Antimicrobial Activity of Essential Oils in Vapor Phase In Vitro and Its Application in Combination with Lactic Acid to Improve Chicken Breast Shelf Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oils and Other Chemicals
2.2. Bacterial Strains and Culture Conditions
2.3. Antimicrobial Activity of Essential Oils in Vapor Phase In Vitro
2.3.1. Screening of Essential Oils’ Antimicrobial Activity
2.3.2. Determination of the Minimal Inhibitory Concentration against P. aeruginosa and E. coli
2.3.3. Determination of the Fractional Inhibitory Concentration Index of Essential Oil Binary Mixtures against P. aeruginosa and E. coli
2.3.4. Determination of the Effect of a Ternary Mixture against P. aeruginosa and E. coli
2.4. Chemical Analysis of Essential Oils in Vapor Phase
2.5. Antimicrobial Activity of Essential Oils in Vapor Phase on Chicken Breast
2.5.1. Chicken Breast Preparation
2.5.2. Microbiological Analysis
2.5.3. Physicochemical Analysis
2.5.4. Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Assays of Antimicrobial Activity of Essential Oils In Vitro
3.1.1. Screening of Essential Oils’ Antimicrobial Activity
3.1.2. Minimal and Fractional Inhibitory Concentrations of Essential Oils in Vapor Phase
3.2. Chemical Composition of Essential Oils in Vapor Phase
3.3. Application of Essential Oils on Chicken Breast
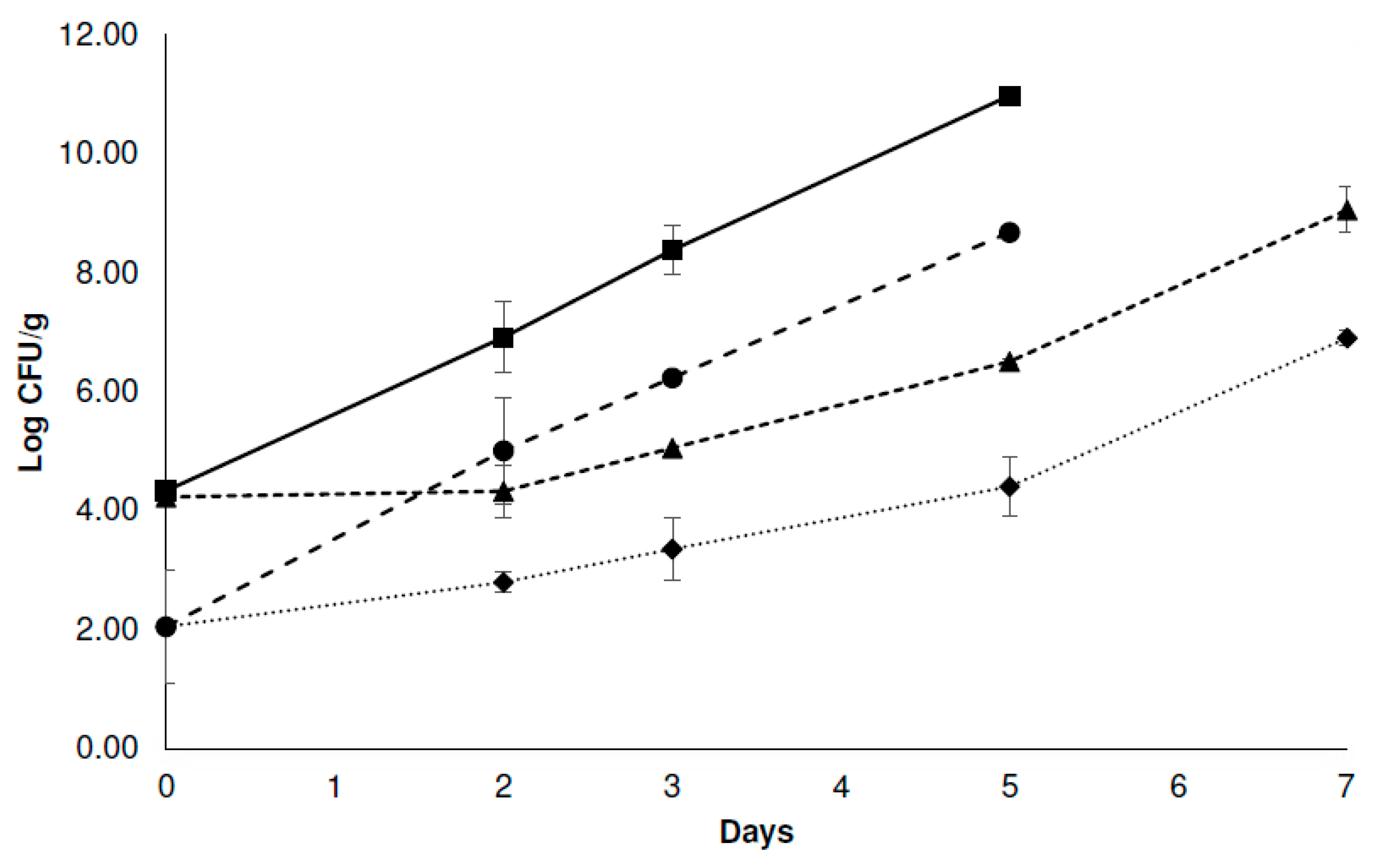
3.3.1. Microbiological Analysis
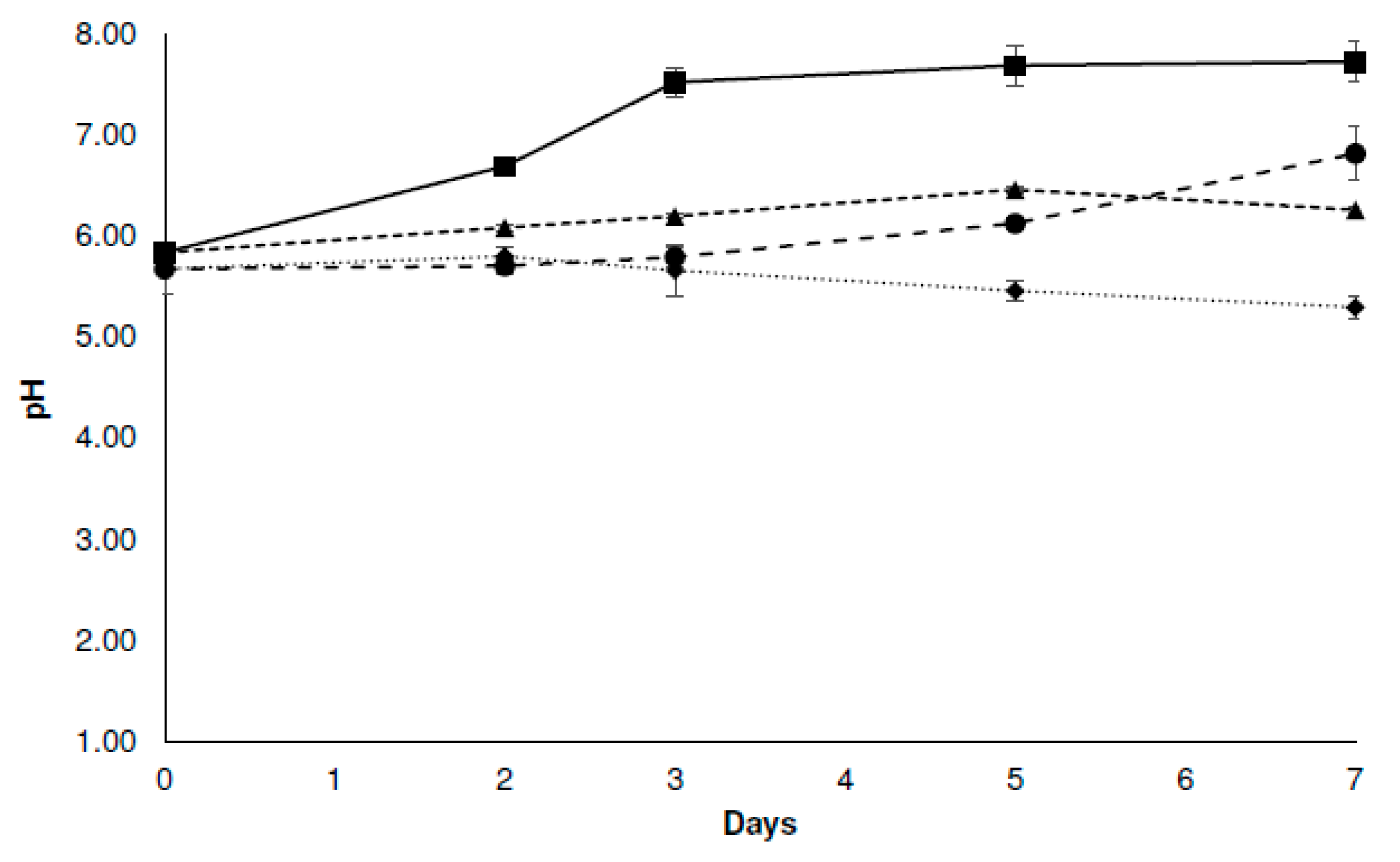
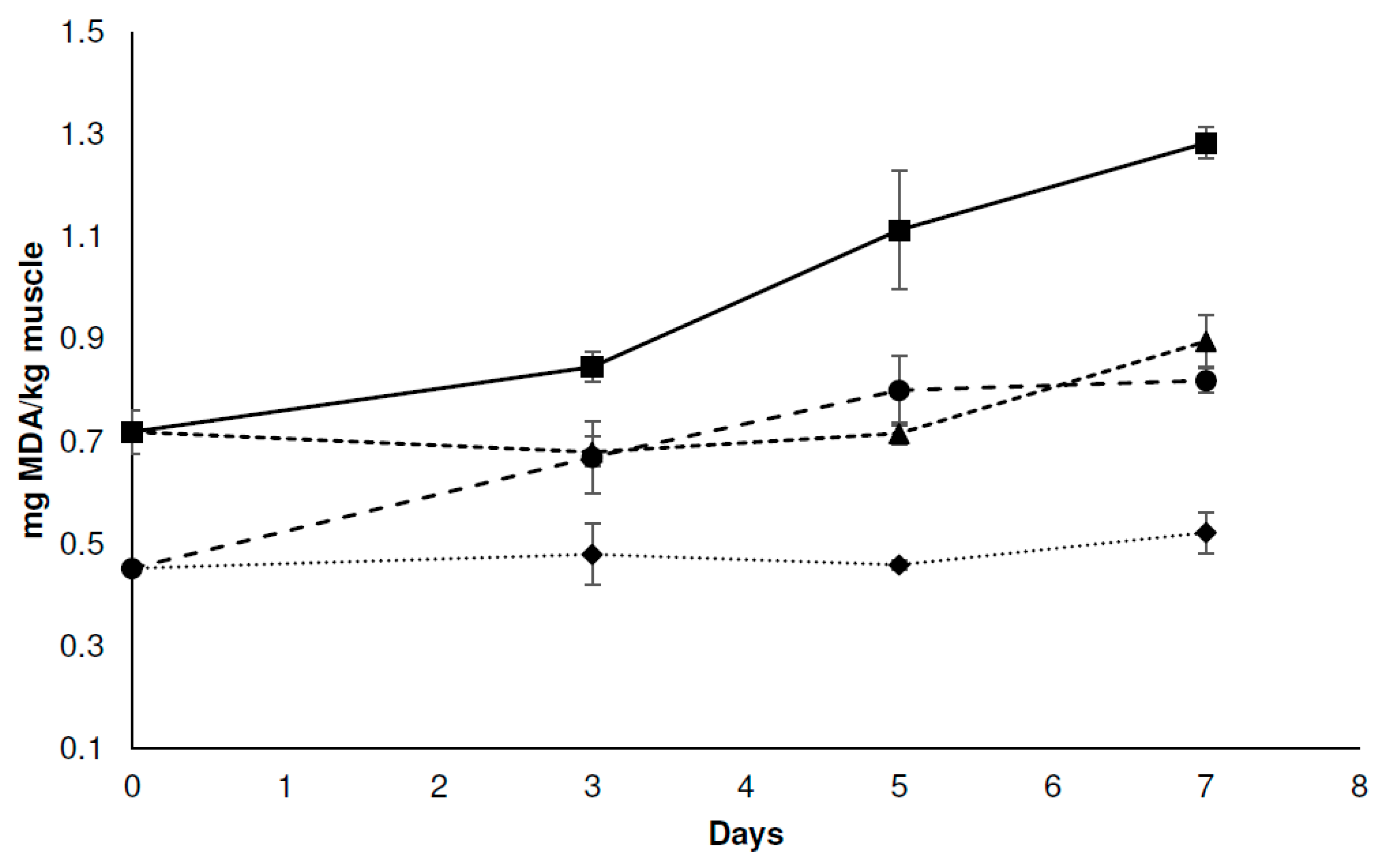
3.3.2. Physicochemical Analysis
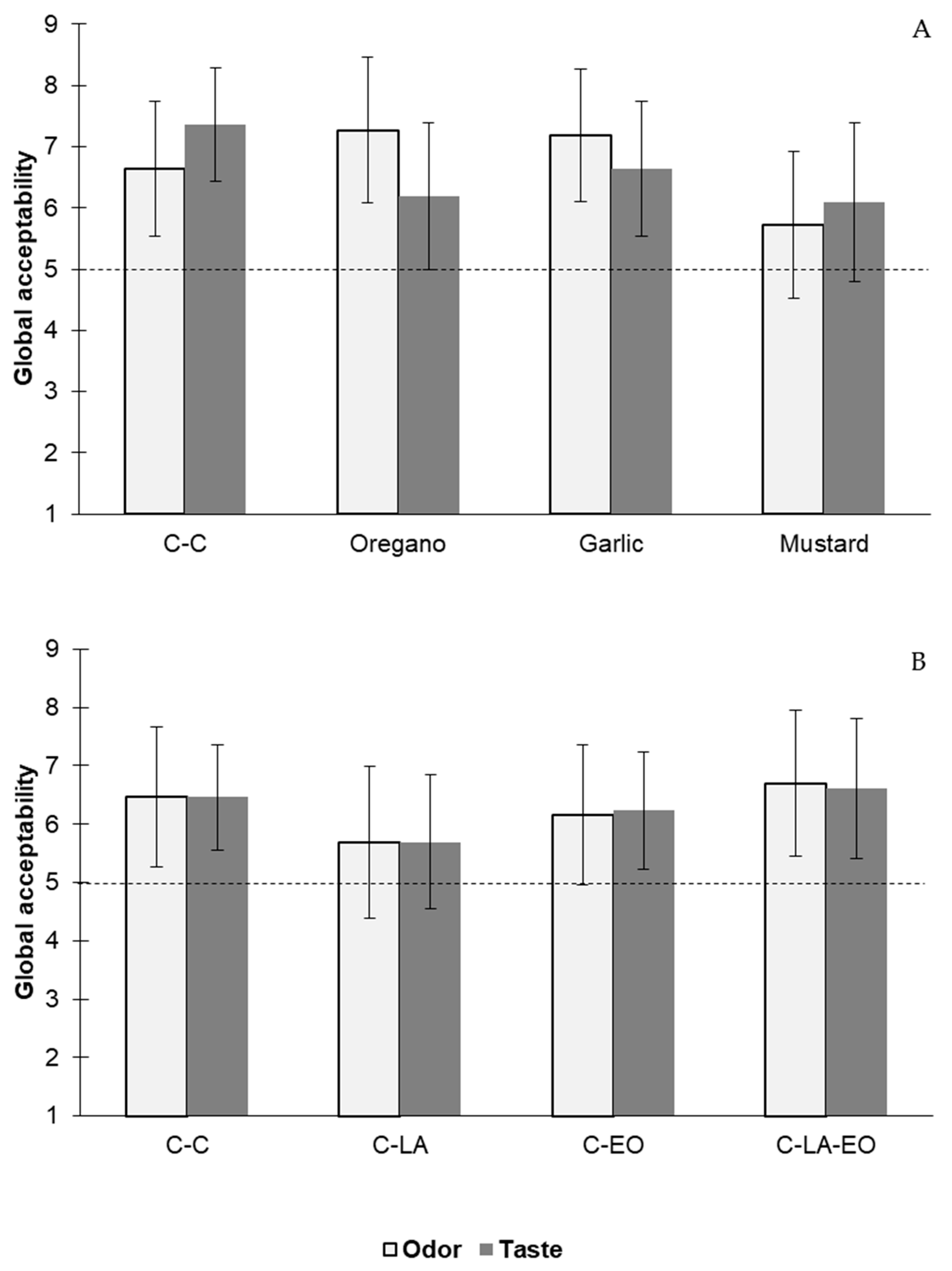
3.3.3. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Food Outlook: Biannual Report on Global Food Markets; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Gleeson, E.; Franco, D.; Cullere, M.; Lorenzo, J.M. Proximate composition, amino acid profile, and oxidative stability of slow-growing indigenous chickens compared with commercial broiler chickens. Foods 2020, 9, 546. [Google Scholar] [CrossRef] [PubMed]
- Bazargani-Gilani, B.; Aliakbarlu, J.; Tajik, H. Effect of pomegranate juice dipping and chitosan coating enriched with Zataria multiflora Boiss essential oil on the shelf-life of chicken meat during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2015, 29, 280–287. [Google Scholar] [CrossRef]
- Albergamo, A.; Vadalà, R.; Metro, D.; Nava, V.; Bartolomeo, G.; Rando, R.; Macri, A.; Messina, L.; Gualtieri, R.; Colombo, N.; et al. Physicochemical, nutritional, microbiological, and sensory qualities of chicken burgers reformulated with Mediterranean plant ingredients and health-promoting compounds. Foods 2021, 10, 2129. [Google Scholar] [CrossRef] [PubMed]
- Radha Krishnan, K.; Babuskin, S.; Azhagu Saravana Babu, P.; Sasikala, M.; Sabina, K.; Archana, G.; Sirvarajan, M.; Sukumar, M. Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. Int. J. Food Microbiol. 2014, 171, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Bolton, D.J.; Meredith, H.; Walsh, D.; McDowell, D.A. The effect of chemical treatments in laboratory and broiler plant studies on the microbial status and shelf-life of poultry. Food Control 2014, 36, 230–237. [Google Scholar] [CrossRef]
- Burfoot, D.; Mulvey, E. Reducing microbial counts on chicken and turkey carcasses using lactic acid. Food Control 2011, 22, 1729–1735. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Food Safety and Inspection Service (USDA-FSIS). Safe and Suitable Ingredients Used in the Production of Meat, Poultry, and Egg Products. FSIS Directive 7120.1, Revision 39. 2019. Available online: https://www.fsis.usda.gov/wps/wcm/connect/bab10e09-aefa-483b-8be8–809a1f051d4c/7120.1.pdf?MOD=AJPERES.2017 (accessed on 19 September 2023).
- Byun, K.H.; Na, K.W.; Ashrafudoulla, M.; Choi, M.W.; Han, S.H.; Kang, I.; Park, S.H.; Ha, S.D. Combination treatment of peroxyacetic acid or lactic acid with UV-C to control Salmonella Enteritidis biofilms on food contact surface and chicken skin. Food Microbiol. 2022, 102, 103906. [Google Scholar] [CrossRef] [PubMed]
- Heir, E.; Solberg, L.E.; Jensen, M.R.; Skaret, J.; Grovlen, M.S.; Holk, A.L. Improved microbial and sensory quality of chicken meat by treatment with lactic acid, organic acid salts and modified atmosphere. Int. J. Food Microbiol. 2022, 362, 109498. [Google Scholar] [CrossRef]
- Ramezani, F.; Nafaji, M.A.; Rahnama, M.; Haddadi, T. Separate and combined effects of lactic acid, chitosan and modified atmosphere packaging on the shelf life of quail carcass under chilled conditions. Int. J. Food Microbiol. 2019, 289, 215–222. [Google Scholar] [CrossRef]
- Ben Amor, N.; Nava, V.; Albergamo, A.; Potortì, A.G.; Lo Turco, V.; Ben Mansour, H.; Di Bella, G. Tunisian essential oils as potential food antimicrobials and antioxidants and screening of their element profile. Eur. Food Res. Technol. 2021, 247, 1221–1234. [Google Scholar] [CrossRef]
- Mirsharifi, S.M.; Sami, M.; Jazaeri, M.; Rezaei, A. Production, characterization, and antimicrobial activity of almond gum/polyvinyl alcohol/chitosan composite films containing thyme essential oil nanoemulsion for extending the shelf-life of chicken breast fillets. Int. J. Biol. Macromol. 2023, 227, 405–415. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Van de Vel, E.; Sampers, I.; Raes, K. A review on influencing factors on the minimum inhibitory concentration of essential oils. Crit. Rev. Food Sci. Nutr. 2019, 59, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Synergistic antimicrobial activities of essential oil vapours against Penicillium corylophilum on a laboratory medium and beef jerky. Int. J. Food Microbiol. 2019, 291, 104–110. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Bárcena-Massberg, Z.; Ramírez-Corona, N.; López-Malo, A.; Palou, E. Fungal inactivation on Mexican corn tortillas by means of thyme essential oil in vapor-phase. Curr. Res. Food Sci. 2022, 5, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Kim, Y.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Antimicrobial activities of gaseous essential oils against Listeria monocytogenes on a laboratory medium and radish sprouts. Int. J. Food Microbiol. 2018, 265, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo Leal, A.C.; Palou, E.; López-Malo, A. Evaluation of the efficiency of allspice, thyme and rosemary essential oils on two foodborne pathogens in-vitro and on alfalfa seeds, and their effect on sensory characteristics of the sprouts. Int. J. Food Microbiol. 2019, 295, 19–24. [Google Scholar] [CrossRef]
- Delcarlo, S.B.; Merly, M.; Gliemmo, M.F.; Vallejo, M.; Schelegueda, L.I.; Campos, C.A. Essential oil in vapor phase in combination with Enterococcus mundtii STw38 to improve refrigerated hake fillets shelf-life. Food Control 2022, 138, 109013. [Google Scholar]
- Friedly, E.C.; Crandall, P.G.; Ricke, S.; OBryan, C.A.; Martin, E.M.; Boyd, L.M. Identification of Listeria innocua surrogates for Listeria monocytogenes in hamburger patties. J. Food Sci. 2008, 73, 174–178. [Google Scholar] [CrossRef]
- Um, M.M.; Brugere, H.; Kérourédan, M.; Oswald, E.; Bibbal, D. Antimicrobial resistance profiles of Enterohemorrhagic and Enteropathogenic Escherichia coli of serotypes O157:H7, O26:H11, O103:H2, O111:H8, O145:H28 compared to Escherichia coli isolated from the same adult cattle. Microb. Drug Resist. 2018, 24, 852–859. [Google Scholar] [CrossRef]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Berenbaum, M.C. A method for testing for synergy with any number of agents. J. Infect. Dis. 1978, 137, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dekker, M.; Heising, J.; Zhao, L.; Fogliano, V. Food matrix design can influence the antimicrobial activity in the food systems: A narrative review. Crit. Rev. Food Sci. 2023, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Gliemmo, M.F.; Montagnani, M.A.; Schelegueda, L.I.; González, M.M.; Campos, C.A. Effect of xantham gum, steviosides, clove, and cinnamon essential oils on the sensory and microbiological quality of a low sugar tomato jam. Food Sci. Technol. Int. 2015, 22, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Vyncke, W. Direct determination of the thiobarbituric acid value in trichloracetic acid extracts of fish as a measure of oxidative rancidity. Eur. J. Lipid Sci. Technol. 1970, 72, 1084–1087. [Google Scholar] [CrossRef]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques; CRC Press Inc.: Boca Raton, FL, USA, 1999. [Google Scholar]
- Reyes-Jurado, F.; Cervantes-Rincón, T.; Bach, H.; López-Malo, A.; Palou, E. Antimicrobial activity of Mexican oregano (Lippia berlandieri), thyme (Thymus vulgaris), and mustard (Brassica nigra) essential oils gaseous phase. Ind. Crops Prod. 2019, 131, 90–95. [Google Scholar] [CrossRef]
- Aguilar-González, A.E.; Palou, E.; López-Malo, A. Response of Aspergillus niger inoculated on tomatoes exposed to vapor phase mustard essential oil for short or long periods and sensory evaluation of treated tomatoes. J. Food Qual. 2017, 2017, 4067856. [Google Scholar] [CrossRef]
- Aguilar-González, A.E.; Palou, E.; López-Malo, A. Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innov. Food Sci. Emerg. Technol. 2015, 32, 181–185. [Google Scholar] [CrossRef]
- Benkeblia, N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). LWT Food Sci. Technol. 2004, 37, 263–268. [Google Scholar] [CrossRef]
- Chao, C.S.; Young, D.G.; Oberg, C.J. Screening for inhibitory activity of essential oils on selected bacteria, fungi and viruses. J. Essent. Oil Res. 2000, 12, 639–649. [Google Scholar] [CrossRef]
- Dobre, A.; Gagiu, V.; Petru, N. Antimicrobial activity of essential oils against food-borne bacteria evaluated by two preliminary methods. Rom. Biotechnol. Lett. 2011, 16, 6. [Google Scholar]
- Lambert, R.J.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Seydim, A.C.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Du, W.-X.; Olsen, C.W.; Avena-Bustillos, R.J.; McHugh, T.H.; Levin, C.E.; Mandrell, R.; Friedman, M. Antibacterial effects of allspice, garlic, and oregano essential oils in tomato films determined by overlay and vapor-phase methods. J. Food Sci. 2009, 74, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Chen, B.; McClements, D.J. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kathuria, A.; Lee, Y.S. Effect of hydrophilic and hydrophobic cyclodextrins on the release of encapsulated allyl isothiocyanate (AITC) and their potential application for plastic film extrusion. J. Appl. Polym. Sci. 2019, 136, 48137. [Google Scholar] [CrossRef]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent Oil. Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Código Alimentario Argentino. Capítulo VI. 2019. Available online: www.argentina.gob.ar/sites/default/files/capitulo_vi_carneosactualiz_2019-09.pdf (accessed on 19 September 2023).
- Swatland, H.J. Structure and Development of Meat Animals and Poultry; Technomic Publishing Company Inc.: Lancaster, PA, USA, 1994. [Google Scholar]
- Jia, B.; Yoon, S.C.; Zhuang, H.; Wang, W.; Li, C. Prediction of pH of fresh chicken breast fillets by VNIR hyperspectral imaging. J. Food Eng. 2017, 208, 57–65. [Google Scholar] [CrossRef]
- Sujiwo, J.; Kim, D.; Jang, A. Relation among quality traits of chicken breast meat during cold storage: Correlations between freshness traits and torrymeter values. Poult. Sci. 2018, 97, 2887–2894. [Google Scholar] [CrossRef]
- Mantel, M.C.; Masson, F.; Talon, R. Bacterial role in flavour development. Meat Sci. 1998, 49, S111–S123. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Mai, T.L. Traditional Preservatives—Organic acdis. Encycl. Food Microbiol. 1999, 3, 119–130. [Google Scholar]
- Fratianni, F.; De Martino, L.; Melone, A.; De Feo, V.; Coppola, R.; Nazzaro, F. Preservation of chicken breast meat treated with thyme and balm essential oils. J. Food Sci. 2010, 75, M528–M535. [Google Scholar] [CrossRef]
- Liu, D.C.; Tsau, R.T.; Lin, Y.C.; Jan, S.S.; Tan, F.J. Effect of various levels of rosemary or Chinese mahogany on the quality of fresh chicken sausage during refrigerated storage. Food Chem. 2009, 117, 106–113. [Google Scholar] [CrossRef]
| Binary Mixture | EO A Concentration | EO B Concentration |
|---|---|---|
| 1 | MIC * | - |
| 2 | - | MIC |
| 3 | 1/2 MIC | 1/2 MIC |
| 4 | 1/2 MIC | 1/4 MIC |
| 5 | 1/4 MIC | 1/2 MIC |
| 6 | 1/4 MIC | 1/4 MIC |
| 7 | 1/4 MIC | 1/8 MIC |
| 8 | 1/8 MIC | 1/4 MIC |
| Microorganisms | Essential Oils | |||||
|---|---|---|---|---|---|---|
| Lemongrass | Ginger | Garlic | Mustard | Oregano | Thyme | |
| L. innocua | 15.0 ± 1.0 | _ | >52.0 | >52.0 | 40.0 ± 2.0 | 30.0 ± 1.0 |
| P. aeruginosa | 21.5 ± 0.5 | _ | >52.0 | >52.0 | _ | _ |
| P. fluorescens | _ | _ | _ | >52.0 | _ | _ |
| L. plantarum | 11.5 ± 0.5 | 21.5 ± 0.5 | >52.0 | >52.0 | _ | _ |
| E. coli ATCC 35218 | _ | _ | _ | >52.0 | >52.0 | 23.0 ± 1.0 |
| E. coli ATCC 25922 | _ | _ | _ | >52.0 | 40.0 ± 1.0 | 31.0 ± 1.0 |
| EO Concentration (MIC Fraction) | FICi | ||
|---|---|---|---|
| Mustard | Garlic | P. aeruginosa | |
| 1 | 0 | No growth | |
| 0 | 1 | No growth | |
| 1/2 | 1/2 | No growth | 1.0 |
| 1/2 | 1/4 | No growth | 0.75 |
| 1/4 | 1/2 | No growth | 0.75 |
| 1/4 | 1/4 | No growth | 0.50 |
| 1/4 | 1/8 | No growth | 0.37 |
| 1/8 | 1/4 | Growth | |
| EO Concentration (MIC Fraction) | FICi | ||
|---|---|---|---|
| Mustard | Oregano | E. coli | |
| 1 | 0 | No growth | |
| 0 | 1 | No growth | |
| 1/2 | 1/2 | No growth | 1.0 |
| 1/2 | 1/4 | Growth | |
| 1/4 | 1/2 | Growth | |
| Compound | % |
|---|---|
| diallyl sulfide | 2.9 |
| allyl isothiocyanate | 10.2 |
| disulfide, methyl-2-propenyl | 0.6 |
| α-pinene | 4.0 |
| camphene | 0.5 |
| myrcene + β-pinene | 6.5 |
| α-phellandrene | 0.5 |
| α-terpinene | 1.9 |
| p-cymene | 30.1 |
| limonene | 4.8 |
| 1,8-cineole | 0.2 |
| γ-terpinene | 9.5 |
| diallyl disulfide | 17.1 |
| linalool | 0.8 |
| trisulfide, methyl-2-propenyl | 0.4 |
| carvacrol | 5.1 |
| trisulfide, di-2-propenyl | 1.5 |
| caryophyllene oxide | 1.2 |
| total | 97.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera de la Cruz, J.F.; Schelegueda, L.I.; Delcarlo, S.B.; Gliemmo, M.F.; Campos, C.A. Antimicrobial Activity of Essential Oils in Vapor Phase In Vitro and Its Application in Combination with Lactic Acid to Improve Chicken Breast Shelf Life. Foods 2023, 12, 4127. https://doi.org/10.3390/foods12224127
Rivera de la Cruz JF, Schelegueda LI, Delcarlo SB, Gliemmo MF, Campos CA. Antimicrobial Activity of Essential Oils in Vapor Phase In Vitro and Its Application in Combination with Lactic Acid to Improve Chicken Breast Shelf Life. Foods. 2023; 12(22):4127. https://doi.org/10.3390/foods12224127
Chicago/Turabian StyleRivera de la Cruz, Jovany Fortino, Laura Inés Schelegueda, Sofía Belén Delcarlo, María Fernanda Gliemmo, and Carmen Adriana Campos. 2023. "Antimicrobial Activity of Essential Oils in Vapor Phase In Vitro and Its Application in Combination with Lactic Acid to Improve Chicken Breast Shelf Life" Foods 12, no. 22: 4127. https://doi.org/10.3390/foods12224127
APA StyleRivera de la Cruz, J. F., Schelegueda, L. I., Delcarlo, S. B., Gliemmo, M. F., & Campos, C. A. (2023). Antimicrobial Activity of Essential Oils in Vapor Phase In Vitro and Its Application in Combination with Lactic Acid to Improve Chicken Breast Shelf Life. Foods, 12(22), 4127. https://doi.org/10.3390/foods12224127





