Occurrence of Mycotoxins in Foods: Unraveling the Knowledge Gaps on Their Persistence in Food Production Systems
Abstract
:1. Introduction
2. Mycotoxin Occurrence in Foodstuffs
3. Cereals and Nuts: Significance and Challenges
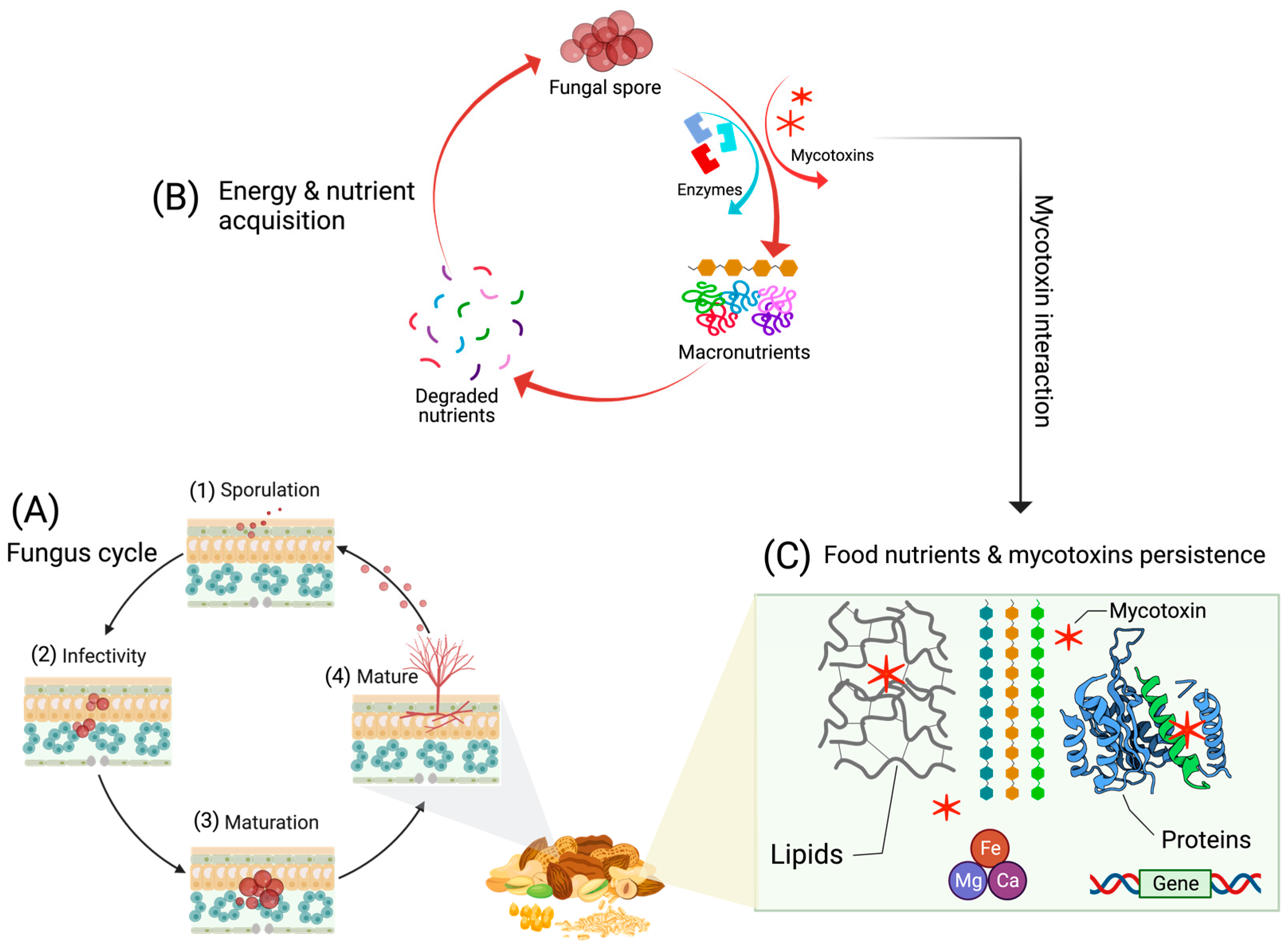
3.1. Fungi and Mycotoxin Persistence in the Food System
3.2. Metabolic Insights Related to the Mycotoxin Persistence in Food
3.3. Genetic and Enzyme Involvement in Toxin Persistence in Food
4. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Rashid Hudu, A.; Gustav Komla, M.; Opoku, N. Influence of Indigenous Processing Methods on Aflatoxin Occurrence in Africa. In Aflatoxins—Occurrence, Detoxification, Determination and Health Risks; IntechOpen: London, UK, 2022. [Google Scholar]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as Human Carcinogens—The IARC Monographs Classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food Chain [CONTAM] Related to Aflatoxin B1 as Undesirable Substance in Animal Feed. EFSA J. 2004, 2, 39. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Britzi, M.; Zakin, V.; Kostyukovsky, M.; Trostanetsky, A.; Quinn, E.; Sionov, E. Rapid Detection and Identification of Mycotoxigenic Fungi and Mycotoxins in Stored Wheat Grain. Toxins 2017, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Buśko, M.; Jeleń, H.; Góral, T.; Chmielewski, J.; Stuper, K.; Szwajkowska-Michałek, L.; Tyrakowska, B.; Perkowski, J. Volatile Metabolites in Various Cereal Grains. Food Addit. Contam. Part A 2010, 27, 1574–1581. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; et al. Risk Assessment of Aflatoxins in Food. EFSA J. 2020, 18, e06040. [Google Scholar] [CrossRef] [PubMed]
- Scafuri, B.; Varriale, A.; Facchiano, A.; D’Auria, S.; Raggi, M.E.; Marabotti, A. Binding of Mycotoxins to Proteins Involved in Neuronal Plasticity: A Combined in Silico/Wet Investigation. Sci. Rep. 2017, 7, 15156. [Google Scholar] [CrossRef] [PubMed]
- Yiannikouris, A.; André, G.; Poughon, L.; François, J.; Dussap, C.-G.; Jeminet, G.; Bertin, G.; Jouany, J.-P. Chemical and Conformational Study of the Interactions Involved in Mycotoxin Complexation with β-d-Glucans. Biomacromolecules 2006, 7, 1147–1155. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Javed, S. Aflatoxin B1 Induced Structural and Conformational Changes in Bovine Serum Albumin: A Multispectroscopic and Circular Dichroism-Based Study. ACS Omega 2021, 6, 18054–18064. [Google Scholar] [CrossRef]
- Bouelet Ntsama, I.; Frazzoli, C.; Pouokam, G.; Colizzi, V. Occurrence and Dietary Risk Assessment of Mycotoxins in Most Consumed Foods in Cameroon: Exploring Current Data to Understand Futures Challenges. Foods 2023, 12, 1713. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin Occurrence in Feed and Feed Raw Materials Worldwide: Long-Term Analysis with Special Focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Oliveira, C.A.F. Co-Occurrence of Mycotoxins in Maize Food and Maize-Based Feed from Small-Scale Farms in Brazil: A Pilot Study. Mycotoxin Res. 2019, 35, 65–73. [Google Scholar] [CrossRef]
- Shi, H.; Li, S.; Bai, Y.; Prates, L.L.; Lei, Y.; Yu, P. Mycotoxin Contamination of Food and Feed in China: Occurrence, Detection Techniques, Toxicological Effects and Advances in Mitigation Technologies. Food Control 2018, 91, 202–215. [Google Scholar] [CrossRef]
- Holanda, D.M.; Kim, S.W. Mycotoxin Occurrence, Toxicity, and Detoxifying Agents in Pig Production with an Emphasis on Deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef]
- Hao, W.; Guan, S.; Li, A.; Wang, J.; An, G.; Hofstetter, U.; Schatzmayr, G. Mycotoxin Occurrence in Feeds and Raw Materials in China: A Five-Year Investigation. Toxins 2023, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.Z.; Nisar, S.; Asi, M.R.; Jinap, S. Natural Incidence of Aflatoxins, Ochratoxin A and Zearalenone in Chicken Meat and Eggs. Food Control 2014, 43, 98–103. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Reddy, P.R.K.; Chilaka, C.A.; Balogun, O.B.; Elghandour, M.M.M.Y.; Rivas-Caceres, R.R.; Salem, A.Z.M. Mycotoxin Toxicity and Residue in Animal Products: Prevalence, Consumer Exposure and Reduction Strategies—A Review. Toxicon 2020, 177, 96–108. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Mycotoxins in Cereals and Related Foodstuffs: A Review on Occurrence and Recent Methods of Analysis. Trends Food Sci. Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, Toxicology, and Exposure Assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Leite, M.; Freitas, A.; Silva, A.S.; Barbosa, J.; Ramos, F. Maize Food Chain and Mycotoxins: A Review on Occurrence Studies. Trends Food Sci. Technol. 2021, 115, 307–331. [Google Scholar] [CrossRef]
- EU Commission Regulation (EC) No 1126/2007 of 28 September 2007 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Fusarium Toxins in Maize and Maize Products. Available online: https://eur-lex.europa.eu/eli/reg/2007/1126/oj (accessed on 24 July 2023).
- Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32023R0915 (accessed on 24 July 2023).
- EU Commission Regulation (EC) No 165/2010 of 26 February 2010 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Aflatoxins. Available online: https://www.legislation.gov.uk/eur/2010/165/contents# (accessed on 22 July 2023).
- FAO/WHO Evaluation of Certain Contaminants in Food: Eighty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives. Available online: https://apps.who.int/iris/handle/10665/254893?show=full (accessed on 25 July 2023).
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Leblanc, J.; Nielsen, E.; Ntzani, E.; et al. Assessment of Information as Regards the Toxicity of Fumonisins for Pigs, Poultry and Horses. EFSA J. 2022, 20, e07534. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Evaluation of the Increase of Risk for Public Health Related to a Possible Temporary Derogation from the Maximum Level of Deoxynivalenol, Zearalenone and Fumonisins for Maize and Maize Products. EFSA J. 2014, 12, 3699. [Google Scholar] [CrossRef]
- Ayeni, K.I.; Sulyok, M.; Krska, R.; Warth, B.; Ezekiel, C.N. Mycotoxins in Complementary Foods Consumed by Infants and Young Children within the First 18 Months of Life. Food Control 2023, 144, 109328. [Google Scholar] [CrossRef]
- Calderón, R.; Palma, P.; Godoy, M.; Vidal, M.; Rivera, A. Co-Occurrence and Estimation of the Risk of Total Aflatoxins (B1, B2, G1, and G2) and Ochratoxin A in Agri-Food Products Consumed in Chile. Food Control 2023, 146, 109493. [Google Scholar] [CrossRef]
- Foerster, C.; Monsalve, L.; Ríos- Gajardo, G. Occurrence of Aflatoxin M1 in Milk and Exposure Estimation for Its Consumption in the Chilean Population. Food Control 2023, 148, 109677. [Google Scholar] [CrossRef]
- Pleadin, J.; Kos, J.; Radić, B.; Vulić, A.; Kudumija, N.; Radović, R.; Janić Hajnal, E.; Mandić, A.; Anić, M. Aflatoxins in Maize from Serbia and Croatia: Implications of Climate Change. Foods 2023, 12, 548. [Google Scholar] [CrossRef]
- Garcia, I.; Valente, D.; Carolino, N.; Dinis, H.; Sousa, R.; Duarte, S.C.; Silva, L.J.G.; Pereira, A.M.P.T.; Pena, A. Occurrence of Zearalenone in Dairy Farms-A Study on the Determinants of Exposure and Risk Assessment. Toxicon 2023, 225, 107051. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Fakhri, Y.; Raeisi, S.; Armoon, B.; Sant’Ana, A.S. Prevalence and Concentration of Ochratoxin A, Zearalenone, Deoxynivalenol and Total Aflatoxin in Cereal-Based Products: A Systematic Review and Meta-Analysis. Food Chem. Toxicol. 2018, 118, 830–848. [Google Scholar] [CrossRef]
- Mahdjoubi, C.K.; Arroyo-Manzanares, N.; Hamini-Kadar, N.; García-Campaña, A.M.; Mebrouk, K.; Gámiz-Gracia, L. Multi-Mycotoxin Occurrence and Exposure Assessment Approach in Foodstuffs from Algeria. Toxins 2020, 12, 194. [Google Scholar] [CrossRef]
- Gab-Allah, M.A.; Tahoun, I.F.; Yamani, R.N.; Rend, E.A.; Shehata, A.B. Natural Occurrence of Deoxynivalenol, Nivalenol and Deoxynivalenol-3-Glucoside in Cereal-Derived Products from Egypt. Food Control 2022, 137, 108974. [Google Scholar] [CrossRef]
- Coppa, C.F.S.C.; Cirelli, A.C.; Gonçalves, B.L.; Barnabé, E.M.B.; Mousavi Khaneghah, A.; Corassin, C.H.; Oliveira, C.A.F. Dietary Exposure Assessment and Risk Characterization of Mycotoxins in Lactating Women: Case Study of São Paulo State, Brazil. Food Res. Int. 2020, 134, 109272. [Google Scholar] [CrossRef] [PubMed]
- Tralamazza, S.M.; Bemvenuti, R.H.; Zorzete, P.; de Souza Garcia, F.; Corrêa, B. Fungal Diversity and Natural Occurrence of Deoxynivalenol and Zearalenone in Freshly Harvested Wheat Grains from Brazil. Food Chem. 2016, 196, 445–450. [Google Scholar] [CrossRef]
- Alim, M.; Iqbal, S.Z.; Mehmood, Z.; Asi, M.R.; Zikar, H.; Chanda, H.; Malik, N. Survey of Mycotoxins in Retail Market Cereals, Derived Products and Evaluation of Their Dietary Intake. Food Control 2018, 84, 471–477. [Google Scholar] [CrossRef]
- Jager, A.V.; Tonin, F.G.; Baptista, G.Z.; Souto, P.C.M.C.; Oliveira, C.A.F. Assessment of Aflatoxin Exposure Using Serum and Urinary Biomarkers in São Paulo, Brazil: A Pilot Study. Int. J. Hydrog. Environ. Health 2016, 219, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.T.; Mousavi Khaneghah, A.; In Lee, S.H.; Fernandes Oliveira, C.A. Biomonitoring of Mycotoxin Exposure Using Urinary Biomarker Approaches: A Review. Toxin Rev. 2021, 40, 383–403. [Google Scholar] [CrossRef]
- Franco, L.T.; Ismail, A.; Amjad, A.; Oliveira, C.A.F. de Occurrence of Toxigenic Fungi and Mycotoxins in Workplaces and Human Biomonitoring of Mycotoxins in Exposed Workers: A Systematic Review. Toxin Rev. 2021, 40, 576–591. [Google Scholar] [CrossRef]
- Njumbe Ediage, E.; Diana Di Mavungu, J.; Song, S.; Wu, A.; Van Peteghem, C.; De Saeger, S. A Direct Assessment of Mycotoxin Biomarkers in Human Urine Samples by Liquid Chromatography Tandem Mass Spectrometry. Anal. Chim. Acta 2012, 741, 58–69. [Google Scholar] [CrossRef]
- Šarkanj, B.; Ezekiel, C.N.; Turner, P.C.; Abia, W.A.; Rychlik, M.; Krska, R.; Sulyok, M.; Warth, B. Ultra-Sensitive, Stable Isotope Assisted Quantification of Multiple Urinary Mycotoxin Exposure Biomarkers. Anal. Chim. Acta 2018, 1019, 84–92. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Talabi, A.O.; Nhamo, N.; Singh, R.K. Nutritional Benefits of Cereals and Pseudo-Cereals. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- USDA Food Composition Database FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 14 March 2023).
- Moure, A.; Sineiro, J.; Domínguez, H.; Parajó, J.C. Functionality of Oilseed Protein Products: A Review. Food Res. Int. 2006, 39, 945–963. [Google Scholar] [CrossRef]
- McKevith, B. Nutritional Aspects of Oilseeds. Nutr. Bull. 2005, 30, 13–26. [Google Scholar] [CrossRef]
- Tabela Brasileira de Composição de Alimentos (TBCA). Version 7.2. São Paulo. 2023. Available online: http://www.fcf.usp.br/tbca (accessed on 14 March 2023).
- Al Juhaimi, F.; Özcan, M.M.; Ghafoor, K.; Babiker, E.E.; Hussain, S. Comparison of Cold-Pressing and Soxhlet Extraction Systems for Bioactive Compounds, Antioxidant Properties, Polyphenols, Fatty Acids and Tocopherols in Eight Nut Oils. J. Food Sci. Technol. 2018, 55, 3163–3173. [Google Scholar] [CrossRef] [PubMed]
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef]
- Ali, S.; Rezende, V.T.; Ullah, S.; de Paiva, E.L.; Tonin, F.G.; Abdullah; Corassin, C.H.; de Oliveira, C.A.F. Food Processing and Challenges in the Food Production and Quality: The Foodomics Approach. Food Biosci. 2023, 56, 103217. [Google Scholar] [CrossRef]
- Kazan, K.; Gardiner, D.M.; Manners, J.M. On the Trail of a Cereal Killer: Recent Advances in Fusarium Graminearum Pathogenomics and Host Resistance. Mol. Plant Pathol. 2012, 13, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; De Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked Mycotoxins: A Review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef]
- Gordon, T.R. Fusarium Oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Paulitz, T.C.; Seaman, W.L.; Dutilleul, P.; Miller, J.D. Head Blight Gradients Caused by Gibberella zeae from Area Sources of Inoculum in Wheat Field Plots. Phytopathology 1997, 87, 414–421. [Google Scholar] [CrossRef]
- Bowles, D.; Lim, E.-K.; Poppenberger, B.; Vaistij, F.E. Glycosyltransferases of Lipophilic Small Molecules. Annu. Rev. Plant Biol. 2006, 57, 567–597. [Google Scholar] [CrossRef]
- Coleman, J.; Blake-Kalff, M.; Davies, E. Detoxification of Xenobiotics by Plants: Chemical Modification and Vacuolar Compartmentation. Trends Plant Sci. 1997, 2, 144–151. [Google Scholar] [CrossRef]
- Bartholomew, D.M.; Van Dyk, D.E.; Lau, S.-M.C.; O’Keefe, D.P.; Rea, P.A.; Viitanen, P.V. Alternate Energy-Dependent Pathways for the Vacuolar Uptake of Glucose and Glutathione Conjugates. Plant Physiol. 2002, 130, 1562–1572. [Google Scholar] [CrossRef]
- Dixon, D.P.; Cummins, I.; Cole, D.J.; Edwards, R. Glutathione-Mediated Detoxification Systems in Plants. Curr. Opin. Plant Biol. 1998, 1, 258–266. [Google Scholar] [CrossRef]
- Wu, Z.D.; Zhang, Q.; Yin, J.; Wang, X.M.; Zhang, Z.J.; Wu, W.F.; Li, F.J. Interactions of Mutiple Biological Fields in Stored Grain Ecosystems. Sci. Rep. 2020, 10, 9302. [Google Scholar] [CrossRef]
- Mohapatra, D.; Kumar, S.; Kotwaliwale, N.; Singh, K.K. Critical Factors Responsible for Fungi Growth in Stored Food Grains and Non-Chemical Approaches for Their Control. Ind. Crops Prod. 2017, 108, 162–182. [Google Scholar] [CrossRef]
- Venkatesh, N.; Keller, N.P. Mycotoxins in Conversation with Bacteria and Fungi. Front. Microbiol. 2019, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Schwake-Anduschus, C.; Proske, M.; Sciurba, E.; Muenzing, K.; Koch, M.; Maul, R. Distribution of Deoxynivalenol, Zearalenone, and Their Respective Modified Analogues in Milling Fractions of Naturally Contaminated Wheat Grains. World Mycotoxin J. 2015, 8, 433–443. [Google Scholar] [CrossRef]
- Moretti, A.; Panzarini, G.; Somma, S.; Campagna, C.; Ravaglia, S.; Logrieco, A.F.; Solfrizzo, M. Systemic Growth of F. graminearum in Wheat Plants and Related Accumulation of Deoxynivalenol. Toxins 2014, 6, 1308–1324. [Google Scholar] [CrossRef]
- Broghagen, M.; Keller, N.P. Signalling Pathways Connecting Mycotoxin Production and Sporulation. Mol. Plant Pathol. 2006, 7, 285–301. [Google Scholar] [CrossRef]
- McLaughlin, J.E.; Bin-Umer, M.A.; Widiez, T.; Finn, D.; McCormick, S.; Tumer, N.E. A Lipid Transfer Protein Increases the Glutathione Content and Enhances Arabidopsis Resistance to a Trichothecene Mycotoxin. PLoS ONE 2015, 10, e0130204. [Google Scholar] [CrossRef]
- Johnson, W.W.; Guengerich, F.P. Reaction of Aflatoxin B1 Exo-8,9-Epoxide with DNA: Kinetic Analysis of Covalent Binding and DNA-Induced Hydrolysis. Proc. Natl. Acad. Sci. USA 1997, 94, 6121–6125. [Google Scholar] [CrossRef]
- Fadl-Allah, E.M.; Mahmoud, M.A.-H.; Abd El-Twab, M.H.; Helmey, R.K. Aflatoxin B 1 Induces Chromosomal Aberrations and 5S RDNA Alterations in Durum Wheat. J. Assoc. Arab. Univ. Basic Appl. Sci. 2011, 10, 8–14. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Utama, G.L.; Suraloka, M.P.A.; Rialita, T.; Balia, R.L. Antifungal and Aflatoxin-Reducing Activity of β-Glucan Isolated from Pichia Norvegensis Grown on Tofu Wastewater. Foods 2021, 10, 2619. [Google Scholar] [CrossRef]
- Bezuidenhout, S.C.; Gelderblom, W.C.A.; Gorst-Allman, C.P.; Horak, R.M.; Marasas, W.F.O.; Spiteller, G.; Vleggaar, R. Structure Elucidation of the Fumonisins, Mycotoxins from Fusarium moniliforme. J. Chem. Soc. Chem. Commun. 1988, 11, 743–745. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant Secondary Metabolites in Cereals: Potential Involvement in Resistance to Fusarium and Mycotoxin Accumulation. Front. Microbiol. 2016, 7, 187515. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Giorni, P.; Cirlini, M.; Reverberi, M.; Gregori, R.; Ludovici, M.; Camera, E.; Fanelli, C.; Battilani, P.; Scala, V. Maize Lipids Play a Pivotal Role in the Fumonisin Accumulation. World Mycotoxin J. 2015, 8, 87–97. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhang, C.; Wu, T.; Ma, Z.; Chen, Y. Phospholipid Homeostasis Plays an Important Role in Fungal Development, Fungicide Resistance and Virulence in Fusarium graminearum. Phytopathol. Res. 2019, 1, 16. [Google Scholar] [CrossRef]
- Alexander, N.J.; McCormick, S.P.; Hohn, T.M. TRI12, a Trichothecene Efflux Pump from Fusarium sporotrichioides: Gene Isolation and Expression in Yeast. Mol. Gen. Genet. 1999, 261, 977–984. [Google Scholar] [CrossRef]
- Del Sorbo, G.; Schoonbeek, H.; De Waard, M.A. Fungal Transporters Involved in Efflux of Natural Toxic Compounds and Fungicides. Fungal Genet. Biol. 2000, 30, 1–15. [Google Scholar] [CrossRef]
- Menke, J.; Weber, J.; Broz, K.; Kistler, H.C. Cellular Development Associated with Induced Mycotoxin Synthesis in the Filamentous Fungus Fusarium graminearum. PLoS ONE 2013, 8, e63077. [Google Scholar] [CrossRef]
- Samayoa, L.F.; Cao, A.; Santiago, R.; Malvar, R.A.; Butrón, A. Genome-Wide Association Analysis for Fumonisin Content in Maize Kernels. BMC Plant Biol. 2019, 19, 166. [Google Scholar] [CrossRef]
- Majumdar, R.; Rajasekaran, K.; Sickler, C.; Lebar, M.; Musungu, B.M.; Fakhoury, A.M.; Payne, G.A.; Geisler, M.; Carter-Wientjes, C.; Wei, Q.; et al. The Pathogenesis-Related Maize Seed (PRms) Gene Plays a Role in Resistance to Aspergillus flavus Infection and Aflatoxin Contamination. Front. Plant Sci. 2017, 8, 1758. [Google Scholar] [CrossRef] [PubMed]
- Korani, W.; Chu, Y.; Holbrook, C.C.; Ozias-Akins, P. Insight into Genes Regulating Postharvest Aflatoxin Contamination of Tetraploid Peanut from Transcriptional Profiling. Genetics 2018, 209, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Kahla, A.; Arunachalam, C.; Perochon, A.; Khan, M.R.; Scofield, S.R.; Doohan, F.M. A Wheat ABC Transporter Contributes to Both Grain Formation and Mycotoxin Tolerance. J. Exp. Bot. 2015, 66, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Gao, L.; Chen, Q.; Pei, H.; Di, Z.; Xiao, J.; Wang, H.; Ma, L.; Chen, P.; Cao, A.; et al. Over-Expressing a UDP-Glucosyltransferase Gene (Ta-UGT 3) Enhances Fusarium Head Blight Resistance of Wheat. Plant Growth Regul. 2018, 84, 561–571. [Google Scholar] [CrossRef]
- Gunupuru, L.R.; Arunachalam, C.; Malla, K.B.; Kahla, A.; Perochon, A.; Jia, J.; Thapa, G.; Doohan, F.M. A Wheat Cytochrome P450 Enhances Both Resistance to Deoxynivalenol and Grain Yield. PLoS ONE 2018, 13, e0204992. [Google Scholar] [CrossRef]
- Brauer, E.K.; Balcerzak, M.; Rocheleau, H.; Leung, W.; Schernthaner, J.; Subramaniam, R.; Ouellet, T. Genome Editing of a Deoxynivalenol-Induced Transcription Factor Confers Resistance to Fusarium graminearum in Wheat. Mol. Plant Microbe Interact. 2020, 33, 553–560. [Google Scholar] [CrossRef]
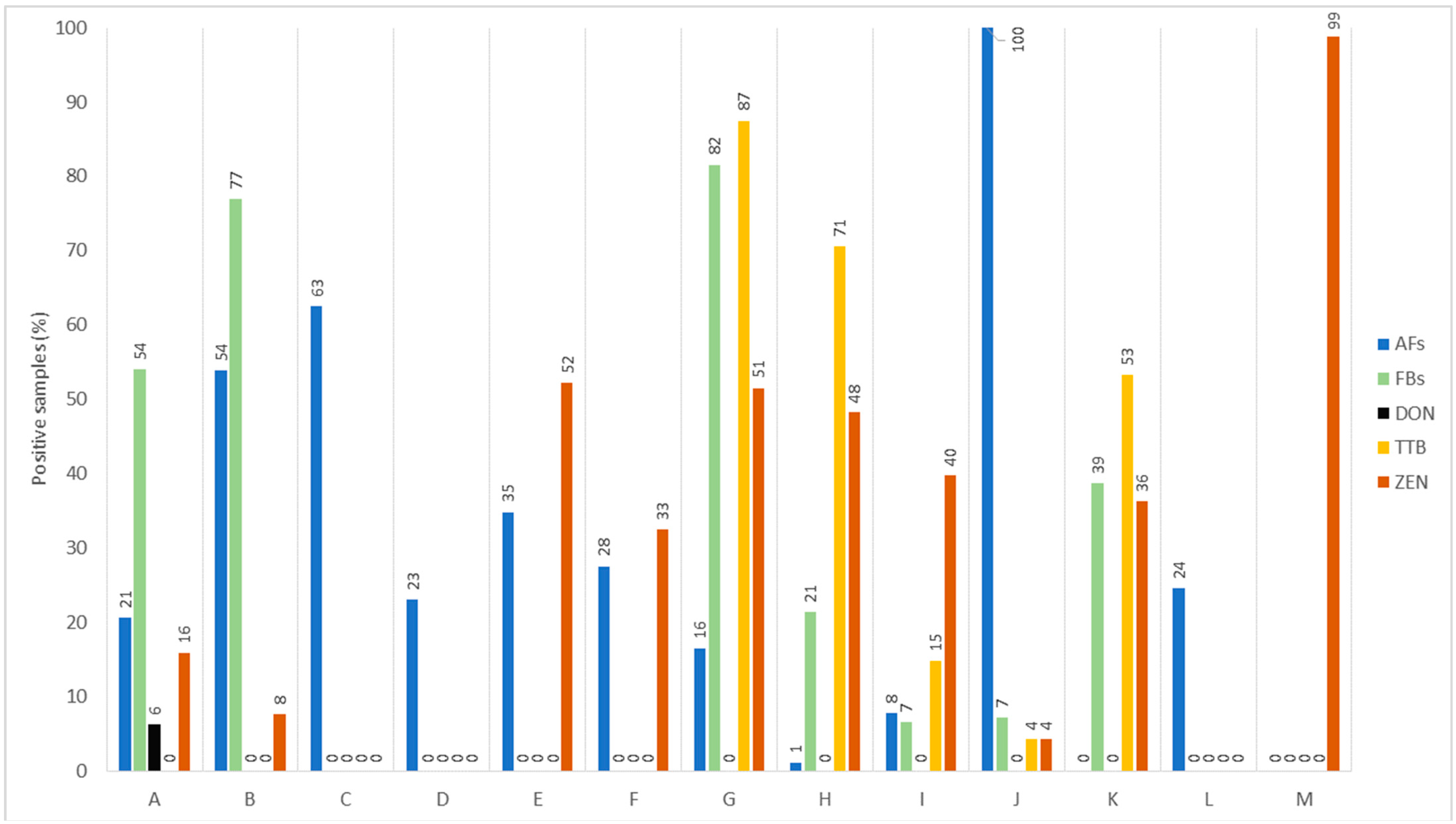
| Country/ Sampling Year | Type of Sample (N) | Mycotoxin | Positive Samples n (%) | Mean (Min–Max) µg/kg | Ref. |
|---|---|---|---|---|---|
| Nigeria | |||||
| 2018–2021 | Cereal-based infant food (63) | 3-NPA | 5 (7.9) | 4.43 (1.24–7.01) | [30] |
| AFB1 | 8 (12.7) | 2.71 (0.36–14.4) | |||
| AFB2 | 3 (4.8) | 0.42 (0.11–0.98) | |||
| AFG1 | 1 (1.6) | 0.62 (0.62–0.62) | |||
| AFs | 8 (12.7) | 2.95 (0.36–15.4) | |||
| AFM1 | 1 (1.6) | 0.57 (<LOD–0.57) | |||
| ALS | 16 (25.4) | 2.85 (1.12–5.97) | |||
| AME | 11 (17.5) | 1.49 (0.26–6.58) | |||
| ALT | 6 (9.5) | 4.71 (1.8–17.1) | |||
| BEA | 44 (69.8) | 0.29 (0.04–1.64) | |||
| Cereulide | 5 (7.9) | 1.07 (0.3–2.34) | |||
| CIT | 14 (22.2) | 106 (5.16–787) | |||
| DON | 4 (6.3) | 39.4 (3.14–110) | |||
| DHC | 7 (11.1) | 18.1 (1.88–54.5) | |||
| EnnA | 3 (4.8) | 0.24 (0.09–0.41) | |||
| EnnA1 | 7 (11.1) | 0.47 (0.1–1.62) | |||
| EnnB | 23 (36.5) | 0.91 (0.02–8.14) | |||
| EnnB1 | 17 (27) | 0.5 (0.06–2.61) | |||
| FB1 | 15 (23.8) | 35.1 (3.99–66.2) | |||
| FB2 | 13 (20.6) | 15.9 (3.54–34.9) | |||
| FB3 | 6 (9.5) | 9.62 (<LOD–9.62) | |||
| FBs | 15 (23.8) | 52.7 (3.99–98.1) | |||
| MON | 6 (9.5) | 3.14 (2.53–6.19) | |||
| OTA | 2 (3.2) | 0.76 (<LOD–0.76) | |||
| ST | 8 (12.7) | 0.29 (0.13–0.96) | |||
| TEN | 4 (6.3) | 1.63 (0.96–2.99) | |||
| ZEN | 10 (15.9) | 1 (0.32–3.22) | |||
| Mixed cereal- and nut-based foods (13) | 3-NPA | 5 (38.5) | 10.1 (4.28–28.2) | ||
| AFB1 | 7 (53.8) | 5.27 (0.36–14.3) | |||
| AFB2 | 4 (30.8) | 1.84 (0.33–3.02) | |||
| AFG1 | 3 (23.1) | 0.48 (0.27–0.89) | |||
| AFs | 7 (53.8) | 6.53 (0.36–17.4) | |||
| AFM1 | 3 (23.1) | 0.56 (0.51–0.66) | |||
| ALS | 7 (53.8) | 1.62 (1.19–3.42) | |||
| AME | 1 (7.7) | 0.26 (<LOD–0.26) | |||
| ALT | 1 (7.7) | 1.8 (<LOD–1.8) | |||
| BEA | 13 (100) | 0.3 (0.04–0.76) | |||
| CIT | 9 (69.2) | 20.1 (6.81–50.2) | |||
| DON | 0 | 0 | |||
| DHC | 4 (30.8) | 20.3 (1.88–38.2) | |||
| EnnA | 0 | 0 | |||
| EnnA1 | 2 (15.4) | 0.26 (0.1–0.43) | |||
| EnnB | 5 (38.5) | 0.46 (0.06–1.13) | |||
| EnnB1 | 3 (23.1) | 0.3 (0.06–0.51) | |||
| FB1 | 9 (69.2) | 27.2 (14.3–60.3) | |||
| FB2 | 9 (69.2) | 6.7 (3.54–15.3) | |||
| FB3 | 1 (7.7) | 9.62 (<LOD–9.62) | |||
| FBs | 10 (76.9) | 31.5 (10.6–85.2) | |||
| MON | 8 (61.5) | 6.72 (2.53–28.6) | |||
| OTA | 1 (7.7) | 2.65 (<LOD–2.65) | |||
| ST | 2 (15.4) | 0.85 (0.73–0.97) | |||
| TEN | 1 (7.7) | 0.4 (<LOD–0.4) | |||
| ZEN | 1 (7.7) | 0.8 (0.8–0.8) | |||
| Chile | |||||
| 2018–2020 | Cocoa (22) | AFs | 0 | ND | [31] |
| OTA | 7 (31.8) | N.I. (ND–4.77) | |||
| Oat (50) | AFs | 0 | ND | ||
| OTA | 1 (2) | N.I. (ND–1.74) | |||
| Cereals (60) | AFs | 0 | ND | ||
| OTA | 1 (1.6) | N.I. (ND–2.51) | |||
| Peanuts (35) | AFs | 0 | ND | ||
| OTA | 0 | ND | |||
| Hazelnut (7) | AFs | 0 | ND | ||
| OTA | 0 | ND | |||
| 2022 | Milk formula (24) | AFM1 | 15 (62.5) | 0.0038 (0.006–0.0117) | [32] |
| Fluid milk (26) | AFM1 | 6 (23.1) | 0.0069 (0.0063–0.0075) | ||
| Chicken meat (115) | AFs | 40 (34.8) | 2.4 (<LOD–8.01) | [18] | |
| OTA | 47 (40.9) | 1.14 (<LOD–4.7) | |||
| ZEN | 60 (52,2) | 2.01 (<LOD–5.01) | |||
| Eggs (80) | AFs | 22 (27.5) | 1.97 (<LOD–4.46) | ||
| OTA | 28 (35) | 1.17 (<LOD–2.98) | |||
| ZEN | 26 (32.5) | 1.58 (<LOD–3.6) | |||
| China | |||||
| 2017–2021 | Corn as feed (2873) | AFs | 474 (16.5) | 63.28 (N.I.–773) | [17] |
| TTB | 2513 (87.47) | 871.28 (N.I.–12,808) | |||
| FBs | 2343 (81.55) | 2618.81 (N.I.–40,090) | |||
| ZEN | 1479 (51.51) | 176.79 (N.I.–4686) | |||
| Wheat (411) | AFs | 5 (1.22) | 2.6 (N.I.–5) | ||
| TTB | 290 (70.56) | 2129.29 (N.I.–59,325) | |||
| FBs | 88 (21.41) | 332.31 (N.I.–910) | |||
| ZEN | 192 (48.18) | 105.5 (N.I.–1205) | |||
| Soybean meal (257) | AFs | 20 (7.78) | 4.65 (N.I.–35) | ||
| TTB | 38 (14.79) | 171.5 (N.I.–597) | |||
| FBs | 17 (6.61) | 760.24 (N.I.–6932) | |||
| ZEN | 102 (39.69) | 45.26 (N.I.–237) | |||
| Peanut meal (69) | AFs | 69 (100) | 417.72 (N.I.–10,091) | ||
| TTB | 3 (4.35) | 77.67 (N.I.–139) | |||
| FBs | 5 (7.25) | 50.4 (N.I.–120) | |||
| ZEN | 3 (4.35) | 37.33 (N.I.–61) | |||
| Oat grass (124) | AFs | 0 (0) | - | ||
| TTB | 66 (53.23) | 1,728.85 (N.I.–9363) | |||
| FBs | 48 (38.68) | 381.76 (N.I.–1986) | |||
| ZEN | 45 (36.29) | 484.53 (N.I.–2622) | |||
| Serbia | |||||
| 2018 | Corn (100) | AFs | 8 (8) | 3.6 (0.8–8.3) | [33] |
| 2019 | Corn (100) | AFs | 11 (11) | 3 (0.6–10.9) | |
| 2020 | Corn (100) | AFs | 5 (5) | 2.1 (1.1–3.0) | |
| 2021 | Corn (100) | AFs | 84 (84) | 38.8 (0.5–246.3) | |
| Croatia | |||||
| 2018 | Corn (110) | AFs | 15 (13.6) | 6.2 (1.6–75.1) | |
| 2019 | Corn (109) | AFs | 17 (15.6) | 2.5 (1.5–26.9) | |
| 2020 | Corn (103) | AFs | 20 (19.4) | 1.6 (1.5–3.3) | |
| 2021 | Corn (111) | AFs | 44 (39.6) | 34.1 (1.5–422.2) | |
| Island of São Miguel (Portugal) | |||||
| 2020 | Dairy milk (22) | ZEN | 22 (100) | 3.53 (1.23–>4.46) | [34] |
| Dairy milk (10) | ZEN | 10 (100) | 1.15 (0.48–2.15) | ||
| Dairy milk (27) | ZEN | 26 (96.3) | 0.48 (<LOD–1.37) | ||
| Dairy milk (25) | ZEN | 25 (100) | 1.15 (0.31–2.43) |
| Target Genes | Host | Mode of Action | Ref. |
|---|---|---|---|
| PRms | Corn | Positively regulates resistance against A. flavus infection and aflatoxin contamination | [82] |
| WRKY | Peanut | Mitigates aflatoxin production | [83] |
| TaBCC3 | Wheat | Positive regulator of DON | [84] |
| UDP-Glucosyl-transferase | Wheat | Positively regulates resistance against Fusarium head blight (FHB) | [85] |
| P450 lanosterol 14α-demethylase (CYP51) | Works against DON contamination in host-induced gene silencing (HIGS) lines | ||
| TaCYP72A | Wheat | Positive regulator of DON | [86] |
| TaNFX1 | Wheat | Negative regulator of resistance against Fusarium head blight | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Freire, L.G.D.; Rezende, V.T.; Noman, M.; Ullah, S.; Abdullah; Badshah, G.; Afridi, M.S.; Tonin, F.G.; de Oliveira, C.A.F. Occurrence of Mycotoxins in Foods: Unraveling the Knowledge Gaps on Their Persistence in Food Production Systems. Foods 2023, 12, 4314. https://doi.org/10.3390/foods12234314
Ali S, Freire LGD, Rezende VT, Noman M, Ullah S, Abdullah, Badshah G, Afridi MS, Tonin FG, de Oliveira CAF. Occurrence of Mycotoxins in Foods: Unraveling the Knowledge Gaps on Their Persistence in Food Production Systems. Foods. 2023; 12(23):4314. https://doi.org/10.3390/foods12234314
Chicago/Turabian StyleAli, Sher, Lucas Gabriel Dionisio Freire, Vanessa Theodoro Rezende, Muhammad Noman, Sana Ullah, Abdullah, Gul Badshah, Muhammad Siddique Afridi, Fernando Gustavo Tonin, and Carlos Augusto Fernandes de Oliveira. 2023. "Occurrence of Mycotoxins in Foods: Unraveling the Knowledge Gaps on Their Persistence in Food Production Systems" Foods 12, no. 23: 4314. https://doi.org/10.3390/foods12234314
APA StyleAli, S., Freire, L. G. D., Rezende, V. T., Noman, M., Ullah, S., Abdullah, Badshah, G., Afridi, M. S., Tonin, F. G., & de Oliveira, C. A. F. (2023). Occurrence of Mycotoxins in Foods: Unraveling the Knowledge Gaps on Their Persistence in Food Production Systems. Foods, 12(23), 4314. https://doi.org/10.3390/foods12234314










