A Critical Review of Risk Assessment Models for Listeria monocytogenes in Dairy Products
Abstract
:1. Introduction
2. Materials and Methods
3. Results
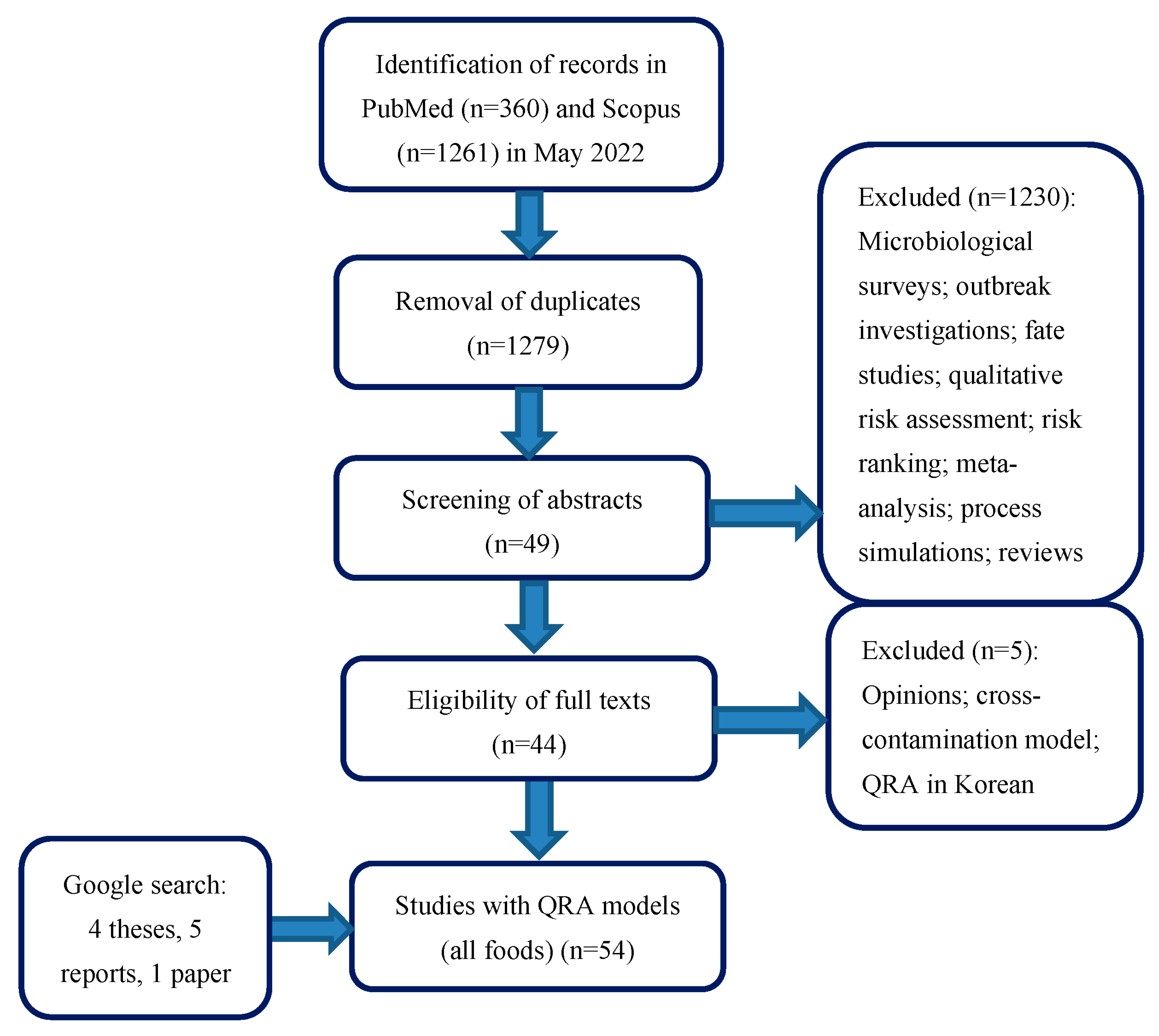
3.1. Systematic Review Process
3.2. Description of the QRA Models in Dairy Products
4. Discussion
4.1. Risk Factors and Control Measures Assessed at Primary Production
4.2. Risk Factors and Control Measures Assessed at Processing
4.3. Cross-Contamination during Processing
4.4. Risk Factors and Control Measures at Retail and Home
4.5. Contributions of Retail and Consumer Practices to the Final Risk of Listeriosis
4.6. L. monocytogenes Growth Kinetic Parameters as Drivers of the Final Risk
4.7. Availability of Models
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- EFSA. The European Union one health 2020 zoonoses report. EFSA J. 2021, 19, 6971. [Google Scholar] [CrossRef]
- Leclercq, A.; Kooh, P.; Augustin, J.-C.; Guillier, L.; Thebault, A.; Cadavez, V.; Gonzales-Barron, U.; Sanaa, M. Risk factors for sporadic listeriosis: A systematic review and meta-analysis. Microb. Risk Anal. 2021, 17, 100128. [Google Scholar] [CrossRef]
- Dass, S. Exposure Assessment of Listeria monocytogenes in Vacuum Packed Cold-Smoked Salmon in the Republic of Ireland. Ph.D. Thesis, Technological University Dublin, Dublin, Ireland, 2011. [Google Scholar]
- Bomfeh, K. Risk Assessment for Listeria monocytogenes in Traditionally Processed Fish from Informal Markets in Accra and Tema. Master’s Thesis, University of Ghana, Accra, Ghana, 2011. [Google Scholar]
- Tang, J. Risk Assessment of Listeria monocytogenes in Ready-to-Eat Meat from Plants to Consumption. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2013. [Google Scholar]
- Guzel, M. Quantitative Risk Assessment for Listeria monocytogenes on Fresh-Cut Lettuce and Fresh-Cut Cantaloupe. Master’s Thesis, Texas A&M University, College Station, TX, USA, 2015. [Google Scholar]
- FDA-FSIS. Quantitative Assessment of Relative Risk to Public Health from Foodborne Listeria monocytogenes among Selected Categories of Ready-to-Eat Foods; Center for Food and Safety and Applied Nutrition, Food and Drug Administration, U.S. Department of Health and Human Services, and Food Safety and Inspection Service, U.S. Department of Agriculture: College Park, MD, USA, 2003; pp. 1–541.
- FAO/WHO. Risk Assessment of Listeria monocytogenes in Ready-to-Eat Foods: Technical Report; World Health Organization and Food and Agriculture Organization of the United Nations: Geneve, Switzerland, 2004; pp. 1–269. [Google Scholar]
- FDA-Health Canada. Joint FDA/Health Canada Quantitative Assessment of the Risk of Listeriosis from Soft-Ripened Cheese Consumption in the United States and Canada: Report; Food Directorate/Direction des Aliments, Health Canada, Santé Canada and Center for Food Safety and Applied Nutrition, Food and Drug Administration (US Department of Health and Human Services): Ottawa, ON, Canada, 2015; pp. 1–177.
- Pérez-Rodríguez, F.; Carrasco, E.; Bover-Cid, S.; Joffré, A.; Valero, A. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready-to-eat (RTE) foods: Activity 2, a quantitative risk characterization on L. monocytogenes in RTE foods; starting from the retail stage. EFSA Support. Publ. 2017, 14, 1252E. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel. Scientific Opinion on the Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, 5134. [Google Scholar] [CrossRef]
- Tenenhaus-Aziza, F.; Daudin, J.-J.; Maffre, A.; Sanaa, M. Risk-based approach for microbiological food safety in the dairy industry: The case of Listeria monocytogenes in soft cheese made from pasteurized milk. Risk Anal. 2014, 34, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Bemrah, N.; Sanaa, M.; Cassin, M.H.; Griffiths, M.W.; Cerf, O. Quantitative risk assessment of human listeriosis from consumption of soft cheese made from raw milk. Prev. Vet. Med. 1998, 37, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, U.; Cummins, E.; Valero, A.; Walsh, D.; Dalmasso, M.; Jordan, K.; Duffy, G. Farm to fork quantitative risk assessment of Listeria monocytogenes contamination in raw and pasteurized milk cheese in Ireland. Risk Anal. 2015, 35, 1140–1153. [Google Scholar] [CrossRef]
- Condoleo, R.; Mezher, Z.; Marozzi, S.; Guzzon, A.; Fischetti, R.; Senese, M.; Sette, S.; Bucchini, L. Risk assessment of human listeriosis from semisoft cheeses made from raw sheep’s milk in Lazio and Tuscany (Italy). Risk Anal. 2017, 37, 661–676. [Google Scholar] [CrossRef]
- Sanaa, M.; Coroller, L.; Cerf, O. Risk assessment of listeriosis linked to the consumption of two soft cheeses made from raw milk: Camembert of Normandy and Brie of Meaux. Risk Anal. 2004, 24, 389–399. [Google Scholar] [CrossRef]
- Campagnollo, F.; Gonzales-Barron, U.; Pilão Cadavez, V.; Sant’Ana, A.; Schaffner, D. Quantitative risk assessment of Listeria monocytogenes in traditional Minas cheeses: The cases of artisanal semi-hard and fresh soft cheeses. Food Control 2018, 92, 370–379. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Pavlis, A.; Nychas, G.-J.E.; Xanthiakos, K. Probabilistic model for Listeria monocytogenes growth during distribution, retail storage, and domestic storage of pasteurized milk. Appl. Environ. Microbiol. 2010, 76, 2181–2191. [Google Scholar] [CrossRef]
- Latorre, A.A.; Pradhan, A.K.; Van Kessel, J.A.S.; Karns, J.S.; Boor, K.J.; Rice, D.H.; Mangione, K.J.; Gröhn, Y.T.; Schukken, Y.H. Quantitative risk assessment of listeriosis due to consumption of raw milk. J. Food Prot. 2011, 74, 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, F.; Bonilauri, P.; Albonetti, S.; Amatiste, S.; Arrigoni, N.; Bianchi, M.; Bertasi, B.; Bilei, S.; Bolzoni, G.; Cascone, G.; et al. Quantitative risk assessment of human salmonellosis and listeriosis related to the consumption of raw milk in Italy. J Food Prot. 2015, 78, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Yoon, K.S. Quantitative microbial risk assessment of Listeria monocytogenes and enterohemorrhagic Escherichia coli in yogurt. Foods 2022, 11, 971. [Google Scholar] [CrossRef] [PubMed]
- Soto-Beltran, M.; Mena, K.; Gerba, C.; Tarwater, P.; Reynolds, K.; Chaidez, C. Risk assessment of Listeria monocytogenes in queso fresco in Culiacan, Mexico. J. Microbiol. Res. 2013, 3, 111–116. [Google Scholar]
- Njage, P.M.K.; Leekitcharoenphon, P.; Hansen, L.T.; Hendriksen, R.S.; Faes, C.; Aerts, M.; Hald, T. Quantitative microbial risk assessment based on Whole Genome Sequencing Data: Case of Listeria monocytogenes. Microorganisms 2020, 8, 1772. [Google Scholar] [CrossRef]
- Farber, J.M.; Ross, W.H.; Harwig, J. Health risk assessment of Listeria monocytogenes in Canada. Int. J. Food Microbiol. 1996, 30, 145–156. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Risk Characterization of Salmonella spp. in Eggs and Broiler Chicken and Listeria monocytogenes in Ready-to-Eat Foods; Joint FAO/WHO Expert Consultation on Risk Assessment of Microbiological Hazards in Foods; FAO Headquarters: Rome, Italy, 2001; pp. 1–40. [Google Scholar]
- Pouillot, R.; Hoelzer, K.; Chen, Y.; Dennis, S.B. Listeria monocytogenes dose response revisited—Incorporating adjustments for variability in strain virulence and host susceptibility. Risk Anal. 2015, 35, 90–108. [Google Scholar] [CrossRef]
- Lakicevic, B.; Nastasijevic, I. Listeria monocytogenes in retail establishments: Contamination routes and control strategies. Food Rev Int. 2016, 33, 247–269. [Google Scholar] [CrossRef]
- Beno, S.M.; Stasiewicz, M.J.; Andrus, A.D.; Ralyea, R.D.; Kent, D.J.; Martin, N.H.; Wiedmann, M.; Boor, K.J. Development and validation of pathogen environmental monitoring programs for small cheese processing facilities. J. Food Prot. 2016, 79, 2095–2106. [Google Scholar] [CrossRef]
- D’amico, D.J. Microbiological quality and safety issues in cheesemaking. Microbiol. Spectr. 2014, 2, 251–309. [Google Scholar] [CrossRef] [PubMed]
- Guillier, L.; Palma, F.; Fritsch, L. Taking account of genomics in quantitative microbial risk assessment: What methods? what issues? Curr. Opin. Food Sci. 2022, 48, 100922. [Google Scholar] [CrossRef]
- Midelet-Bourdin, G.; Leleu, G.; Copin, S.; Roche, S.M.; Velge, P.; Malle, P. Modification of a virulence-associated phenotype after growth of Listeria monocytogenes on food. J. Appl. Microbiol. 2006, 101, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Pricope-Ciolacu, L.; Nicolau, A.I.; Wagner, M.; Rychli, K. The effect of milk components and storage conditions on the virulence of Listeria monocytogenes as determined by a Caco-2 cell assay. Int. J. Food Microbiol. 2013, 166, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Moller-Nielsen, E.M.; Björkman, J.T.; Kiil, K.; Grant, K.; Dallman, T.; Painset, A.; Amar, C.; Roussel, S.; Guillier, L.; Benjamin, F.; et al. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready-to-eat (RTE) foods: Activity 3, the comparison of isolates from different compartments along the food chain, and from humans using whole genome sequencing (WGS) analysis. EFSA Support. Publ. 2017, 14, 1151E. [Google Scholar]
- Painset, A.; Björkman, J.T.; Kiil, K.; Guillier, L.; Mariet, J.F.; Félix, B.; Amar, C.; Rotariu, O.; Roussel, S.; Perez-Reche, F.; et al. LiSEQ–whole-genome sequencing of a cross-sectional survey of Listeria monocytogenes in ready-to-eat foods and human clinical cases in Europe. Microb. Genom. 2019, 5, e000257. [Google Scholar] [CrossRef]
- Fritsch, L.; Guillier, L.; Augustin, J.C. Next generation quantitative microbiological risk assessment: Refinement of the cold smoked salmon-related listeriosis risk model by integrating genomic data. Microb. Risk Anal. 2018, 10, 20–27. [Google Scholar] [CrossRef]
- Plaza-Rodríguez, C.; Haberbeck, L.U.; Desvignes, V.; Dalgaard, P.; Sanaa, M.; Nauta, M.; Filter, M.; Guillier, L. Towards transparent and consistent exchange of knowledge for improved microbiological food safety. Curr. Opin. Food Sci. 2018, 19, 129–137. [Google Scholar] [CrossRef]
- Filter, M.; Nauta, M.; Pires, S.M.; Guillier, L.; Buschhardt, T. Towards efficient use of data, models and tools in food microbiology. Curr. Opin. Food Sci. 2022, 46, 100834. [Google Scholar] [CrossRef]
- Rougier, N.P.; Hinsen, K.; Alexandre, F.; Arildsen, T.; Barba, L.A.; Benureau, F.C.; Brown, C.T.; de Buyl, P.; Caglayan, O.; Davison, A.P.; et al. Sustainable computational science: The ReScience initiative. PeerJ Comput. Sci. 2017, 3, e142. [Google Scholar] [CrossRef]
- Perkel, J.M. Challenge to scientists: Does your ten-year-old code still run? Nature 2020, 584, 656–659. [Google Scholar] [CrossRef] [PubMed]
| Scope | Food | RTE | Cross-Contamination | DR—Endpoint | Type of DR Model | DR Sub-Populations | Strain Variability | Temp Profiles/ Lag Time | Country | Source |
|---|---|---|---|---|---|---|---|---|---|---|
| Farm-to-table | Raw milk soft cheese | Yes | No | WG—I, D | Farber et al. [24] | High-risk/Low-risk | Proportion of virulent strains | No/No | France | Bemrah et al. [13] |
| Soft-ripened cheese | Yes | No | Exp—I | FAO/WHO [8] | High-risk/Low-risk | Strain diversity implicit in r; Tmin and EGR20 represent strain variability | No/Yes | North America | FDA-HealthCanada [9] | |
| Raw/pasteurized milk cheese | Yes | Yes: processing, cheese smearing stage | WG—I | Bemrah et al. [13] | High-risk/Low-risk | Proportion of virulent strains | No/No | Ireland | Tiwari et al. [14] | |
| Sheep’s raw milk semi-soft cheese | Yes | No | Exp—I | FAO/WHO [8] | High-risk/Low-risk | Strain diversity implicit in r; challenge test data from a mixture of strains | No/No | Italy | Condoleo et al. [15] | |
| Processing-to-table | Raw milk cheeses: Camembert of Normandy and Brie of Meau | Yes | No | Exp—I | FAO/WHO [25] | High-risk/Low-risk | Tmin and pHmin represent strain variability | No/No | France | Sanaa et al. [16] |
| Raw milk semi-hard cheese and pasteurized milk soft cheese | Yes | No | Exp—I | FAO/WHO [8] | High-risk/Low-risk | Strain diversity implicit in r | No/No | Brazil | Campagnollo et al. [17] | |
| Pasteurized milk soft cheese | Yes | Yes: cheese making (pasteurized milk, cheese surface); ripening (cross-contamination, cheese surface); packaging | Exp—I | FAO/WHO [8] | Generic | Strain diversity implicit in r; lag time distribution | Yes/Yes | France | Tenenhaus-Aziza et al. [12] | |
| End Process-to-table | Pasteurized milk | Yes | No | . | . | . | Tmin represents strain variability | Yes/Yes | Greece | Koutsoumanis et al. [18] |
| Raw milk | No | No | Exp—I | FAO/WHO [8] | Multiple | Strain diversity implicit in r | No/No | USA | Latorre et al. [19] | |
| Retail-to-table | Soft/semi-soft cheese | Yes | No | Exp—I | EFSA BIOHAZ [11] based on Pouillot et al. [26] | Multiple (sex/age group) | Challenge test data from a mixture of strains; strain virulence and host susceptibility explicit in r distribution | No/No | Non-specific | EFSA BIOHAZ [11] |
| Various dairy products | Yes | No | Mouse Epi—I | FDA-FSIS [7] | Multiple | Variability in the virulence of different strains represented in DR | No/No | USA | FDA-FSIS [7] | |
| Pasteurized milk | Yes | No | Exp—I | FAO/WHO [8] | High-risk/Low-risk | Strain diversity implicit in r | No/No | Non-specific | FAO-WHO [8] | |
| Ice cream | Yes | No | Exp—I | FAO/WHO [8] | High-risk/Low-risk | Strain diversity implicit in r | No/No | Non-specific | FAO-WHO [8] | |
| Raw milk | No | No | Exp—I | FAO/WHO [8] | High-risk/Low-risk | Strain diversity implicit in r | No/No | Italy | Giacometti et al. [20] | |
| Soft/semi-soft cheeses | Yes | No | Exp—I | Pouillot et al. [26] | Multiple | Challenge test data from a mixture of strains; strain virulence and host susceptibility explicit in r distribution | Yes/Yes | EU | Pérez-Rodríguez et al. [10] | |
| Yogurt | Yes | No | Exp—I | FAO/WHO [8] | High-risk/Low-risk | Challenge test data from a mixture of strains; strain diversity implicit in r | No/No | Korea | Yang and Yoon [21] | |
| Consumption | Raw milk cheese (Queso fresco) | Yes | No | WG—I | Farber et al. [24] | High-risk/Low-risk | Proportion of virulent strains | No/No | Mexico | Soto-Beltrán et al. [22] |
| Cultured milk | Yes | No | Exp—I | FAO/WHO [25] | Multiple | Strain variability modeled by class (cold, acid, salt, desiccation stressed) from WGS data | No/No | Denmark | Njage et al. [23] |
| Scope | Food | Predictive Microbiology Models | What-If Scenarios | Sensitivity | Complexity | Source |
|---|---|---|---|---|---|---|
| Farm-to-table | Raw milk soft cheese | - | (1) Excluding mastitis source of LM decreases median counts in cheese from 2.53 to 1.88 CFU/g; (2) reducing mean prevalence of contaminated farms from 3% to 2% decreases median counts in cheese from 2.53 to 0.024 CFU/g; (3) excluding mastitis source of LM and reducing the mean prevalence of contaminated farms from 3% to 2% reduces the mean incidence of listeriosis by 80%; (4) decreasing servings/person/year from 50 to 20 reduces the mean number of cases by 60%. | - | Low | Bemrah et al. [13] |
| Soft-ripened cheese | Growth (linear EGR and square root, RLT); inactivation | (1) Consuming an artisanal raw milk soft-ripened cheese increases the mean risk per serving 157 times in comparison to the pasteurized one; (2) the mean risk per raw milk soft-ripened cheese serving is ~24–37 times smaller when every milk collection (bulk tank) is tested for LM, than when no testing is conducted; (3) reducing LM in raw milk by 3 log CFU/mL at the beginning of cheese manufacturing reduces the mean risk by a factor of 7–10 compared to baseline raw milk cheese; (4) testing batches of cheeses and removing non-compliant ones reduces the risk by 7–12 times that of the mean risk of non-tested pasteurized cheeses. | (1) Halving the EGR20 of LM reduces the mean risk per contaminated serving by a factor of ~8. Doubling the EGR20 multiplies the mean risk by a factor of ~4; (2) an increase of 1 °C in the home fridge temperature increases the mean risk per contaminated serving by a factor of 1.7; (3) shortening the maximum duration of the home storage from 56 to 28 days reduces the mean risk per contaminated serving by a factor of 1.4. Outcome—risk per serving: LM in contaminated cheese after home storage (r = 0.95); after retail storage (r = 0.83); after transport (r = 0.75); after aging (r = 0.64); LPD (r = −0.54); EGR20 (r = 0.45) | High: Meta-analysis, previous adjustment of mixed distributions, models for mixing and partition, lag phase modeled as “work to be done” | FDA-HealthCanada [9] | |
| Raw/pasteurized milk cheese | Growth (Gompertz) | (1) An increase in the initial LM in raw milk at farm level from 0.03 to 10 CFU/mL up to a maximum of 100 CFU/mL increases the final mean concentration by 35% in raw cheese and by 45% in pasteurized cheese; (2) when there is no further contamination during retail storage (only cross-contamination through smearing), the counts decrease by 24% in raw cheeses and 97% in pasteurized ones; (3) improper storage temperature above 4 °C at retail increases by 39% LM in raw cheeses and by 64% in pasteurized ones. | Outcome—counts in raw/pasteurized milk cheese: Temperature at retail (r = 0.65/0.75); cheese consumption (r = 0.28/0.48); storage time at retail (r = 0.15/0.20); fecal/silage/farm contamination factors (r = 0.15/0.27); cross-contamination from smearing machine (r = 0.05/0.12) | Medium: a separate Bayesian model to estimate sources of contamination on farms; discrete differential equation modeled transmission during smearing from (1) contaminated cheese to machine, (2) machine to cheese, and (3) machine to the surrounding environment and environment impact on the cheese | Tiwari et al. [14] | |
| Sheep’s raw milk semi-soft cheese | Growth (linear and EGR square root) | (1) Cheese from mastitis-free flocks decreased concentration of LM in bulk tank milk by 24%, in comparison to contaminated random flocks; (2) flocks with a single mastitis case increase risk per contaminated serving seven times that of contaminated random flocks; (3) cheeses from mastitis free flocks present 0.07 times the risk per contaminated serving; (4) cheeses from family flocks (10 animals maximum) have a risk eight times higher. | - | Low | Condoleo et al. [15] | |
| Processing-to-table | Raw milk cheeses: Camembert of Normandy and Brie of Meau | Growth/no growth, Growth (modified logistic, cardinal models for temperature and pH) | - | - | Medium: growth was computed in the core and rind of cheeses considering modeled pH profiles | Sanaa et al. [16] |
| Raw milk semi-hard cheese and pasteurized milk soft cheese | Growth (empirical functions) | (1) Anti-listerial LAB reduced from 7.7 log CFU LM/g of raw milk semi-hard cheese in the baseline scenario to 1.1 log CFU/g of cheese, reducing risk by >6 log RR; (2) addition of anti-listerial LAB to milk contaminated with LM at 1 log CFU/mL (the same concentration used in the baseline) reduced the risk 4.6-fold in pasteurized milk soft cheese in the general and vulnerable population. | - | Low | Campagnollo et al. [17] | |
| Pasteurized milk soft cheese | Growth (logistic growth model with delay and growth rate as secondary cardinal growth model with interactions) | (1) When the initial number of cells in the ripening room environment decreases from 2000 to 500 cells, the risk is divided by 3.7; (2) when the primo-contamination event occurs on the smearing machine, instead of during cheese-making, with 500 cells, the risk is divided by 350; (3) when the generation time of LM in the environment extends from 24 (base) to 48 h, the risk of listeriosis is divided by 546. | - | High: many recontamination and cross-contamination modules | Tenenhaus-Aziza et al. [12] | |
| End Process-to-table | Pasteurized milk | Growth (linear model, polynomial functions for growth rate and lag phase duration) | (1) Changing domestic storage temperature from 5 to 4 °C increases the proportion of milk cartoons with no growth from 55 to 59%; (2) excluding the door shelf from the fridge increases the proportion of cartoons with no growth from 55% to 62%. | Outcome—counts in milk at consumption: Domestic storage time (r = 0.482); domestic temperature door-shelf (r = 0.288); retail storage temperature (r = 0.181); retail storage time (r = 0.174) | Medium: temperature profiles from the Greek chill chain of pasteurized milk, including transportation to retail, retail storage, and domestic storage; the lag time at a certain temperature was calculated based on the h0 physiological state parameter | Koutsoumanis et al. [18] |
| Raw milk | Growth (linear model, square root for growth rate) | (1) Increasing LM prevalence in bulk tank milk from 6% to 25% increases the mean risk four times; (2) a five-fold decrease in the median listeriosis cases per year is observed if a raw milk testing program was in place (i.e., conducting monthly testing of one sample of milk and recall of milk). | Outcome—probability of illness: temperature of the home refrigerator (r = 0.55–0.77); temperature of retail/farm fridge (r = 0.55); storage time in the home refrigerator (r = 0.27–0.36); serving size for raw milk purchased directly from milk tanks and milk consumed on farms (r = 0.19–0.30) | Low | Latorre et al. [19] | |
| Retail-to-table | Soft and semi-soft cheese | Growth (Rosso model, EGR 5 °C) | Probability of cheeses containing > 2.0 log CFU/g is 0.022 at retail and 0.024 at consumption (USA data) | Sensitivity analysis was conducted, taking together various RTE food classes. | Low: generic model; only demands some knowledge in R to utilize it | EFSA BIOHAZ [11] |
| Various dairy products | Growth (linear model, square root model for EGR) | (1) If the maximum refrigerator temperature is set at 7 °C (instead of 16 °C in the baseline), the number of cases of listeriosis is reduced by 69%, and limiting the refrigerator temperature to 5 °C further reduces the number of cases to >98%; (2) reducing the maximum storage time from the 14-day baseline to 4 days reduced the annual incidence of listeriosis cases by 43.6%; (3) in queso fresco, the risk per serving is 43 times greater for the perinatal population and 36 times greater for the elderly population if cheeses were made from raw milk compared to pasteurized milk. | - | Medium: ten different dairy products considered; dose–response models developed for three subpopulations | FDA-FSIS [7] | |
| Pasteurized milk | Growth (linear model, square root growth model) | (1) If all milk were consumed immediately after purchase at retail, the number of cases in both susceptible and healthy populations would decrease 1000-fold; (2) if temperature distribution was shifted so the median increased from 3.4 to 6.2 °C, the mean number of illnesses increased > 10-fold for both populations; (3) when the storage time distribution was shifted from a median of 5.3 days to 6.7 days, the mean rate of illnesses increased 4.5-fold and 1.2-fold for the healthy and susceptible populations. | - | Medium: dose–response models for healthy and susceptible populations developed; a hierarchical beta-binomial model for the prevalence of LM in pasteurized milk | FAO-WHO [8] | |
| Ice cream | No growth/no death | - | - | Medium: D–R models for healthy and susceptible populations developed; a hierarchical beta-binomial model for the prevalence of LM in ice cream | FAO-WHO [8] | |
| Raw milk | Growth (linear model) | - | - | Low | Giacometti et al. [20] | |
| Soft/semi-soft cheeses | Growth (Baranyi model with Jameson effect, EGR 5 °C) | (1) Slicing the cheese increases the risk of infection by two times; (2) increasing storage temperature by 3–4 °C produces an increase of 530% cases per million servings; (3) decreasing storage temperature did not produce a substantial variation in the incidence of listeriosis (~4%) since temperature conditions in the baseline scenario did not allow for growth of LM in RTE cheese; (4) decreasing maximum mean initial LM counts values from 5 to 3 log CFU/g produces a decrease in up to 98% the cases; (5) decreasing time to consumption by 25% produced a decrease of 33% in the incidence of listeriosis cases per million servings; (6) adding the lag time effect produced a reduction of 30% in the number of cases per million servings. | - | Medium: time–temperature dynamic profiles from retail to consumption, and microbial competition models used solved with the RK4 algorithm | Pérez-Rodríguez et al. [10] | |
| Drinking yogurt/regular yogurt | Survival (Weibull model, secondary polynomial model) | - | Outcome—risk of illness from drinking and regular yogurt: prevalence of LM (r = 0.67, 0.21), storage time at market (r = −0.31,--0.35), consumption (r = 0.08, 0.02) | Low | Yang and Yoon [21] | |
| Consumption | Queso fresco | - | - | - | Low | Soto-Beltrán et al. [22] |
| Cultured milk | Growth (linear model) | - | The increase in the proportion of tolerant LM resulted in an increased association between the estimated cases per million and an increase in the concentration of the pathogen during consumer storage. This is due to the increase in the concentration of the pathogen during storage for the scenarios involving 0%, 25%, and 75% tolerant proportion groups, which were 236 ± 139, 255 ± 150, and 293 ± 172 CFU/g, respectively, compared to 274 ± 161 for the 50% tolerant proportion | High: WGS data was used to model population heterogeneity in microbial phenotypic stress responses to integrate it into predictive models | Njage et al. [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzales-Barron, U.; Cadavez, V.; Guillier, L.; Sanaa, M. A Critical Review of Risk Assessment Models for Listeria monocytogenes in Dairy Products. Foods 2023, 12, 4436. https://doi.org/10.3390/foods12244436
Gonzales-Barron U, Cadavez V, Guillier L, Sanaa M. A Critical Review of Risk Assessment Models for Listeria monocytogenes in Dairy Products. Foods. 2023; 12(24):4436. https://doi.org/10.3390/foods12244436
Chicago/Turabian StyleGonzales-Barron, Ursula, Vasco Cadavez, Laurent Guillier, and Moez Sanaa. 2023. "A Critical Review of Risk Assessment Models for Listeria monocytogenes in Dairy Products" Foods 12, no. 24: 4436. https://doi.org/10.3390/foods12244436
APA StyleGonzales-Barron, U., Cadavez, V., Guillier, L., & Sanaa, M. (2023). A Critical Review of Risk Assessment Models for Listeria monocytogenes in Dairy Products. Foods, 12(24), 4436. https://doi.org/10.3390/foods12244436






