinotti, A. Electrospun Nanofibers of Poly(Vinyl Alcohol) and Chitosan-Based Emulsions Functionalized with Cabreuva Essential Oil. Int. J. Biol. Macromol. 2020, 160, 307–318. [Google Scholar] [CrossRef]
- Bruzaud, J.; Tarrade, J.; Celia, E.; Darmanin, T.; Taffin de Givenchy, E.; Guittard, F.; Herry, J.-M.; Guilbaud, M.; Bellon-Fontaine, M.-N. The Design of Superhydrophobic Stainless Steel Surfaces by Controlling Nanostructures: A Key Parameter to Reduce the Implantation of Pathogenic Bacteria. Mater. Sci. Eng. C 2017, 73, 40–47. [Google Scholar] [CrossRef]
| Social Behavior | Species | Effects | References |
|---|---|---|---|
| Cooperative interaction | Listeria monocytogenes and Salmonella Typhimurium | Metabolic collaboration during biofilm formation | [17] |
| Competitive interaction | L. monocytogenes and Bacillus cereus | Restrained L. monocytogenes growth and biofilm formation by Bacillus cereus | [18] |
| Competitive interaction | P. putida strains and Salmonella java | Mutual inhibition, potential use of P. putida as biocontrol agents against S. java | [19] |
| Competitive interaction | Escherichia coli, Vibrio cholerae, Bdellovibrio bacteriovorus | Predation—B. bacteriovorus is predator whereas E. coli and V. cholerae are prey | [20] |
| Cooperative interaction | P. aeruginosa and Staphylococcus aureus | Mutual defense and metabolic cooperation against antibiotics from these cystic fibrosis-adapted strains | [21] |
| Competitive interaction | Salmonella Typhimurium wild type and mutant with E. coli | Outgrowth of Salmonella strains and suppression of matrix production by E. coli within the biofilm | [22] |
| Cooperative interaction | Streptococcus oralis, Actinomyces oris, Candida albicans | Promotion of biofilms and planktonic environments among all three species | [23] |
| Competitive interaction | probiotic E. coli, shiga-toxigenic E. coli, P. aeruginosa, S. aureus, and Staphylococcus epidermidis | Suppression of E. coli as well as S. aureus and S. epidermidis biofilms by probiotic E. coli strain | [24] |
| Flow Conditions | Flow System | Bacteria | Flow Parameters | Results | References |
|---|---|---|---|---|---|
| Stagnant and shaken fluid, laminar | Flow chamber | Pseudomonas fluorescens | Shear stress: 1.39 × 10−4 and 8.33 × 10−4 Pa for laminar flow | Clumps in biofilm under shaking fluid conditions; higher shear stress promotes EPS formation and dense biofilms | [43] |
| Laminar and turbulent | Closed-loop system | Seawater bacterial consortium | Flow rates: laminar—0.023 m/s, turbulent—0.052 m/s | Highly prevalent bio-corrosion on weld joints under laminar flow | [44] |
| Laminar | Flow chamber | Shewanella oneidensis | Flow rate—0.1 to 0.8 mL/min (equivalent shear stress: 2 to 16 mPa) | Higher rate of biofilm removal at higher flow rates | [45] |
| Static, laminar, and turbulent | Parallel flow cell system | Bacillus sp. | Shear stress of 0.23, 0.68, 1.39, 2.30 Pa | Complexly structured biofilms at lower shear stress; biofilms with dense and smooth structures and higher adhesive strength under turbulent flow | [46] |
| Laminar | Microfluidic device | E. coli and S. aureus | Flow rates: 0.015, 0.03, 0.04, and 0.05 mL/min | Higher shear force required to inhibit biofilm formations on hydrophilic surfaces | [47] |
| Laminar | PDMS microcha-nnels | S. aureus | Shear stress: 0.015 to 0.15 Pa | Tower-like structures formed during biofilm formation at ~0.06 Pa shear stress | [48] |
| Turbulent | Rotating cylinder reactor | Bacillus cereus and P. fluorescens | Shear stress: 0.70, 1.66,5.50, 10.9, 17.7 Pa | Higher rate of biofilm removal under low shear stress | [49] |
| Laminar and Turbulent | Microfluidic flow channel | S. mutans, S. epidermidis, P. aeruginosa | Shear stress: 0.6, 4.1, 11.5, 23.8, 35.5, 55.3 Pa | Source of microspray affects crescent or curved shapes of ripples in biofilms; wrinkled surface with P. aeruginosa biofilm | [50] |
| NA | Rotating disc system | Candida albicans | Shear stress: 0.003, 0.110, 0.198 Pa | Shear stress and growth phases affect biofilm formation | [51] |
| Turbulent | Simulated cooling water system | P. fluorescens | Flow rate: 0.6,1.0,1.6 m/s | Adhesive strength of biofilms increases with flow rate | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabhukhot, G.S.; Eggleton, C.D.; Patel, J. Multispecies Bacterial Biofilms and Their Evaluation Using Bioreactors. Foods 2023, 12, 4495. https://doi.org/10.3390/foods12244495
Prabhukhot GS, Eggleton CD, Patel J. Multispecies Bacterial Biofilms and Their Evaluation Using Bioreactors. Foods. 2023; 12(24):4495. https://doi.org/10.3390/foods12244495
Chicago/Turabian StylePrabhukhot, Grishma S., Charles D. Eggleton, and Jitendra Patel. 2023. "Multispecies Bacterial Biofilms and Their Evaluation Using Bioreactors" Foods 12, no. 24: 4495. https://doi.org/10.3390/foods12244495
APA StylePrabhukhot, G. S., Eggleton, C. D., & Patel, J. (2023). Multispecies Bacterial Biofilms and Their Evaluation Using Bioreactors. Foods, 12(24), 4495. https://doi.org/10.3390/foods12244495





