Degradation of Pesticide Residues in Water, Soil, and Food Products via Cold Plasma Technology
Abstract
:1. Introduction
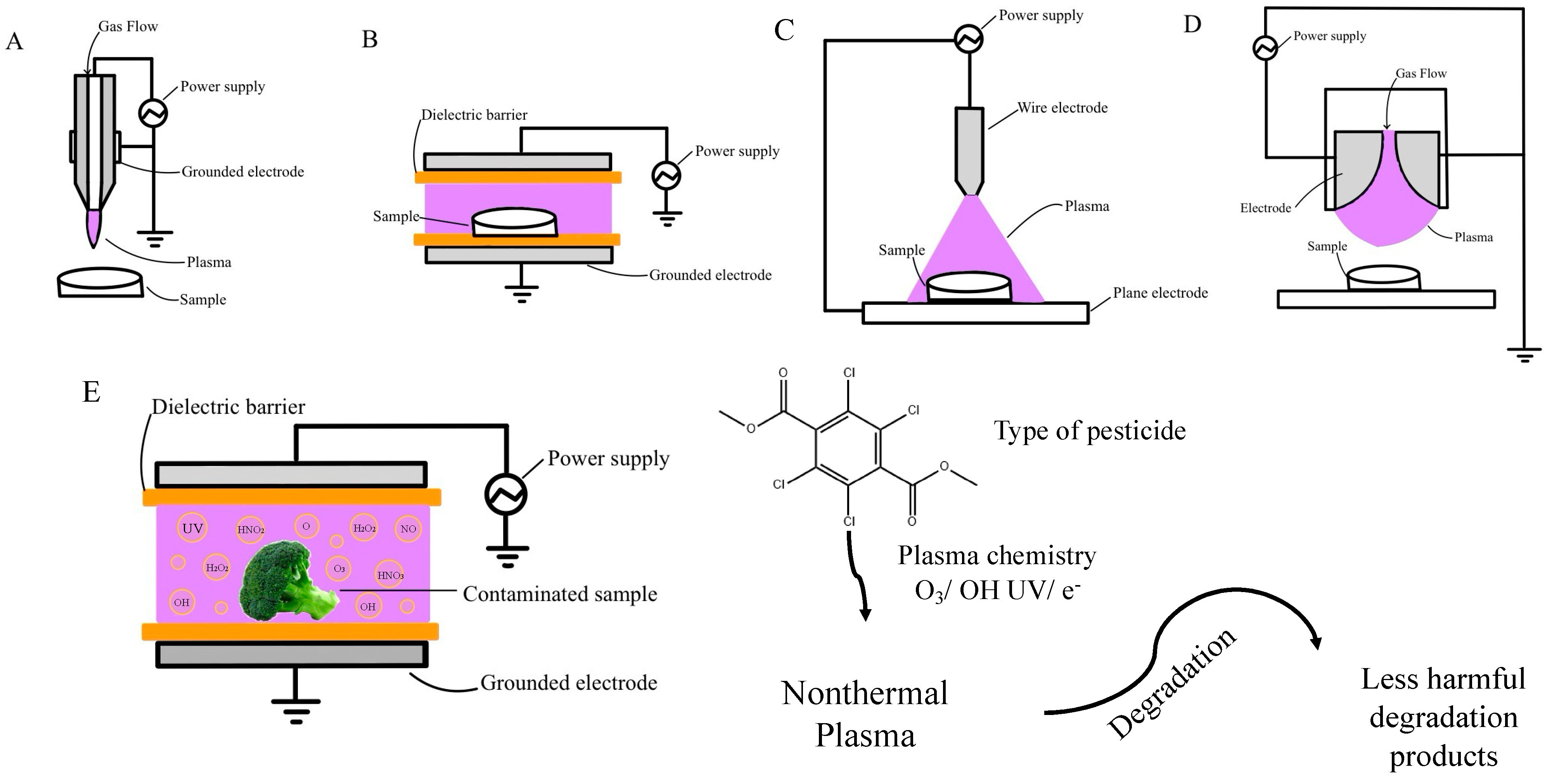
2. Types of Nonthermal Plasma
2.1. Gas Plasma
2.1.1. Atmospheric Pressure Plasma-Jet Discharge
2.1.2. Dielectric Barrier Discharge
2.1.3. Corona Discharge
2.1.4. Gliding Arc Discharge
2.2. Plasma-Activated Water
2.2.1. Discharge over Water Surface
2.2.2. Discharge under the Water Surface
3. Pesticide Degradation in Cold Plasma
3.1. Degradation of Pesticide Residues in Water
| Pesticide | Plasma System | Plasma Configuration | Key Findings | Reference |
|---|---|---|---|---|
| 2,4-dinitrophenol (DNP) | Dielectric barrier discharge | Working gas: air Input power: 150 W (AC source) Discharge time: 60 s Voltage: 100 V Dielectric barrier: quartz | Degradation value: 83.6% Fe2+ is conducive to DNP degradation The pH value decreases with increasing discharge time. | [149] |
| Atrazine, chlorfenvinphos, 2,4-dibromophenol, and lindane | DBD (a conventional batch reactor) | Dielectric barriers: Pyrex glass containers Working gas: helium Frequency: 100 kHz Power: 30 W Voltage: 20 kV Distance between both electrodes: 16 mm High-voltage electrodes: metallic cylinders | Kinetic constant (k) 0.534 min−1 for atrazine 0.567 min−1 for chlorfenvinphos 0.802 min−1 for 2,4-dibromophenol 0.389 min−1 for lindane The efficiency declines when the solution to be treated contains high concentrations of organic matter and mineral salts. | [157] |
| DBD (a coaxial thin-falling water-film reactor) | High-voltage electrode: copper mesh Dielectric barrier: glass vessel Grounded electrode: stainless-steel tube Working gas: helium High-voltage DC pulses: 12 kV Power: 24 W Repetition frequency: 94 kHz | Kinetic constant (k) 0.104 min−1 for atrazine 0.523 min−1 for chlorfenvinphos 0.273 min−1 for 2,4-dibromophenol 0.294 min−1 for lindane | ||
| Dimethoate | Dielectric barrier discharge | Applied power: 85 W Airgap distance: 5 mm Current: 0–1.2 A Voltage: 0–250 V Frequency: 5–35 kHz Electrodes: stainless steel Dielectric barrier: quartz plate | Degradation efficiency: >96% The degradation efficiency is improved by adding radical promoters. The hydroxyl radical (•OH) plays an important role in the degradation pathways. | [159] |
| Dichlorvos and dimethoate | Dielectric barrier discharge | Frequency: 5–35 kHz Voltage: 0–250 V Current: 0–1.2 A Electrodes: stainless steel Dielectric barrier: quartz plate | The degradation efficiency increases with a higher discharge power and a shorter airgap distance. Hydroxyl radicals are most likely the main drivers of the degradation process. | [158] |
| Nitenpyram | Dielectric barrier discharge | Optimum voltage: 80 V Current: 1–2.5 A Dielectric barrier: quartz glass Distance between the barrier and the solution surface: 8 mm Input power: 200 W Treatment time: 180 min | NTP can be effectively removed from the aqueous solution. Increasing the input power improves the degradation efficiency. A suitable catalyst improves the degradation process. The pH of NTP reduces with discharge time. Decomposition of NTP: 82.7% | [160] |
| Mesotrione | Dielectric barrier discharge | Dielectric barrier: glass tube Inner electrode: stainless steel Outer electrode: stainless-steel mesh Apply voltage: 17 kV Frequency: 300 Hz Power: 65 W | Catalytic systems are more efficient than noncatalytic DBD treatment. Most efficient catalytic system: 5 ppm Fe2+/DBD Highest mineralization efficiency (71%): system 10 mM H2O2/DBD In terms of global toxicity, samples after degradation in each catalytic system can be considered nontoxic. | [156] |
| Endosulfan | Dielectric barrier discharge | The gap between electrodes: 3.5 mm Inner electrode: stainless-steel rod Ground electrode: silver plate Dielectric barrier: quartz tube Voltage: 1–40 kV Working gas: air | Best performance: adding catalyst CeO2 The conversion increases with a higher input power, but decreases with increasing ES concentration. Conversion rate: 82% Mineralization: 15% The combination of cerium oxide catalyst increases the conversion to 94% and the mineralization to 48%. | [154] |
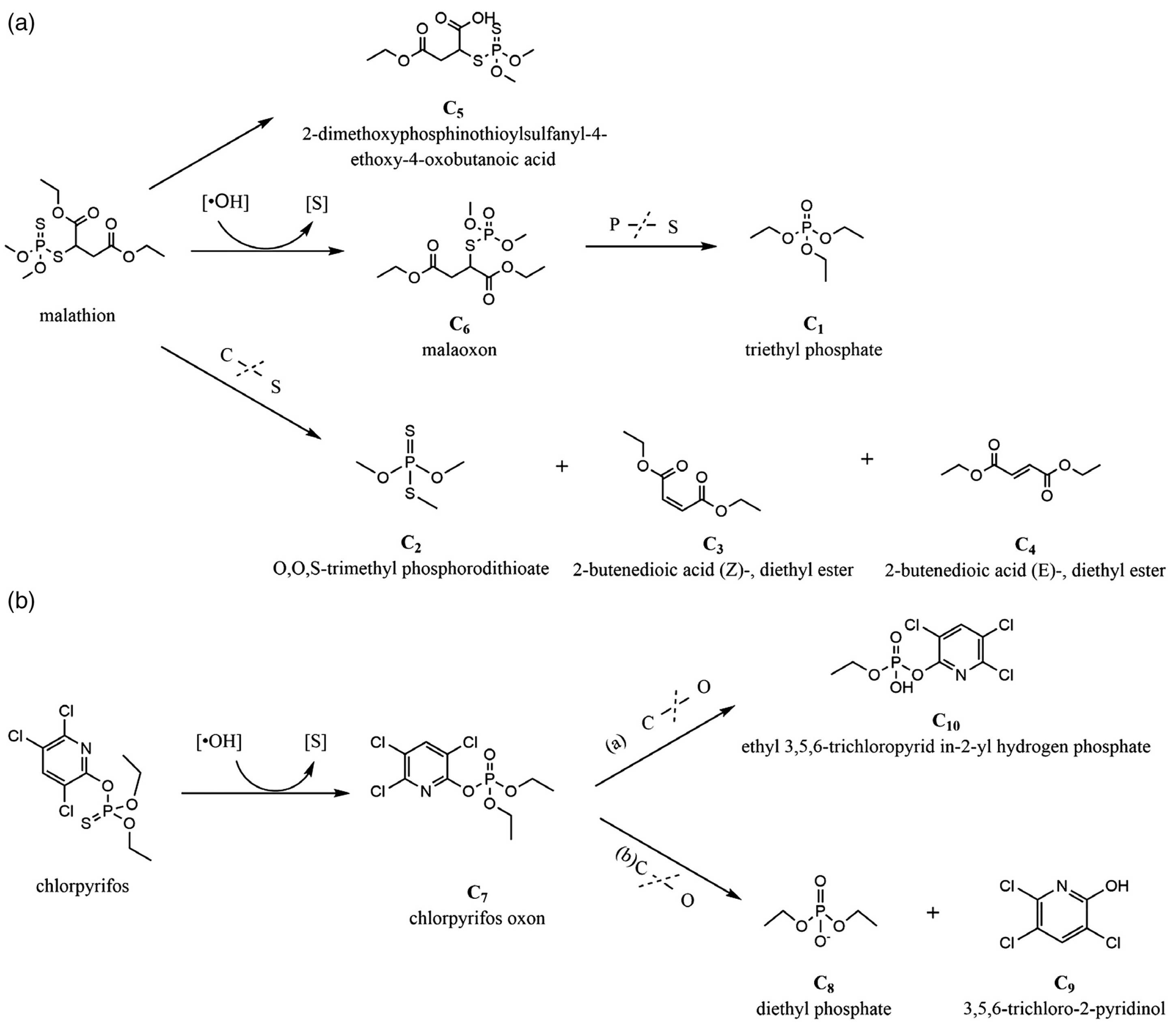
| Dichlorvos, malathion, and endosulfan | Dielectric barrier discharge | Working gas: atmospheric air Electrodes: aluminum plate Input voltage: 230 V Frequency: 50 Hz Dielectric barrier: polypropylene container | Degradation efficacy 78.98 ± 0.81% for dichlorvos 69.62 ± 0.14% for malathion 57.71 ± 0.58% for endosulfan The degraded compounds and intermediates formed were less toxic than the parent pesticide. | [155] |
| Chlorophenoxyacetic herbicide 2,4-D | Pulsed corona discharge | Working gas: oxygen Solution layer depth: 5 mm Pulse repetition rate: 25 Hz High-voltage electrode: copper wire | Apparent reaction rate: 0.195 min−1 Mineralization: more than 90% after 60 min Performance enhancement is attributed to the formation of other reactive oxidizing species besides the ozone. Improvement in the energy efficiency: optimization of the electrical characteristics of the discharge. | [151] |
| Bisphenol A (BPA), estrone (E1), and 17b-estradiol (E2) | Dielectric barrier discharge | Working gas: air Electrode: aluminum plate High-voltage electrode: acrylic sheet Input voltage: 230 V Frequency: 50 Hz | Degradation efficiency 93% for BPA 83% for E1 86% for E2 Oxygen radicals play a key role in the degradation process. | [153] |
| 2,4-dichlorophenoxyacetic acid | Pulsed corona discharge | Water depth: 2 cm Height of high-voltage electrodes: 5 mm above liquid Pulse voltage: 140 kV | A higher degradation of 2,4-D was observed under acidic pH conditions. Toxicity: 10 mg/L Complete degradation was within 6 min with a yield of 0.9 g/kWh | [161] |
| Carbamate (carbaryl, methiocarb and aminocarb) | Dielectric barrier discharge | Optimal voltage: 90 kV Optimal duration: 5 min Working gas: dry air Electrodes: circular aluminum plate Dielectric barrier: Plexiglass and polypropylene Distance between the electrodes: 49 mm | Maximum degradation 50.5% in carbaryl 99.6% in methiocarb 99.3% in aminocarb | [152] |
| Organophosphorus pesticides (chlorpyrifos, chlorpyrifos oxone, and diazinon) and an organochlorine pesticide (DDT solution) | Microplasma discharge water | Applied voltage: 30 kV Power ingestion: 153.7 ± 0.57 W Working gas: air | Nitrogen oxide plays the main role in degrading organophosphorus pesticides. Dissolved ozone and hydroxyl radical play a key role in the degradation of organochlorine pesticide. Degraded pesticide molecules transform to several smaller molecular components | [162] |
| Dimethoate | Plasma needle | Working gas: argon Power supply: 2.5 kV The tip of the power electrode: 5 mm below the surface of sample Gas flow rate: 0.5 slm Treatment time: 30 min | Dimethoate reduction: 1 × 10−4 M Degradation product: dimethoate oxo-analogue omethoate The degradation product is more toxic than parent dimethoate. | [163] |
3.2. Degradation of Pesticide Residues in Soil
3.3. Degradation of Pesticide Residues in Food
4. Mechanism of Nonthermal Plasma on Pesticide Degradation
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- UN. World Population Prospects: The 2017 Revision; UN: New York, NY, USA, 2017. [Google Scholar]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.J. Introduction to pesticides and wildlife. In Pesticides and Wildlife; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2000; Volume 771, pp. 1–5. [Google Scholar]
- Hoffman, R.S.; Capel, P.D.; Larson, S.J. Comparison of pesticides in eight U.S. urban streams. Environ. Toxicol. Chem. 2000, 19, 2249–2258. [Google Scholar] [CrossRef]
- Cassou, E. Agricultural Pollution; U.S. Geological Survey: Washington, DC, USA, 2018.
- FAO. FAOSTAT Analytical Briefs; FAO: Rome, Italy, 2021; 22p. [Google Scholar]
- Ritchie, H.; Roser, M.; Rosado, P. Pesticides. Available online: https://ourworldindata.org/pesticides (accessed on 19 November 2022).
- Doumeizel, V. Foresight Review of Food Safety; Lloyd’s Register Foundation: London, UK, 2019. [Google Scholar]
- Peivasteh Roudsari, L.; Barzegar-Bafrouei, R.; Sharifi, K.; Azimisalim, S.; Karami, M.; Abedinzadeh, S.; Asadinezhad, S.; Tajdar-oranj, B.; Mahdavi, V.; Mirza Alizadeh, A.; et al. Origin, dietary exposure, and toxicity of endocrine-disrupting food chemical contaminants: A comprehensive review. Heliyon 2023, 9, e18140. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Semchuk, K.M.; Love, E.J.; Lee, R.G. Parkinson’s disease and exposure to agricultural work and pesticide chemicals. Neurology 1992, 42, 1328–1335. [Google Scholar] [CrossRef]
- Thakur, D.S.; Khot, R.; Joshi, P.P.; Pandharipande, M.; Nagpure, K. Glyphosate poisoning with acute pulmonary edema. Toxicol. Int. 2014, 21, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, M.; Kerr, K.J.; Sanin, L.H.; Cole, D.C.; Bassil, K.L.; Vakil, C. Non-cancer health effects of pesticides: Systematic review and implications for family doctors. Can. Fam. Physician 2007, 53, 1712–1720. [Google Scholar]
- World Health Organization; United Nations Environment Programme. Public Health Impact of Pesticides Used in Agriculture; United Nations Environment Programme: Nairobi, Kenya, 1990. [Google Scholar]
- Mnif, W.; Hassine, A.I.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor pesticides: A review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef]
- Rawn, D.F.K.; Quade, S.C.; Sun, W.-F.; Fouguet, A.; Bélanger, A.; Smith, M. Captan residue reduction in apples as a result of rinsing and peeling. Food Chem. 2008, 109, 790–796. [Google Scholar] [CrossRef]
- Lentza-Rizos, C.; Avramides, E.J.; Kokkinaki, K. Residues of Azoxystrobin from Grapes to Raisins. J. Agric. Food Chem. 2006, 54, 138–141. [Google Scholar] [CrossRef]
- Yang, T.; Doherty, J.; Zhao, B.; Kinchla, A.J.; Clark, J.M.; He, L. Effectiveness of Commercial and Homemade Washing Agents in Removing Pesticide Residues on and in Apples. J. Agric. Food Chem. 2017, 65, 9744–9752. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Valverde, A.; Camacho, F.; Boulaid, M.; García-Fuentes, L. Effect of household processing and unit to unit variability of azoxystrobin, acrinathrin and kresoxim methyl residues in zucchini. Food Control 2012, 25, 594–600. [Google Scholar] [CrossRef]
- Kontou, S.; Tsipi, D.; Tzia, C. Stability of the dithiocarbamate pesticide maneb in tomato homogenates during cold storage and thermal processing. Food Addit. Contam. 2004, 21, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Marudov, G.; Radeva, S.; Pietrowska, E.; Nikolov, I. Influence of processing peaches into puree and nectar on contents of pesticides residues. Fluessiges-OBST 1999, 66, 171–172. [Google Scholar]
- Duhan, A.; Kumari, B.; Gulati, R. Effect of household processing on fenazaquin residues in okra fruits. Bull. Environ. Contam. Toxicol. 2010, 84, 217–220. [Google Scholar] [CrossRef]
- Calvo Crespo, H.; Redondo, D.; Remón, S.; Venturini, M.; Arias, E. Efficacy of electrolyzed water, chlorine dioxide and photocatalysis for disinfection and removal of pesticide residues from stone fruit. Postharvest Biol. Technol. 2019, 148, 22–31. [Google Scholar] [CrossRef]
- Cámara, M.A.; Cermeño, S.; Martínez, G.; Oliva, J. Removal residues of pesticides in apricot, peach and orange processed and dietary exposure assessment. Food Chem. 2020, 325, 126936. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Mir, M.M.; Sofi, S.A.; Shah, M.A.; Sidiq, T.; Sunooj, K.V.; Hamdani, A.M.; Mousavi Khaneghah, A. Current strategies for the reduction of pesticide residues in food products. J. Food Compos. Anal. 2022, 106, 104274. [Google Scholar] [CrossRef]
- Chung, S.W. How effective are common household preparations on removing pesticide residues from fruit and vegetables? A review. J. Sci. Food Agric. 2018, 98, 2857–2870. [Google Scholar] [CrossRef]
- Kim, S.-W.; Abd El-Aty, A.M.; Choi, J.-H.; Lee, Y.-J.; Lieu, T.T.B.; Chung, H.S.; Rahman, M.M.; Choi, O.-J.; Shin, H.-C.; Rhee, G.-S.; et al. Contributing effect of various washing procedures and additives on the decline pattern of diethofencarb in crown daisy, a model of leafy vegetables. Food Chem. 2016, 201, 153–159. [Google Scholar] [CrossRef]
- Radwan, M.A.; Abu-Elamayem, M.M.; Shiboob, M.H.; Abdel-Aal, A. Residual behaviour of profenofos on some field-grown vegetables and its removal using various washing solutions and household processing. Food Chem. Toxicol. 2005, 43, 553–557. [Google Scholar] [CrossRef]
- Delsart, C.; Franc, C.; Grimi, N.; de Revel, G.; Vorobiev, E.; Peuchot, M.M. Effects of pulsed electric fields on four residual fungicides in white wines. In Proceedings of the 1st World Congress on Electroporation and Pulsed Electric Fields in Biology, Medicine and Food & Environmental Technologies, Portoroz, Slovenia, 6–10 September 2015; pp. 124–127. [Google Scholar]
- Chen, F.; Zeng, L.; Zhang, Y.; Liao, X.; Ge, Y.; Hu, X.; Jiang, L. Degradation behaviour of methamidophos and chlorpyrifos in apple juice treated with pulsed electric fields. Food Chem. 2009, 112, 956–961. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, Y.; Zhang, Y.; Chen, J.; Chen, F.; Liao, X.; Hu, X. Reduction of diazinon and dimethoate in apple juice by pulsed electric field treatment. J. Sci. Food Agric. 2012, 92, 743–750. [Google Scholar] [CrossRef]
- Chowdhury, M.A.Z.; Jahan, I.; Karim, N.; Alam, M.K.; Rahman, M.A.; Moniruzzaman, M.; Gan, S.H.; Fakhruddin, A.N.M. Determination of carbamate and organophosphorus pesticides in vegetable samples and the efficiency of gamma-radiation in their removal. BioMed Res. Int. 2014, 2014, 145159. [Google Scholar] [CrossRef] [PubMed]
- Thihara Rodrigues, F.; Marchioni, E.; Lordel-Madeleine, S.; Kuntz, F.; Casañas Haasis Villavicencio, A.L.; Julien-David, D. Degradation of profenofos in aqueous solution and in vegetable sample by electron beam radiation. Radiat. Phys. Chem. 2020, 166, 108441. [Google Scholar] [CrossRef]
- Ciarrocchi, I.R.; Mendes, K.F.; Pimpinato, R.F.; Spoto, M.H.F.; Tornisielo, V.L. The effect of radiation in the degradation of carbendazim and azoxystrobin in strawberry. Radiat. Phys. Chem. 2021, 179, 109269. [Google Scholar] [CrossRef]
- Iizuka, T.; Shimizu, A. Removal of pesticide residue from Brussels sprouts by hydrostatic pressure. Innov. Food Sci. Emerg. Technol. 2014, 22, 70–75. [Google Scholar] [CrossRef]
- Cengiz, M.F.; Başlar, M.; Basançelebi, O.; Kılıçlı, M. Reduction of pesticide residues from tomatoes by low intensity electrical current and ultrasound applications. Food Chem. 2018, 267, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Karaca, H. The effects of ozone-enriched storage atmosphere on pesticide residues and physicochemical properties of table grapes. Ozone Sci. Eng. 2019, 41, 404–414. [Google Scholar] [CrossRef]
- Rodrigues, A.A.Z.; de Queiroz, M.E.L.R.; Neves, A.A.; de Oliveira, A.F.; Prates, L.H.F.; de Freitas, J.F.; Heleno, F.F.; Faroni, L.R.D.A. Use of ozone and detergent for removal of pesticides and improving storage quality of tomato. Food Res. Int. 2019, 125, 108626. [Google Scholar] [CrossRef]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomasevic, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Shahbaz, H.M.; Kim, J.U.; Kim, D.-H.; Yoon, S.; Jeong, S.H.; Park, J.; Lee, D.-U. Photolysis and TiO2 photocatalytic treatment under UVC/VUV irradiation for simultaneous degradation of pesticides and microorganisms. Appl. Sci. 2020, 10, 4493. [Google Scholar] [CrossRef]
- Chakka, A.K.; Sriraksha, M.S.; Ravishankar, C.N. Sustainability of emerging green non-thermal technologies in the food industry with food safety perspective: A review. LWT Food Sci. Technol. 2021, 151, 112140. [Google Scholar] [CrossRef]
- Hogan, E.; Kelly, A.; Sun, D. High pressure processing of foods: An overview. In Emerging Technologies for Food Processing; Sun, D.W., Ed.; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Rastogi, N.; Raghavarao, K.; Balasubramaniam, V.; Niranjan, K.; Knorr, D. Opportunities and challenges in high pressure processing of foods. Crit. Revi Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef] [PubMed]
- Lozowicka, B.; Jankowska, M.; Hrynko, I.; Kaczynski, P. Removal of 16 pesticide residues from strawberries by washing with tap and ozone water, ultrasonic cleaning and boiling. Environ. Monit. Assess. 2016, 188, 51. [Google Scholar] [CrossRef]
- Misra, N.N.; Schlüter, O.; Cullen, P.J. Chapter 1—Plasma in food and agriculture. In Cold Plasma in Food and Agriculture; Misra, N.N., Schlüter, O., Cullen, P.J., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 1–16. [Google Scholar]
- Phan, K.T.K.; Phan, H.T.; Boonyawan, D.; Intipunya, P.; Brennan, C.S.; Regenstein, J.M.; Phimolsiripol, Y. Non-thermal plasma for elimination of pesticide residues in mango. Innov. Food Sci. Emerg. Technol. 2018, 48, 164–171. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, J.; Yang, Y.; Guo, L.; Zhang, C. Degradation of organophosphorus pesticide induced by oxygen plasma: Effects of operating parameters and reaction mechanisms. Chemosphere 2010, 81, 408–414. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Tataraki, D.; Rassias, G. Degradation of atrazine in soil by dielectric barrier discharge plasma—Potential singlet oxygen mediation. Chem. Eng. J. 2018, 347, 682–694. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma-activated water: Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Gao, Y.; Francis, K.; Zhang, X. Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture. Food Res. Int. 2022, 157, 111246. [Google Scholar] [CrossRef]
- Bruggeman, P.; Schram, D.C. On OH production in water containing atmospheric pressure plasmas. Plasma Sources Sci. Technol. 2010, 19, 045025. [Google Scholar] [CrossRef]
- Misnal, M.F.I.; Redzuan, N.; Zainal, M.N.F.; Ahmad, N.; Raja Ibrahim, R.K.; Agun, L. Cold plasma: A potential alternative for rice grain postharvest treatment management in Malaysia. Rice Sci. 2022, 29, 1–15. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Xiang, Q.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Lethal and sublethal effect of a dielectric barrier discharge atmospheric cold plasma on Staphylococcus aureus. J. Food Prot. 2017, 80, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabová, B.; Sersenová, D.; Janda, M.; Hensel, K. Chemical and antibacterial effects of plasma activated water: Correlation with gaseous and aqueous reactive oxygen and nitrogen species, plasma sources and air flow conditions. J. Phys. D Appl. Phys. 2018, 52, 034002. [Google Scholar] [CrossRef]
- Lukes, P.; Locke, B.R. Plasmachemical oxidation processes in a hybrid gas–liquid electrical discharge reactor. J. Phys. D Appl. Phys. 2005, 38, 4074. [Google Scholar] [CrossRef]
- Teodoru, S.; Kusano, Y.; Bogaerts, A. The effect of O2 in a humid O2/N2/NOx gas mixture on NOx and N2O remediation by an atmospheric pressure dielectric barrier discharge. Plasma Process Polym. 2012, 9, 652–689. [Google Scholar] [CrossRef]
- Bolouki, N.; Kuan, W.-H.; Huang, Y.-Y.; Hsieh, J.-H. Characterizations of a plasma-water system generated by repetitive microsecond pulsed discharge with air, nitrogen, oxygen, and argon gases species. Appl. Sci. 2021, 11, 6158. [Google Scholar] [CrossRef]
- Kopuk, B.; Gunes, R.; Palabiyik, I. Cold plasma modification of food macromolecules and effects on related products. Food Chem. 2022, 382, 132356. [Google Scholar] [CrossRef]
- LÜ, Y.-J.; Yan, W.-J.; Hu, S.-H.; Wang, B.-W. Hydrogen production by methanol decomposition using gliding arc gas discharge. J. Fuel Chem. Technol. 2012, 40, 698–706. [Google Scholar] [CrossRef]
- Fanelli, F.; Fracassi, F. Atmospheric pressure non-equilibrium plasma jet technology: General features, specificities and applications in surface processing of materials. Surf. Coat. Technol. 2017, 322, 174–201. [Google Scholar] [CrossRef]
- Kostov, K.G.; Nishime, T.M.C.; Castro, A.H.R.; Toth, A.; Hein, L.R.O. Surface modification of polymeric materials by cold atmospheric plasma jet. Appl. Surf. Sci. 2014, 314, 367–375. [Google Scholar] [CrossRef]
- Zhu, W.-C.; Wang, B.-R.; Xi, H.-L.; Pu, Y.-K. Decontamination of VX surrogate malathion by atmospheric pressure radio-frequency plasma jet. Plasma Chem. Plasma Process 2010, 30, 381–389. [Google Scholar] [CrossRef]
- Yi, Z.; Chen, L.; Ren, Y.; Li, Y.; Liu, Z.; Wu, J.; Zhu, A. Decontamination of aniline and malathion on material surface by array cold atmospheric pressure plasma jet: Mechanism and decontamination pathways. J. Environ. Chem. Eng. 2022, 10, 107383. [Google Scholar] [CrossRef]
- Fernández, A.; Shearer, N.; Wilson, D.R.; Thompson, A. Effect of microbial loading on the efficiency of cold atmospheric gas plasma inactivation of Salmonella enterica serovar Typhimurium. Int. J. Food Microbiol. 2012, 152, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; In, J.; Chung, M.-S.; Min, S.C. Microbial decontamination of particulate food using a pilot-scale atmospheric plasma jet treatment system. J. Food Eng. 2021, 294, 110436. [Google Scholar] [CrossRef]
- Kim, C.; Lee, T.; Puligundla, P.; Mok, C. Effect of relative humidity on the inactivation of foodborne pathogens by corona discharge plasma jet (CDPJ). LWT Food Sci. Technol. 2020, 127, 109379. [Google Scholar] [CrossRef]
- Dimitrakellis, P.; Gogolides, E. Hydrophobic and superhydrophobic surfaces fabricated using atmospheric pressure cold plasma technology: A review. Adv. Colloid. Interface Sci. 2018, 254, 1–21. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Brandenburg, R.; von Woedtke, T.; Ehlbeck, J.; Foest, R.; Stieber, M.; Kindel, E. Antimicrobial treatment of heat sensitive products by miniaturized atmospheric pressure plasma jets (APPJs). J. Phys. D Appl. Phys. 2008, 41, 194008. [Google Scholar] [CrossRef]
- Surowsky, B.; Fröhling, A.; Gottschalk, N.; Schlüter, O.; Knorr, D. Impact of cold plasma on Citrobacter freundii in apple juice: Inactivation kinetics and mechanisms. Int. J. Food Microbiol. 2014, 174, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Baniya, H.B.; Guragain, R.P.; Baniya, B.; Subedi, D.P. Cold atmospheric pressure plasma jet for the improvement of wettability of polypropylene. Int. J. Polym. Sci. 2020, 2020, 3860259. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Wang, S.; Lv, L.; Li, M.; Shi, L. Effect of metal mesh addition on polymer surface etching by an atmospheric pressure plasma jet. Appl. Surf. Sci. 2021, 570, 151258. [Google Scholar] [CrossRef]
- Iseki, S.; Ohta, T.; Aomatsu, A.; Ito, M.; Kano, H.; Higashijima, Y.; Hori, M. Rapid inactivation of Penicillium digitatum spores using high-density nonequilibrium atmospheric pressure plasma. Appl. Phys. Lett. 2010, 96, 153704. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-Y.; Chang, C.-R.; Chang, T.-J.; Chang, Y.-J.; Liew, Y.; Chau, C.-F. Changes in physicochemical properties of corn starch upon modifications by atmospheric pressure plasma jet. Food Chem. 2019, 283, 46–51. [Google Scholar] [CrossRef]
- Fadhlalmawla, S.A.; Mohamed, A.-A.H.; Almarashi, J.Q.M.; Boutraa, T. The impact of cold atmospheric pressure plasma jet on seed germination and seedlings growth of fenugreek (Trigonella foenum-graecum). Plasma Sci. Technol. 2019, 21, 105503. [Google Scholar] [CrossRef]
- Nicol, M.J.; Brubaker, T.R.; Honish, B.J.; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D.; et al. Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species. Sci. Rep. 2020, 10, 3066. [Google Scholar] [CrossRef]
- Kong, F.; Zhang, P.; Yu, W.; Zhang, C.; Liu, J.; Ren, C.; Shao, T. Enhanced surface insulating performance for polystyrene by atmospheric pressure plasma jet deposition. Appl. Surf. Sci. 2020, 527, 146826. [Google Scholar] [CrossRef]
- Waghmare, R. Cold plasma technology for fruit based beverages: A review. Trends Food Sci. Technol. 2021, 114, 60–69. [Google Scholar] [CrossRef]
- Guo, C.A.; Tang, F.; Chen, J.; Wang, X.; Zhang, S.; Zhang, X. Development of dielectric-barrier-discharge ionization. Anal. Bioanal. Chem. 2015, 407, 2345–2364. [Google Scholar] [CrossRef] [PubMed]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; Von Woedtke, T.; Brandenburg, R.; Von dem Hagen, T.; Weltmann, K. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D Appl. Phys. 2010, 44, 013002. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Han, Z.; Cheng, J.-H. Microwave-assisted food processing technologies for enhancing product quality and process efficiency: A review of recent developments. Trends Food Sci. Technol. 2017, 67, 58–69. [Google Scholar] [CrossRef]
- Okyere, A.Y.; Rajendran, S.; Annor, G.A. Cold plasma technologies: Their effect on starch properties and industrial scale-up for starchmodification. Curr. Res. Food Sci. 2022, 5, 451–463. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 2017, 26, 053001. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Li, J.; Liu, S.; Zhang, H.; Bai, Y. Effects of dielectric barrier discharge plasma on the inactivation of Zygosaccharomyces rouxii and quality of apple juice. Food Chem. 2018, 254, 201–207. [Google Scholar] [CrossRef]
- Samanta, K.K.; Joshi, A.G.; Jassal, M.; Agrawal, A.K. Hydrophobic functionalization of cellulosic substrate by tetrafluoroethane dielectric barrier discharge plasma at atmospheric pressure. Carbohydr. Polym. 2021, 253, 117272. [Google Scholar] [CrossRef]
- Park, Y.; Oh, K.S.; Oh, J.; Seok, D.C.; Kim, S.B.; Yoo, S.J.; Lee, M.-J. The biological effects of surface dielectric barrier discharge on seed germination and plant growth with barley. Plasma Process Polym. 2018, 15, 1600056. [Google Scholar] [CrossRef]
- Tial, M.K.S.; Mitsugi, F. Fundamental study on soil treatment using dielectric barrier discharge plasma for sustainable agriculture. In Proceedings of the 2021 5th International Conference Electrical, Telecommunication and Computer Engineering (ELTICOM), Medan, Indonesia, 15–16 September 2021; pp. 55–59. [Google Scholar]
- Kim, Y.H.; Lee, C.; Lee, S.-J.; Yoon, K.S. Reduction of E. coli O157: H7 and Bacillus cereus levels in red pepper powder using dielectric barrier discharge (DBD) plasma for enhanced quality. Innov. Food Sci. Emerg. Technol. 2022, 76, 102916. [Google Scholar] [CrossRef]
- Chang, J.; Lawless, P.A.; Yamamoto, T. Corona discharge processes. IEEE Trans. Plasma Sci. 1991, 19, 1152–1166. [Google Scholar] [CrossRef]
- Turner, M. Chapter 2—Physics of cold plasma. In Cold Plasma in Food and Agriculture; Misra, N.N., Schlüter, O., Cullen, P.J., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 17–51. [Google Scholar]
- Gasparik, R.; Yamabe, C.; Ihara, S.; Satoh, S. Comparison of copper and stainless steel used for low voltage electrode in wire-to-plane electrode configuration for NOx treatment. JAP J. Appl. Phys. 1998, 37, 5786–5788. [Google Scholar] [CrossRef]
- Pignata, C.; D’Angelo, D.; Fea, E.; Gilli, G. A review on microbiological decontamination of fresh produce with nonthermal plasma. J. Appl. Microbiol. 2017, 122, 1438–1455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, D.; Qiu, R.; Tang, Y.; Du, C. Non-thermal plasma technology for organic contaminated soil remediation: A review. Chem. Eng. J. 2017, 313, 157–170. [Google Scholar] [CrossRef]
- Li, M.-W.; Hu, Z.; Wang, X.-Z.; Wu, Q.; Chen, Y.; Tian, Y.-L. Low-temperature synthesis of carbon nanotubes using corona discharge plasma at atmospheric pressure. Diam. Relat. Mater. 2004, 13, 111–115. [Google Scholar] [CrossRef]
- Puligundla, P.; Lee, T.; Mok, C. Effect of intermittent corona discharge plasma treatment for improving microbial quality and shelf life of kumquat (Citrus japonica) fruits. LWT Food Sci. Technol. 2018, 91, 8–13. [Google Scholar] [CrossRef]
- Tyurikov, K.; Alexandrov, S.; Iankevich, G. Corona discharge plasma application for the deposition of nanocomposite coatings. Mater. Today Proc. 2020, 30, 404–407. [Google Scholar] [CrossRef]
- Song, K.; Wang, H.; Jiao, Z.; Qu, G.; Chen, W.; Wang, G.; Wang, T.; Zhang, Z.; Ling, F. Inactivation efficacy and mechanism of pulsed corona discharge plasma on virus in water. J. Hazard. Mater. 2022, 422, 126906. [Google Scholar] [CrossRef]
- Niemira, B.; Gutsol, A.; Fridman, A. Cold, atmospheric pressure plasma reduces Listeria innocua on the surface of apples. In Proceedings of the International Association for Food Protection Annual Meeting, Baltimore, MD, USA, 14–17 August 2005; pp. 2–40. [Google Scholar]
- Tiya-Djowe, A.; Acayanka, E.; Mbouopda, A.P.; Boyom-Tatchemo, W.; Laminsi, S.; Gaigneaux, E.M. Producing oxide catalysts by exploiting the chemistry of gliding arc atmospheric plasma in humid air. Catal. Today 2019, 334, 104–112. [Google Scholar] [CrossRef]
- Feng, R.; Li, J.; Wu, Y.; Zhu, J.; Song, X.; Li, X. Experimental investigation on gliding arc discharge plasma ignition and flame stabilization in scramjet combustor. Aerosp. Sci. Technol. 2018, 79, 145–153. [Google Scholar] [CrossRef]
- Kim, H.-S.; Wright, K.C.; Hwang, I.-W.; Lee, D.-H.; Rabinovich, A.; Fridman, A.; Cho, Y. Concentration of hydrogen peroxide generated by gliding arc discharge and inactivation of E. coli in water. Int. Commun. Heat. Mass. Transf. 2013, 42, 5–10. [Google Scholar] [CrossRef]
- Cerny, P.; Bartos, P.; Olsan, P.; Spatenka, P. Hydrophobization of cotton fabric by gliding arc plasma discharge. Curr. Appl. Phys. 2019, 19, 128–136. [Google Scholar] [CrossRef]
- Phan, K.T.K.; Phan, H.T.; Brennan, C.S.; Regenstein, J.M.; Jantanasakulwong, K.; Boonyawan, D.; Phimolsiripol, Y. Gliding arc discharge non-thermal plasma for retardation of mango anthracnose. LWT Food Sci. Technol. 2019, 105, 142–148. [Google Scholar] [CrossRef]
- Jelínek, P.; Polášková, K.; Jeník, F.; Jeníková, Z.; Dostál, L.; Dvořáková, E.; Cerman, J.; Šourková, H.; Buršíková, V.; Špatenka, P.; et al. Effects of additives on atmospheric pressure gliding arc applied to the modification of polypropylene. Surf. Coat. Technol. 2019, 372, 45–55. [Google Scholar] [CrossRef]
- Miraei Ashtiani, S.-H.; Rafiee, M.; Mohebi Morad, M.; Khojastehpour, M.; Khani, M.R.; Rohani, A.; Shokri, B.; Martynenko, A. Impact of gliding arc plasma pretreatment on drying efficiency and physicochemical properties of grape. Innov. Food Sci. Emerg. Technol. 2020, 63, 102381. [Google Scholar] [CrossRef]
- Wang, Q.; Salvi, D. Recent progress in the application of plasma-activated water (PAW) for food decontamination. Curr. Opin. Food Sci. 2021, 42, 51–60. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.-D.; von Woedtke, T. The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Process Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Neretti, G.; Taglioli, M.; Colonna, G.; Borghi, C.A. Characterization of a dielectric barrier discharge in contact with liquid and producing a plasma activated water. Plasma Sources Sci. Technol. 2016, 26, 015013. [Google Scholar] [CrossRef]
- Trizio, I.; Sardella, E.; Francioso, E.; Dilecce, G.; Rizzi, V.; Cosma, P.; Schmidt, M.; Hänsch, M.; von Woedtke, T.; Favia, P.; et al. Investigation of air-DBD effects on biological liquids for in vitro studies on eukaryotic cells. Clin. Plasma Med. 2015, 3, 62–71. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, R.; Tian, Y.; Su, B.; Wang, K.; Yu, S.; Zhang, J.; Fang, J. Sterilization efficiency of a novel electrochemical disinfectant against Staphylococcus aureus. Environ. Sci. Technol. 2016, 50, 3184–3192. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Patange, A.; Sun, D.-W.; Tiwari, B. Plasma-activated water: Physicochemical properties, microbial inactivation mechanisms, factors influencing antimicrobial effectiveness, and applications in the food industry. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3951–3979. [Google Scholar] [CrossRef] [PubMed]
- Oehmigen, K.; Winter, J.; Hähnel, M.; Wilke, C.; Brandenburg, R.; Weltmann, K.-D.; von Woedtke, T. Estimation of possible mechanisms of Escherichia coli inactivation by plasma treated sodium chloride solution. Plasma Process Polym. 2011, 8, 904–913. [Google Scholar] [CrossRef]
- Guo, J.; Qin, D.; Li, W.; Wu, F.; Li, L.; Liu, X. Inactivation of Penicillium italicum on kumquat via plasma-activated water and its effects on quality attributes. Int. J. Food Microbiol. 2021, 343, 109090. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.X.; Liu, Z.C.; Chen, C.; Yang, A.J.; Li, D.; Rong, M.Z.; Chen, H.L.; Kong, M.G. Aqueous reactive species induced by a surface air discharge: Heterogeneous mass transfer and liquid chemistry pathways. Sci. Rep. 2016, 6, 23737. [Google Scholar] [CrossRef] [PubMed]
- Sysolyatina, E.V.; Lavrikova, A.Y.; Loleyt, R.A.; Vasilieva, E.V.; Abdulkadieva, M.A.; Ermolaeva, S.A.; Sofronov, A.V. Bidirectional mass transfer-based generation of plasma-activated water mist with antibacterial properties. Plasma Process Polym. 2020, 17, 2000058. [Google Scholar] [CrossRef]
- Kovačević, V.V.; Dojčinović, B.P.; Jović, M.; Roglić, G.M.; Obradović, B.M.; Kuraica, M.M. Measurement of reactive species generated by dielectric barrier discharge in direct contact with water in different atmospheres. J. Phys. D Appl. Phys. 2017, 50, 155205. [Google Scholar] [CrossRef]
- Uchida, G.; Nakajima, A.; Ito, T.; Takenaka, K.; Kawasaki, T.; Koga, K.; Shiratani, M.; Setsuhara, Y. Effects of nonthermal plasma jet irradiation on the selective production of H2O2 and NO2− in liquid water. J. Appl. Phys. 2016, 120, 203302. [Google Scholar] [CrossRef]
- Ma, R.; Wang, G.; Tian, Y.; Wang, K.; Zhang, J.; Fang, J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar] [CrossRef]
- Wang, Q.; Salvi, D. Evaluation of plasma-activated water (PAW) as a novel disinfectant: Effectiveness on Escherichia coli and Listeria innocua, physicochemical properties, and storage stability. LWT Food Sci. Technol. 2021, 149, 111847. [Google Scholar] [CrossRef]
- Kang, J.H.; Han, J.-Y.; Lee, H.S.; Ryu, S.; Kim, S.B.; Cho, S.; Kang, D.-H.; Min, S.C. Plasma-activated water effectively decontaminates steamed rice cake. LWT Food Sci. Technol. 2022, 157, 112838. [Google Scholar] [CrossRef]
- Kumar, N.; Attri, P.; Dewilde, S.; Bogaerts, A. Inactivation of human pancreatic ductal adenocarcinoma with atmospheric plasma treated media and water: A comparative study. J. Phys. D Appl. Phys. 2018, 51, 255401. [Google Scholar] [CrossRef]
- Laurita, R.; Gozzi, G.; Tappi, S.; Capelli, F.; Bisag, A.; Laghi, G.; Gherardi, M.; Cellini, B.; Abouelenein, D.; Vittori, S.; et al. Effect of plasma activated water (PAW) on rocket leaves decontamination and nutritional value. Innov. Food Sci. Emerg. Technol. 2021, 73, 102805. [Google Scholar] [CrossRef]
- Sharmin, N.; Sone, I.; Walsh, J.L.; Sivertsvik, M.; Fernández, E.N. Effect of citric acid and plasma activated water on the functional properties of sodium alginate for potential food packaging applications. Food Packag. Shelf Life 2021, 29, 100733. [Google Scholar] [CrossRef]
- Barrales Astorga, J.; Hadinoto, K.; Cullen, P.; Prescott, S.; Trujillo, F.J. Effect of plasma activated water on the nutritional composition, storage quality and microbial safety of beef. LWT Food Sci. Technol. 2022, 154, 112794. [Google Scholar] [CrossRef]
- Guo, D.; Liu, H.; Zhou, L.; Xie, J.; He, C. Plasma-activated water production and its application in agriculture. J. Sci. Food Agric. 2021, 101, 4891–4899. [Google Scholar] [CrossRef] [PubMed]
- Piskarev, I. Water activated by air spark plasma radiation. High Energy Chem. 2019, 53, 71–75. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, R.; Zhang, Q.; Feng, H.; Liang, Y.; Zhang, J.; Fang, J. Assessment of the physicochemical properties and biological effects of water activated by non-thermal plasma above and beneath the water surface. Plasma Process Polym. 2015, 12, 439–449. [Google Scholar] [CrossRef]
- Herianto, S.; Hou, C.-Y.; Lin, C.-M.; Chen, H.-L. Nonthermal plasma-activated water: A comprehensive review of this new tool for enhanced food safety and quality. Compr. Rev. Food Sci. Food Saf. 2021, 20, 583–626. [Google Scholar] [CrossRef]
- Herianto, S.; Shih, M.-K.; Lin, C.-M.; Hung, Y.-C.; Hsieh, C.-W.; Wu, J.-S.; Chen, M.-H.; Chen, H.-L.; Hou, C.-Y. The effects of glazing with plasma-activated water generated by a piezoelectric direct discharge plasma system on whiteleg shrimp (Litopenaeus vannamei). LWT Food Sci. Technol. 2022, 154, 112547. [Google Scholar] [CrossRef]
- Hou, C.-Y.; Lai, Y.-C.; Hsiao, C.-P.; Chen, S.-Y.; Liu, C.-T.; Wu, J.-S.; Lin, C.-M. Antibacterial activity and the physicochemical characteristics of plasma activated water on tomato surfaces. LWT Food Sci. Technol. 2021, 149, 111879. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, X.; Ma, T. Properties of plasma-activated water with different activation time and its effects on the quality of button mushrooms (Agaricus bisporus). LWT Food Sci. Technol. 2021, 147, 111633. [Google Scholar] [CrossRef]
- Liu, B.; Honnorat, B.; Yang, H.; Arancibia, J.; Rajjou, L.; Rousseau, A. Non-thermal DBD plasma array on seed germination of different plant species. J. Phys. D Appl. Phys. 2018, 52, 025401. [Google Scholar] [CrossRef]
- Guo, J.; Huang, K.; Wang, X.; Lyu, C.; Yang, N.; Li, Y.; Wang, J. Inactivation of yeast on grapes by plasma-activated water and its effects on quality attributes. J. Food Prot. 2017, 80, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, C.; Jiang, A.; Guan, Q.; Sun, X.; Liu, S.; Hao, K.; Hu, W. the effects of cold plasma-activated water treatment on the microbial growth and antioxidant properties of fresh-cut pears. Food Bioprocess. Technol. 2019, 12, 1842–1851. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, S.; Dang, J.; Wang, S.; Liu, Z.; Fang, J.; Han, P.; Zhang, J. Reduction of phoxim pesticide residues from grapes by atmospheric pressure non-thermal air plasma activated water. J. Hazard. Mater. 2019, 377, 98–105. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Oliveira, M.; Burgess, C.M.; Kerry, J.P.; Tiwari, B.K. Plasma-activated water as an alternative nitrite source for the curing of beef jerky: Influence on quality and inactivation of Listeria innocua. Innov. Food Sci. Emerg. Technol. 2020, 59, 102276. [Google Scholar] [CrossRef]
- Panda, N.R.; Sahu, D. Enhanced hydrogen generation efficiency of methanol using dielectric barrier discharge plasma methodology and conducting sea water as an electrode. Heliyon 2020, 6, e04717. [Google Scholar] [CrossRef]
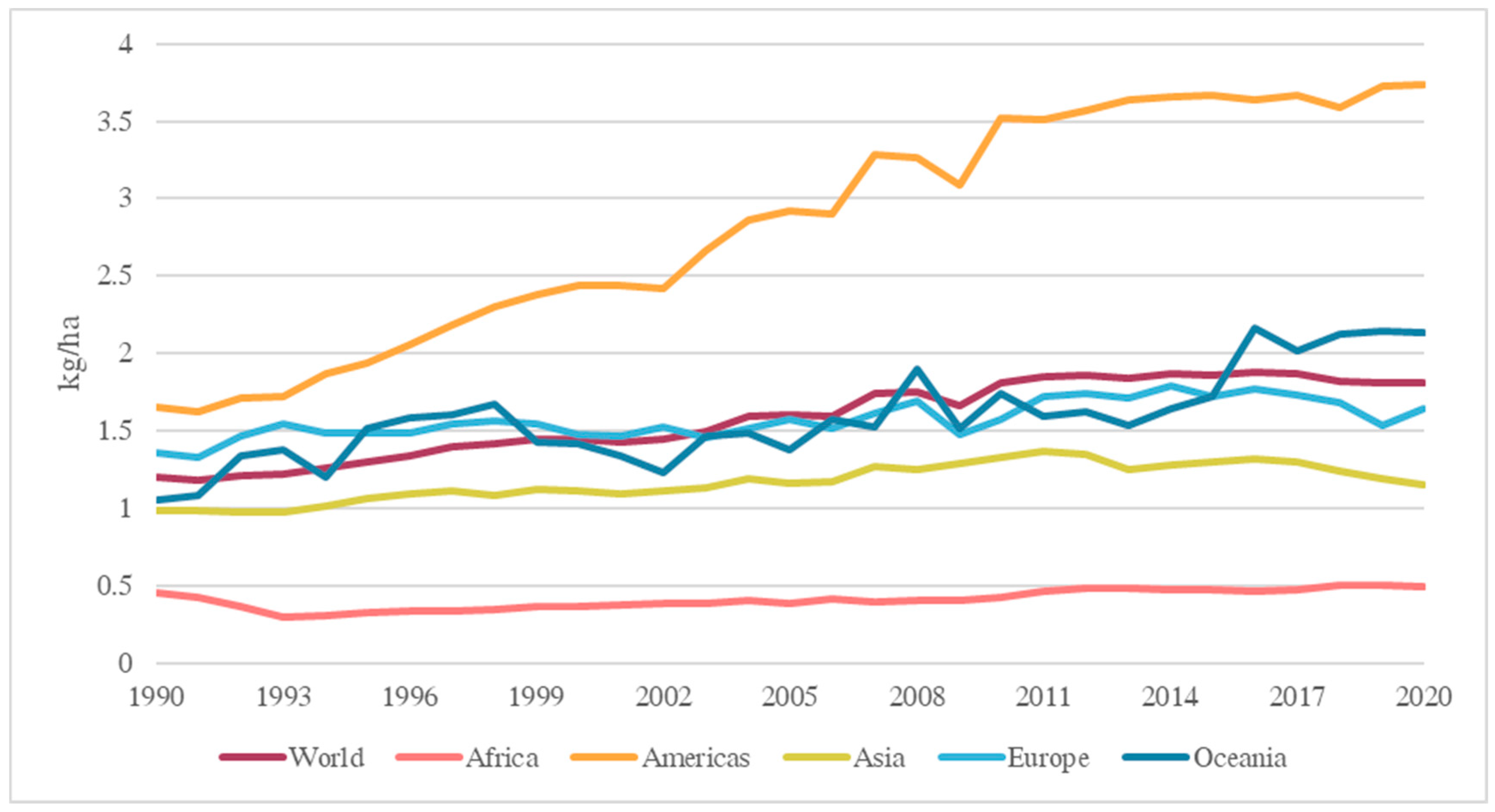
- FAO. Pesticides Use, Pesticides Trade and Pesticides Indicators—Global, Regional and Country Trends, 1990–2020; FAOSTAT Analytical Briefs, no. 46; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Murtaza, B.; Bibi, I.; Naeem, M.A.; Niazi, N.K. A critical review of different factors governing the fate of pesticides in soil under biochar application. Sci. Total Environ. 2020, 711, 134645. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.H.; Kang, B.-K. Decomposition reaction of organophosphorus nerve agents on solid surfaces with atmospheric radio frequency plasma generated gaseous species. Langmuir 2007, 23, 8074–8078. [Google Scholar] [CrossRef]
- Lundqvist, J.; von Brömssen, C.; Rosenmai, A.K.; Ohlsson, Å.; Le Godec, T.; Jonsson, O.; Kreuger, J.; Oskarsson, A. Assessment of pesticides in surface water samples from Swedish agricultural areas by integrated bioanalysis and chemical analysis. Environ. Sci. Eur. 2019, 31, 53. [Google Scholar] [CrossRef]
- Topolovec, B.; Škoro, N.; Puаč, N.; Petrovic, M. Pathways of organic micropollutants degradation in atmospheric pressure plasma processing—A review. Chemosphere 2022, 294, 133606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, Z.; Zhang, Y.; Feng, J.; Li, J. Low-temperature plasma-induced degradation of aqueous 2,4-dinitrophenol. J. Hazard. Mater. 2008, 154, 506–512. [Google Scholar] [CrossRef]
- Singh, R.K.; Fernando, S.; Baygi, S.F.; Multari, N.; Thagard, S.M.; Holsen, T.M. Breakdown products from perfluorinated alkyl substances (PFAS) degradation in a plasma-based water treatment process. Environ. Sci. Technol. 2019, 53, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Bradu, C.; Magureanu, M.; Parvulescu, V.I. Degradation of the chlorophenoxyacetic herbicide 2,4-D by plasma-ozonation system. J. Hazard. Mater. 2017, 336, 52–56. [Google Scholar] [CrossRef]
- Moutiq, R.; Pankaj, S.K.; Wan, Z.; Mendonca, A.; Keener, K.; Misra, N.N. atmospheric pressure cold plasma as a potential technology to degrade carbamate residues in water. Plasma Chem. Plasma Process. 2020, 40, 1291–1309. [Google Scholar] [CrossRef]
- Sarangapani, C.; Danaher, M.; Tiwari, B.; Lu, P.; Bourke, P.; Cullen, P.J. Efficacy and mechanistic insights into endocrine disruptor degradation using atmospheric air plasma. Chem. Eng. J. 2017, 326, 700–714. [Google Scholar] [CrossRef]
- Manoj Kumar Reddy, P.; Mahammadunnisa, S.; Subrahmanyam, C. Catalytic non-thermal plasma reactor for mineralization of endosulfan in aqueous medium: A green approach for the treatment of pesticide contaminated water. Chem. Eng. J. 2014, 238, 157–163. [Google Scholar] [CrossRef]
- Sarangapani, C.; Misra, N.; Milosavljevic, V.; Bourke, P.; O’Regan, F.; Cullen, P. Pesticide degradation in water using atmospheric air cold plasma. J. Water Process Eng. 2016, 9, 225–232. [Google Scholar] [CrossRef]
- Jović, M.S.; Dojčinović, B.P.; Kovačević, V.V.; Obradović, B.M.; Kuraica, M.M.; Gašić, U.M.; Roglić, G.M. Effect of different catalysts on mesotrione degradation in water falling film DBD reactor. Chem. Eng. J. 2014, 248, 63–70. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Molina, R.; Schikora, H.; Müller, M.; Bayona, J.M. Removal of priority pollutants from water by means of dielectric barrier discharge atmospheric plasma. J. Hazard. Mater. 2013, 262, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.M.; Bai, Y.H.; Yu, H.; Zhang, C.H.; Chen, J.R. Degradation of selected organophosphate pesticides in wastewater by dielectric barrier discharge plasma. Bull. Environ. Contam. Toxicol. 2013, 91, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Bai, Y.; Li, X.; Chen, J. Application of dielectric barrier discharge plasma for degradation and pathways of dimethoate in aqueous solution. Sep. Purif. Technol. 2013, 120, 191–197. [Google Scholar] [CrossRef]
- Li, S.P.; Jiang, Y.Y.; Cao, X.H.; Dong, Y.W.; Dong, M.; Xu, J. Degradation of nitenpyram pesticide in aqueous solution by low-temperature plasma. Environ. Technol. 2013, 34, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Philip, L.; Ramanujam, S. Removal of 2,4-dichlorophenoxyacetic acid in aqueous solution by pulsed corona discharge treatment: Effect of different water constituents, degradation pathway and toxicity assay. Chemosphere 2017, 184, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.I.; Lee, N.R.; Ahn, J.; Kim, J.Y.; Kim, J.H.; Kwon, K.H.; Kim, Y.-J. Degradation of different pesticides in water by microplasma: The roles of individual radicals and degradation pathways. Environ. Sci. Pollut. Res. 2021, 28, 8296–8309. [Google Scholar] [CrossRef] [PubMed]
- Mitrović, T.; Lazović, S.; Nastasijević, B.; Pašti, I.A.; Vasić, V.; Lazarević-Pašti, T. Non-thermal plasma needle as an effective tool in dimethoate removal from water. J. Environ. Manag. 2019, 246, 63–70. [Google Scholar] [CrossRef]
- Salihu, S.; Iyya, Z. Assessment of physicochemical parameters and organochlorine pesticide residues in selected vegetable farmlands soil in Zamfara state, Nigeria. Sci. Prog. Res. 2022, 2, 559–566. [Google Scholar]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, G.; Atreya, K.; Scheepers, P.T.J.; Geissen, V. Concentration and distribution of pesticide residues in soil: Non-dietary human health risk assessment. Chemosphere 2020, 253, 126594. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, A.J.; Dalla Valle, M.; Prevedouros, K.; Jones, K.C. The role of soil organic carbon in the global cycling of persistent organic pollutants (POPs): Interpreting and modelling field data. Chemosphere 2005, 60, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.C.; Lu, N.; Li, J.; Wu, Y. Degradation of pentachlorophenol in soil by pulsed corona discharge plasma. J. Hazard. Mater. 2010, 180, 436–441. [Google Scholar] [CrossRef]
- Rostami, S.; Jafari, S.; Moeini, Z.; Jaskulak, M.; Keshtgar, L.; Badeenezhad, A.; Azhdarpoor, A.; Rostami, M.; Zorena, K.; Dehghani, M. Current methods and technologies for degradation of atrazine in contaminated soil and water: A review. Environ. Technol. Innov. 2021, 24, 102019. [Google Scholar] [CrossRef]
- Wang, T.; Lu, N.; Li, J.; Wu, Y. Plasma-TiO2 catalytic method for high-efficiency remediation of p-nitrophenol contaminated soil in pulsed discharge. Environ. Sci. Technol. 2011, 45, 9301–9307. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A. Recent advances of cold plasma technology for water and soil remediation: A critical review. Chem. Eng. J. 2022, 428, 131657. [Google Scholar] [CrossRef]
- Wang, T.; Ren, J.; Qu, G.; Liang, D.; Hu, S. Glyphosate contaminated soil remediation by atmospheric pressure dielectric barrier discharge plasma and its residual toxicity evaluation. J. Hazard. Mater. 2016, 320, 539–546. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Sun, Y.; Zhang, W.; Mu, R.; Li, X.; Chen, H.; Gao, P.; Xue, G.; Ognier, S. Degradation of p-nitrophenol in soil by dielectric barrier discharge plasma. Water Air Soil Pollut. 2015, 226, 419. [Google Scholar] [CrossRef]
- Hatzisymeon, M.; Tataraki, D.; Rassias, G.; Aggelopoulos, C.A. Novel combination of high voltage nanopulses and in-soil generated plasma micro-discharges applied for the highly efficient degradation of trifluralin. J. Hazard. Mater. 2021, 415, 125646. [Google Scholar] [CrossRef]
- Wang, T.; Lu, N.; Li, J.; Wu, Y. Evaluation of the potential of pentachlorophenol degradation in soil by pulsed corona discharge plasma from soil characteristics. Environ. Sci. Technol. 2010, 44, 3105–3110. [Google Scholar] [CrossRef]
- Wang, T.; Lu, N.; Li, J.; Wu, Y.; Su, Y. Enhanced degradation of p-nitrophenol in soil in a pulsed discharge plasma-catalytic system. J. Hazard. Mater. 2011, 195, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.C.; Qu, G.; Li, J.; Liang, D. Remediation of p-nitrophenol and pentachlorophenol mixtures contaminated soil using pulsed corona discharge plasma. Sep. Purif. Technol. 2014, 122, 17–23. [Google Scholar] [CrossRef]
- World Health Organization. Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 19 November 2023).
- Bai, Y.; Chen, J.; Mu, H.; Zhang, C.; Li, B. Reduction of dichlorvos and omethoate residues by O2 plasma treatment. J. Agric. Food Chem. 2009, 57, 6238–6245. [Google Scholar] [CrossRef] [PubMed]
- Ranjitha Gracy, T.K.; Gupta, V.; Mahendran, R. Influence of low-pressure nonthermal dielectric barrier discharge plasma on chlorpyrifos reduction in tomatoes. J. Food Process Eng. 2019, 42, e13242. [Google Scholar] [CrossRef]
- Hanley, T.R., Jr.; Carney, E.W.; Johnson, E.M. Developmental toxicity studies in rats and rabbits with 3, 5, 6-trichloro-2-pyridinol, the major metabolite of chlorpyrifos. Toxicol. Sci. 2000, 53, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Pankaj, S.K.; Walsh, T.; O’Regan, F.; Bourke, P.; Cullen, P.J. In-package nonthermal plasma degradation of pesticides on fresh produce. J. Hazard. Mater. 2014, 271, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Dorraki, N.; Mahdavi, V.; Ghomi, H.; Ghasempour, A. Elimination of diazinon insecticide from cucumber surface by atmospheric pressure air-dielectric barrier discharge plasma. Biointerphases 2016, 11, 041007. [Google Scholar] [CrossRef]
- Cong, L.; Huang, M.; Zhang, J.; Yan, W. Effect of dielectric barrier discharge plasma on the degradation of malathion and chlorpyrifos on lettuce. J. Sci. Food Agric. 2021, 101, 424–432. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Imani, S.; Dorranian, D.; Larijani, K.; Shojaee, M. Effect of cold plasma on degradation of organophosphorus pesticides used on some agricultural products. J. Plant Prot. Res. 2017, 57, 26–35. [Google Scholar] [CrossRef]
- Sarangapani, C.; O’Toole, G.; Cullen, P.; Bourke, P. Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innov. Food Sci. Emerg. Technol. 2017, 44, 235–241. [Google Scholar] [CrossRef]
- Feng, X.; Ma, X.; Liu, H.; Xie, J.; He, C.; Fan, R. Argon plasma effects on maize: Pesticide degradation and quality changes. J. Sci. Food Agric. 2019, 99, 5491–5498. [Google Scholar] [CrossRef]
- Tappi, S.; Gozzi, G.; Vannini, L.; Berardinelli, A.; Romani, S.; Ragni, L.; Rocculi, P. Cold plasma treatment for fresh-cut melon stabilization. Innov. Food Sci. Emerg. Technol. 2016, 33, 225–233. [Google Scholar] [CrossRef]
- Lacombe, A.; Niemira, B.A.; Gurtler, J.B.; Fan, X.; Sites, J.; Boyd, G.; Chen, H. Atmospheric cold plasma inactivation of aerobic microorganisms on blueberries and effects on quality attributes. Food Microbiol. 2015, 46, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Guo, D.M.; Feng, X.X. Plasma degradation of pesticides on the surface of corn and evaluation of its quality changes. Sustainability 2021, 13, 8830. [Google Scholar] [CrossRef]
- Sarangapani, C.; Scally, L.; Gulan, M.; Cullen, P.J. Dissipation of pesticide residues on grapes and strawberries using plasma-activated water. Food Bioprocess. Technol. 2020, 13, 1728–1741. [Google Scholar] [CrossRef]
- Sawangrat, C.; Phimolsiripol, Y.; Leksakul, K.; Thanapornpoonpong, S.-N.; Sojithamporn, P.; Lavilla, M.; Castagnini, J.M.; Barba, F.J.; Boonyawan, D. Application of pinhole plasma jet activated water against Escherichia coli, colletotrichum gloeosporioides, and decontamination of pesticide residues on chili (Capsicum annuum L.). Foods 2022, 11, 2859. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, R.; Yu, F.; Xi, D.; Wang, P.; Li, J.; Wang, X.; Zhang, X.; Bazaka, K.; Ostrikov, K. Removal of organophosphorus pesticide residues from Lycium barbarum by gas phase surface discharge plasma. Chem. Eng. J. 2018, 342, 401–409. [Google Scholar] [CrossRef]
- Xie, S.; Feng, H.; Yang, F.; Zhao, Z.; Hu, X.; Wei, C.; Liang, T.; Li, H.; Geng, Y. Does dual reduction in chemical fertilizer and pesticides improve nutrient loss and tea yield and quality? A pilot study in a green tea garden in Shaoxing, Zhejiang Province, China. Environ. Sci. Poll. Res. 2019, 26, 2464–2476. [Google Scholar] [CrossRef]
- Ali, M.; Cheng, J.-H.; Sun, D.-W. Effect of plasma activated water and buffer solution on fungicide degradation from tomato (Solanum lycopersicum) fruit. Food Chem. 2021, 350, 129195. [Google Scholar] [CrossRef]
- Sawangrat, C.; Leksakul, K.; Bonyawan, D.; Anantana, T.; Jomjunyong, S. Decontamination of pesticide residues on tangerine fruit using non-thermal plasma technology. IOP Conf. Ser. Earth Environ. Sci. 2019, 347, 012048. [Google Scholar] [CrossRef]
- Ranjitha Gracy, T.; Gupta, V.; Mahendran, R. Effect of plasma activated water (PAW) on chlorpyrifos reduction in tomatoes. Int. J. Chem. Stud. 2019, 7, 5000–5006. [Google Scholar]
- Ali, M.; Sun, D.-W.; Cheng, J.-H.; Johnson Esua, O. Effects of combined treatment of plasma activated liquid and ultrasound for degradation of chlorothalonil fungicide residues in tomato. Food Chem. 2022, 371, 131162. [Google Scholar] [CrossRef]
- Gavahian, M.; Sarangapani, C.; Misra, N. Cold plasma for mitigating agrochemical and pesticide residue in food and water: Similarities with ozone and ultraviolet technologies. Food Res. Int. 2021, 141, 110138. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Philip, L.; Ramanujam, S. Rapid removal of carbofuran from aqueous solution by pulsed corona discharge treatment: Kinetic study, oxidative, reductive degradation pathway, and toxicity assay. Ind. Eng. Chem. Res. 2016, 55, 7201–7209. [Google Scholar] [CrossRef]
- Chamberlain, E.; Shi, H.; Wang, T.; Ma, Y.; Fulmer, A.; Adams, C. Comprehensive screening study of pesticide degradation via oxidation and hydrolysis. J. Agric. Food Chem. 2012, 60, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Li, J.; Hui, W.; Li, M.; Ma, Q. Research progress of application of ozone technology in the disinfection and preservation. Sci. Technol. Food Ind. 2012, 33, 432–435. [Google Scholar]
- Khan, M.S.I.; Lee, S.H.; Kim, Y.-J. A mechanistic and kinetic study of diazinone degradation under the influence of microplasma discharge water. J. Water Process Eng. 2020, 36, 101310. [Google Scholar] [CrossRef]
- Bennett, C.; Ngamrung, S.; Ano, V.; Umongno, C.; Mahatheeranont, S.; Jakmunee, J.; Nisoa, M.; Leksakul, K.; Sawangrat, C.; Boonyawan, D. Comparison of plasma technology for the study of herbicide degradation. RSC Adv. 2023, 13, 14078–14088. [Google Scholar] [CrossRef]
- Mori, T.; Sudo, S.; Kawagishi, H.; Hirai, H. Biodegradation of diuron in artificially contaminated water and seawater by wood colonized with the white-rot fungus Trametes versicolor. J. Wood Sci. 2018, 64, 690–696. [Google Scholar] [CrossRef]

| Working Gas | Reaction | References |
|---|---|---|
| Argon | [59] | |
| Oxygen | [60] | |
| Nitrogen | [60] | |
| Air | [61] | |
| Gas Type | Design Parameter | Applications | References |
|---|---|---|---|
| Ar | Voltage: 6 kV Frequency: 60 Hz Argon gas flow: 3 slm Exposure time: 1.7 min | Spore inactivation | [76] |
| Ar | High-voltage power supply: 5.5 kV Frequency: 20 kHz Gas flow rate: 3 L/min Temperature: 27 °C | Wettability improvement | [74] |
| Air | Power: 400–800 W Exposure time: 30 min | Corn starch modifications | [77] |
| Ar | Voltage: 16 kV Frequency: 24 kHz Argon flow rate: 2 slm Distance between the tube and treated seed: 2 mm Exposure time: 10 min | Seed germination | [78] |
| He + O2 | Applied voltage: 17–20 kVpp Frequency: 5 kHz Helium gas: 1–2 standard liters per minute (slm) Oxygen gas flow rate: 0.01–0.08 slm Distance of jet from sample: 10–30 mm | Antibacterial effects | [79] |
| Ar + N2 | Deposition time: 30 or 60 s Distance between the nozzle and substrate: 53 mm Power: 360 W Frequency: 20 kHz | Surface insulation performance improvement | [80] |
| He + O2 | Helium flow rate: 200 sccm Oxygen flow rate: 0–30 sccm Voltage: 8–18 kV Frequency: 20 kHz Distance between the tube and film surface: 1 mm Sample etching time: 40 s | Polymer film treatment | [75] |
| Gas Type | Configuration | Applications | References |
|---|---|---|---|
| Air | Input power: 90 W Dielectric barrier: quartz plate Electrodes: steel Treatment time: 140 s Gap distance: 10 mm | Inactivation of yeast spoilage | [88] |
| N2 + air | Power: 400 W Plasma-generating area: 91.875 cm2 Electrode area: 110.25 cm2 Dielectric barrier: lumina ceramic plate | Seed germination and plant growth | [90] |
| Helium/tetrafluoroethane | Voltage: 6.0 kV Frequency: 17.4 ± 0.74 kHz Dielectric material: Teflon or glass Gap distance: 2.1 mm Electrode material: aluminum | Hydrophobic functionalization of cellulosic fabric | [89] |
| Air | Dielectric barrier: alumina ceramic plates Gap distance: 1 mm Power source: 10 kV, 12 kHz | Soil treatment | [91] |
| Argon | Distance between electrodes: 10 mm. Peak-to-peak voltage: 0–11.86 kV Dielectric barrier: circular acrylic plate Electrode: stainless steel | Bacterial reduction | [92] |
| Carrier Gas Type | Configuration | Applications | References |
|---|---|---|---|
| Gas mixture (CH4 + H2) | Upper electrode: tungsten wire Lower tungsten: circular-plate stainless steel Voltage: 8 kV Frequency: 25 kHz Power 40 W | Synthesis of carbon nanotubes | [98] |
| Air | Output voltage: 8 kV Frequency: 20 kHz Electrode: ring-shaped stainless steel | Improving microbial quality and shelf life | [99] |
| Air | High voltage: 20 kV Input current: 1.5 A Frequency: 58 kHz High-voltage electrode: tungsten | Inactivation of foodborne pathogens | [70] |
| Helium | Frequency: 27 kHz High-voltage electrode: tungsten Voltage: 1.8–2.2 kV Discharge power: 40–90 W Gas flow rate: 0–1 L/min | Deposition of nanocomposite coatings | [100] |
| Air | Low-voltage electrode: stainless-steel circular plate High-voltage electrode: stainless-steel multi-needle Peak voltage: 19 kV Frequency: 80 Hz | Virus inactivation | [101] |
| Gas Type | Configuration | Applications | References |
|---|---|---|---|
| Air | Power supply: 200 W Electrode: circular stainless-steel disk Gap between electrodes: 2.5 mm Maximum voltage: 3 kV | Inactivation of Escherichia coli | [105] |
| Compressed air | Frequency: 50 Hz Power input: 750 W Electrode: copper | Hydrophobization of cotton fabric | [106] |
| Argon | Voltage: 8 kV Power: 600 W Current: 0.6 A | Retardation of mango anthracnose | [107] |
| Compressed dry air | Voltage: 10 kV Frequency: 50 Hz Electrode: stainless steel Power: 500 W | Modification of polypropylene | [108] |
| Air | Electrode: stainless steel Gap between electrodes: 8.16–20.18 mm Gas flow rate: 10 L/min Frequency: 50 Hz Applied power: 300 W Peak-to-peak voltage: 27 kV | Drying efficiency | [109] |
| Gas Type | Configuration | Applications | References |
|---|---|---|---|
| Argon/Oxygen (Ar:O2 = 98%:2%) | Plasma system: plasma jet High-voltage source: 18 kV peak-to-peak voltage Frequency: 10 kHz Flow rate: 5 L/min Distance from the liquid surface: 2 cm | Inactivation of foodborne pathogens on strawberries | [122] |
| Argon gas | Plasma source: plasma jet High-frequency sinusoidal voltage: 2–6 kVp-p Frequency: 2.5 MHz Maximum power: 3.5 W Gas flow rate: 3 l pm Distance between the nozzle and water surface: 10 mm | Inactivation of human pancreatic ductal adenocarcinoma | [125] |
| Air | Plasma system: corona discharge Pin-electrode: stainless steel Distance from the liquid surface: 5 mm Peak voltage: 9 kV Frequency: 5 kHz | Decontamination and nutritional value | [126] |
| Compressed air | Plasma system: plasma jet Input power: 295 V Frequency: 22.5 kHz Distance from the liquid surface: 5 cm | Inactivation of E. coli and Listeria innocua | [123] |
| Room air | Plasma system: surface barrier discharge Gap between the liquid and electrode: 44.8 mm Frequency: 18 kHz | Food packaging | [127] |
| Air | Plasma system: DBD Dielectric barrier: aluminum oxide (Al2O3) Power: 51.7 W Frequency: 14.4 kHz Voltage: 8 kV | Inactivation of aerobic bacteria and coliform bacteria | [124] |
| Atmospheric air | Plasma system: spark discharge Resonance frequency: 60 kHz Duty cycle: 50 μs Electrode: copper | Nutritional composition, storage quality, and microbial safety | [128] |
| Gas Type | Configuration | Applications | References |
|---|---|---|---|
| Air | Plasma system: plasma jet Current: 1.1–1.3 mA Voltage: 8.2 kV Air flow rate: 1.2 L/min Inoculation time: 30 min Activation time: 60 min | Inactivation of yeast on a grape | [137] |
| Air | Plasma source: DBD Flow rate: 1.0 L/min Peak voltage (Vp): 0–20 kV AC frequency: 9 kHz | Maintaining the antioxidant activity | [138] |
| Air | Plasma system: plasma jet Peak voltage: 25 kV Frequency: 20 kHz | Pesticide residue reduction | [139] |
| N2, O2, and air | Plasma system: DBD High-voltage electrode: stainless-steel wires Gas flow rate: 1.5 slm Ground electrode: annular aluminum | Enhancement of seed germination | [136] |
| Ambient air and compressed N2 | Plasma source: plasma jet Gas flow rate: 1 L/min Discharge time: 10 min Temperature: 150–200 ℃ | Beef curing | [140] |
| Air | Plasma system: APPJ discharge Voltage: 3.0 kV Frequency: 16 kHz Power: 60 W | Antibacterial activity | [134] |
| Atmospheric-pressure air | Plasma system: DBD High-voltage electrode: copper spring Grounding electrode: copper mesh Discharge voltage: 2.8 kV Frequency: 10 kHz | Microbial inactivation | [135] |
| N2 + O2 | Plasma system: piezoelectric direct discharge plasma Power: 60–70 W Air flow rate: 20 L/min Activation time: 20 min | Glazing agent on a shrimp | [133] |
| Pesticide | Plasma System | Plasma Configuration | Key Findings | Reference |
|---|---|---|---|---|
| Pentachlorophenol (PCP) | Pulsed corona discharge | Working gas with optimal efficacy: oxygen High-voltage pulses: 0–50 kV Pulse frequency: 0–150 Hz High-voltage electrode: nine stainless-steel hypodermic pinheads Ground electrode: wire netting Distance between electrodes: 12 mm | The degradation increases with an increase in the peak pulse voltage or pulse frequency. The ozone plays an important role in PCP degradation. Maximum PCP degradation efficiency: 92% | [175] |
| p-Nitrophenol (PNP) | Pulsed discharge plasma | Catalyst: TiO2 Optimum amount of TiO2: 2% Pulse frequency: 100 Hz Pulsed discharge voltage: 20 kV Pulse-forming capacitance: 200 pF Input energy per pulse: 0.023 J | PNP degradation: 88.8% Higher TiO2 amount has an inhibitive effect. A higher air moisture content enhances PNP removal. | [176] |
| Contaminant mixture containing p-nitrophenol and pentachlorophenol | Pulsed corona discharge plasma | High-voltage electrode: 19 stainless-steel hypodermic hollow needles Ground electrode: wire netting Distance between adjacent needles: 12.5 mm Distance between electrodes: 16 mm Pulse frequency: 50 Hz Pulsed discharge voltage: 18 kV Pulse-forming capacitance: 200 pF | PNP degradation: 86% PCP degradation: 94.1% Energy yield: 18.3% Degradation efficiency decreases with increasing initial pollutant concentration. | [177] |
| p-nitrophenol (PNP) | Dielectric barrier discharge | Voltage: 38.2 kV High-voltage electrode: stainless steel Dielectric barrier: quartz glass Working gas: air | PNP degradation: 63.2% The treatment time, applied discharge voltage, and soil pH value have a positive effect on the degradation efficiency. Airflow is harmful to the decomposition process. The ozone plays an important role as an active species in gas form. | [173] |
| Glyphosate | Dielectric barrier discharge | Optimal discharge voltage: 28 kV Power-frequency discharge: 50 Hz Distance between probe and ground electrode: 5 mm | Glyphosate degradation: 93.9% Energy yield: 0.47 g kWh−1 Increasing the discharge voltage and decreasing the organic matter content of the soil facilitate glyphosate degradation. | [172] |
| Atrazine | Dielectric barrier discharge | High-voltage electrode: stainless-steel disc Dielectric barrier: quartz Ground electrode: stainless-steel grid Voltage power supply: 34.2–44.8 kV Working gas: dry compressed air | Degradation efficiency: 86.9% and 98.1% for initial concentrations of 100 and 10 mg/kg, respectively. A low soil moisture content (5–10%) enhances atrazine degradation. Atrazine mineralization: 65.5% Main oxidizing agents: OH·, H2O2, or O3 | [49] |
| Trifluralin | Dielectric barrier discharge | High voltage: 20 kV High-voltage and grounded electrode: stainless steel Dielectric barrier: quartz tube Gas flow rate: 0.075 L/min Working gas: compressed air | The degradation of trifluralin is feasible, even in thicker soil. The degradation efficiency decreases by 30% with increasing soil moisture. The energy efficiency is up to three orders of magnitude. | [174] |
| Pesticide | Food Product | Plasma System | Plasma Configuration | Key Findings | Reference |
|---|---|---|---|---|---|
| Dichlorvos and omethoate | Maize | Radiofrequency (RF) discharge | Working gas: oxygen Power supply: 500 W, 13.56 MHz Reaction chamber: cylindrical Pyrex glass tube | This treatment was significantly effective in the degradation of original DDVP and omethoate. The degradation efficiency mainly depends on the related operating parameters and chemical structures of the pesticides. DDVP and omethoate molecules are degraded into less toxic compounds. | [179] |
| Azoxystrobin, cyprodinil, fludioxonil, and pyriproxyfen | Strawberries | Dielectric barrier discharge | High-voltage electrode: Perspex Ground electrode: polypropylene Package container: polyethylene terephthalate (PET) High-voltage output: 0–120 kV Frequency: 50 Hz Working gas: atmospheric air | Maximum decrease (5 min, 80 kV) 69% of azoxystrobin 45% of cyprodinil 71% of fludioxonil 46% of pyriproxyfen Plasma treatment is a means of ensuring chemical food safety and microbicidal effects. | [163] |
| Diazinon | Cucumber | Dielectric barrier discharge | Working gas: air Upper electrode: copper Dielectric barrier: quartz Second electrode: stainless-steel mesh Pulsed high voltage: 0–14 kV Frequency: 6 kHz | Degradation efficiency depends on the plasma treatment time, discharge power, and pesticide concentration. The produced organophosphate pesticides are harmless and less hazardous compounds. | [183] |
| Diazinon and chlorpyrifos | Apples and cucumbers | Dielectric barrier discharge | Frequency: 13 kHz Distance between electrodes: 7 mm Exposure time: 10 min Voltage: 13 kV | Cold plasma considerably reduces the amount of pesticide residues without leaving any trace of harmful or toxic substances. No undesirable effects on the color or texture of the samples were noted. The efficiency increases with a higher voltage and a longer exposure time. | [185] |
| Boscalid and Imidacloprid | Blueberry | Dielectric barrier discharge | Electrodes: aluminum plate Package container: polyethylene terephthalate (PET) Dielectric barrier: PET Working gas: atmospheric air High voltage output: 80 kV Treatment time: 5 min | Degradation efficiency: 80.18% for boscalid 75.62% for imidacloprid The total phenol and flavonoid contents of blueberries increase significantly after plasma treatment. There is no significant effect on physical parameters. | [186] |
| Omethoate and dichlorvos | Goji (Lycium barbarum) | Gas-phase surface discharge (GPSD) | GPSD setup comprises tungsten wires (150 µm), hollow-core quartz fibers, and a bipolar high AC voltage Plasma exposure time: 30 min Discharge voltage: 10 kV | The degradation depends significantly on the applied voltage and the plasma exposure time. Omethoate degradation: 99.55% Dichlorvos degradation: 96.83% Omethoate and DDVP molecules can be completely degraded into nontoxic species without compromising the quality of Lycium barbarum | [193] |
| Chlorpyrifos and carbaryl | Maize | Dielectric barrier | Two aluminum electrodes Two glass dielectric barriers Distance between electrodes: 6 mm Working gas: argon | Chlorpyrifos degradation: 91.5% Carbaryl degradation: 73.1% This treatment improved the hydrophilicity of the treated maize. No significant change in the vitamin B2 content of maize was noted. A significant increase in the acid value and a decrease in the moisture and starch contents was observed. | [194] |
| Chlorpyrifos and carbaryl | Grapes and strawberries | Pin-to-plate atmospheric plasma discharge | High-voltage electrode: pin array Ground electrode: flat plate Distance between the pins and the ground electrode: 7 cm A resonant frequency: 55.51 kHz A discharge voltage: 32 kV Input power: 5.66 W | Chlorpyrifos degradation: 79% on grapes and 69% on strawberries Carbaryl degradation: 86% on grapes and 73% on strawberries Important factors for pesticide dissipation include nitrates, nitrites, and hydrogen peroxide. No significant changes in the key physical attributes (color and firmness) were noted. Slight changes in the ascorbic acid levels were observed. | [191] |
| Chlorothalonil (CTL) and thiram (THM) | Tomato (Solanum lycopersicum) fruit | PAW and plasma-activated buffer solution (PABS) | Working gas: atmospheric air | CTL degradation: 85.3% with PAW and 74.2% with PABS THM degradation: 79.47% in PAW and 72.21% in PABS Increasing the activation time results in a significant reduction in the amount of fungicide residues. Oxidation–reduction potential (ORP) and electrical conductivity (EC) improve significantly after plasma treatment, while the pH value decreases with the activation time. No notable negative impact was observed on tomatoes. | [195] |
| Chlorpyrifos and carbaryl | Corn | Dielectric barrier discharge | Two aluminum electrodes Dielectric barrier: glass Working gas: air Gap between two electrodes: 6 mm Plasma treatment time: 60 s Air flow rate: 1000 mL/min Power: 20 W Frequency: 1200 Hz | Chlorpyrifos degradation: 86.2% Carbaryl degradation: 66.6% A remarkable decrease in the moisture and starch contents was noted. The vitamin B2 content of treated corn does not show a significant difference from that of untreated corn. | [190] |
| Chlorpyrifos and cypermethrin | Mango | Gliding arc discharge | Plasma treatment time: 5 min Working gas: argon Ar flow rate: 5 L/min Transformer power: 600 W | Chlorpyrifos degradation: 74.0% Cypermethrin degradation: 62.9% A significant decrease in titratable acidity and total phenolic content was noted. There was an increases in carotenoid content. Total soluble solid, color, and texture parameters were not significantly different. | [47] |
| Cypermethrin | Tangerine | Pinhole plasma jet | DC power supply: 15 kV. Acrylic container: 410 × 290 × 90 mm Electric power: 125 W Working gas: air Air flow rate: 15 L/min. Discharge time: 60 min | Cypermethrin reduction: 0.75 ppm Tangerine exhibits longer shelf-life after treatment. No significant differences were noted in appearance, acid flavor, sweetness, and smell. | [196] |
| Phoxim | Grapes | Plasma jet | Plasma discharge time: 30 min Treatment time: 10 min Working gas: air Power supply: alternating current Frequency: 20 kHz Air flow rate: 5 L/min Plasma jet under water: 2 cm | Phoxim degradation: 73.60%. Acidic PAW environment: pH < 3. Oxidation capacity: >500 mV. Treatment does not significantly affect the qualities of grapes, including color, firmness, sugar content, vitamin C, and SOD. | [139] |
| Chlorpyrifos | Tomato | Dielectric barrier discharge | Treatment time: 15 min Air flow rate: 10 L/h. Initial concentration: 0.8 mg/kg. Input voltage: 200 V. Working gas: air. | Maximum reduction in chlorpyrifos: 51.97%. The total color index was increased significantly. The texture of the tomato was unaffected after PAW treatment. | [197] |
| Chlorpyrifos | Tomato | Dielectric barrier discharge | Electrodes: aluminum Glass dielectric: 2 mm Frequency: 50 Hz Distance between electrodes: 5 cm Plasma exposure time: 6 min Plasma reactor size: 350 × 350 × 350 cm | Maximum reduction of chlorpyrifos: 89.19% Initial concentration: 0.6 ppm The color index (TI) was significantly enhanced. Firmness, bio yield point, carotenoids, and total phenolic contents were decreased considerably. | [180] |
| Malathion and chlorpyrifos | Lettuce | Dielectric barrier discharge | Frequency input: 50 Hz High voltage output: 0–130 kV Distance between electrodes: 40 mm Treatment time: 180 s | Malathion degradation: 64.6% Chlorpyrifos degradation: 62.7% No significant damage was noted in regards to color and chlorophyll content. Ascorbic acid decreased significantly during long-term treatment. | [184] |
| Chlorothalonil fungicide | Tomato | Plasma-activated water (PAW) and plasma-activated buffer solution (PABS) | Power output: 600–1000 W Operating voltage: 2–7 kV Working gas: dry air Air flow rate: 20 L/min Distance between nozzle exit and liquid surface: 30 mm. Treatment time: 15 min. | Chlorothalonil reduction: 89.28% (PAW10-U) Chlorothalonil reduction 80.23% (PABS10-U) Degradation products: 2,4,5-trichloroisophthalonitrile, 2,4-dichloroisophthalonitrile, 4-chloroisophthalonitrile, isophthalonitrile and phenylacetonitrile. No negative effects were observed regarding tomato quality. | [198] |
| Carbendazim and chlorpyrifos | Chili | Pinhole plasma jet-activated water | Working gas: argon and 2% oxygen Anode electrode: tungsten Cathode electrode: aluminum blade Gas flow rate: 10 L/min | The efficiency of pesticide degradation is higher on the chili surface than in the solution. Carbendazim and chlorpyrifos degradation rates of 57% and 54% were noted in the solution, respectively. Carbendazim and chlorpyrifos degradation rates of 80% and 65% were observed on the chili surface. | [192] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sojithamporn, P.; Leksakul, K.; Sawangrat, C.; Charoenchai, N.; Boonyawan, D. Degradation of Pesticide Residues in Water, Soil, and Food Products via Cold Plasma Technology. Foods 2023, 12, 4386. https://doi.org/10.3390/foods12244386
Sojithamporn P, Leksakul K, Sawangrat C, Charoenchai N, Boonyawan D. Degradation of Pesticide Residues in Water, Soil, and Food Products via Cold Plasma Technology. Foods. 2023; 12(24):4386. https://doi.org/10.3390/foods12244386
Chicago/Turabian StyleSojithamporn, Phanumas, Komgrit Leksakul, Choncharoen Sawangrat, Nivit Charoenchai, and Dheerawan Boonyawan. 2023. "Degradation of Pesticide Residues in Water, Soil, and Food Products via Cold Plasma Technology" Foods 12, no. 24: 4386. https://doi.org/10.3390/foods12244386
APA StyleSojithamporn, P., Leksakul, K., Sawangrat, C., Charoenchai, N., & Boonyawan, D. (2023). Degradation of Pesticide Residues in Water, Soil, and Food Products via Cold Plasma Technology. Foods, 12(24), 4386. https://doi.org/10.3390/foods12244386






