Abstract
Salmonella contamination in poultry meat is an important food safety issue as this pathogen can lead to serious illness and economic losses worldwide. In poultry meat processing, a variety of strong bacteriostatic agents has been introduced for controlling Salmonella including bacteriophages (phages), organic acids, and modified atmosphere packaging (MAP). In our study, two selected phages including vB_SenM_P7 and vB_SenP_P32 were used in combination with propionic acid (PA) and MAP for controlling Salmonella of multiple serovars on chicken meat under storage at 4 °C. The two phages showed strong lytic activity against over 72 serovars of Salmonella tested (25.0 to 80.6%). Phages, vB_SenM_P7 and vB_SenP_P32 showed 40% and 60% survival rates, respectively, after the exposure to temperatures up to 70 °C. Both phages remained active, with nearly 100% survival at a wide range of pH (2 to 12) and 15% NaCl (w/v). The available chlorine up to 0.3% (v/v) led to a phage survival rate of 80–100%. A combination of Salmonella phage cocktail and 0.5% PA could reduce Salmonella counts in vitro by 4 log CFU/mL on day 3 whereas a phage cocktail and 0.25% PA showed a 4-log reduction on day 5 during storage at 4 °C. For the phage treatment alone, a 0.3-log reduction of Salmonella was observed on day 1 of storage at 4 °C. In the chicken meat model, treatment by a phage cocktail and PA at both concentrations in MAP conditions resulted in a complete reduction of Salmonella cells (4–5 log unit/g) on day 2 of storage whereas each single treatment under MAP conditions showed a complete cell reduction on day 4. For the meat sensory evaluation, chicken meat treated with a phage cocktail-PA (0.5%) in MAP condition showed the highest preference scores, suggesting highly acceptability and satisfactory. These findings suggest that a combined treatment using a phage cocktail and PA in MAP conditions effectively control Salmonella in poultry meat during storage at low temperature to improve the quality and safety of food.
1. Introduction
Poultry meat is often linked with contamination issues by Salmonella spp., which inhabits the gastrointestinal tract of live poultry. Contamination in meat can occur during slaughtering and processing [1]. Consumption of food contaminated with Salmonella leads to illness. Infectious disease with Salmonella, called salmonellosis, is among the most common foodborne diseases reported worldwide. In 2021, salmonellosis affected 60,050 people in EU member states. With most salmonellosis outbreaks caused by S. Enteritidis (54.6%), poultry meat is still major source of infection [2]. S. Typhimurium was the second most common serovar infecting humans in the EU (11.7%), North America, Australia and New Zealand, and other countries whereas S. Infantis, S. Hadar, and S. Virchow have been listed as the most frequently found serovar [2,3,4]. Overall, salmonellosis causes significant economic losses, with EPSA estimating the total economic burden of human salmonellosis at EUR 3 billion per year [5]. According to the Commission Regulation (EU) No 200/2010 Implementing Regulation (EC) No 2160/2003 of the European Parliament and of the Council, as regards a target for the reduction of the prevalence of Salmonella serovars in adult breeding flocks of Gallus gallus, 5 serovars as described above were prioritized by EU member states for control of poultry and poultry products entry [6].
During processing at slaughterhouses and processing plants, meat can become contaminated by microflora presented in processing areas and equipment because of unhygienic management. Several methods have been established including chemical methods (organic acids, chloride, phosphate) [7,8,9], physical methods (X-ray, steam, radiation, UV, etc.) [10,11], and natural and synthetic antimicrobials (ionic antimicrobials and bacteriophages) [10,12,13,14]. However, each of these methods alone might not be sufficient to eliminate contaminated microflora that are present on foods or food surfaces. Therefore, the combined methods are of interest for the investigation of a synergistic effect in controlling Salmonella populations.
Bacteriophages or phages are the bacteria-killing viruses that have been accepted by the United States Food and Drug Administration (US FDA) as being generally recognized as safe (GRAS) for use as an antibacterial food additive in poultry meat products [15,16]. Phages are extensively studied by researchers due to their strong bactericidal effect against specific bacteria without dangerous impacts on human and animal health. Phages can be applied to reduce Salmonella in live poultry at the beginning of cultivation on farms and in poultry meat in processing plants [17,18]. Phage cocktails can expand their lytic ability covering several serovars and prevents bacterial co-infection [19,20]. Similar to phages, propionic acid (PA) has also been approved as GRAS by the US FDA and is widely used as a food additive in several human and animal foods, cosmetics, and pharmaceuticals [21]. PA penetrates microbial cells and decreases the internal pH level—preventing the growth of bacteria and finally killing them [22]. PA is also an alternative to antibiotics used in poultry diets for reducing avian pathogens and diseases [23]. For product shelf-life extension, modified atmosphere packaging (MAP) is commonly used in the poultry industry. MAP has been shown to prevent and control Salmonella, other foodborne pathogens, and spoilage micro-organisms in food products [24]. Modified gases within packaging can inhibit bacterial growth (CO2), anaerobic growth (O2), and prevent lipid oxidation of meat (N2) [25].
The current study aimed to evaluate the synergistic effects of a phage cocktail, PA, and MAP in reducing Salmonella counts in artificially contaminated chicken meat during storage at low temperature. The physical changes through color change and the meat sensory evaluation after it was treated with the combined treatments were also investigated. Results obtained in the current study will be useful for the poultry industry to consider using the combined treatments as an alternative approach to improve the safety of foods.
2. Materials and Methods
2.1. Salmonella Phages, Phage Lysate and Phage Cocktail Preparation
Two Salmonella phages including vB_SenM_P7 and vB_SenP_P32 were included in this study. Phages vB_SenM_P7 and vB_SenP_P32 were previously isolated from wastewater of animal farm and wastewater treatment station using S. Agona H2-016 and S. Enteritidis S5-371 as the natural hosts, respectively. Salmonella phage stocks were used to prepare 10-fold serial dilutions in salt magnesium (SM) buffer for further overlay preparation on the host lawns following the protocols of Pelyuntha & Vongkamjan (2022) [17]. The overlay was harvested using 5 mL of SM buffer stirred in a horizontal shaker at room temperature for 1–2 h, followed by centrifugation at 6000 rpm for 15 min at 4 °C. The supernatant was filtrated through 0.20 µm syringe filters and phage lysates were kept at 4 °C. The titer of each phage was counted by the forming plaques present on plates of desired dilutions. The phage cocktail was prepared using the same concentration (8 log PFU/mL) of both phages at a ratio of 1:1. Phage titer was also determined by counting plaques as previously described by Pelyuntha et al. (2021) [20].
2.2. Salmonella Strains and Phage Host Range Determination
The host range of phages vB_SenM_P7 and vB_SenP_P32 was determined by spotting 10 µL of phage lysate on lawn cultures of each Salmonella serovar (72 serovars). These serovars were obtained from different sources that linked to the food production system including foods, broiler farms, broiler slaughterhouses and processing plants, and human sources [20,26]. The overlay lawn was observed for the formation of the clear plaques after growth at 37 °C for 18 to 24 h. Host range determination was performed in triplicate [27].
2.3. Phage Stability Tests
2.3.1. Effect of Temperature on Salmonella Phage Titer
The effect of different temperatures on phage stability was investigated using a modified method from Ateba & Akindolire (2019) [28]. Each phage suspension (8 log PFU/mL) was incubated at 25, 37, 45, 50, 55, 60, 65, 80, and 100 °C for 1 h. Each treatment was dropped on the double overlay agar plate containing the suspension of each bacterial host. A phage lysate at 4 °C was included as a control. Titer of the phages was monitored following the method previously described.
2.3.2. Effect of pH on Salmonella Phage Titer
The pH of the phage suspension (8 log PFU/mL) was adjusted with 1 M HCl or 1 M NaOH to obtain the pH values ranging from 2 to 12. Each treatment was left at 25 °C for 24 h. Titer of the phages was monitored following the method previously described.
2.3.3. Effect of Salinity on Salmonella Phage Titer
The suspension of phage (8 log PFU/mL) was blended with NaCl solution to obtain the final concentrations ranging 0.5 to 20.0% (w/v) and incubated at 25 °C for 24 h. A phage lysate without NaCl treatment was included as a control. Titer of the phages was monitored following the method previously described.
2.3.4. Effect of Free Available Chlorine on Salmonella Phage Titer
The suspension of phage (8 log PFU/mL) was blended with free available chorine solution at 0.1, 0.25, 0.5, 1, 2.5, 5% and incubated for 24 h at 25 °C. A phage lysate without chorine treatment was included as a control. Titer of the phages was monitored following the method previously described.
2.4. Minimum Inhibition Concentration of Propionic Acid
The broth micro-dilution method was performed to determine the MIC value of PA. Filter-sterile PA was diluted in 96-well microtiter plates containing TSB broth to obtain the final PA concentrations ranging from 0.1 to 1% (v/v). The inoculum of mixed serovars of Salmonella suspension (10 µL) containing 4 log CFU/mL was added to the wells. The fresh TSB broth well was used as the control (no Salmonella added), and the inoculum viability (no PA) was used as a positive control. All microplates were incubated at 37 °C for 24 h. MIC values were defined as the lowest concentration of PA that had no visible growth in observed wells [29].
2.5. Effect of a Phage Cocktail and Propionic Acid on Salmonella Reduction In Vitro
An overnight culture of each Salmonella (S. Enteritidis S5-370, S. Hadar PPI-013, S. Infantis S5-506, S. Typhimurium S5-371, and S. Virchow H2-117) was mixed and resuspended in TSB and diluted to obtain a final concentration of 4 log CFU/mL. Phage cocktail stock was diluted with TSB to achieve a final phage concentration of 7 log PFU/mL. A 20 mL suspension of Salmonella and 20 mL of a phage cocktail or phage cocktail with PA (final concentration at 0.25% and 0.5% v/v) were mixed at a ratio of 1:1 by volume and incubated at 4 °C. Only mixed culture was served as a control. The number of viable cells from each treatment and control was enumerated at day 0, 1, 2, 3, 4 and 5 by a spread plate on TSA [20]. If the result of viable cell count was at an undetectable level (ND), the presence and absence of any remaining Salmonella in the culture broth (25 mL) were also confirmed by the modified ISO 6579: 2017 according to the protocol provided by Biomérieux company and re-streaked on Xylose Lysine Deoxycholate (XLD) agar.
2.6. Treatment of Chicken Meat with a Phage Cocktail and PA
Chicken breast was purchased from a supermarket and stored at 4 °C prior to analysis. The chicken breast was cut into a piece of approximately 100 g. To decontaminate the microflora, chicken meat was soaked in 50 ppm of available chlorine solution for 5 min and subsequently washed with sterile distilled water for 5 min three times to remove any available chlorine residue. Chicken meat was soaked in mixed Salmonella suspension (S. Enteritidis, S. Hadar, S. Infantis, S. Typhimurium, and S. Virchow; 4 log CFU/g) for 10 min to allow bacterial attachment. Chicken meat was transferred into linear low-density polyethylene (LLDPE) bags. Then, 1 mL of a phage cocktail, 0.25% (v/v) PA, 0.5% (v/v) PA, a phage cocktail with 0.25% (v/v) PA, a phage cocktail with 0.5% (v/v) PA, or PBS (control) was added to each bag. All bags were filled with N2 at a sample/gas volume ratio of 1:4 (w/v) connected to an N2 cylinder and heat-sealed (tecnovac® Tecnova, Grassobbio BG, Italy). All samples were kept at 4 °C and were examined for microbiological changes (day 0 to 5), physical change (day 0, 3, and 5), and meat sensory evaluation (day 0 and 5).
2.7. Monitoring of Salmonella Reduction and Phage Titers in Chicken Meat during Storage
Chicken meat samples were collected on day 0, 1, 2, 3, 4, and 5. Each chicken breast treated was aseptically cut into a piece (approximately 25 g) and mixed with 225 mL of buffered peptone water (BPW) in sterile stomacher bag. The solution in bag was homogenized using stomacher machine with a speed of 220 rpm for 2 min. The mixture was diluted with the same buffer to obtain the appropriate dilution that was spread on XLD agar plates [17]. All plates were incubated at 37 °C for 18 h. The formation of colonies with black centers was observed and recoded. All tests were run in triplicate.
To determine the amount of Salmonella phages, the homogenized solution was collected and centrifuged at 6000 rpm for 15 min to settle debris. The supernatant was filtered through 0.20 µm syringe filters and serially 10-fold diluted to obtain the appropriate concentrations. Titer of the phages was monitored following the method previously described.
2.8. Evaluation of the Color Change in Chicken Meat
The color change of the chicken meat during storage was evaluated as described by Kim et al. (2014) [30]. Each sample was cut into pieces 2 cm in height and measured with a colorimeter (ColorFlex® EZ, HunterLAB, Hunter Associates Laboratory, Inc., Reston, VA, USA). Lightness (L*) and yellowness (b*) were evaluated. The color was measured five times per sample. Whiteness was calculated using the following equation: Whiteness = L* − 3b*
2.9. Sensory Preference Evaluation in Chicken Meat
A total of 50 panelists from the Faculty of Agro-Industry, Prince of Songkla University were included for the evaluation of the overall preference of the meat products. Chicken meat was cut into a small piece (2 cm × 3 cm × 2 cm) and individually kept in a plastic bag. Sample codes with three-digit numbers were presented in random order to avoid carryover effects. Appearance, color, odor, texture, juiciness, and overall liking were the attributes evaluated by each panelist (Table 1).

Table 1.
Description of sensory attributes decided upon by the panelists.
Liking scores were given for appearance, color, odor, texture, juiciness, and overall liking of samples using a nine-point hedonic scale (1 = dislike extremely, 2 = dislike very much, 3 = dislike moderately, 4 = dislike slightly, 5 = neither like or dislike, 6 = like slightly, 7 = like moderately, 8 = like very much, and 9 = like extremely). Chicken meat treated with MAP alone (control), MAP with a phage cocktail-0.25% PA, and MAP with a phage cocktail-0.5% PA were selected for evaluation based on the 100% reduction of Salmonella count both in vitro and in chicken meat. A meat sensory preference evaluation was carried out at day 0 and 5 of meat storage.
2.10. Statistical Analysis
Statistical analysis was performed using SPSS (Version 22.0) of Windows statistics software (SPSS Inc., Chicago, IL, USA). The data were subjected to one-way analysis of variance followed by Tukey’s range test. A significant difference between control and treatments was calculated using the independent-samples t-test. A difference was also considered statistically significant at the p-value < 0.05.
3. Results
3.1. Phage Host Range Determination
As shown in Figure 1, two selected phages showed different lysis activity. Phage vB_SenM_P7 showed lower lytic ability against Salmonella, presenting 25.0% (lysed 18 serovars) whereas phage vB_SenP_P32 showed the lytic ability as high as 80.6% (lysed 58 serovars). Both phages had strong lytic ability against the two most common serovars in outbreaks worldwide, including S. Enteritidis and S. Typhimurium. In addition, phage vB_SenM_P7 could lyse S. Infantis and S. Virchow, which represent the most concerning serovars in the EU [6].
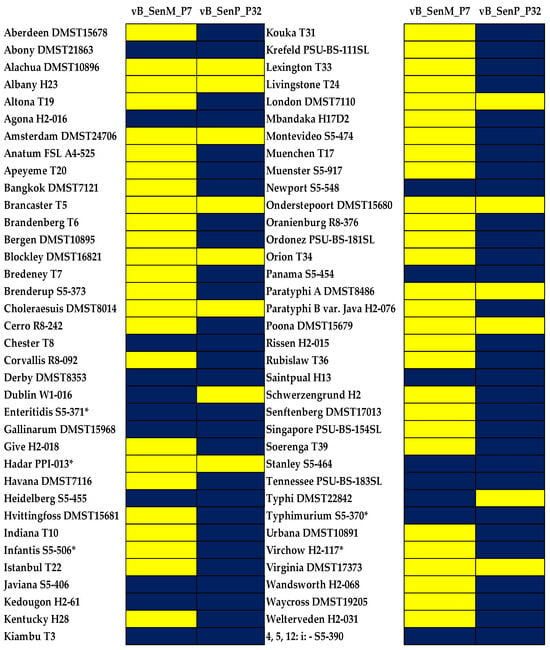
Figure 1.
Heatmap representation of the lytic activity of Salmonella phages vB_SenM_P7 and vB_SenP_P32 on different Salmonella serovars. Serovars with the asterisk (*) indicates those that particularly concern the EU. Blue indicates lysis (+). Yellow indicates non-lysis (−).
3.2. Phage Stability
The stability of each phage included in the phage cocktail for further applications was evaluated. Phage vB_SenM_P7 could be recovered up to 35.5% when exposed to a wide range of temperatures between 4 and 70 °C for 1 h (Figure 2a). However, this phage was inactive when exposed to the temperature above 75 °C. Phage vB_SenP_P32 showed 32% survival when exposed to temperatures between 4 and 50 °C (Figure 2a). Phage vB_SenM_P7 remained up to 80% survival when exposed to pH ranging from 2 to 12 while phage vB_SenP_P32 showed high survival rate of nearly 100% when exposed to pH 2 to 12 (Figure 2b). Both phages also showed high survival rates when exposed to NaCl solution up to 15% (Figure 2c). When exposed to chlorine 0.1% and 0.5% for 24 h, phage vB_SenM_P7 showed survival rates of 94% and 30%, respectively, while phage vB_SenP_P32 showed survival rates of 93% and 17%, respectively (Figure 2d). Overall, both phages showed similar survival rates against a wide range of temperatures, pH, salinity, and chlorine concentrations.
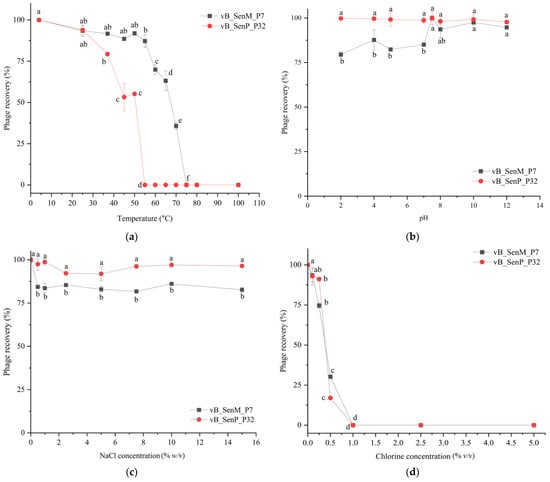
Figure 2.
The stability of phages vB_SenM_P7 and vB_SenP_P32 in different conditions; (a) temperatures, (b) pH (c) NaCl concentrations, and (d) chlorine concentrations. All values provided as mean ± standard deviation of triplicate. Different lowercase letters indicate the significant difference at the p-value of 0.05.
3.3. Effect of a Phage Cocktail and Propionic Acid on Salmonella Reduction In Vitro
Propionic acid showed the MIC value of 0.25% (v/v) on Salmonella tested. The concentrations of MIC and 2-fold MIC (0.5% v/v) were used in this study. Reduction of Salmonella cells by different treatments was monitored at 1-day intervals for 5 days at 4 °C. A combination of phage cocktail-0.5% PA could completely reduce Salmonella count by 4 log CFU/mL on day 3 whereas a combination of phage cocktail-0.25% PA completely reduced Salmonella cells later on day 5 (Table 2). Overall, the combination (a phage cocktail with PA) was more effective for controlling Salmonella than the single treatment, i.e., phage cocktail or PA alone. On day 5, up to 3-log reduction of Salmonella cells was observed in the treatments by a phage cocktail or PA alone when compared to a control (p < 0.05).

Table 2.
Monitoring of Salmonella populations (log CFU/mL) by different treatments at 4 °C.
3.4. Reduction of Salmonella in Chicken Meat Treated with a Phage Cocktail and Propionic Acid during Storage at 4 °C in MAP Condition
The significant reduction of Salmonella cells between 0.6 to 1.7 log units was observed in all treatments on day 1 of storage (Table 3). Combined treatments of a phage cocktail and PA (0.25% or 0.5%) could completely reduce Salmonella cells by 100% (4 log units) on day 2 whereas the PA-treated groups showed a complete reduction of Salmonella cells on day 4 of storage. However, a phage cocktail showed greater efficacy in controlling Salmonella in MAP condition than PA as indicated by a complete reduction of Salmonella cells a day earlier than that observed in PA-treated groups.

Table 3.
Monitoring of Salmonella populations (log CFU/g) by different treatments in chicken meat during storage at 4 °C in MAP condition.
In addition, the titers of a phage cocktail remained nearly constant from day 0 to 5 of storage in the treatment with or without PA at 4 °C in MAP condition (Table 4). After 5 days of storage, the titers changed from 7.5 to 7.1 log PFU/g, 6.9 to 6.5 log PFU/g and 6.1 to 6.8 log PFU/g for phages in MAP only, phages with 0.25% PA and phages with 0.5% PA, respectively.

Table 4.
Phage titers (log PFU/g) of during storage in various phage treatments.
3.5. Change of the Meat Color during Storage at 4 °C in MAP Condition
The whiteness was calculated and considered as the typical parameter affecting color changes in chicken meat. The increase of whiteness value in chicken meat was only observed in the MAP group (control), which increased from 9.6 ± 0.0 to 17.2 ± 0.1 on day 5 of storage (Table 5). However, the reduction of whiteness value was observed in all treatments. The whiteness values of chicken meat treated with a phage cocktail only, 0.25% PA, 0.5% PA, a phage cocktail + 0.25% PA and a phage cocktail + 0.5% PA significantly reduced (p < 0.05) to 10.7 ± 0.1, 10.3 ± 0.1, 14.5 ± 0.1, 11.8 ± 0.3 and 11.5 ± 0.1, respectively.

Table 5.
Color change of the chicken meat during storage at 4 ºC in MAP condition.
3.6. Meat Sensory Evaluation
On day 0, the highest preference scores were observed in the treatment with a phage cocktail-0.5% PA. This sample showed a score of appearance, color, odor, juiciness, and overall liking as high as 6.9 ± 0.9, 7.0 ± 1.0, 5.7 ± 1.4, 6.8 ± 1.2, and 6.6 ± 1.1, respectively (Table 6). In MAP conditions after 5 days of storage at 4 °C, scores for the meat appearance, color, and texture preference in the treatment with a phage cocktail-0.5% PA were 7.1 ± 0.9, 7.0 ± 1.0, and 6.8 ± 1.2, respectively. These were higher than the control that showed scores for the meat appearance, color, and texture preference as 6.7 ± 1.2, 5.7 ± 1.4, and 6.7 ± 1.2, respectively. Scores for samples in the treatment with a phage cocktail-0.5% PA were also higher than that in the treatment with a phage cocktail-0.25% PA which showed scores for the meat appearance, color, and texture preference as 6.8 ± 1.3, 6.4 ± 1.3, and 6.6 ± 1.3, respectively. The highest preference for odor was found in the treatment with a phage cocktail-0.25% PA (6.4 + 1.5), followed by a phage cocktail-0.5% PA (6.3 ± 1.2) and the control (5.9 ± 1.5). The highest juiciness preference score was observed in the control (7.1 ± 1.0) whereas the lowest juiciness was found in the treatment with a phage cocktail-0.25% PA (6.6 ± 1.3). From the overall liking, the treatment with a phage cocktail-0.5% PA was still the most accepted from day 0 until day 5 of storage as indicated by a likely score of 6.9 ± 1.0, followed by control (6.5 ± 1.2) and the treatment with a phage cocktail-0.25% PA (6.4 ± 1.3).

Table 6.
Meat sensory preference evaluation of the chicken meat kept in linear low-density polyethylene (LLDPE) packaging during storage at 4 °C in MAP condition for 0 and 5 days.
4. Discussion
Salmonella phages isolated from various sources showed differences in the ability to lyse Salmonella serovars. Previous studies included 8–29 major serovars of Salmonella of concern for food production [17,20,31]. In the present study, 72 major serovars linked to poultry and poultry meat production were included. Although the two selected phages showed differences in the lysis ability against the major serovars included, when they were combined as a phage cocktail, this phage cocktail could cover a broad spectrum of Salmonella serovars of concern in the food production from different sources [20,26]. The phage cocktail in the present study could lyse up to four serovars that most concern the EU, including S. Enteritidis, S. Infantis, S. Typhimurium, and S. Virchow.
The lytic ability of phages is one of the major criteria when selecting phages for developing a biocontrol targeting foodborne pathogens [32]. Similar studies employed this approach for combining several phages as a phage cocktail to target a wide range of Salmonella serovars present in the poultry industry, poultry produce, or other food products [33,34,35,36,37] In addition, previous studies suggest that the use of a phage cocktail can improve the lytic activity against a wide range of multiple serovars. Treatment with a single phage may lead to the development of phage-resistance in bacteria. This is the benefit of using a phage cocktail: if bacteria develop a resistance to one phage, they might be vulnerable to other phages present in a cocktail [18,20].
The two selected phages in the cocktail showed high stability over a wide range of conditions, suggesting the suitability of a phage cocktail to be used on meat products, especially at the post-harvest stage where other harsh processes including some heat treatment, acid-base treatment, chlorine washing, or salt stress may be involved. However, the use of a single approach as in a phage cocktail may still result in the incomplete elimination of Salmonella and other bacterial cells in the food matrices [33,38]. In addition, some Salmonella cells are resistant and could survive under storage at low temperature [39]. For a phage cocktail treatment in meat products, reduction of Salmonella cells between 0.9 and 2.2 out of 6 log units of S. Enteritidis and S. Typhimurium on chicken breasts were reported after treatment with a cocktail composed of three strong lytic phages (UAB_Phi20, UAB_Phi78, and UAB_Phi87) at 4 °C for 72 h [40]. Another study reported a maximum reduction of 1 log unit on chicken meat after 3 h treated with a phage cocktail (ENT101 and TYM10), while at the end of study, a total of 0.7 log unit reduction was observed when compared to control [41]. Due to the limitation of inhibition by phage cocktail, the remaining bacteria can grow and constantly contaminate until the end of storage.
Organic acids can reduce the growth of several foodborne bacteria present on meat surfaces during storage at low temperature (−16 to 4 °C) [17,42]. PA at 1% and 2% (v/v) achieved a maximum reduction of Listeria monocytogenes in poultry leg after being dipped on the PA solution during storage for 7 days by 2.6 and 2.7 log unit, respectively [43]. In the present study, the single treatment of PA at 0.25% and 0.5% achieved the maximum reduction between 0.3 to 0.5 log units. The combination between a phage cocktail and PA were effective when compared to a single treatment applied in this study. The combined phage cocktail and PA at both concentrations reduced Salmonella counts to undetectable levels in vitro and complete elimination of Salmonella cells was observed in the meat model after 3 days of storage at 4 °C. Overall, the synergistic effect between the phage and propionic acid treatments could be observed here. Previous studies showed that a phage cocktail combined with lactic acid, paracetic acid, cetylpyridinium chloride, lauric arginate, and peroxyacetic acid could reduce the Salmonella counts in chicken breast fillets and freshly trimmed meat [10,44].
The addition of modified gas might play a major role in inhibiting the remaining bacteria present in packaging where a single strategy is used. Consequently, Salmonella counts could not be detected on day 3 to 5 of storage. Moreover, the titer of phages presented in each combination was not affected by PA and gas used in the treatment. Phages could survive and remained active until the end of storage. MAP is introduced into the food-processing step in order to improve the product shelf-life by reducing the growth of foodborne and spoilage bacteria in meat, especially Salmonella. Djordjević et al. (2018) reported that MAP conditions with 20% O2: 50% CO2: 30% N2 and 20% O2: 30% CO2: 50% N2 reduced Salmonella count by more than 2 log CFU/g in minced meat at day 6 of storage [25]. Authors also suggested that MAP containing 50% CO2 was more effective than a regular vacuum atmosphere (VA) and MAP with 30% CO2. Other strategies are also introduced to combine with MAP such as irradiation, ultraviolet light, essential oils, organic acids, and bacteriophages for increasing the efficacy of MAP to reduce Salmonella in foods [10,45,46,47,48].
Regarding the combination of combined phages, propionic acid, and MAP, a synergistic effect was observed in the reduction of Salmonella count in chicken meat. Phages can destroy the peptidoglycan present on the bacterial cell wall upon the activity of endolysins. Phage progenies continually reproduced after infecting the same neighbor cells [49]. PA is commonly used as a food preservative and is characterized as an antibacterial compound. PA is superior by inhibiting the growth of Gram-negative and Gram-positive bacteria, as well as yeasts and molds. The mode of action of PA is pH-dependent, which directly affects the redox reduction of NADPH formation, the source of required energy in bacteria [29,50]. MAP is a non-thermal process used for food preservation. O2, N2, and CO2 are typical gases used in MAP packages. O2 can be used for inhibiting anaerobic bacteria but does not reduce oxidation, resulting in food spoilage if moisture is present; N2 is used to displace oxygen from food packaging. In addition, CO2 slows down the growth of different microorganisms. It can freely diffuse into cells, form bicarbonate ions, and release protons. Upon the multiple reactions of CO2, the physiological changes of bacteria were observed by alteration of cell membrane function, decrease in the function of enzymes, and change in the physiology of proteins and internal pH [51].
Color of the meat is the one of the most important characteristics for a customer’s purchase decision. The color of chicken meat depends on the level of protein denaturation, storage temperature and pH, and lipid oxidation activity [52,53]. The lower whiteness scores indicate the overall acceptable of the quality of the meat color change. In the present study, the whiteness values of the meat treated with the combined phage cocktail and PA treatments were lower than that in meat from the control group during a 5-day storage. In addition, other sensory preference scores in phage–acid-treated groups were satisfactory without significant difference (p > 0.05) when compared to control group, suggesting they were as acceptable as if the meat had never been treated. As pH increases, the negatively charged ions begin to accumulate in higher concentration and cause the repulsion of muscle proteins. When repulsion occurred, the proteins in meat turned from light- to dark-colored. In addition to PA (positively charged ions from the acid) in the meat package, the proteins are not being repulsed, thus the appearance of the meat is pale [53]. Overall, the use of a phage cocktail, PA and MAP for controlling Salmonella in chicken meat as a combination did not affect the quality and characteristics of the meat.
5. Conclusions
In our study, the combined approach between a phage cocktail and PA provided us with a potential alternative to control Salmonella in artificially contaminated poultry meat. These methods did not change the characteristic of the meat, while all examined sensory preferences of the meat were still acceptable. The combination with MAP conditions can be consequently developed to extend the shelf-life of raw chicken meat and related products in large-scale production in the meat industry and to increase the safety of foods. In addition, the whole-genome sequence study of two phages will be in our future plan to ensure the safety of the phages in food production.
Author Contributions
Conceptualization, W.P. and K.V.; methodology, W.P. and K.V.; investigation, W.P.; resources, K.V.; data curation, W.P.; writing—original draft preparation, W.P.; writing—review and editing, K.V.; visualization, W.P.; supervision, K.V.; project administration, W.P.; funding acquisition, K.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Agricultural Research Development Agency (ARDA), Thailand to KV (No. CRP6305031030).
Institution Review Board Statement
The study was conducted according to the guidelines and approval of the Research Ethics Committee of Prince of Songkla University (ethical clearance number. 56/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors acknowledge the Department of Biotechnology, Faculty of Agro-Industry, Kasetsart University, Bangkok, and the Faculty of Agro-Industry, Prince of Songkla University, Hat Yai, Songkhla for providing facilities to conduct the research study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nair, D.V.T.; Johny, A.K. Salmonella in poultry meat production. In Food Safety in Poultry Meat Production; Venkitanarayanan, K., Thakur, S., Ricke, S.C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–24. [Google Scholar]
- European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC). The European Union one health 2021 zoonoses report. EFSA J. 2022, 20, 7666. [Google Scholar]
- The OzFoodNet Working Group. Monitoring the incidence and causes of disease potentially transmitted by food in Australia: Annual report of the OzFoodNet network, 2017. Commun. Dis. Intell. 2022, 46, 1–68. [Google Scholar]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/salmonella/backyardpoultry-06-22/index.html (accessed on 6 August 2023).
- EPSA Explains Zoonotic Diseases: Food-Borne Zoonotic Diseases. Available online: https://www.efsa.europa.eu/en/corporate/pub/factsheetfoodbornezoonoses2014#documents (accessed on 6 August 2023).
- European Commission. Commission regulation (EU) No 200/2010 implementing regulation (EC) No 2160/2003 of the European Parliament and of the Council as regards a Union target for the reduction of the prevalence of Salmonella serovars in adult breeding flocks of Gallus gallus. Off. J. Eur. Union 2010, L61, 1–9. [Google Scholar]
- Agirdemir, O.; Yurdakul, O.; Keyvan, E.; Sen, E. Effects of various chemical decontaminants on Salmonella Typhimurium survival in chicken carcasses. Food Sci. Technol. 2020, 41, 335–342. [Google Scholar] [CrossRef]
- Nkosi, D.V.; Bekker, J.L.; Hoffman, L.C. The use of organic acids (lactic and acetic) as a microbial decontaminant during the slaughter of meat animal species: A review. Foods 2021, 10, 2293. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.A.M.; Link, D.T.; Bertolini, A.B.; Tobias, F.L.; Mioni, M.D.S.R. A descriptive review of the use of organic acids and peracetic acid as a decontaminating strategy for meat. eFood 2023, 4, e104. [Google Scholar] [CrossRef]
- Yeh, Y.; De Moura, F.H.; Van Den Broek, K.; De Mello, A.S. Effect of ultraviolet light, organic acids, and bacteriophage on Salmonella populations in ground beef. Meat Sci. 2018, 139, 44–48. [Google Scholar] [CrossRef]
- Albert, T.; Braun, P.G.; Saffaf, J.; Wiacek, C. Physical methods for the decontamination of meat surfaces. Curr. Clin. Microbiol. Rep. 2021, 8, 9–20. [Google Scholar] [CrossRef]
- Hawkins, J.L.; Vimini, B.; Schwarz, J.G.; Nichols, P.; Parveen, S. Application of antimicrobial agents via commercial spray cabinet to inactivate Salmonella on skinless chicken meat. J. Food Prot. 2016, 79, 569–573. [Google Scholar] [CrossRef]
- Ma, Q.; Davidson, P.M.; Zhong, Q. Properties and potential food applications of lauric arginate as a cationic antimicrobial. Int. J. Food Microbiol. 2020, 315, 108417. [Google Scholar] [CrossRef]
- Don, A.J.P.; Parveen, S.; Schwarz, J.; Hamill, L.; Nindo, C.; Hall, P.; Vimini, B. Efficacy and quality attributes of antimicrobial agent application via a commercial electrostatic spray cabinet to inactivate Salmonella on chicken thigh meat inactivate Salmonella on chicken meat. J. Food Prot. 2021, 84, 2221–2228. [Google Scholar]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Woolston, J.; Sulakvelidze, A. Phage biocontrol applications in food production and processing. Curr. Issues Mol. Biol. 2021, 40, 267–302. [Google Scholar] [CrossRef] [PubMed]
- Pelyuntha, W.; Vongkamjan, K. Combined effects of Salmonella phage cocktail and organic acid for controlling Salmonella Enteritidis in chicken meat. Food Control. 2022, 133, 108653. [Google Scholar] [CrossRef]
- Pelyuntha, W.; Yafa, A.; Ngasaman, R.; Yingkajorn, M.; Chukiatsiri, K.; Champoochana, N.; Vongkamjan, K. Oral administration of a phage cocktail to reduce Salmonella colonization in broiler gastrointestinal tract—A pilot study. Animals 2022, 12, 3087. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, L.D. Designing phage therapeutics. Curr. Pharm. Biotechnol. 2010, 11, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Pelyuntha, W.; Ngasaman, R.; Yingkajorn, M.; Chukiatsiri, K.; Benjakul, S.; Vongkamjan, K. Isolation and characterization of potential Salmonella phages targeting multidrug-resistant and major serovars of Salmonella derived from broiler production chain in Thailand. Front. Microbiol. 2021, 12, 662461. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. CFR-Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=184 (accessed on 6 August 2023).
- Gonzalez-Garcia, R.A.; McCubbin, T.; Navone, L.; Stowers, C.; Nielsen, L.K.; Marcellin, E. Microbial propionic acid production. Fermentation 2017, 3, 21. [Google Scholar] [CrossRef]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 2018, 5, 216. [Google Scholar] [CrossRef]
- Caleb, O.J.; Mahajan, P.V.; Al-Said, F.A.J.; Opara, U.L. Modified atmosphere packaging technology of fresh and fresh-cut produce and the microbial consequences—A review. Food Bioprocess Technol. 2013, 6, 303–329. [Google Scholar] [CrossRef]
- Djordjević, J.; Bošković, M.; Starčević, M.; Ivanović, J.; Karabasil, N.; Dimitrijević, M.; Lazić, I.B.; Baltić, M.Ž. Survival of Salmonella spp. in minced meat packaged under vacuum and modified atmosphere. Braz. J. Microbiol. 2018, 49, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Sripaurya, B.; Ngasaman, R.; Benjakul, S.; Vongkamjan, K. Virulence genes and antibiotic resistance of Salmonella recovered from a wet market in Thailand. J. Food Saf. 2019, 39, e12601. [Google Scholar] [CrossRef]
- Pelyuntha, W.; Sanguankiat, A.; Kovitvadhi, A.; Vongkamjan, K. Broad lytic spectrum of novel Salmonella phages on ciprofloxacin-resistant Salmonella contaminated in the broiler production chain. Vet. World 2022, 15, 2039. [Google Scholar] [CrossRef] [PubMed]
- Ateba, C.N.; Akindolire, M.A. Isolation and characterisation of bacteriophages with lytic activity against virulent Escherichia coli O157: H7: Potential bio-control agents. Preprints 2019, 2019010132. [Google Scholar] [CrossRef]
- Pelyuntha, W.; Chaiyasut, C.; Kantachote, D.; Sirilun, S. Cell-free supernatants from cultures of lactic acid bacteria isolated from fermented grape as biocontrol against Salmonella Typhi and Salmonella Typhimurium virulence via autoinducer-2 and biofilm interference. PeerJ 2019, 7, e7555. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.W.R.; Pak, W.M.; Kang, J.E.; Park, H.M.; Kim, B.R.; Anh, D.H. Effects of chicken breast meat on quality properties of Mackerel (Scomber japonicus) sausage. Korean J. Food Sci. Anim. Resour. 2014, 34, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Petsong, K.; Benjakul, S.; Chaturongakul, S.; Switt, A.I.M.; Vongkamjan, K. Lysis profiles of Salmonella phages on Salmonella isolates from various sources and efficiency of a phage cocktail against S. enteritidis and S. typhimurium. Microorganisms 2019, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Montso, P.K.; Mlambo, V.; Ateba, C.N. Characterization of lytic bacteriophages infecting multidrug-resistant shiga toxigenic atypical Escherichia coli O177 strains isolated from cattle feces. Front. Public. Health 2019, 7, 355. [Google Scholar] [CrossRef]
- Islam, M.S.; Zhou, Y.; Liang, L.; Nime, I.; Liu, K.; Yan, T.; Wang, X.; Li, J. Application of a phage cocktail for control of Salmonella in foods and reducing biofilms. Viruses 2019, 11, 841. [Google Scholar] [CrossRef]
- Sritha, K.S.; Bhat, S.G. In vitro efficiency evaluation of phage cocktail for biocontrol of Salmonella spp. in food products. Arch. Microbiol. 2021, 203, 5445–5452. [Google Scholar]
- Aguilera, M.; Martínez, S.; Tello, M.; Gallardo, M.J.; García, V. Use of cocktail of bacteriophage for Salmonella typhimurium control in chicken meat. Foods 2022, 11, 1164. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Malik, H.; Dubal, Z.B.; Jaiswal, R.K.; Kumar, S.; Kumar, B.; Agarwal, R.K. Isolation and characterization of Salmonella phages and phage cocktail mediated biocontrol of Salmonella enterica serovar Typhimurium in chicken meat. LWT 2022, 155, 112957. [Google Scholar]
- Tayyarcan, E.K.; Evran, S.; Akin, P.A.; Soykut, E.A.; Boyaci, I.H. The use of bacteriophage cocktails to reduce Salmonella Enteritidis in hummus. LWT 2022, 154, 112848. [Google Scholar] [CrossRef]
- Wójcicki, M.; Świder, O.; Gientka, I.; Błażejak, S.; Średnicka, P.; Shymialevich, D.; Cieślak, H.; Wardaszka, A.; Emanowicz, P.; Sokołowska, B.; et al. Effectiveness of a phage cocktail as a potential biocontrol agent against saprophytic bacteria in ready-to-eat plant-based food. Viruses 2023, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.L.D.; Vieira, B.S.; Carvalho, F.T.; Carvalho, R.C.T.; Figueiredo, E.E.D.S. Salmonella behavior in meat during cool storage: A systematic review and meta-analysis. Animals 2022, 12, 2902. [Google Scholar] [CrossRef] [PubMed]
- Spricigo, D.A.; Bardina, C.; Cortés, P.; Llagostera, M. Use of a bacteriophage cocktail to control Salmonella in food and the food industry. Int. J. Food Microbiol. 2013, 165, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Abhisingha, M.; Dumnil, J.; Pitaksutheepong, C. Efficiency of phage cocktail to reduce Salmonella Typhimurium on chicken meat during low temperature storage. LWT 2020, 129, 109580. [Google Scholar] [CrossRef]
- Cosansu, S.; Ayhan, K. Effects of lactic and acetic acid on survival of Salmonella enteritidis during refrigerated and frozen storage of chicken meats. Food Bioprocess Technol. 2012, 5, 372–377. [Google Scholar] [CrossRef]
- Gonzalez-Fandos, E.; Herrera, B. Efficacy of propionic acid against Listeria monocytogenes attached to poultry skin during refrigerated storage. Food Control 2013, 34, 601–606. [Google Scholar] [CrossRef]
- Sukumaran, A.T.; Nannapaneni, R.; Kiess, A.; Sharma, C.S. Reduction of Salmonella on chicken meat and chicken skin by combined or sequential application of lytic bacteriophage with chemical antimicrobials. Int. J. Food Microbiol. 2015, 207, 8–15. [Google Scholar] [CrossRef]
- Michaelsen, A.R.; Sebranek, J.G.; Dickson, J.S. Effects of microbial inhibitors and modified atmosphere packaging on growth of Listeria monocytogenes and Salmonella enterica Typhimurium and on quality attributes of injected pork chops and sliced cured ham. J. Food Prot. 2006, 69, 2671–2680. [Google Scholar] [CrossRef] [PubMed]
- Kudra, L.L.; Sebranek, J.G.; Dickson, J.S.; Mendonca, A.F.; Zhang, Q.; Jackson-Davis, A.; Prusa, K.J. Control of Salmonella enterica Typhimurium in chicken breast meat by irradiation combined with modified atmosphere packaging. J. Food Prot. 2011, 74, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Park, S.H.; Choi, S.R.; Kim, J.S.; Chun, H.H. The combined effects of ultraviolet-C irradiation and modified atmosphere packaging for inactivating Salmonella enterica serovar Typhimurium and extending the shelf life of cherry tomatoes during cold storage. Food Packag. Shelf Life 2015, 3, 19–30. [Google Scholar] [CrossRef]
- Sukumaran, A.T.; Nannapaneni, R.; Kiess, A.; Sharma, C.S. Reduction of Salmonella on chicken breast fillets stored under aerobic or modified atmosphere packaging by the application of lytic bacteriophage preparation SalmoFreshTM. Poult. Sci. 2016, 95, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lin, H.; Ji, X.; Yan, G.; Lei, L.; Han, W.; Gu, J.; Huang, J. Therapeutic applications of lytic phages in human medicine. Microb. Pathog. 2020, 142, 104048. [Google Scholar] [CrossRef] [PubMed]
- Pelyuntha, W.; Chaiyasut, C.; Kantachote, D.; Sirilun, S. Organic acids and 2, 4-Di-tert-butylphenol: Major compounds of Weissella confusa WM36 cell-free supernatant against growth, survival and virulence of Salmonella Typhi. PeerJ 2020, 8, e8410. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Martin, A.; Sachsenröder, J.; Bandick, N. Effects of modified atmosphere packaging on an extended-spectrum beta-lactamase–producing Escherichia coli, the microflora, and shelf life of chicken meat. Poult. Sci. 2020, 99, 7004–7014. [Google Scholar] [CrossRef]
- Carvalho, R.; Shimokomaki, M.; Estévez, M. Poultry meat color and oxidation. In Poultry Quality Evaluation: Quality Attributes and Consumer Values; Petracci, M., Berri, C., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 133–157. [Google Scholar]
- Hinkle, J.B. Acid Marination for Tenderness Enhancement of Beef Bottom Round. Master’s Thesis, University of Nebraska, Lincoln, NE, USA, 2010. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).