The Leptospermum scoparium (Mānuka)-Specific Nectar and Honey Compound 3,6,7-Trimethyllumazine (LepteridineTM) That Inhibits Matrix Metalloproteinase 9 (MMP-9) Activity
Abstract
:1. Introduction
2. Materials and Methods
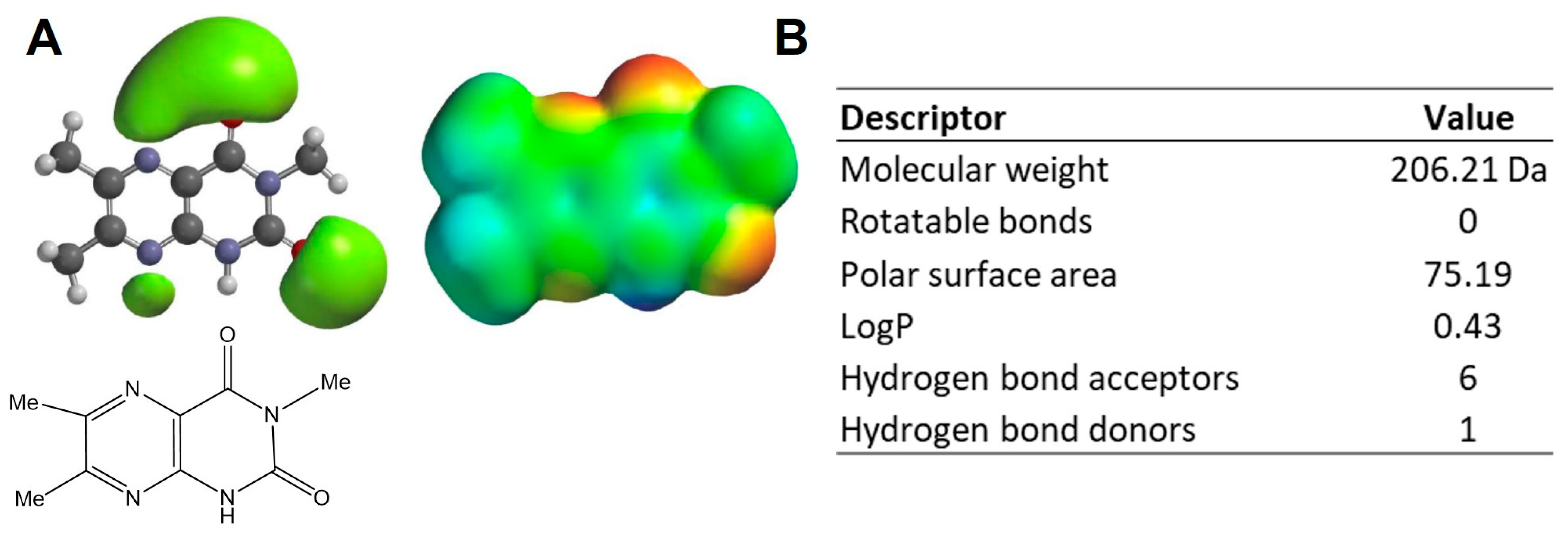
2.1. Physicochemical Properties
2.2. Molecular Docking
2.3. MMP-9 Fluorescent and Colorimetric Activity Assays
2.4. Mass Spectrometry Activity Analyses
2.5. Expression in E. coli, Denaturing Purification, and On-Column Refolding of Recombinant Human Matrix Metalloproteinase 9 (MMP-9) in Its Latent Form (His-Tagged proMMP-9cat)
2.6. Protein Quantitation
2.7. SDS/PAGE Electrophoresis and Western Blotting
2.8. Generation of Activated Human MMP-9cat from His-Tagged proMMP-9cat
2.9. His-Tagged proMMP-9cat and MMP-9cat Gelatinase Activity Assay
2.10. LepteridineTM-Simulated Digestion
2.11. Statistical Analysis
3. Results
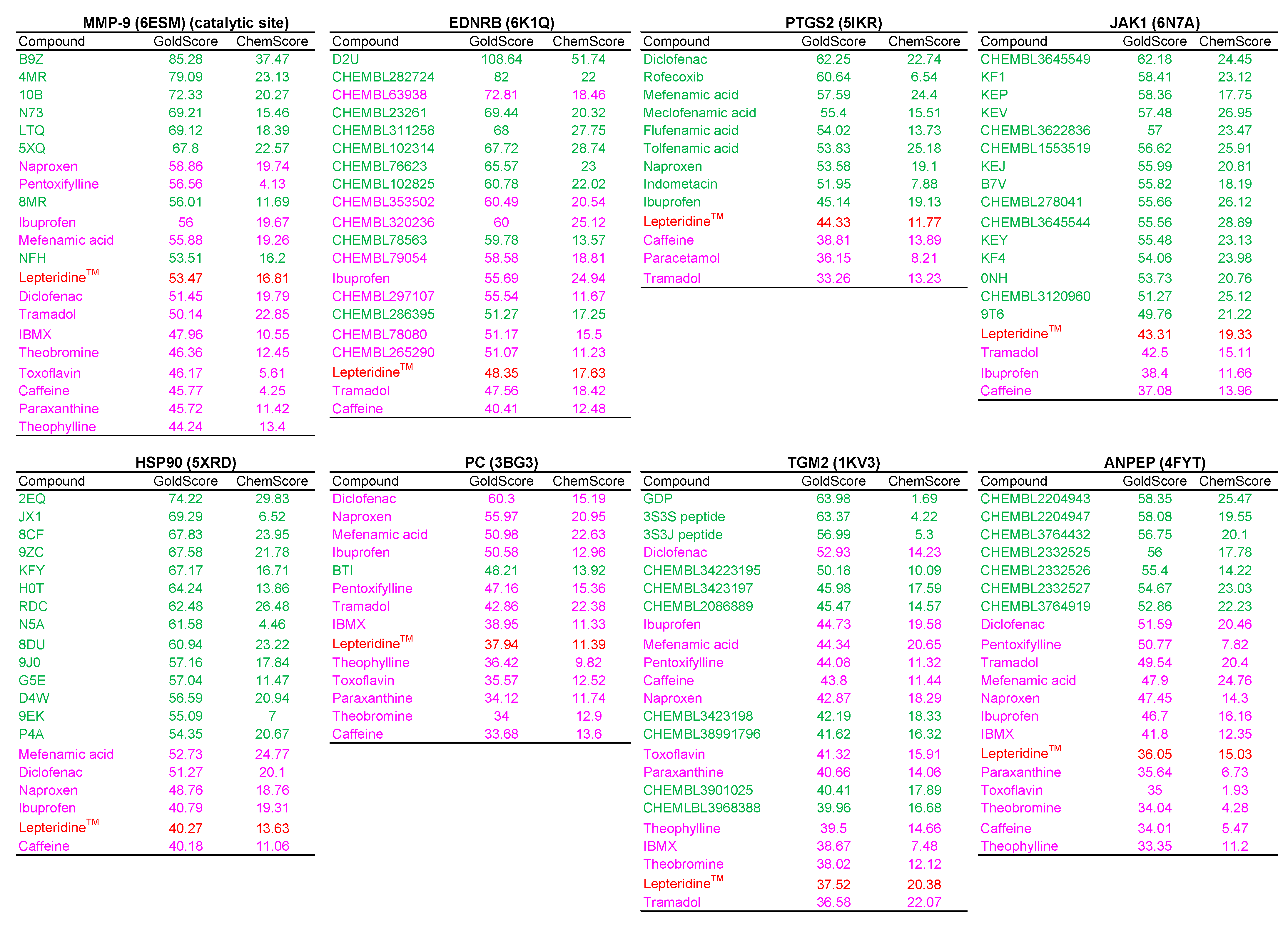
3.1. Modelling of LepteridineTM as a Putative Ligand for MMP-9 and Other Inflammatory Mediators
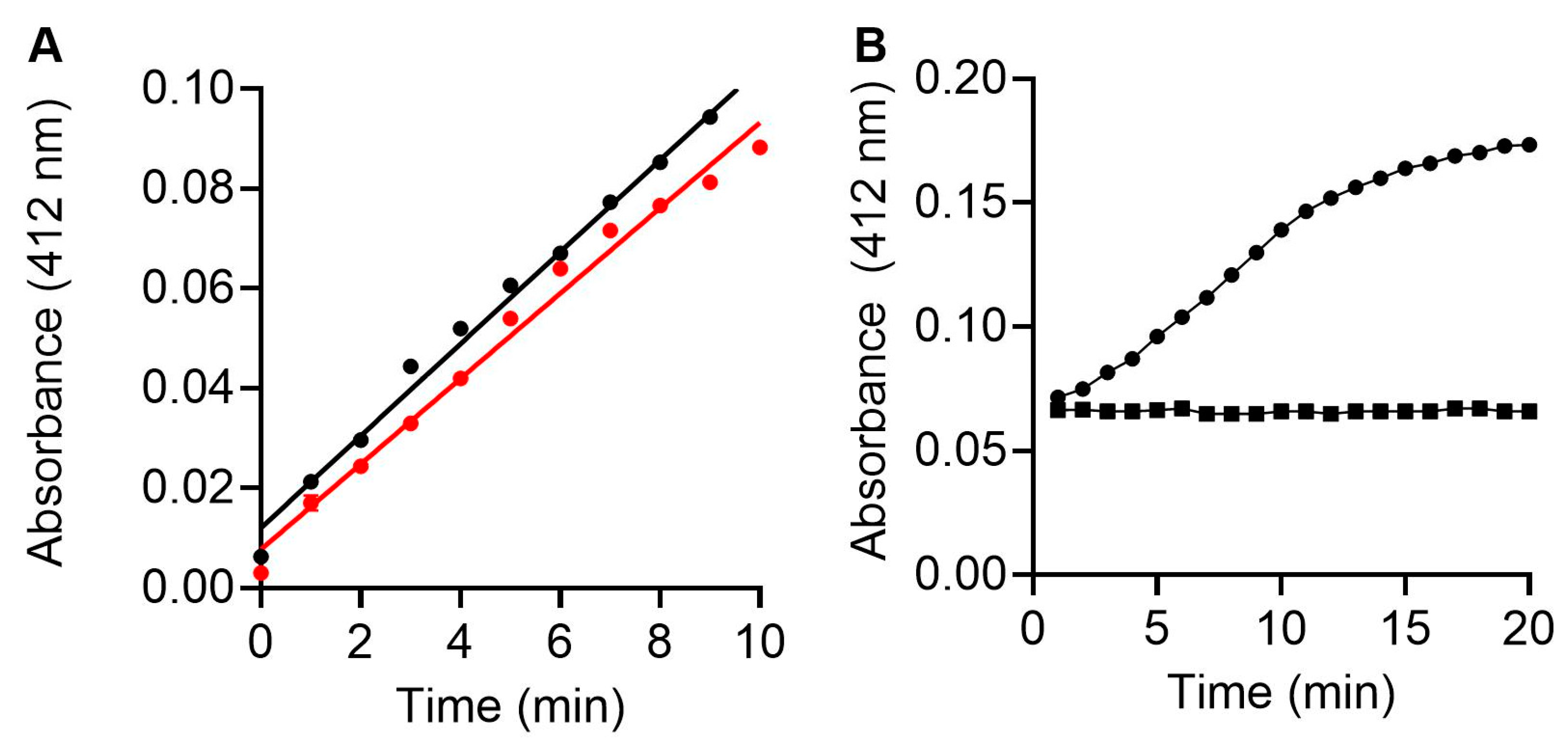
3.2. Fluorescence and Colorimetric Spectrometry Assessment of LepteridineTM’s Ability to Inhibit MMP-9 Activity
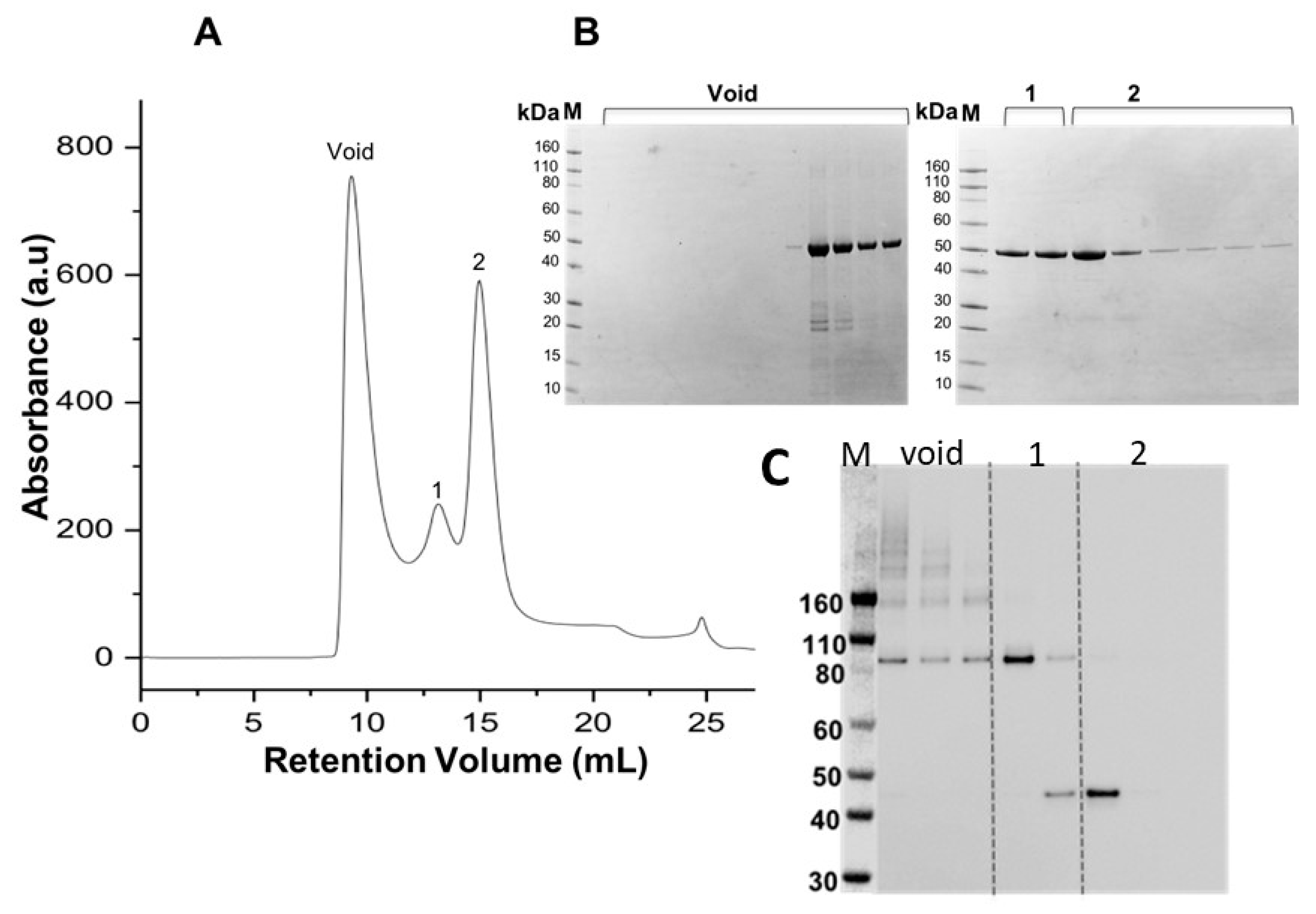
3.3. Purification and Characterisation of Recombinant proMMP-9cat
3.4. Gelatin Zymography Assessment of LepteridineTM and Its Ability to Inhibit His-Tagged proMMP-9cat and MMP-9cat Activity
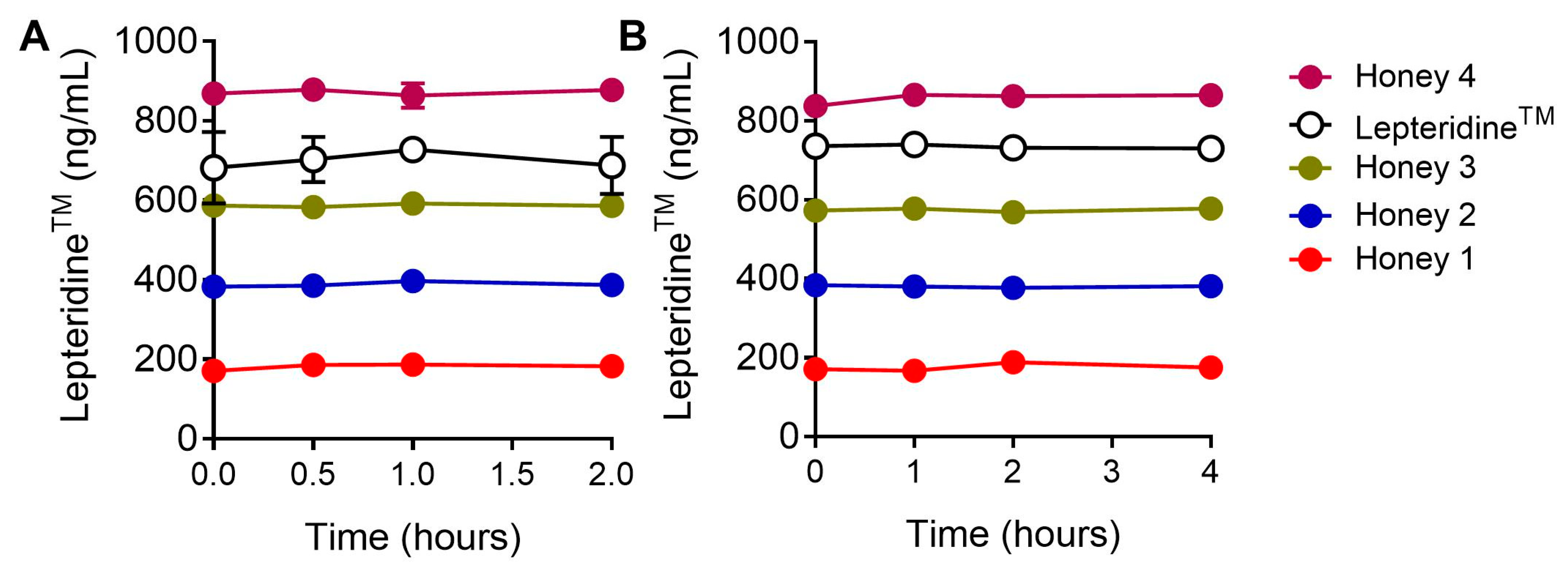
3.5. Gastric Stability of LepteridineTM
4. Discussion
5. Conclusions
6. Intellectual Property
- WO/2017/099612—Marker Compounds of Leptospermum Honeys and Methods of Isolation and Assaying Thereof.
- WO/2021/002763—Use of a Composition Comprising 3,6,7-Trimethyllumazine for Preventing, Ameliorating, or Treating MMP-9-Associated Conditions and Inflammation.
- LepteridineTM is a trademark of Comvita Limited for 3,6,7-trimethyllumazine. All references to the Lepteridine trademark in this article are with the authorisation of Comvita Limited.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Candidate | Rationale | Citation |
|---|---|---|
| MMP-9 | Key role in extracellular matrix remodelling, cell proliferation, and inflammatory pathways including cytokine and chemokine processing. Associated with gastric mucosal damage and ulcer formation. | [31,58,65] |
| EDNRB | Receptor for ET-1 produced by gastrointestinal tract. Evokes dysregulation of cytokine production and a feature of inflammatory bowel disease. | [66] |
| PTGS2 | Intestinal immune response regulation. | [67] |
| JAK1 | Receptor for several inflammatory cytokines. Target of some ulcerative colitis medications. | [68] |
| HSP90 | Upregulated in inflammation and coordinates transcriptional response. Potential inflammatory bowel disease treatment target. | [69] |
| PC | Potential mediator of metabolic stress-driven inflammation and oxidative stress. | [70] |
| TGM2 | Can regulate cytokine-induced gene transcription via nuclear factor-κB. Upregulated in an inflammatory state and wounds. | [71,72] |
| ANPEP | Removes amino acids from peptide substrates. Plays key roles in hormone regulation, maturation, and degradation of bioactive peptides including inflammatory cytokines. | [73] |
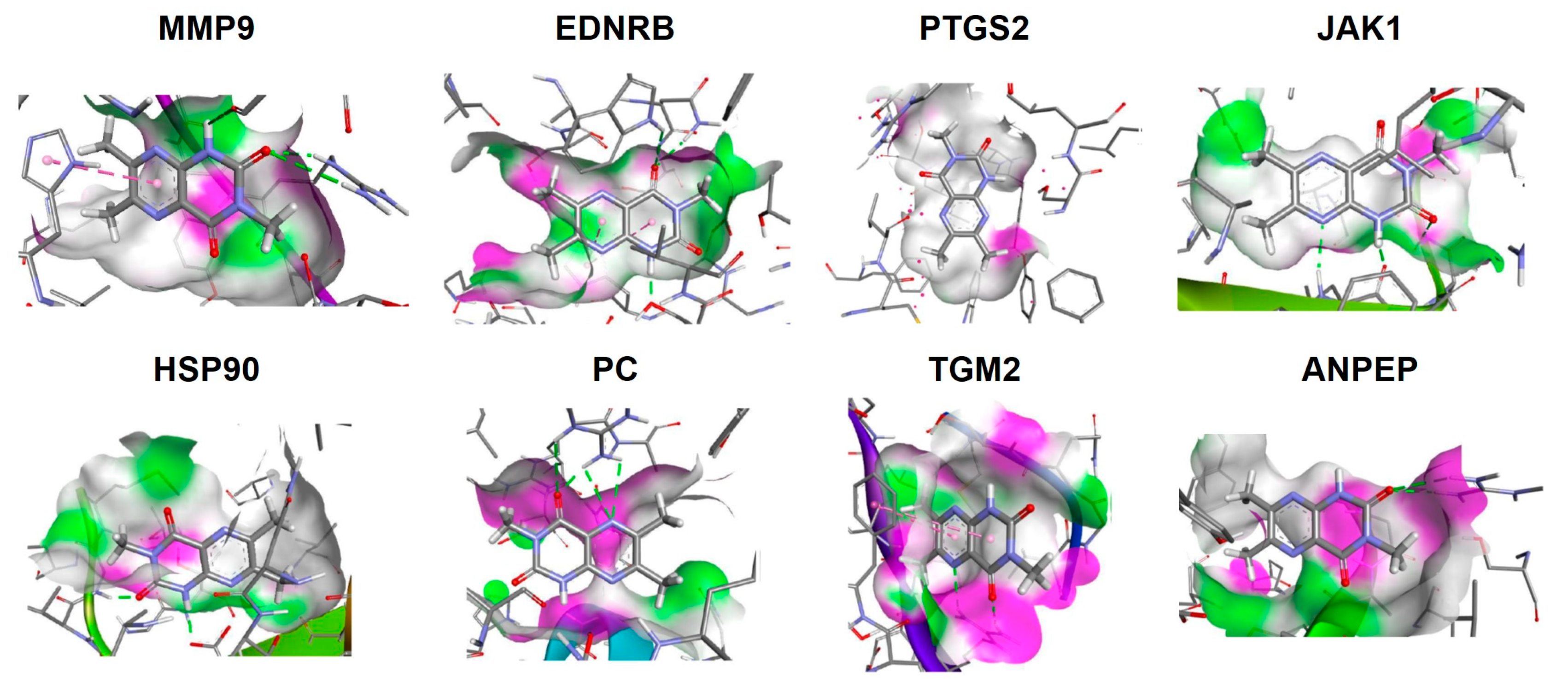

References
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef]
- Scepankova, H.; Combarros-Fuertes, P.; Fresno, J.M.; Tornadijo, M.E.; Dias, M.S.; Pinto, C.A.; Saraiva, J.A.; Estevinho, L.M. Role of Honey in Advanced Wound Care. Molecules 2021, 26, 4784. [Google Scholar] [CrossRef]
- Carter, D.A.; Blair, S.E.; Cokcetin, N.N.; Bouzo, D.; Brooks, P.; Schothauer, R.; Harry, E.J. Therapeutic Manuka Honey: No Longer So Alternative. Front. Microbiol. 2016, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- Molan, P.C. The evidence supporting the use of honey as a wound dressing. Int. J. Low. Extrem. Wounds 2006, 5, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Cokcetin, N.N.; Pappalardo, M.; Campbell, L.T.; Brooks, P.; Carter, D.A.; Blair, S.E.; Harry, E.J. The Antibacterial Activity of Australian Leptospermum Honey Correlates with Methylglyoxal Levels. PLoS ONE 2016, 11, e0167780. [Google Scholar] [CrossRef]
- Daniels, B.J.; Prijic, G.; Meidinger, S.; Loomes, K.M.; Stephens, J.M.; Schlothauer, R.C.; Furkert, D.P.; Brimble, M.A. Isolation, Structural Elucidation, and Synthesis of Lepteridine from Mānuka (Leptospermum scoparium) Honey. J. Agric. Food Chem. 2016, 64, 5079–5084. [Google Scholar] [CrossRef]
- Stephens, J.M.; Schlothauer, R.C.; Morris, B.D.; Yang, D.; Fearnley, L.; Greenwood, D.R.; Loomes, K.M. Phenolic compounds and methylglyoxal in some New Zealand manuka and kanuka honeys. Food Chem. 2010, 120, 78–164. [Google Scholar] [CrossRef]
- Kato, Y.; Umeda, N.; Maeda, A.; Matsumoto, D.; Kitamoto, N.; Kikuzaki, H. Identification of a novel glycoside, leptosin, as a chemical marker of manuka honey. J. Agric. Food Chem. 2012, 60, 3418–3423. [Google Scholar] [CrossRef]
- Yao, L.; Datta, N.; Tomás-Barberán, F.A.; Ferreres, F.; Martos, I.; Singanusong, R. Flavonoids, phenolic acids and abscisic acid in Australian and New Zealand Leptospermum honeys. Food Chem. 2003, 81, 159–168. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Mazzoni, L.; Giampieri, F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S.B.; Abbas, A.T.; Al-Hindi, R.R.; El-Shitany, N.A.; Abdel-Dayem, U.A.; Ali, S.S.; Saleh, R.M.; Al Jaouni, S.K.; Kamal, M.A.; Harakeh, S.M. Manuka Honey Exerts Antioxidant and Anti-Inflammatory Activities That Promote Healing of Acetic Acid-Induced Gastric Ulcer in Rats. Evid.-Based Complement. Altern. Med. 2017, 2017, 5413917. [Google Scholar] [CrossRef] [PubMed]
- Medhi, B.; Prakash, A.; Avti, P.K.; Saikia, U.N.; Pandhi, P.; Khanduja, K.L. Effect of Manuka honey and sulfasalazine in combination to promote antioxidant defense system in experimentally induced ulcerative colitis model in rats. Indian J. Exp. Biol. 2008, 46, 583–590. [Google Scholar]
- Prakash, A.; Medhi, B.; Avti, P.K.; Saikia, U.N.; Pandhi, P.; Khanduja, K.L. Effect of different doses of Manuka honey in experimentally induced inflammatory bowel disease in rats. Phytother. Res. 2008, 22, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Ferrari, D.; Collina, S.; Curti, V. Influence of in vitro simulated gastroduodenal digestion on methylglyoxal concentration of Manuka (Lectospermum scoparium) honey. J. Agric. Food Chem. 2013, 61, 2140–2145. [Google Scholar] [CrossRef]
- Degen, J.; Vogel, M.; Richter, D.; Hellwig, M.; Henle, T. Metabolic transit of dietary methylglyoxal. J. Agric. Food Chem. 2013, 61, 10253–10260. [Google Scholar] [CrossRef]
- Lin, B.; Daniels, B.J.; Middleditch, M.J.; Furkert, D.P.; Brimble, M.A.; Bong, J.; Stephens, J.M.; Loomes, K.M. Utility of the Leptospermum scoparium Compound Lepteridine as a Chemical Marker for Manuka Honey Authenticity. ACS Omega 2020, 5, 8858–8866. [Google Scholar] [CrossRef]
- Carmona-Martinez, V.; Ruiz-Alcaraz, A.J.; Vera, M.; Guirado, A.; Martinez-Esparza, M.; Garcia-Penarrubia, P. Therapeutic potential of pteridine derivatives: A comprehensive review. Med. Res. Rev. 2019, 39, 461–516. [Google Scholar] [CrossRef]
- De Jonghe, S.; Marchand, A.; Gao, L.J.; Calleja, A.; Cuveliers, E.; Sienaert, I.; Herman, J.; Clydesdale, G.; Sefrioui, H.; Lin, Y.; et al. Synthesis and in vitro evaluation of 2-amino-4-N-piperazinyl-6-(3,4-dimethoxyphenyl)-pteridines as dual immunosuppressive and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2011, 21, 145–149. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Patsilinakos, A.; Tran, T.M.; Marson, C.M. Pteridine-2,4-diamine derivatives as radical scavengers and inhibitors of lipoxygenase that can possess anti-inflammatory properties. Future Med. Chem. 2015, 7, 1937–1951. [Google Scholar] [CrossRef]
- Shen, C.; Dillissen, E.; Kasran, A.; Lin, Y.; Herman, J.; Sienaert, I.; De Jonghe, S.; Kerremans, L.; Geboes, K.; Boon, L.; et al. Immunosuppressive activity of a new pteridine derivative (4AZA1378) alleviates severity of TNBS-induced colitis in mice. Clin. Immunol. 2007, 122, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.C.; Nebert, D.W.; Vasiliou, V. Update of human and mouse matrix metalloproteinase families. Hum. Genom. 2010, 4, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Widgerow, A.D. Nanocrystalline silver, gelatinases and the clinical implications. Burns 2010, 36, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Engers, J.; Haque, M.; King, S.; Al-Omari, D.; Ma, T.Y. Matrix Metalloproteinase-9 (MMP-9) induced disruption of intestinal epithelial tight junction barrier is mediated by NF-kappaB activation. PLoS ONE 2021, 16, e0249544. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Meijer, M.J.; Kubben, F.J.; Sier, C.F.; Kruidenier, L.; van Duijn, W.; van den Berg, M.; van Hogezand, R.A.; Lamers, C.B.; Verspaget, H.W. Expression of matrix metalloproteinases-2 and -9 in intestinal tissue of patients with inflammatory bowel diseases. Dig. Liver Dis. 2005, 37, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Baugh, M.D.; Perry, M.J.; Hollander, A.P.; Davies, D.R.; Cross, S.S.; Lobo, A.J.; Taylor, C.J.; Evans, G.S. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology 1999, 117, 814–822. [Google Scholar] [CrossRef]
- Garg, P.; Vijay-Kumar, M.; Wang, L.; Gewirtz, A.T.; Merlin, D.; Sitaraman, S.V. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 296, G175–G184. [Google Scholar] [CrossRef]
- Moore, B.A.; Manthey, C.L.; Johnson, D.L.; Bauer, A.J. Matrix metalloproteinase-9 inhibition reduces inflammation and improves motility in murine models of postoperative ileus. Gastroenterology 2011, 141, 1283–1292.e4. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, X.; Shapiro, S.D.; Shipley, J.M.; Twining, S.S.; Diaz, L.A.; Senior, R.M.; Werb, Z. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell 2000, 102, 647–655. [Google Scholar] [CrossRef]
- Van den Steen, P.E.; Proost, P.; Wuyts, A.; Van Damme, J.; Opdenakker, G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 2000, 96, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Van den Steen, P.E.; Proost, P.; Brand, D.D.; Kang, A.H.; Van Damme, J.; Opdenakker, G. Generation of glycosylated remnant epitopes from human collagen type II by gelatinase B. Biochemistry 2004, 43, 10809–10816. [Google Scholar] [CrossRef] [PubMed]
- Bong, J.; Loomes, K.M.; Lin, B.; Stephens, J.M. New approach: Chemical and fluorescence profiling of NZ honeys. Food Chem. 2018, 267, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Aitken, H.; Johannes, M.; Loomes, K.M.; Brimble, M.A. Synthesis of leptosin, a glycoside isolated from mānuka honey. Tetrahedron Lett. 2013, 54, 6916–6919. [Google Scholar] [CrossRef]
- MacPherson, L.J.; Bayburt, E.K.; Capparelli, M.P.; Carroll, B.J.; Goldstein, R.; Justice, M.R.; Zhu, L.; Hu, S.; Melton, R.A.; Fryer, L.; et al. Discovery of CGS 27023A, a non-peptidic, potent, and orally active stromelysin inhibitor that blocks cartilage degradation in rabbits. J. Med. Chem. 1997, 40, 2525–2532. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbour Laboratory: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Smith, P.; Krohn, R.; Hermanson, G.; Malia, A.; Gartner, F. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Duncan, M.E.; Richardson, J.P.; Murray, G.I.; Melvin, W.T.; Fothergill, J.E. Human matrix metalloproteinase-9: Activation by limited trypsin treatment and generation of monoclonal antibodies specific for the activated form. Eur. J. Biochem. 1998, 258, 37–43. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Murphy, G.; Nagase, H. Progress in matrix metalloproteinase research. Mol. Asp. Med. 2008, 29, 290–308. [Google Scholar] [CrossRef]
- Overall, C.M. Molecular determinants of metalloproteinase substrate specificity: Matrix metalloproteinase substrate binding domains, modules, and exosites. Mol. Biotechnol. 2002, 22, 51–86. [Google Scholar] [CrossRef] [PubMed]
- Collier, I.E.; Krasnov, P.A.; Strongin, A.Y.; Birkedal-Hansen, H.; Goldberg, G.I. Alanine scanning mutagenesis and functional analysis of the fibronectin-like collagen-binding domain from human 92-kDa type IV collagenase. J. Biol. Chem. 1992, 267, 6776–6781. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Chen, J.; Khalil, R.A. Zymography as a Research Tool in the Study of Matrix Metalloproteinase Inhibitors. Methods Mol. Biol. 2017, 1626, 79–102. [Google Scholar] [CrossRef] [PubMed]
- Woessner, J.F., Jr. Quantification of matrix metalloproteinases in tissue samples. Methods Enzymol. 1995, 248, 510–528. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Maekawa, R.; Tanaka, H.; Matsumoto, M.; Takeda, Y.; Tamura, Y.; Nemori, R.; Yoshioka, T. Inhibition of gelatinolytic activity in tumor tissues by synthetic matrix metalloproteinase inhibitor: Application of film in situ zymography. Clin. Cancer Res. 2000, 6, 3290–3296. [Google Scholar]
- Kleiner, D.E.; Stetler-Stevenson, W.G. Quantitative zymography: Detection of picogram quantities of gelatinases. Anal. Biochem. 1994, 218, 325–329. [Google Scholar] [CrossRef]
- Van den Steen, P.E.; Dubois, B.; Nelissen, I.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit. Rev. Biochem. Mol. Biol. 2002, 37, 375–536. [Google Scholar] [CrossRef]
- Lin, B.; Loomes, K.M.; Prijic, G.; Schlothauer, R.; Stephens, J.M. Lepteridine as a unique fluorescent marker for the authentication of manuka honey. Food Chem. 2017, 225, 175–180. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Lauer-Fields, J.L.; Fields, G.B.; Steffensen, B. Contributions of the MMP-2 collagen binding domain to gelatin cleavage. Substrate binding via the collagen binding domain is required for hydrolysis of gelatin but not short peptides. Matrix Biol. 2004, 23, 171–181. [Google Scholar] [CrossRef]
- Murphy, G.; Nguyen, Q.; Cockett, M.I.; Atkinson, S.J.; Allan, J.A.; Knight, C.G.; Willenbrock, F.; Docherty, A.J. Assessment of the role of the fibronectin-like domain of gelatinase A by analysis of a deletion mutant. J. Biol. Chem. 1994, 269, 6632–6636. [Google Scholar] [CrossRef]
- O’Farrell, T.J.; Pourmotabbed, T. The fibronectin-like domain is required for the type V and XI collagenolytic activity of gelatinase B. Arch. Biochem. Biophys. 1998, 354, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Fields, G.B. New strategies for targeting matrix metalloproteinases. Matrix Biol. 2015, 44–46, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Kukacka, J.; Krizkova, S.; Huska, D.; Adam, V.; Masarik, M.; Prusa, R.; Kizek, R. Matrix metalloproteinases. Curr. Med. Chem. 2010, 17, 3751–3768. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, M.; Mohanty, P.S. Molecular docking in organic, inorganic, and hybrid systems: A tutorial review. Monatshefte Chem.—Chem. Mon. 2023, 154, 683–707. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.; Meadows, M.B.; Cherukuri, K.; Smeltzer, M.P.; Smith, R.A.; Radic, M.Z.; Bowlin, G.L. The Effect of Manuka Honey on dHL-60 Cytokine, Chemokine, and Matrix-Degrading Enzyme Release under Inflammatory Conditions. Med. One 2019, 4, e190005. [Google Scholar] [CrossRef]
- Tsang, K.K.; Kwong, E.W.; To, T.S.; Chung, J.W.; Wong, T.K. A Pilot Randomized, Controlled Study of Nanocrystalline Silver, Manuka Honey, and Conventional Dressing in Healing Diabetic Foot Ulcer. Evid.-Based Complement. Altern. Med. 2017, 2017, 5294890. [Google Scholar] [CrossRef]
- Li, S.L.; Zhao, J.R.; Ren, X.Y.; Xie, J.P.; Ma, Q.Z.; Rong, Q.H. Increased expression of matrix metalloproteinase-9 associated with gastric ulcer recurrence. World J. Gastroenterol. WJG 2013, 19, 4590–4595. [Google Scholar] [CrossRef]
- Schonbeck, U.; Mach, F.; Libby, P. Generation of biologically active IL-1 beta by matrix metalloproteinases: A novel caspase-1-independent pathway of IL-1 beta processing. J. Immunol. 1998, 161, 3340–3346. [Google Scholar] [CrossRef]
- Jacobsen, J.A.; Major Jourden, J.L.; Miller, M.T.; Cohen, S.M. To bind zinc or not to bind zinc: An examination of innovative approaches to improved metalloproteinase inhibition. Biochim. Biophys. Acta 2010, 1803, 72–94. [Google Scholar] [CrossRef]
- Saghatelian, A.; Jessani, N.; Joseph, A.; Humphrey, M.; Cravatt, B.F. Activity-based probes for the proteomic profiling of metalloproteases. Proc. Natl. Acad. Sci. USA 2004, 101, 10000–10005. [Google Scholar] [CrossRef]
- Becker, D.P.; Barta, T.E.; Bedell, L.J.; Boehm, T.L.; Bond, B.R.; Carroll, J.; Carron, C.P.; Decrescenzo, G.A.; Easton, A.M.; Freskos, J.N.; et al. Orally active MMP-1 sparing alpha-tetrahydropyranyl and alpha-piperidinyl sulfone matrix metalloproteinase (MMP) inhibitors with efficacy in cancer, arthritis, and cardiovascular disease. J. Med. Chem. 2010, 53, 6653–6680. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.T. The importance of estimating the therapeutic index in the development of matrix metalloproteinase inhibitors. Cardiovasc. Res. 2006, 69, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.M.; Wold, J.P.; Engelsen, S.B. Auto fluorescence Spectroscopy in Food Analysis. In Handbook of Food Analysis Instruments; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Caron, A.; Desrosiers, R.R.; Beliveau, R. Ischemia injury alters endothelial cell properties of kidney cortex: Stimulation of MMP-9. Exp. Cell Res. 2005, 310, 105–116. [Google Scholar] [CrossRef]
- Angerio, A.D.; Bufalino, D.; Bresnick, M.; Bell, C.; Brill, S. Inflammatory bowel disease and endothelin-1: A review. Crit. Care Nurs. Q. 2005, 28, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dubois, R.N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 2010, 29, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, F.R.; Colbert, R.A.; Gadina, M. JAK1: Number one in the family; number one in inflammation? Rheumatology 2021, 60, ii3–ii10. [Google Scholar] [CrossRef]
- Hoter, A.; Naim, H.Y. The Functions and Therapeutic Potential of Heat Shock Proteins in Inflammatory Bowel Disease—An Update. Int. J. Mol. Sci. 2019, 20, 5331. [Google Scholar] [CrossRef]
- Hughey, C.C.; Crawford, P.A. Pyruvate Carboxylase Wields a Double-Edged Metabolic Sword. Cell Metab. 2019, 29, 1236–1238. [Google Scholar] [CrossRef]
- Mirza, A.; Liu, S.L.; Frizell, E.; Zhu, J.; Maddukuri, S.; Martinez, J.; Davies, P.; Schwarting, R.; Norton, P.; Zern, M.A. A role for tissue transglutaminase in hepatic injury and fibrogenesis, and its regulation by NF-kappaB. Am. J. Physiol. 1997, 272, G281–G288. [Google Scholar] [CrossRef]
- Agnihotri, N.; Kumar, S.; Mehta, K. Tissue transglutaminase as a central mediator in inflammation-induced progression of breast cancer. Breast Cancer Res. 2013, 15, 202. [Google Scholar] [CrossRef]
- Lu, C.; Amin, M.A.; Fox, D.A. CD13/Aminopeptidase N Is a Potential Therapeutic Target for Inflammatory Disorders. J. Immunol. 2020, 204, 3–11. [Google Scholar] [CrossRef] [PubMed]



| Candidate | Docking Structure | GoldScore | ChemScore | Compound Similarity | Likely Binding, or Interacting with Inhibitor Residues/Domains |
|---|---|---|---|---|---|
| MMP9 | 6ESM | 53.47 | 16.81 | Active/Inactive | No |
| EDNRB | 6K1Q | 48.35 | 17.63 | NA | Unclear |
| PTGS2 | 5IKR | 44.33 | 11.77 | Inactive | No |
| JAK1 | 6N7A | 43.32 | 19.33 | Active/Inactive | Weak |
| HSP90 | 5XRD | 40.27 | 13.63 | Inactive | Weak |
| PC | 3BG3 | 37.94 | 11.39 | Inactive | No |
| TGM2 | 1KV3 | 37.52 | 20.38 | Active/Inactive | Weak |
| ANPEP | 4FYT | 36.05 | 15.03 | Inactive | Weak |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, B.; Nair, S.; Fellner, D.M.J.; Nasef, N.A.; Singh, H.; Negron, L.; Goldstone, D.C.; Brimble, M.A.; Gerrard, J.A.; Domigan, L.; et al. The Leptospermum scoparium (Mānuka)-Specific Nectar and Honey Compound 3,6,7-Trimethyllumazine (LepteridineTM) That Inhibits Matrix Metalloproteinase 9 (MMP-9) Activity. Foods 2023, 12, 4072. https://doi.org/10.3390/foods12224072
Lin B, Nair S, Fellner DMJ, Nasef NA, Singh H, Negron L, Goldstone DC, Brimble MA, Gerrard JA, Domigan L, et al. The Leptospermum scoparium (Mānuka)-Specific Nectar and Honey Compound 3,6,7-Trimethyllumazine (LepteridineTM) That Inhibits Matrix Metalloproteinase 9 (MMP-9) Activity. Foods. 2023; 12(22):4072. https://doi.org/10.3390/foods12224072
Chicago/Turabian StyleLin, Bin, Smitha Nair, Daniel M. J. Fellner, Noha Ahmed Nasef, Harjinder Singh, Leonardo Negron, David C. Goldstone, Margaret A. Brimble, Juliet A. Gerrard, Laura Domigan, and et al. 2023. "The Leptospermum scoparium (Mānuka)-Specific Nectar and Honey Compound 3,6,7-Trimethyllumazine (LepteridineTM) That Inhibits Matrix Metalloproteinase 9 (MMP-9) Activity" Foods 12, no. 22: 4072. https://doi.org/10.3390/foods12224072
APA StyleLin, B., Nair, S., Fellner, D. M. J., Nasef, N. A., Singh, H., Negron, L., Goldstone, D. C., Brimble, M. A., Gerrard, J. A., Domigan, L., Evans, J. C., Stephens, J. M., Merry, T. L., & Loomes, K. M. (2023). The Leptospermum scoparium (Mānuka)-Specific Nectar and Honey Compound 3,6,7-Trimethyllumazine (LepteridineTM) That Inhibits Matrix Metalloproteinase 9 (MMP-9) Activity. Foods, 12(22), 4072. https://doi.org/10.3390/foods12224072








