3.1. Spirulina Effects on Mozzarella Cheese’s Chemical Composition
The milk composition was affected by Spirulina supplementation only in terms of protein, lowering the percentage from 4.59 to 4.31 (SEM 0.07,
p < 0.05), while the fat and lactose contents were not influenced by Spirulina inclusion in the buffalo diets. The same trend was observed in the mozzarella cheese’s chemical composition; the fat, lactose, and moisture contents were not different among the two groups, while the protein amount was tendentially higher in the C group ((18.01 vs. 17.12 ± 0.33%;
p < 0.10 (mean ± SEM)) than the S group. Our results do not agree with the increases in protein, fat, and lactose found by Šimkus et al. [
40] as effects of Spirulina supplementation in cows, and the findings of Boeckaert et al. [
41] and Stamey et al. [
42], who registered an increase in milk fat. Despite the small differences in the protein content, the AA profiles of the mozzarella cheese samples did not change with Spirulina supplementation (
Table 3). Among the EAAs, LEU and LYS are the most abundant in both products, tending to be higher in the S samples, which could be due to the AA profile of the added Spirulina. Microalgae in general are good sources of EAAs. The milk from the S group contained 27% more EAAs and 23% more NEAAs than the C group. The presence of EAAs in milk and dairy products, especially branched-chain amino acids, is important in terms of their benefits for human health [
43]. Recently, Cacciola et al. [
44,
45] reported interesting results about a possible beneficial effect of delactosed buffalo milk whey by-product on colorectal human carcinogenesis. It is well known that primary proteolysis occurs during mozzarella processing [
46], and the effect of the composition of milk protein fractions (relative contents of alpha S1 casein and beta casein) on milk coagulation properties has been widely studied [
47,
48].
Table 4 shows no significant differences in the FA profiles of the mozzarella cheese samples between the C and S groups, nor in the calculated nutritional index. Manzocchi et al. [
49] did not observe any significant differences in the dairy cow milk FA content when substituting 5% of soybean meal with Spirulina in their diets, except for the total n-6 FA, which was higher in the control milk samples. This highlights that the diet supplemented with Spirulina lowered the apparent transfer efficiency of the total n-6 FA. In contrast, numerous authors have reported that replacing feed with Spirulina yields a significant improvement in the FA profile of ruminants’ milk. Christodoulou et al. [
50] examined the effects of three levels of Spirulina substitution (i.e., 5%, 10%, and 15%) in the diets of ewes. Their findings revealed that only the highest level of substitution increased the PUFA and ω-3 PUFA contents, while a mere 5% of substitution was adequate to decrease the AI. Christaki et al. [
51] added 40 g of powdered Spirulina to cow diets and observed a decrease in the SFA amount, while the MUFA and PUFA contents in the milk increased at the 45th day of experimentation. As stated by many authors, species and breeds strongly affect the animals’ responses to diets [
14,
15]. This might partially explain why the buffalo mozzarella cheese chemical compositions did not differ among the two groups in our study. Moreover, the amount of Spirulina included in the S diets for the buffaloes could be too low to see changes/improvements in the milk and cheese compositions. To date, most of the studies in which microalgae were tested for animal feeding showed inconsistent and contradictory effects, probably due to the high variability among the studies, the doses of microalgae and species, the experimental period, and the percentage of forage in the diet [
14]. However, microalgae use in animal feeding originated with the objective of improving the nutritional quality, particularly in terms of the FA profile, of milk and dairy products [
15].
The mozzarella cheese’s mineral content was not affected by the diets, as shown in
Table 5. As it is well known, the major mineral that is present in buffalo milk, and thus in buffalo mozzarella cheese, is calcium, while the second most predominant mineral is phosphorus. Gulzar et al. [
52] reported that the mineral content is influenced by the cheese process, with the ash content reducing proportionally as the milling pH decreases. They explained that acid development solubilises micellar calcium phosphate, leading to mineral expulsion in whey. Specifically, the calcium levels decrease in line with the milling pH reduction, although there is uncertainty about the influence of this cheese-making step on the potassium and sodium levels. The buffalo milk mineral content is subject to variation due to several factors, including the season, environment, diet, stage of lactation, animal breed, and genetics [
53]. The Mediterranean buffalo has been reported to possess the highest magnesium content in milk, whilst the greatest amount of minerals found in buffalo milk occurred during the summer season [
54]. In our study, it was found that the mineral content was consistent across the samples because both groups were homogeneous, consisting of the same breed, and were fed the same diet. Therefore, the inclusion of Spirulina powder did not affect the mineral percentage in the buffalo mozzarella cheese.
The results of the analysis on VOCs can be found in
Table 6. In total, 94 compounds were identified, and the most prevalent class in both groups is “others”. This group covers all of the individual VOCs that do not belong to the main classes considered in this study. Although the less abundant classes are the same in both samples, including esters, aldehydes, halogenated hydrocarbons, sulphur, and nitrogen compounds (from highest to lowest), the most abundant VOC classes are different between the two groups. In the C samples, the most and least frequent classes are alcohols, ketones, hydrocarbons, and aromatic hydrocarbons, in that order. In contrast, in the S samples, the most frequent classes are hydrocarbons, alcohols, aromatic hydrocarbons, and ketones, in that order. The only class that significantly differs from the others is aromatic hydrocarbons, which are higher in the C samples than the S samples, despite being ranked fifth on the list of VOC classes for the C group (in descending order) and fourth for the S group. However, the individual VOCs that were found to be significantly different (only 4% of the VOCs detected) do not belong to this class. Specifically, toluene (
p < 0.05), isobutyl acetate (
p < 0.05), and acetic acid 2-phenylethyl ester (
p < 0.01) were found at higher levels in product C, while 1 Pentanol (
p < 0.01) was detected at lower levels (
p < 0.01) (
Table 6). Unlike the study by Sabia et al. [
10], where the most prevalent VOC class was ketones, particularly in the cheese from buffalos that were fed wrapped ryegrass silage, our study found that ketones ranked third and fifth in the VOC lists of the C and S groups, respectively. Moreover, the level of ketones was higher in the C samples compared to the S samples (though not significantly). This is similar to the study by Sacchi et al. [
3], where ketones were more abundant in the control sample than the experimental ones. Ketones are common in many dairy products, and their origin can be attributed to SFA β-oxidation. Some studies suggest that these compounds are derived from animal feeds, and silage is the primary source of ketones [
3]. Further, animals consuming silage-based diets may result in the production of alcohols [
55]. There are numerous metabolic pathways implicated in the alcohol biosynthesis found in cheese. These pathways include the reduction of methyl ketones and aldehydes, the degradation of linoleic and linolenic acids, as well as AA and lactose metabolism.
Table 6 shows that the alcohol class is higher in the C samples in comparison to the S samples, although not significantly different, and they are the same as the ketones levels. Our study involved two groups of animals that were fed the same diet, with the same amount of silage. Therefore, the variations in the levels of ketones and alcohols could be attributed to the addition of Spirulina, even in small quantities. Although there is no significant difference in the levels of halogenated hydrocarbons between the two groups, it should be noted that the level of this class is four times greater in the C samples than in the S samples. The mozzarella cheese production process impacts both the quantity and quality of the VOC composition. High temperatures during stretching can cause compound loss, while microflora activity during curd ripening can generate new compounds. Additionally, enzymes and milk bacteria are involved in the development of the cheese’s flavour. The aroma of cheese is also the result of the interaction of volatile and non-volatile chemical compounds, mainly concentrated in the water-soluble fraction, resulting from the transformation of the primary components (fat, proteins, and carbohydrates) and the action of bacteria present during milk processing. Therefore, understanding the production process is crucial to regulate the VOC composition of mozzarella cheese [
3]. However, the flavour of mozzarella cheese is not determined by the quantity of a particular compound/VOC, but by the equilibrium of all of the VOCs present.
The results about the instrumental colour of mozzarella showed that Spirulina integration was responsible of the higher values of the L*, a*, and b* indices in both the external and internal surfaces (
Table 7). It is likely that the blue phycocyanin, one of the two pigmented antioxidants found in Spirulina, may have contributed to an increased lightness with a blue tint. However, it is worth noting that Park et al. [
56] did not find a significant correlation between the L* value, pigment content, or antioxidant activity of Spirulina. If this finding is confirmed in future studies, it would be a favourable outcome, considering the high demand for porcelain white mozzarella among consumers.
A correct instrumental measurement of colour is useful at the stage of analysing consumer preferences for the research, development, and improvement of cheese-making methods. When observed under a light source, PDO buffalo mozzarella cheese should have a porcelain white colour and a glowing appearance. The white colour is given by casein micelles, while the yellowish hues are imparted by carotenoids in green fodder, which are not absorbed in the cattle species and are therefore released in the milk. In contrast, buffaloes, sheep, and goats are able to assimilate and transform these compounds, so the colour of their milk tends to remain white.
3.2. Spirulina Effects on Mozzarella Cheese’s Sensory Quality
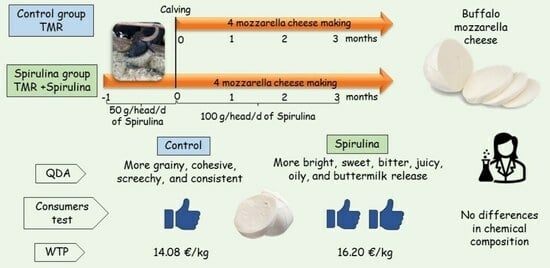
As for the QDA, no significant product x replication or product x assessor interactions were observed, suggesting that the training program and the reference frame used in this study were efficacious in ensuring the high reliability of the panel (i.e., products were not evaluated differently in different replications or by different assessors).
Table 8 shows a significant diet effect on the sensory profiles of mozzarella cheeses. The samples from the S group were evaluated as being brighter (
p < 0.05) with a lower white inner colour (
p < 0.01) than the C group. The S mozzarella cheeses were perceived with a higher butter odour (
p < 0.01), a higher whey flavour, and a sweeter and more bitter taste than the samples from the C group (
p < 0.05). Furthermore, the panellists perceived higher oiliness (
p < 0.01) and moisture (
p < 0.0001) intensities in the S samples with a greater milk release when cutting (
p < 0.001) and a tendentially higher tenderness (
p < 0.10) than in the mozzarella cheeses from the C group, which, on the contrary, were perceived as being more grainy, cohesive, screechy, and having a greater consistency when cutting than the S mozzarella cheeses (
p < 0.01).
In a recent study on buffalo mozzarella cheese [
11], changes in texture were attributed, at least in part, to the corresponding fatty acid composition, which was lower in saturated fatty acids, such as C14:0, and richer in unsaturated fatty acids, such as C18:1, in products obtained from animals that were fed fresh fodder. The lower melting point of unsaturated fatty acids can produce softer cheeses. Furthermore, some attributes (e.g., bitter taste, flakiness, and grainy texture) may be influenced by more intense proteolysis that occurs in the summer, which, in turn, may contribute to the softening of the mozzarella and may have a direct effect on the flavour through the production of short-chain peptides and amino acids [
57].
The greater oiliness could also be due to a different acid composition in the fat. For example, in Friesian cattle, Christaki et al. [
51], following the administration of 40 g/head/d of Spirulina, observed a reduction in the content of saturated fatty acids in milk, with a consequent increase in MUFA and PUFA compared to the control. The sensory characteristics of fresh mozzarella largely rely on the raw materials and production techniques used. Further investigation is required to evaluate the influence of diverse components, including VOCs, AAs, FAs, and peptides, on each sensory attribute.
The data on the distribution of individual preferences (
Table 9) show that the information received is able to direct the consumers’ judgements.
The consumers in blind conditions (P) evaluated both samples from the C and S groups to be greater than the middle value (i.e., 5) (
Table 10), meaning there were satisfactory sensory properties in both products. Moreover, the blind evaluation was not influenced by the animals’ diet, nor by the ages and sexes of the consumers. No difference was observed between the two groups for expected (E) liking. Likewise, the actual (A) liking was only influenced by diet, with a higher rate for the S group samples (7.29) than the C group samples (6.85).
Table 10 shows that for both groups, the expected liking was significantly higher than the blind evaluation (
p < 0.01), which means there was a negative disconfirmation: consumers evaluate the mozzarella cheese to be lower than their expectations. There was no difference between the actual and perceived liking of the C group samples, which means that the information does not increase the product evaluation; on the contrary, for the S mozzarella cheese, the information strongly influenced the actual liking (
p < 0.001), as it was close to the expected acceptability. This means that there was a complete assimilation, because the information given to the consumers brought them to similarly evaluate the samples in both the actual and expected conditions (i.e., with and without tasting the mozzarella cheese).
Many aspects of the product can be used by consumers to make their food choices. Grunert et al. [
58] identified four main dimensions of quality for dairy products: hedonism, health, convenience, and process. A few of them can be experienced before purchase (e.g., colour), while most of them can be perceived after purchase (e.g., sensory properties), or may never be perceived (e.g., healthiness and appearance ethical). The latter must be communicated to the consumer, as they are characteristics that cannot be perceived before nor after the purchase [
59]. Therefore, regarding these characteristics, consumers are forced to develop expectations to guide their food choices. In particular, to develop expectations on product quality attributes, consumers can use both intrinsic (e.g., holes in the cheese, colour of the external rind, etc.) and extrinsic (e.g., price) characteristics. However, consumers tend to rely mostly on extrinsic characteristics provided to them in the form of product information [
60]. In particular, ethical concerns, such as environmental pollution and animal welfare, are becoming increasingly important in the hierarchy of purchasing motivations for animal products. Blockhuis et al. [
61] highlighted that animal welfare is increasingly recognised as an important component of quality for consumers of animal products. Numerous studies have been conducted on the effect of information on food liking [
62,
63]. For example, the effect of information relating to animal welfare on lamb [
64] and beef [
65] liking has been studied, as well as the effect of information relating to organic production on the acceptability of foods and beverages [
66,
67]. All of these studies have shown that information-induced expectations can influence the perception of quality. Therefore, if expectations receive a positive disconfirmation (when the satisfaction score of the product tasted without external information is higher than expected) or a negative one (when the product is worse than expected), the assimilation model is generally applicable. According to this model, when external information is provided, the hedonic tests highlight a shift in acceptability towards expectations and reach different values from those obtained by tasting the same food without external information [
68]. In both groups, the expected liking was significantly higher than the liking expressed in the blind conditions (
p < 0.01), thus indicating that a negative disconfirmation occurred: the consumers found the mozzarella to be less pleasant than expected. In these conditions, generally, actual liking moves in the direction of expected liking for group C; no significant difference was observed between the actual and perceived liking. Thus, in this case, there was no assimilation, as the information did not improve the acceptability of the product (
Table 10). Conversely, regarding the mozzarella cheese obtained from the S group, the information greatly improved the actual liking (
p < 0.001), which moved in the direction of the expectations. According to Blokhuis et al. [
61], the perception of food quality is determined by the welfare of the animals producing that food together with the overall nature and safety of the final product. In particular, the assimilation in this case was complete, since no difference was observed between the actual liking (expressed by the consumers with both sensory stimuli and information available) and expected liking (expressed by the consumers with only the information available). The complete assimilation observed for the S product is probably due to the important role played by information in determining the actual liking of mozzarella with Spirulina. This information is able to respond to some of the main and most current consumer concerns, such as the nutraceutical properties of products and animal welfare.
Napolitano et al. [
64] showed that consumers are influenced by animal welfare information and shift their WTP in the direction of their expectations. In particular, the discrepancy between the expected liking and the actual willingness to pay was not fully assimilated, indicating that it was also expressed in relation to other aspects (for example, the sensorial properties of the products). Experimental auctions are able to place consumers in real situations where they can show their true preferences. In particular, the second-price Vickrey’s auction has been widely used to assess consumers’ WTP for real goods, including food [
38], and the values that consumers place on food safety [
69] and animal welfare [
39]. Under this specific type of auction, consumers are individually asked to submit a sealed bid corresponding to the highest price they would agree to pay for a particular product. The highest bidder (i.e., the winner), by paying the second highest price, has the opportunity to purchase a product at a price that is equal to or, more often, lower than the value they attribute to the product [
70]. Currently, consumers are not looking for the cheapest food, but the best quality/price ratio, i.e., the maximum benefit for what they are willing to spend [
71]. In our study, it was observed that gender had no influence on the overall offer, while it was found that the consumers offered EUR 4.05 (16.20 EUR/kg) for a 250 g pack of Spirulina mozzarella cheese compared to EUR 3.52 (14.08 EUR/kg) for the control mozzarella cheese (F1,128 = 8.73;
p = 0.0074).