A Study on the Structural and Digestive Properties of Rice Starch–Hydrocolloid Complexes Treated with Heat–Moisture Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Rice Starch
2.3. HMT Treatment of Samples
2.4. Determination of RS Content
2.5. GI Measurement
2.6. Observation of Particle Morphology
2.7. X-ray Diffraction (XRD)
2.8. Fourier Transform Infrared (FTIR)
2.9. Measurement of Pasting Characteristics
2.10. Thermal Characterization
2.11. Determination of Solubility and Swelling Power
2.12. Rheological Characterization
2.12.1. Dynamic Rheology
2.12.2. Static Rheology
2.13. Statistics and Analysis of Data
3. Results and Discussion
3.1. In Vitro Digestibility Analysis
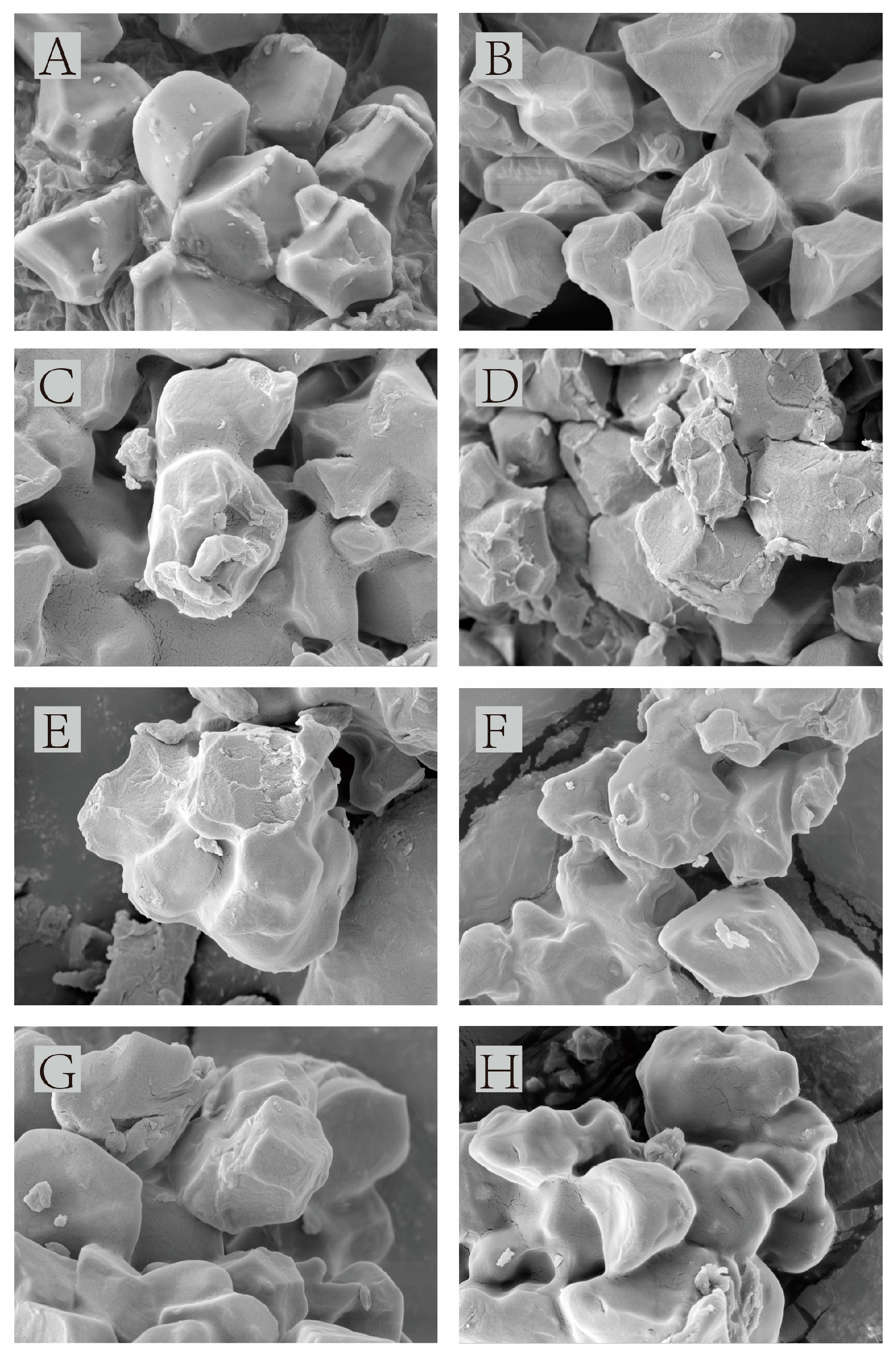
3.2. Scanning Electron Microscopy Analysis
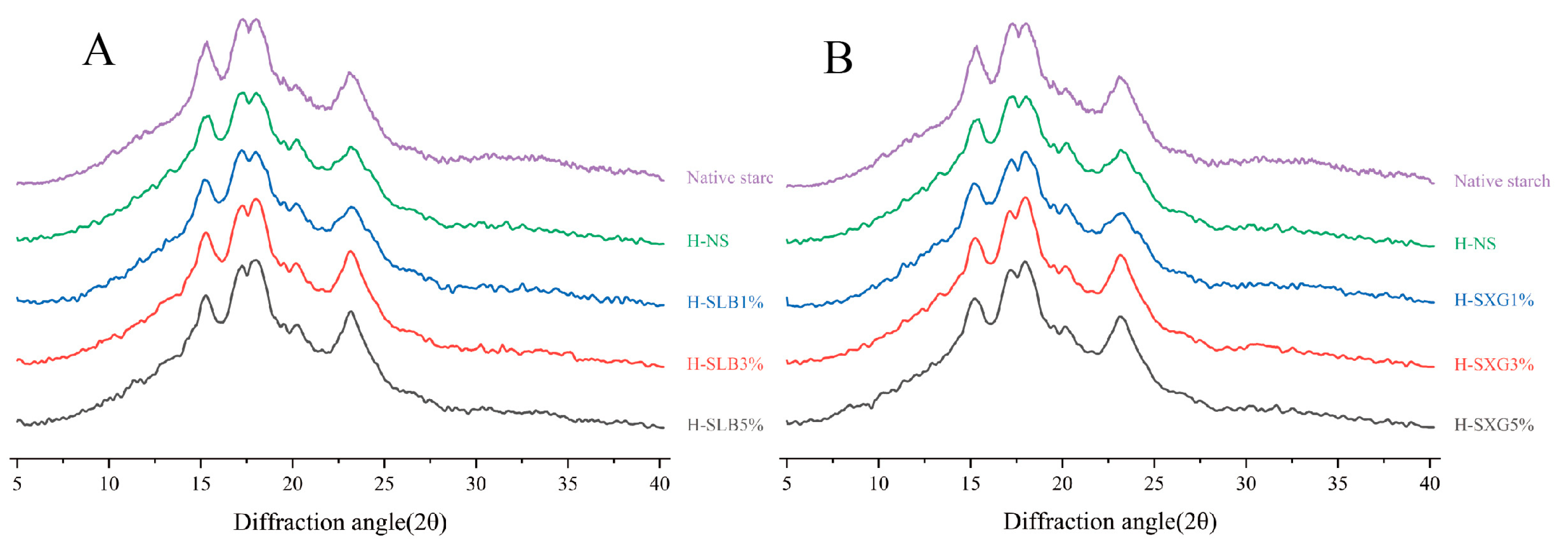
3.3. X-ray Diffraction Analysis
3.4. Fourier Transform Infrared (FTIR) Spectral Analysis
3.5. Analysis of Pasting Properties
3.6. Thermal Performance Analysis
3.7. Swelling Power and Solubility Analysis
3.8. Analysis of Rheological Properties
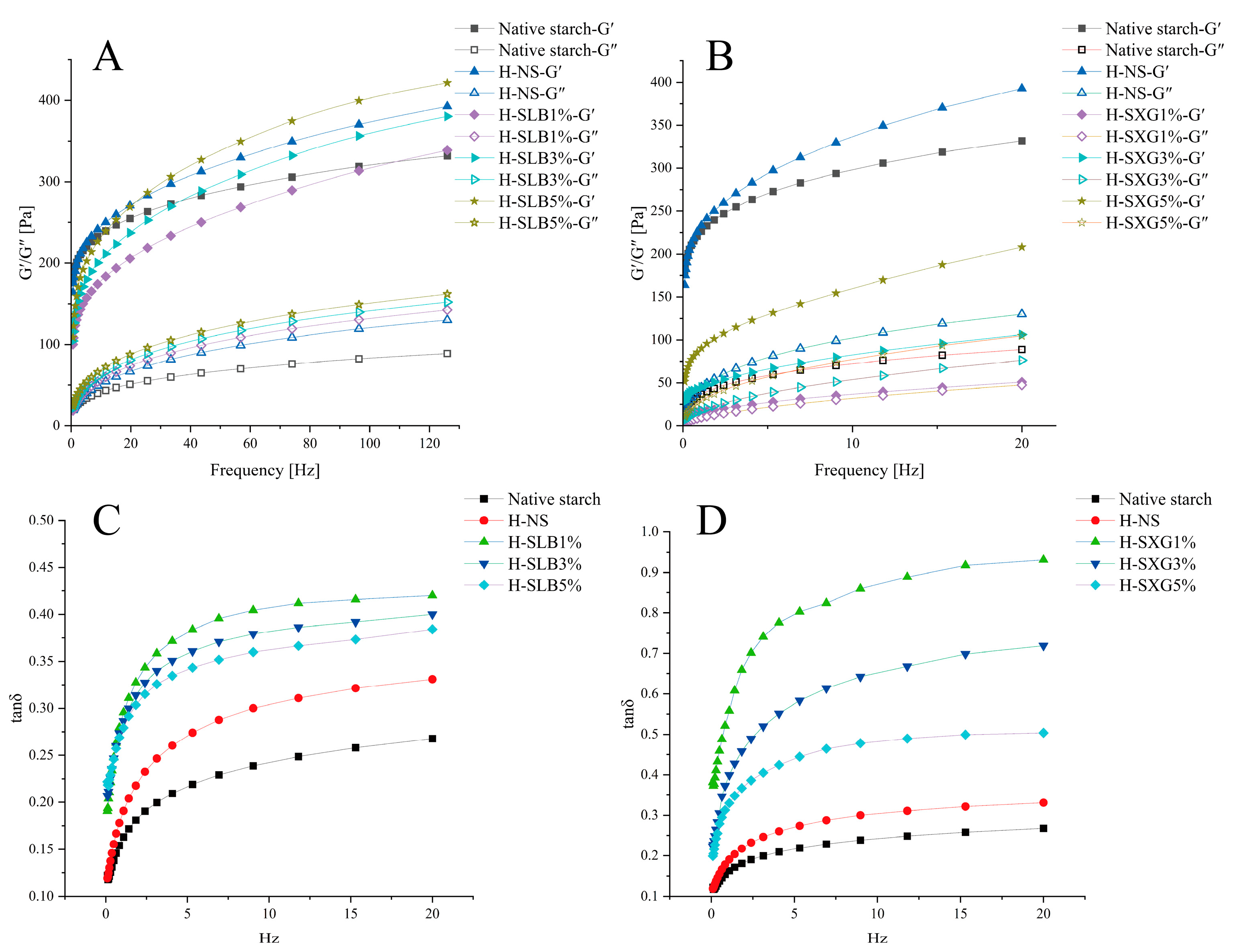
3.8.1. Dynamic Rheological Properties
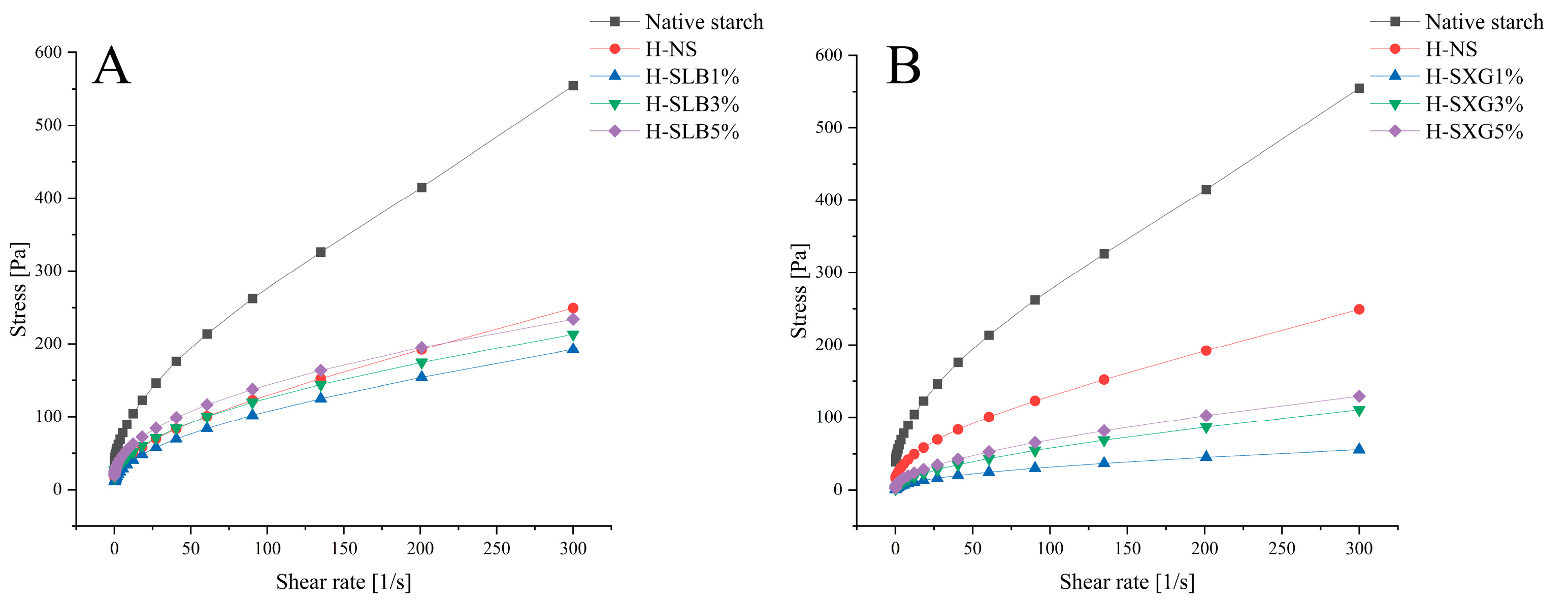
3.8.2. Static Rheology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geng, D.-H.; Tang, N.; Zhang, X.; Zhao, M.; Jia, X.; Cheng, Y. Insights into the textural properties and starch digestibility on rice noodles as affected by the addition of maize starch and rice starch. LWT 2023, 173, 114265. [Google Scholar] [CrossRef]
- Huang, H.; Liu, C.; Ma, X.; Wu, J.; Wang, F.; Liu, Y.; Li, X. Structural evolution, digestibility and inhibition on starch digestion of rice glutelin fibril aggregates as affected by incubation. Int. J. Biol. Macromol. 2022, 214, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and Measurement of Nutritionally Important Starch Fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. S2), S33–S50. [Google Scholar] [PubMed]
- Atkin, N.J.; Abeysekera, R.M.; Robards, A.W. The events leading to the formation of ghost remnants from the starch granule surface and the contribution of the granule surface to the gelatinization endotherm. Carbohydr. Polym. 1998, 36, 193–204. [Google Scholar] [CrossRef]
- Chater, P.I.; Wilcox, M.D.; Pearson, J.P.; Brownlee, I.A. The impact of dietary fibres on the physiological processes governing small intestinal digestive processes. Bioact. Carbohydr. Diet. Fibre 2015, 6, 117–132. [Google Scholar] [CrossRef]
- Giacco, R.; Costabile, G.; Riccardi, G. Metabolic effects of dietary carbohydrates: The importance of food digestion. Food Res. Int. 2016, 88, 336–341. [Google Scholar] [CrossRef]
- Chi, C.; Lian, S.; Zou, Y.; Chen, B.; He, Y.; Zheng, M.; Zhao, Y.; Wang, H. Preparation, multi-scale structures, and functionalities of acetylated starch: An updated review. Int. J. Biol. Macromol. 2023, 249, 126142. [Google Scholar] [CrossRef]
- Guo, L.; Yang, N.; Gao, W.; Tao, H.; Cui, B.; Liu, P.; Zou, F.; Lu, L.; Fang, Y.; Wu, Z. Self-healing properties of retrograded starch films with enzyme-treated waxy maize starch as healing agent. Carbohydr. Polym. 2023, 299, 120238. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Halal, S.L.M.E.; Dias, A.R.G.; da Rosa Zavareze, E. Physical modification of starch by heat-moisture treatment and annealing and their applications: A review. Carbohydr. Polym. 2021, 274, 118665. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Y.; Chai, Z.; Liu, H.; Wei, X.; Ye, X.; Tian, J.; Fang, H. Physicochemical properties and in vitro digestibility of proso millet starch modified by heat-moisture treatment and annealing processing. Int. J. Biol. Macromol. 2023, 235, 123829. [Google Scholar] [CrossRef]
- Kunyanee, K.; Luangsakul, N. The impact of heat moisture treatment on the physicochemical properties and in vitro glycemic index of rice flour with different amylose contents and associated effects on rice dumpling quality. LWT 2022, 154, 112694. [Google Scholar] [CrossRef]
- Piecyk, M.; Domian, K. Effects of heat–moisture treatment conditions on the physicochemical properties and digestibility of field bean starch (Vicia faba var. minor). Int. J. Biol. Macromol. 2021, 182, 425–433. [Google Scholar] [CrossRef]
- Xiang, G.; Li, J.; Lin, Q.; Zhang, Y.; Ding, Y.; Guo, X.; Pan, Q.; Liu, Q.; Fu, X.; Yang, Y.; et al. The effect of heat-moisture treatment changed the binding of starch, protein and lipid in rice flour to affect its hierarchical structure and physicochemical properties. Food Chem. X 2023, 19, 100785. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Su, P.; Pan, N.; Liu, X.; Zhang, Y.; Zhang, H. Insights into the aggregation structure and physicochemical properties of heat-moisture treated wheat starch and its associated effects on noodle quality. J. Cereal Sci. 2023, 112, 103704. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Liu, C.; Zheng, X. Heat-moisture modified blue wheat starch: Physicochemical properties modulated by its multi-scale structure. Food Chem. 2022, 386, 132771. [Google Scholar] [CrossRef]
- Ali, N.A.; Dash, K.K.; Routray, W. Physicochemical characterization of modified lotus seed starch obtained through acid and heat moisture treatment. Food Chem. 2020, 319, 126513. [Google Scholar] [CrossRef]
- Schafranski, K.; Ito, V.C.; Lacerda, L.G. Impacts and potential applications: A review of the modification of starches by heat-moisture treatment (HMT). Food Hydrocoll. 2021, 117, 106690. [Google Scholar] [CrossRef]
- Mahmood, K.; Kamilah, H.; Shang, P.L.; Sulaiman, S.; Ariffin, F.; Alias, A.K. A review: Interaction of starch/non-starch hydrocolloid blending and the recent food applications. Food Biosci. 2017, 19, 110–120. [Google Scholar] [CrossRef]
- Feng, L.; Wu, J.; Cai, L.; Li, M.; Dai, Z.; Li, D.; Liu, C.; Zhang, M. Effects of different hydrocolloids on the water migration, rheological and 3D printing characteristics of β-carotene loaded yam starch-based hydrogel. Food Chem. 2022, 393, 133422. [Google Scholar] [CrossRef]
- Liu, W.; Wang, R.; Li, J.; Xiao, W.; Rong, L.; Yang, J.; Wen, H.; Xie, J. Effects of different hydrocolloids on gelatinization and gels structure of chestnut starch. Food Hydrocoll. 2021, 120, 106925. [Google Scholar] [CrossRef]
- Taziki Shams-abadi, S.; Razavi, S.M.A. Cress seed gum improves rheological, textural and physicochemical properties of native wheat starch-sucrose mixture. Int. J. Biol. Macromol. 2021, 181, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Sotome, I.; Okadome, H. In vitro starch digestibility and in vivo glucose response of gelatinized potato starch in the presence of non-starch polysaccharides. Starch Int. J. Investig. Process. Use Carbohydr. Deriv. 2015, 67, 415–423. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Xu, Z.; Liu, W.; Gao, B.; Xie, J.; Chen, T.; Li, E.; Li, B.; Li, C. The addition of crosslinked corn bran arabinoxylans with different gelling characteristics was associated with the pasting, rheological, structural, and digestion properties of corn starch. Int. J. Biol. Macromol. 2023, 236, 123906. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zheng, B.; Rao, C.; Chen, L. Effect of extrusion with hydrocolloid-starch molecular interactions on retrogradation and in vitro digestibility of chestnut starch and processing properties of chestnut flour. Food Hydrocoll. 2023, 140, 108633. [Google Scholar] [CrossRef]
- Azeem, M.; Mu, T.-H.; Zhang, M. Effects of hydrocolloids and proteins on dough rheology and in vitro starch digestibility of sweet potato-wheat bread. LWT 2021, 142, 110970. [Google Scholar] [CrossRef]
- Kang, J.; Yue, H.; Li, X.; He, C.; Li, Q.; Cheng, L.; Zhang, J.; Liu, Y.; Wang, S.; Guo, Q. Structural, rheological and functional properties of ultrasonic treated xanthan gums. Int. J. Biol. Macromol. 2023, 246, 125650. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D. Locust bean gum: Processing, properties and food applications—A review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef]
- Goldsmith, F.; Guice, J.; Page, R.; Welsh, D.A.; Taylor, C.M.; Blanchard, E.E.; Luo, M.; Raggio, A.M.; Stout, R.W.; Carvajal-Aldaz, D.; et al. Obese ZDF rats fermented resistant starch with effects on gut microbiota but no reduction in abdominal fat. Mol. Nutr. Food Res. 2017, 61, 201501025. [Google Scholar] [CrossRef]
- Xie, H.; Gao, J.; Xiong, X.; Gao, Q. Effect of heat-moisture treatment on the physicochemical properties and in vitro digestibility of the starch-guar complex of maize starch with varying amylose content. Food Hydrocoll. 2018, 83, 213–221. [Google Scholar] [CrossRef]
- Chung, H.-J.; Liu, Q.; Hoover, R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohydr. Polym. 2009, 75, 436–447. [Google Scholar] [CrossRef]
- Jang, H.L.; Bae, I.Y.; Lee, H.G. In vitro starch digestibility of noodles with various cereal flours and hydrocolloids. LWT—Food Sci. Technol. 2015, 63, 122–128. [Google Scholar] [CrossRef]
- Sasaki, T.; Kohyama, K. Influence of non-starch polysaccharides on the in vitro digestibility and viscosity of starch suspensions. Food Chem. 2012, 133, 1420–1426. [Google Scholar] [CrossRef]
- Slaughter, S.L.; Ellis, P.R.; Jackson, E.C.; Butterworth, P.J. The effect of guar galactomannan and water availability during hydrothermal processing on the hydrolysis of starch catalysed by pancreatic α-amylase. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2002, 1571, 55–63. [Google Scholar] [CrossRef]
- Jia, R.; McClements, D.J.; Dai, L.; He, X.; Li, Y.; Ji, N.; Qin, Y.; Xiong, L.; Sun, Q. Improvement of pasting and gelling properties of potato starch using a direct vapor-heat moisture treatment. Int. J. Biol. Macromol. 2022, 219, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, V.; Singhal, R.S.; Kulkarni, P.R. Effect of salts on interactions of starch with guar gum. Food Hydrocoll. 1996, 10, 329–334. [Google Scholar] [CrossRef]
- Jain, S.; Winuprasith, T.; Suphantharika, M. Design and synthesis of modified and resistant starch-based oil-in-water emulsions. Food Hydrocoll. 2019, 89, 153–162. [Google Scholar] [CrossRef]
- Yu, B.; Li, J.; Tao, H.; Zhao, H.; Liu, P.; Cui, B. Physicochemical properties and in vitro digestibility of hydrothermal treated Chinese yam (Dioscorea opposita Thunb.) starch and flour. Int. J. Biol. Macromol. 2021, 176, 177–185. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, H.; Wu, Y.; Liu, Y.; Ouyang, J. Influence of moisture and amylose on the physicochemical properties of rice starch during heat treatment. Int. J. Biol. Macromol. 2021, 168, 656–662. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Hoover, R.; Donner, E.; Liu, Q. Starch chain interactions within the amorphous and crystalline domains of pulse starches during heat-moisture treatment at different temperatures and their impact on physicochemical properties. Food Chem. 2014, 143, 175–184. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Li, X.; Chen, L.; Zhang, B. Multi-scale structure, pasting and digestibility of heat moisture treated red adzuki bean starch. Int. J. Biol. Macromol. 2017, 102, 162–169. [Google Scholar] [CrossRef]
- Turnbaugh, P.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Asranudin; Holilah; Syarifin, A.N.K.; Purnomo, A.S.; Ansharullah; Fudholi, A. The effect of heat moisture treatment on crystallinity and physicochemical-digestibility properties of purple yam flour. Food Hydrocoll. 2021, 120, 106889. [Google Scholar] [CrossRef]
- Lin, S.; Liu, X.; Cao, Y.; Liu, S.; Deng, D.; Zhang, J.; Huang, G. Effects of xanthan and konjac gums on pasting, rheology, microstructure, crystallinity and in vitro digestibility of mung bean resistant starch. Food Chem. 2021, 339, 128001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, Z.; Zhu, L.; Hong, Y. Comparative study on the interaction between native corn starch and different hydrocolloids during gelatinization. Int. J. Biol. Macromol. 2018, 116, 136–143. [Google Scholar] [CrossRef]
- Chung, H.-J.; Liu, Q.; Hoover, R. Effect of single and dual hydrothermal treatments on the crystalline structure, thermal properties, and nutritional fractions of pea, lentil, and navy bean starches. Food Res. Int. 2010, 43, 501–508. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Williams, P.A. Effect of Galactomannans on the Thermal and Rheological Properties of Sago Starch. J. Agric. Food Chem. 2001, 49, 1578–1586. [Google Scholar] [CrossRef]
- Mathobo, V.M.; Silungwe, H.; Ramashia, S.E.; Anyasi, T.A. Effects of heat-moisture treatment on the thermal, functional properties and composition of cereal, legume and tuber starches—A review. J. Food Sci. Technol. 2021, 58, 412–426. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Zheng, X. Recent advances in heat-moisture modified cereal starch: Structure, functionality and its applications in starchy food systems. Food Chem. 2021, 344, 128700. [Google Scholar] [CrossRef]
- McClements, D.J. Food hydrocolloids: Application as functional ingredients to control lipid digestion and bioavailability. Food Hydrocoll. 2021, 111, 106404. [Google Scholar] [CrossRef]
- Zavareze, E.D.R.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Kulicke, W.M.; Eidam, D.; Kath, F.; Kix, M.; Kull, A.H. Hydrocolloids and rheology: Regulation of visco-elastic characteristics of waxy rice starch in mixtures with galactomannans. Starch-Stärke 1996, 48, 105–114. [Google Scholar] [CrossRef]
- Yang, X.; Chi, C.; Liu, X.; Zhang, Y.; Zhang, H.; Wang, H. Understanding the structural and digestion changes of starch in heat-moisture treated polished rice grains with varying amylose content. Int. J. Biol. Macromol. 2019, 139, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Kennedy, J.F.; Li, B.; Xu, X.; Jie, B.J. Characters of rice starch gel modified by gellan, carrageenan, and glucomannan: A texture profile analysis study. Carbohydr. Polym. 2007, 69, 411–418. [Google Scholar] [CrossRef]
- Funami, T.; Kataoka, Y.; Omoto, T.; Goto, Y.; Asia, I.; Nishinari, K. Effects of non-ionic polysaccharides on the gelatinization and retrogradation behavior of wheat starch. Food Hydrocoll. 2005, 19, 1–13. [Google Scholar] [CrossRef]
- Ji, X.; Rong, L.; Yu, Q.; Chen, Y.; Li, J.; Xie, J. Gallic acid and heat moisture treatment improve pasting, rheological, and microstructure properties of Chinese yam starch-chitosan gels: A comparative study. Int. J. Biol. Macromol. 2022, 222, 114–120. [Google Scholar] [CrossRef]
- Yang, K.; Luo, X.; Zhai, Y.; Liu, J.; Chen, K.; Shao, X.; Wu, X.; Li, Y.; Chen, Z. Influence of sodium alginate on the gelatinization, rheological, and retrogradation properties of rice starch. Int. J. Biol. Macromol. 2021, 185, 708–715. [Google Scholar] [CrossRef]
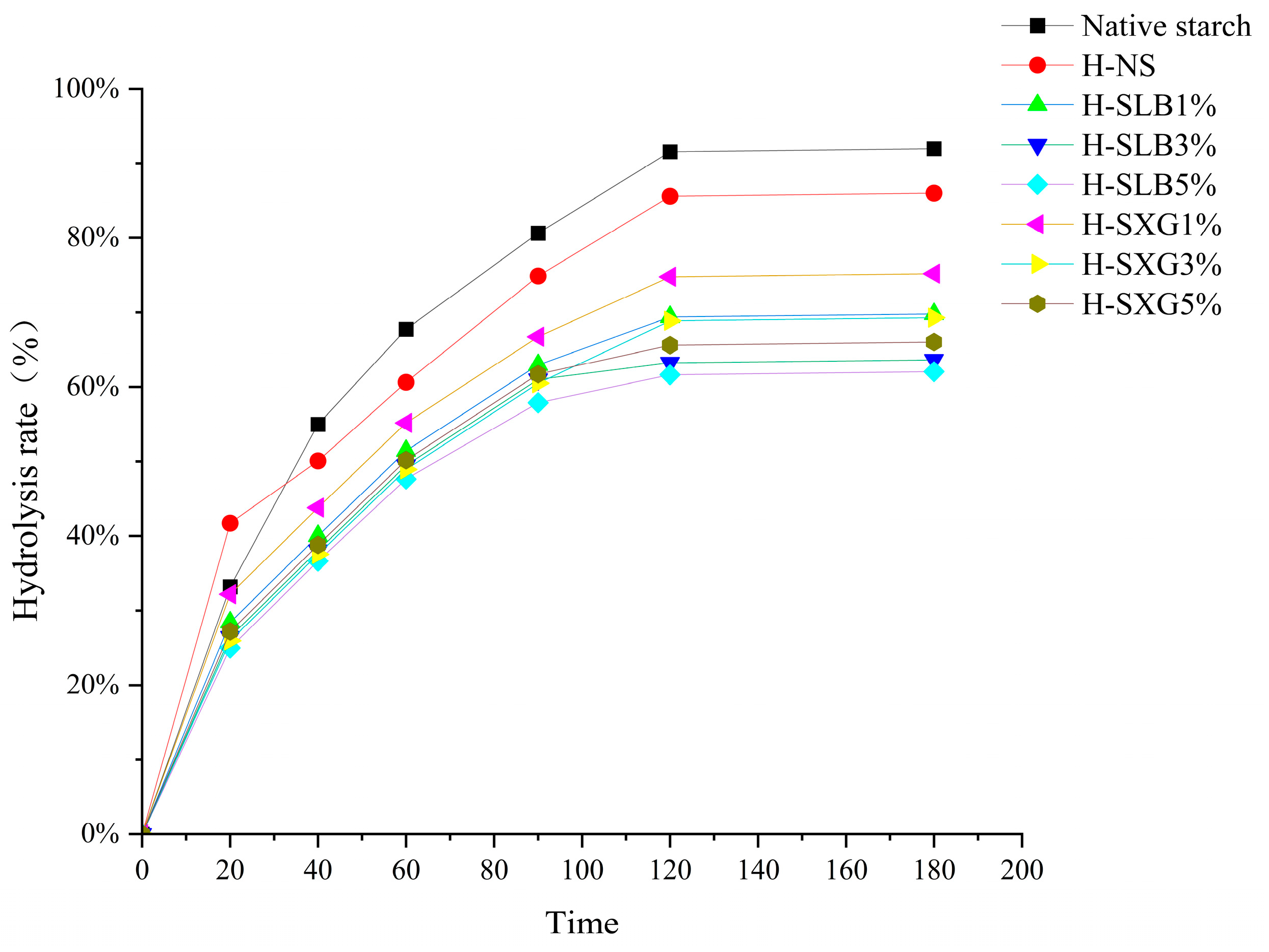



| RDS (%) | SDS (%) | RS (%) | C∞ (%) | HI | eGI | R2 | |
|---|---|---|---|---|---|---|---|
| Native starch | 33.18 ± 0.52 c | 58.4 ± 0.9 d | 8.42 ± 0.39 a | 95.83 ± 0.99 g | 94.89 ± 0.6 g | 89.99 ± 0.65 g | 0.9987 |
| H-NS | 41.71 ± 0.6 d | 43.87 ± 1.22 c | 14.42 ± 0.73 b | 86.96 ± 0.13 f | 88.94 ± 0.3 f | 84.86 ± 0.29 f | 0.9963 |
| H-SLB1% | 28.42 ± 1.27 b | 40.96 ± 1.54 abc | 30.62 ± 2.59 cd | 72.53 ± 0.35 e | 72.45 ± 0.31 d | 70.65 ± 0.19 d | 0.9948 |
| H-SLB3% | 26.47 ± 2.96 ab | 36.77 ± 5.08 a | 36.76 ± 1.44 e | 66.26 ± 0.32 b | 67.85 ± 0.27 b | 66.68 ± 0.94 b | 0.9939 |
| H-SLB5% | 24.66 ± 1.92 a | 36.98 ± 2.43 ab | 38.36 ± 3.69 e | 64.56 ± 0.1 a | 65.45 ± 0.5 a | 64.62 ± 0.59 a | 0.9928 |
| H-SXG1% | 32.19 ± 0.95 c | 42.6 ± 2.51 bc | 25.21 ± 1.62 c | 77.36 ± 0.31 | 78.13 ± 0.06 e | 75.54 ± 0.11 e | 0.9971 |
| H-SXG3% | 25.94 ± 2.18 ab | 42.92 ± 3.58 c | 31.14 ± 1.67 d | 71.5 ± 0.15 d | 72.3 ± 0.58 d | 70.52 ± 0.12 d | 0.9932 |
| H-SXG5% | 27.19 ± 1.16 ab | 38.43 ± 4.2 abc | 34.39 ± 4.11 de | 68.66 ± 0.49 c | 69.61 ± 0.14 c | 68.2 ± 0.43 c | 0.9944 |
| RC (%) | R1047/1022 | |
|---|---|---|
| Native starch | 33.72 ± 0.31 d | 0.8657 ± 0.0026 a |
| H-NS | 26.88 ± 0.54 a | 0.9377 ± 0.005 b |
| H-SLB1% | 27.12 ± 0.42 a | 1.0182 ± 0.0068 c |
| H-SLB3% | 32.64 ± 0.56 c | 1.0194 ± 0.0179 c |
| H-SLB5% | 33.17 ± 0.48 cd | 1.0564 ± 0.0127 c |
| H-SXG1% | 28.62 ± 0.37 b | 1.0264 ± 0.0769 c |
| H-SXG3% | 33.29 ± 0.33 cd | 1.023 ± 0.0954 c |
| H-SXG5% | 33.24 ± 0.52 cd | 1.025 ± 0.0743 c |
| Test | Peak Viscosity (cP) | Trough Viscosity (cP) | Breakdown (cP) | Final Viscosity (cP) | Setback (cP) | Pasting Temperature (°C) |
|---|---|---|---|---|---|---|
| Native starch | 2536 ± 8.91 g | 1585 ± 14.21 g | 951 ± 11.06 e | 2851 ± 5.58 h | 1266 ± 12.66 h | 73.47 ± 0.47 a |
| H-NS | 1062 ± 13.99 f | 947 ± 11.16 f | 115 ± 11.36 d | 1298 ± 8.93 g | 351 ± 3.51 f | 75.61 ± 0.31 b |
| H-SLB1% | 774 ± 12.63 c | 682 ± 11.49 c | 92 ± 5.25 c | 936 ± 8.25 d | 254 ± 2.54 d | 78.32 ± 0.48 d |
| H-SLB3% | 874 ± 14.17 d | 773 ± 7.56 d | 101 ± 9.37 cd | 1099 ± 14.63 e | 326 ± 3.26 e | 79.12 ± 0.45 e |
| H-SLB5% | 974 ± 7.6 e | 864 ± 10.41 e | 110 ± 8.64 d | 1262 ± 12.4 f | 398 ± 3.98 g | 79.92 ± 0.3 f |
| H-SXG1% | 365 ± 6.1 a | 358 ± 13.89 a | 7 ± 2.78 a | 428 ± 9.12 a | 70 ± 0.7 a | 77.34 ± 0.47 c |
| H-SXG3% | 427 ± 6.87 b | 403 ± 9.27 b | 24 ± 6.96 b | 525 ± 14.46 b | 122 ± 1.22 b | 78.64 ± 0.42 de |
| H-SXG5% | 433 ± 13.85 b | 407 ± 14.66 b | 26 ± 8.02 b | 604 ± 12.91 c | 197 ± 1.97 c | 78.83 ± 0.47 de |
| T0/°C | Tp/°C | Tc/°C | Tc − T0/°C | ΔH/(J/g) | |
|---|---|---|---|---|---|
| Native starch | 63.34 ± 0.17 a | 71.68 ± 0.15 a | 80.61 ± 0.38 a | 17.27 ± 0.5 a | 15.98 ± 0.08 e |
| H-NS | 66.56 ± 0.24 bc | 75.11 ± 0.06 b | 85.92 ± 0.42 b | 19.36 ± 0.4 b | 13.38 ± 0.5 a |
| H-SLB1% | 66.9 ± 0.11 cd | 75.82 ± 0.29 c | 86.91 ± 0.43 c | 20.01 ± 0.09 c | 13.72 ± 0.12 ab |
| H-SLB3% | 66.67 ± 0.44 bcd | 77.175 ± 0.13 d | 89.46 ± 0.36 d | 22.79 ± 0.09 d | 14.64 ± 0.16 c |
| H-SLB5% | 66.42 ± 0.2 b | 76.96 ± 0.29 d | 89.4 ± 0.17 d | 22.98 ± 0.3 de | 15.26 ± 0.4 d |
| H-SXG1% | 67.09 ± 0.2 d | 75.89 ± 0.33 c | 87.19 ± 0.46 c | 20.1 ± 0.43 c | 13.21 ± 0.47 a |
| H-SXG3% | 67.68 ± 0.28 e | 78.475 ± 0.1 e | 91.15 ± 0.19 e | 23.47 ± 0.3 e | 14.07 ± 0.33 b |
| H-SXG5% | 68.2 ± 0.22 f | 79.005 ± 0.25 f | 91.72 ± 0.29 e | 23.52 ± 0.41 e | 15.23 ± 0.18 d |
| τ0 | K | n | R2 | |
|---|---|---|---|---|
| Native starch | 40.679 ± 0.2 a | 11.167 ± 0.06 b | 0.667 ± 0.04 a | 0.9987 |
| H-NS | 15.789 ± 0.27 b | 6.702 ± 0.16 e | 0.619 ± 0.03 ab | 0.9993 |
| H-SLB1% | 8.293 ± 0.26 e | 7.972 ± 0.15 d | 0.549 ± 0.03 cd | 0.9998 |
| H-SLB3% | 14.953 ± 0.24 d | 9.573 ± 0.1 c | 0.531 ± 0.02 cde | 0.9989 |
| H-SLB5% | 16.766 ± 0.09 c | 13.548 ± 0.1 a | 0.486 ± 0.03 de | 0.9999 |
| H-SXG1% | 0.03 ± 0.01 h | 3.048 ± 0.08 h | 0.507 ± 0.02 e | 0.9999 |
| H-SXG3% | 0.218 ± 0.09 g | 4.104 ± 0.18 f | 0.575 ± 0.04 bc | 0.9997 |
| H-SXG5% | 3.147 ± 0.14 f | 4.717 ± 0.14 g | 0.575 ± 0.03 bc | 0.9994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Dou, B.; Jia, J.; Liu, Y.; Zhang, N. A Study on the Structural and Digestive Properties of Rice Starch–Hydrocolloid Complexes Treated with Heat–Moisture Treatment. Foods 2023, 12, 4241. https://doi.org/10.3390/foods12234241
Zhang Y, Dou B, Jia J, Liu Y, Zhang N. A Study on the Structural and Digestive Properties of Rice Starch–Hydrocolloid Complexes Treated with Heat–Moisture Treatment. Foods. 2023; 12(23):4241. https://doi.org/10.3390/foods12234241
Chicago/Turabian StyleZhang, Yu, Boxin Dou, Jianhui Jia, Ying Liu, and Na Zhang. 2023. "A Study on the Structural and Digestive Properties of Rice Starch–Hydrocolloid Complexes Treated with Heat–Moisture Treatment" Foods 12, no. 23: 4241. https://doi.org/10.3390/foods12234241
APA StyleZhang, Y., Dou, B., Jia, J., Liu, Y., & Zhang, N. (2023). A Study on the Structural and Digestive Properties of Rice Starch–Hydrocolloid Complexes Treated with Heat–Moisture Treatment. Foods, 12(23), 4241. https://doi.org/10.3390/foods12234241






