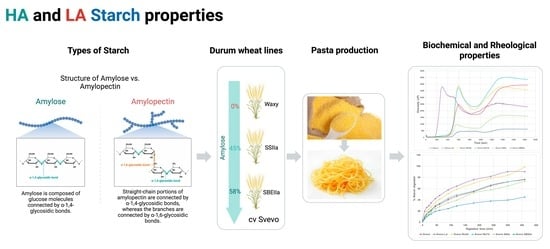
Impact of Variation in Amylose Content on Durum Wheat cv. Svevo Technological and Starch Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Field Trials
2.2. Semolina Analyses
2.3. Pasta Analyses
2.4. Starch Preparation and Analyses
2.5. Statistical Analysis
3. Results and Discussion
3.1. Impact of Amylose Variation on Semolina and Dough Properties
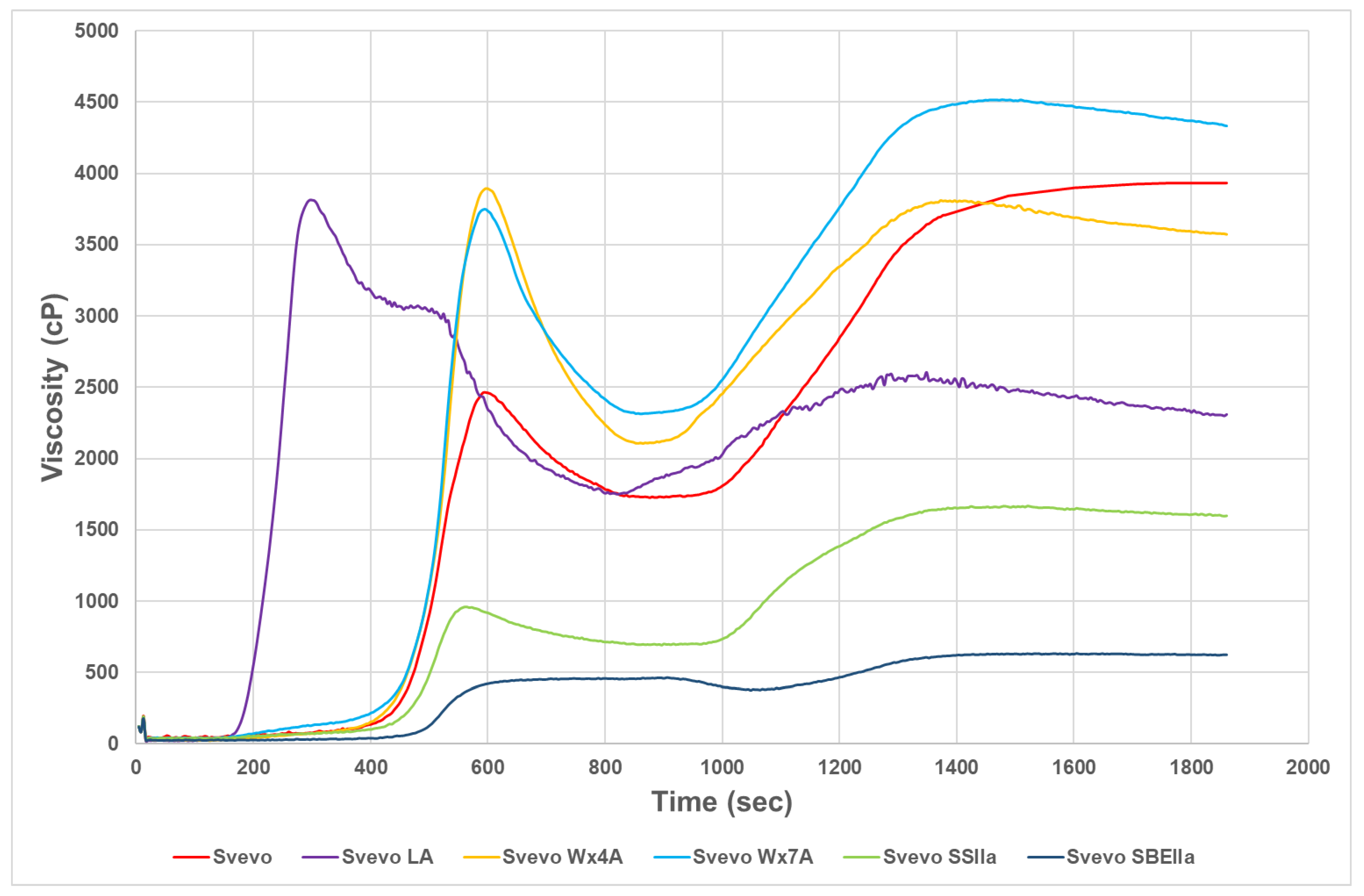
3.2. Impact of Amylose Variation on Pasta Technological Properties and Starch Pasting Viscosity
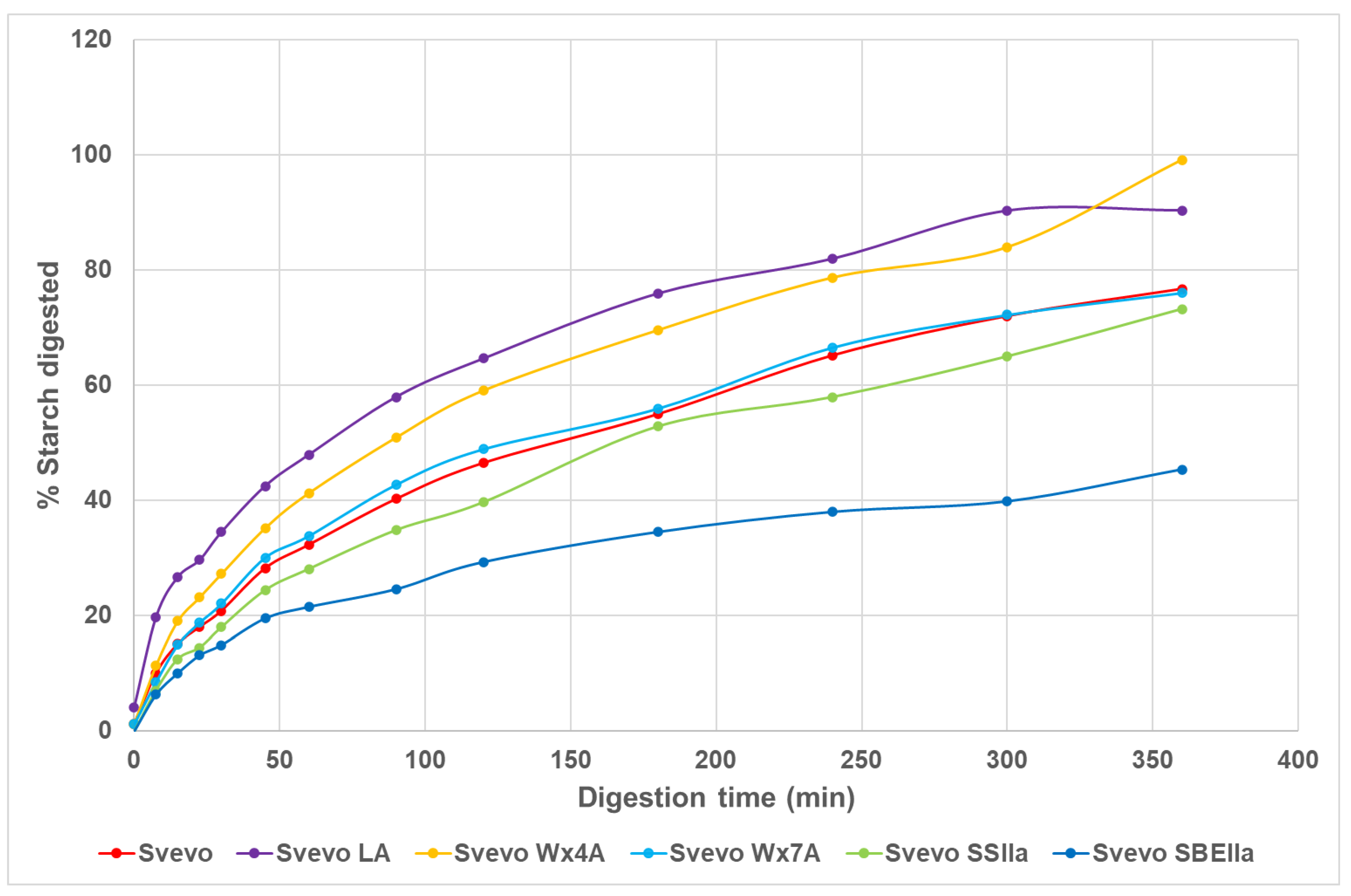
3.3. Impact of Amylose Variation on Pasta In Vitro Starch Digestion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its metabolism, evolution, and biotechnological modification in plants. Ann. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef] [PubMed]
- Botticella, E.; Savatin, D.V.; Sestili, F. The triple jags of dietary fibers in cereals: How biotechnology is longing for high fiber grains. Front. Plant Sci. 2021, 12, 745579. [Google Scholar] [CrossRef] [PubMed]
- Hazard, B.; Zhang, X.; Colasuonno, P.; Uaauy, C.; Beckles, D.M.; Dubcovsky, J. Induced mutations in the starch branching enzyme ii (sbeii) genes increase amylose and resistant starch content in durum wheat. Crop Sci. 2012, 52, 1754–1766. [Google Scholar] [CrossRef] [PubMed]
- Slade, A.J.; McGuire, C.; Loeler, D.; Mullenberg, J.; Skinner, W.; Fazio, G.; Holm, A.; Brandt, K.M.; Steine, M.N.; Goodstal, J.F.; et al. Development of high amylose wheat through TILLING. BMC Plant Biol. 2012, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Botticella, E.; Sestili, F.; Sparla, F.; Moscatello, S.; Marri, L.; Cuesta-Seijo, J.A.; Falini, G.; Battistelli, A.; Trost, P.; Lafiandra, D. Combining mutations at genes encoding key enzymes involved in starch synthesis affects the amylose content, carbohydrate allocation and hardness in the wheat grain. Plant Biotechnol. J. 2018, 16, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Chia, T.; Chirico, M.; King, R.; Ramirez-Gonzales, R.; Saccomanno, B.; Seung, D.; Simmonds, J.; Trick, M.; Uauy, C.; Verhoeven, T.; et al. A carbohydrate-binding protein, B-GRANULE CONTENT 1, influences starch granule size distribution in a dose-dependent manner in polyploid wheat. J. Exp. Bot. 2020, 71, 105–115. [Google Scholar] [CrossRef]
- Esch, L.; Ngai, Q.Y.; Barclay, J.E.; McNelly, R.; Hayta, S.; Smedley, M.A.; Smith, A.M.; Seung, D. Increasing amyloplast size in wheat endosperm through mutation of PARC6 affects starch granule morphology. New Phytol. 2023, 240, 224–241. [Google Scholar] [CrossRef]
- Miura, H.; Tanii, S.; Nakamura, T.; Watanabe, N. Genetic control of amylose content in wheat endosperm starch and differential effects of three Wx genes. Theor. Appl. Genet. 1994, 89, 276–280. [Google Scholar] [CrossRef]
- Yamamori, M.; Nakamura, T.; Endo, T.R.; Nagamine, T. Waxy protein deficiency and chromosomal location of coding genes in common wheat. Theor. Appl. Genet. 1994, 89, 179–184. [Google Scholar] [CrossRef]
- Graybosch, R.A.; Peterson, C.J.; Hansen, L.E.; Rahman, S.; Mili, A.; Skerritt, J.H. Identification and characterization of U.S. wheats carrying nulli alleles at the wx loci. Cereal Chem. 1998, 75, 162–165.40. [Google Scholar] [CrossRef]
- Ellis, R.P.; Cochrane, M.P.; Dale, M.F.B.; Duffus, C.M.; Lynn, A.; Morrison, I.M.; Prentice, R.D.M.; Swanston, J.S.; Tiller, S.A. Starch production and industrial use. J. Sci. Food Agric. 1998, 77, 289–311. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Erazo-Castrejón, S.V.; Doehlert, D.C.; McMullen, M.S. Staling of bread as affected by waxy wheat flour blends. Cereal Chem. 2002, 79, 178–182. [Google Scholar] [CrossRef]
- Morita, N.; Maeda, T.; Miyazaki, M.; Yamamori, M.; Miura, H.; Phtsuka, I. Dough and baking properties of high amylose and waxy wheat flours. Cereal Chem. 2002, 79, 491–495. [Google Scholar] [CrossRef]
- Jonnala, R.S.; MacRitchie, F.; Smail, V.W.; Seabourn, B.W.; Tilley, M.; Lafiandra, D.; Urbano, M. Protein and quality characterization of complete and partial near-isogenic lines of waxy wheat. Cereal Chem. 2010, 87, 538–545. [Google Scholar] [CrossRef]
- Yi, J.; Johnson, J.W.; Kerr, W.L. Properties of bread made from frozen dough containing waxy wheat flour. J. Cereal Sci. 2009, 50, 364–369. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat starch production, structure, functionality and applications—A review. Int. J. Food Sci. Technol. 2017, 52, 38–58. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Nugent, A.P. Health properties of resistant starch. Nutr. Bull. 2005, 30, 27–54. [Google Scholar] [CrossRef]
- Luo, J.; Ahmed, R.; Kosar-Hashemi, B.; Larroque, O.; Butardo, V.M.; Tanner, G.J.; Colgrave, M.L.; Upadhyaya, N.M.; Tetlow, I.J.; Millar, T.; et al. The different effects of starch synthase IIa mutations or variation on endosperm amylose content of barley, wheat and rice are determined by the distribution of starch synthase I and starch branching enzyme IIb between the starch granule and amyloplast stroma. Theor. Appl. Genet. 2015, 128, 1407–1419. [Google Scholar]
- Yamamori, M.; Fujita, S.; Hayakawa, K.; Matsuki, J.; Yasui, T. Genetic elimination of starch granule protein, SGP-1, of wheat generates an altered starch with apparent high amylose. Theor. Appl. Genet. 2000, 101, 21–29. [Google Scholar] [CrossRef]
- Hogg, A.C.; Gause, K.; Hofer, P.; Martin, J.M.; Graybosch, R.A.; Hansen, L.E.; Giroux, M.J. Creation of a high-amylose durum wheat through mutagenesis of starch synthase II (SSIIa). J. Cereal Sci. 2013, 57, 377–383. [Google Scholar] [CrossRef]
- Botticella, E.; Sestili, F.; Ferrazzano, G.; Mantovani, P.; Cammerata, A.; D’Egidio, M.G.; Lafiandra, D. The impact of the SSIIa null mutations on grain traits and composition in durum wheat. Breed. Sci. 2016, 66, 572–579. [Google Scholar] [CrossRef]
- Lafiandra, D.; Sestili, F.; D’Ovidio, R.; Janni, M.; Botticella, E.; Ferrazzano, G.; Silvestri, M.; Ranieri, R.; DeAmbrogio, E. Approaches for modification of starch composition in durum wheat. Cereal Chem. 2010, 87, 28–34. [Google Scholar] [CrossRef]
- Sestili, F.; Janni, M.; Doherty, A.; Botticella, E.; D’Ovidio, R.; Mascci, S.; Jones, H.D.; Lafiandra, D. Increasing the amylose content of durum wheat through silencing of the SBEIIa genes. BMC Plant Biol. 2010, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Hazard, B.; Zhang, X.; Naemeh, R.; Hamilton, M.K.; Rust, B.; Raybould, H.E.; Newman, J.W.; Martin, R.; Dubcovsky, J. Mutations in durum wheat SBEII genes conferring increased amylose and resistant starch affect grain yield components, semolina and pasta quality and fermentation responses in rats. Crop Sci. 2015, 55, 2813–2825. [Google Scholar] [CrossRef] [PubMed]
- Sestili, F.; Palombieri, S.; Botticella, E.; Mantovani, P.; Bovina, R.; Lafiandra, D. TILLING mutants of durum wheat result in a high amylose phenotype and provide information on alternative splicing mechanisms. Plant Sci. 2015, 233, 127–133. [Google Scholar]
- Sissons, M.; Sestili, F.; Botticella, E.; Masci, S.; Lafiandra, D. Can manipulation of durum wheat amylose content reduce the glycaemic index of spaghetti? Foods 2020, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- AACC International. Approved Methods of Analysis, 11th ed.; Method 26-41.01., 44-15A; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Horneck, D.A.; Miller, R.O. Determination of total nitrogen in plant tissue. Handb. Ref. Methods Plant Anal. 1998, 2, 75–83. [Google Scholar]
- Sissons, M.; Ovenden, B.; Adorada, D.; Milgate, A. Durum wheat quality in high input irrigation systems in South Eastern Australia. Crop Pasture Sci. 2014, 65, 411–422. [Google Scholar] [CrossRef]
- Marti, A.; Cecchini, C.; D’Egidio, M.G.; Dreisoemer, J.; Pagani, M.A. Characterization of durum wheat semolina by means of a rapid shear-based method. Cereal Chem. 2014, 91, 542–547. [Google Scholar] [CrossRef]
- Sissons, M.; Egan, N. Effect of variation in starch B-granule content in durum wheat on technological properties and in vitro starch digestion of spaghetti. Cereal Chem. 2023, 100, 873–886. [Google Scholar] [CrossRef]
- Grant, L.A.; Vignaux, N.; Doehlert, D.C.; McMullen, M.S.; Elias, E.M.; Kianian, S. Starch characteristics of waxy and nonwaxy tetraploid (Triticum turgidum L. var. durum) wheats. Cereal Chem. 2001, 78, 590–595. [Google Scholar] [CrossRef]
- Tomoko, S.; Matsuki, J. Effect of wheat starch structure on swelling power. Cereal Chem. 1998, 75, 525–529. [Google Scholar]
- Ribeiro, N.C.B.V.; Ramer-Tait, A.E.; Cazarin, C.B.B. Resistant starch: A promising ingredient and health promoter. PharmaNutrition 2022, 21, 100304. [Google Scholar] [CrossRef]
- Taghouti, M.; Gaboun, F.; Nsarellah, N.; Rhrib, R.; El-Haila, M.; Kamar, M.; Abbad-Andaloussi, F.; Udupa, S.M. Genotype x Environment interaction for quality traits in durum wheat cultivars adapted to different environments. Afr. J. Biotechnol. 2010, 9, 3054–3062. [Google Scholar]
- Grant, L.A.; Doehlert, D.C.; McMullen, M.S.; Vignaux, N. Spaghetti cooking quality of waxy and non-waxy durum wheats and blends. J. Sci. Food Agric. 2004, 84, 190–196. [Google Scholar] [CrossRef]
- Fu, B.X.; Wang, K.; Dupuis, B.; Taylor, D.; Nam, S. Kernel vitreousness and protein content: Relationship, interaction and synergistic effects on durum wheat quality. J. Cereal Sci. 2018, 79, 210–217. [Google Scholar] [CrossRef]
- Hogg, A.C.; Martin, L.M.; Manthey, F.A.; Giroux, M.J. Nutritional and quality traits of pasta made from SSIIa null high amylose durum wheat. Cereal Chem. 2015, 92, 395–400. [Google Scholar] [CrossRef]
- Soh, H.N.; Sissons, M.J.; Turner, M.A. Effect of starch granule size distribution and elevated amylose content on durum dough rheology and spaghetti cooking quality. Cereal Chem. 2006, 83, 513–519. [Google Scholar] [CrossRef]
- Fardet, A.; Hoebler, C.; Baldwin, P.M.; Bouchet, B.; Gallant, D.J.; Barry, J.L. Involvement of the protein network in the in vitro degradation of starch from spaghetti and lasagne: A microscopic and enzymic study. J. Cereal Sci. 1998, 27, 133–145. [Google Scholar] [CrossRef]
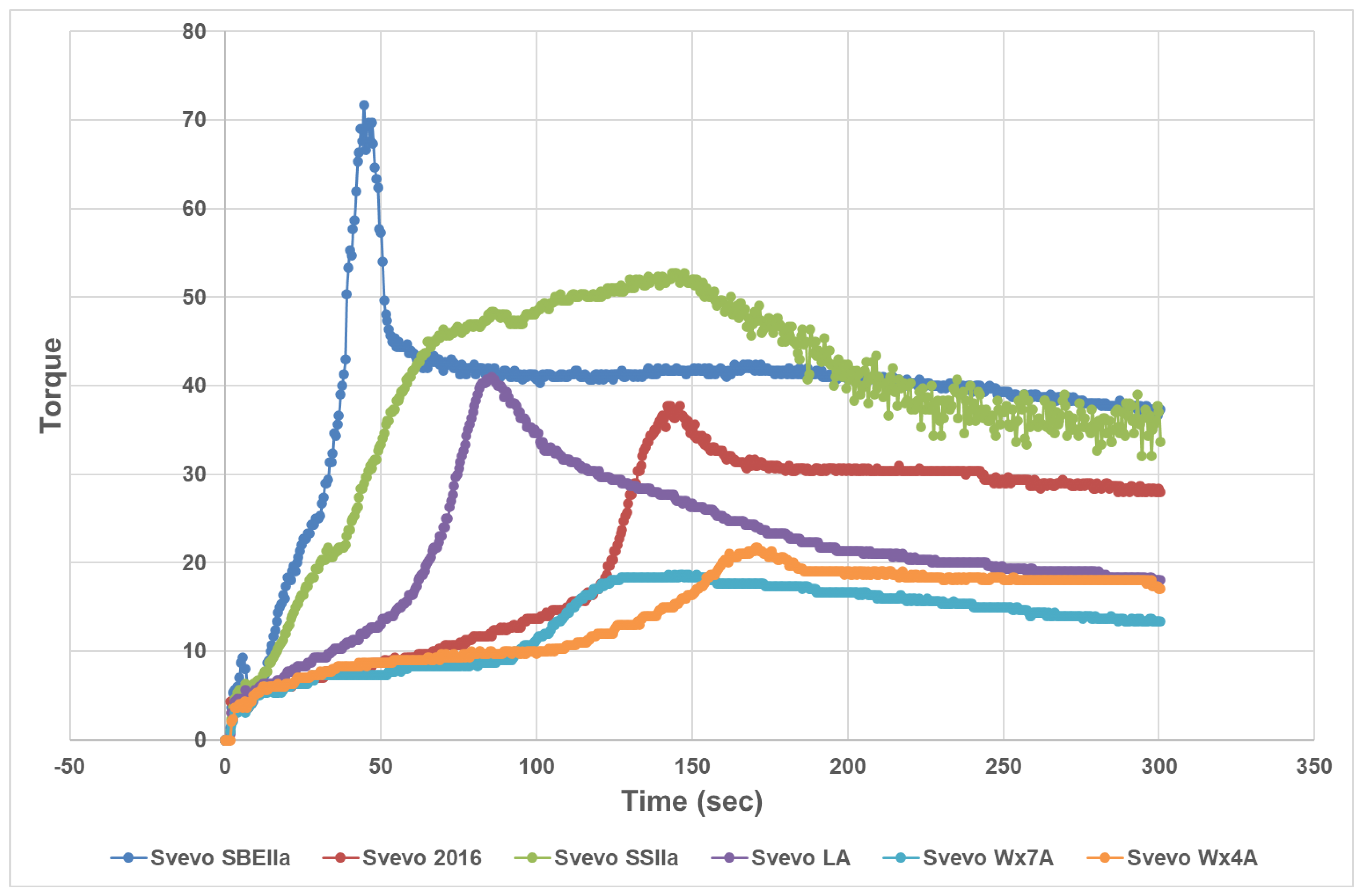
| Genotype | Sowing Year | Amylose (% dmb) | SP | Protein * (14% mb) | S-L* | S-a* | S-b* | FWA% (14% mb) | MPT (Min) | RBD | PMT (s) | Torque (AU) | Area | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Svevo LA | 2016 | 14.9 a | 10.6 d | 12.8 | 83.4 b | −1.4 d | 20.3 b | 67.6 d | 4.8 b | 58 c | 85 b | 41.7 d | 1833 a | |
| Svevo Wx4A | 2016 | 30.0 b | 10.9 d | 10.9 | 84.2 d | −1.8 c | 21.3 c | 58.7 a | 7.6 c | 13 a | 170 d | 21.7 b | 2132 b | |
| Svevo Wx7A | 2016 | 31.7 c | 11.5 d | 11.0 | 83.4 b | −0.8 e | 17.9 a | 59.4 b | 4.6 b | 75 c | 143 c | 18.7 a | 1716 a | |
| Svevo | 2016 | 34.0 d | 8.9 c | 13.4 | 84.9 e | −2.5 b | 24.0 e | 62.0 c | 4.8 b | 34 b | 145 c | 37.7 c | 2424 c | |
| Svevo SSIIa | 2016 | 43.5 e | 6.6 b | 14.7 | 81.7 a | −1.7 c | 22.6 d | 74.2 e | 3.9 ab | 68 c | 144 c | 53.0 e | 6016 d | |
| Svevo SBEIIa | 2017 | 57.8 f | 5.1 a | 15.9 | 82.2 a | −3.4 a | 31.0 f | 77.4 f | 2.5 a | 72 c | 47 a | 72.3 f | 1953 ab | |
| p < 0.005 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| LSD | 1.33 | 1.41 | 0.53 | 0.15 | 0.34 | 0.44 | 1.37 | 20.35 | 7.89 | 2.37 | 292 |
| Genotype (Amylose%) | Sow Year | RS (% dmb) | TS (% dmb) | DPL* | DPa* | DPb* | FCT (s) | Firmness PH (g) | Firmness Area (g/s) | Overcooking Tolerance | S-PH (g) | S-Area (g/s) | CL% | WABS% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Svevo LA (14.9) | 2016 | 0.11 | 70.2 | 65.6 | 3.5 | 32.9 | 551 | 1104 | 356 | 74 | 27.6 | 11.8 | 5.8 | 142 |
| Svevo Wx4A (30) | 2016 | 0.22 | 73.9 | 67.5 | 3.0 | 34.6 | 680 | 826 | 401 | 51 | 23.0 | 12.8 | 5.5 | 156 |
| Svevo Wx7A (31.7) | 2016 | 0.20 | 74.5 | 66.9 | 3.1 | 35.2 | 675 | 864 | 439 | 50 | 19.9 | 10.6 | 5.4 | 154 |
| Svevo (34) | 2016 | 0.73 | 73.4 | 70.1 | 0.3 | 44.6 | 664 | 1334 | 615 | 52 | 17.0 | 9.3 | 4.6 | 146 |
| Svevo SSIIa (43.5) | 2016 | 2.06 | 67.3 | 67.0 | 2.2 | 38.3 | 578 | 1125 | 566 | 51 | 14.7 | 5.4 | 6.9 | 123 |
| Svevo SBEIIa (57.8) | 2017 | 7.36 | 65.3 | 64.5 | 1.5 | 39.9 | 675 | 1124 | 544 | 49 | 15.9 | 5.7 | 6.6 | 120 |
| p < 0.005 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.010 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| LSD | 0.19 | 1.8 | 1.30 | 0.58 | 1.34 | 102.6 | 39.19 | 21.48 | 2.0 | 2.29 | 1.90 | 0.52 | 3.58 |
| Kinetics | |||||||
|---|---|---|---|---|---|---|---|
| Genotype | Amylose% | C∞ % I | k1 | C∞ % II | k2 | Total Area under Digestion Curve | Normalised Area |
| Svevo LA | 14.9 | 50.4 | 0.0402 | 92.8 | 0.0097 | 25,621 | 1.29 |
| Svevo Wx4A | 30.0 | 49.6 | 0.0278 | 101.3 | 0.0069 | 23,575 | 1.19 |
| Svevo Wx7A | 31.7 | 44.1 | 0.0239 | 79.9 | 0.0076 | 19,590 | 0.99 |
| Svevo | 34.0 | 38.9 | 0.0276 | 83.2 | 0.0065 | 19,851 | 1.00 |
| Svevo SSIIa | 43.5 | 35.4 | 0.0255 | 81.0 | 0.0057 | 18,652 | 0.94 |
| Svevo SBEIIa | 57.8 | 24.3 | 0.0347 | 45.2 | 0.0083 | 12,231 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sissons, M.; Palombieri, S.; Sestili, F.; Lafiandra, D. Impact of Variation in Amylose Content on Durum Wheat cv. Svevo Technological and Starch Properties. Foods 2023, 12, 4112. https://doi.org/10.3390/foods12224112
Sissons M, Palombieri S, Sestili F, Lafiandra D. Impact of Variation in Amylose Content on Durum Wheat cv. Svevo Technological and Starch Properties. Foods. 2023; 12(22):4112. https://doi.org/10.3390/foods12224112
Chicago/Turabian StyleSissons, Mike, Samuela Palombieri, Francesco Sestili, and Domenico Lafiandra. 2023. "Impact of Variation in Amylose Content on Durum Wheat cv. Svevo Technological and Starch Properties" Foods 12, no. 22: 4112. https://doi.org/10.3390/foods12224112
APA StyleSissons, M., Palombieri, S., Sestili, F., & Lafiandra, D. (2023). Impact of Variation in Amylose Content on Durum Wheat cv. Svevo Technological and Starch Properties. Foods, 12(22), 4112. https://doi.org/10.3390/foods12224112