Observation and Measurement of Ice Morphology in Foods: A Review
Abstract
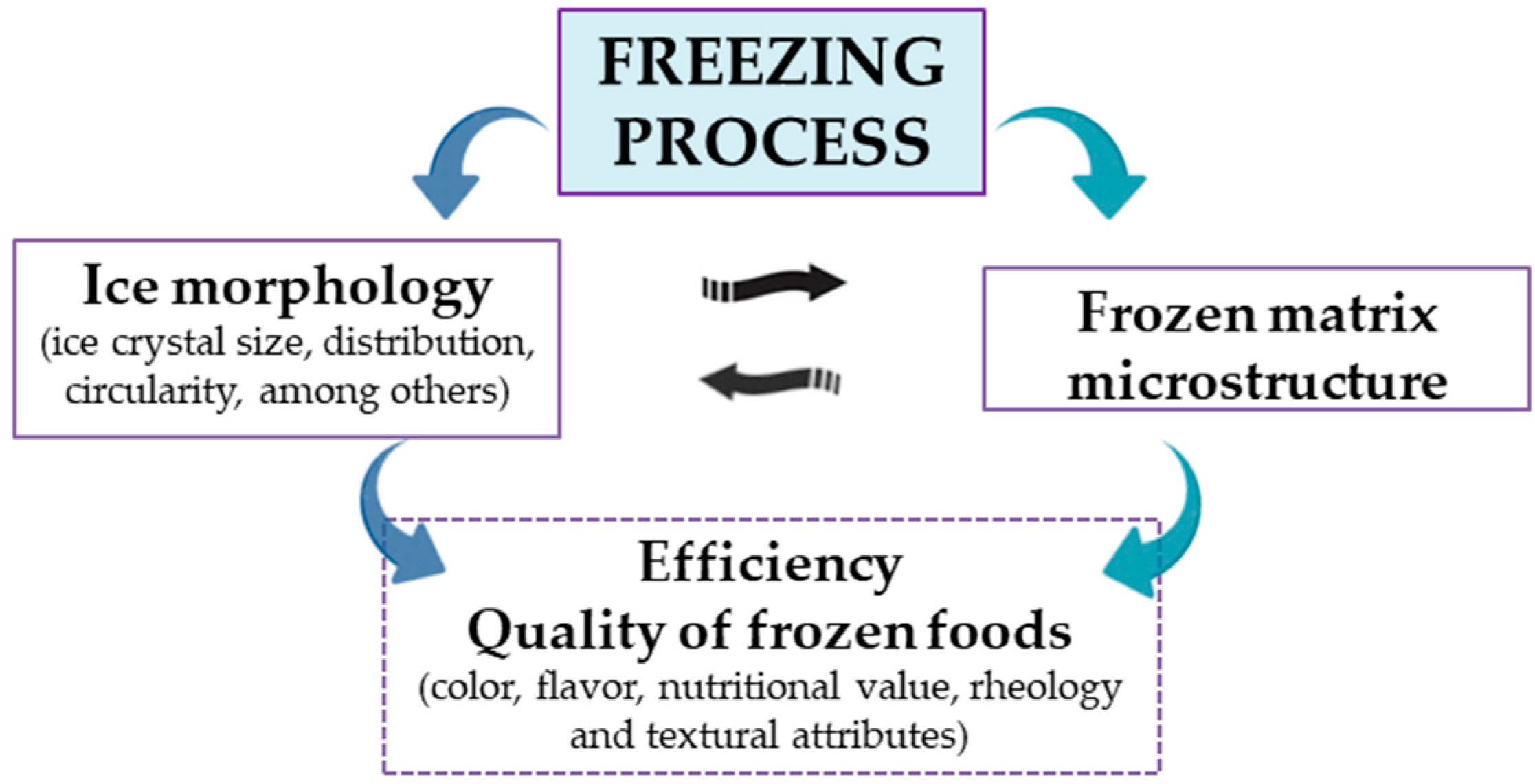
:1. Introduction
2. Methodology
3. Optical Microscopy Applied to Frozen Foods
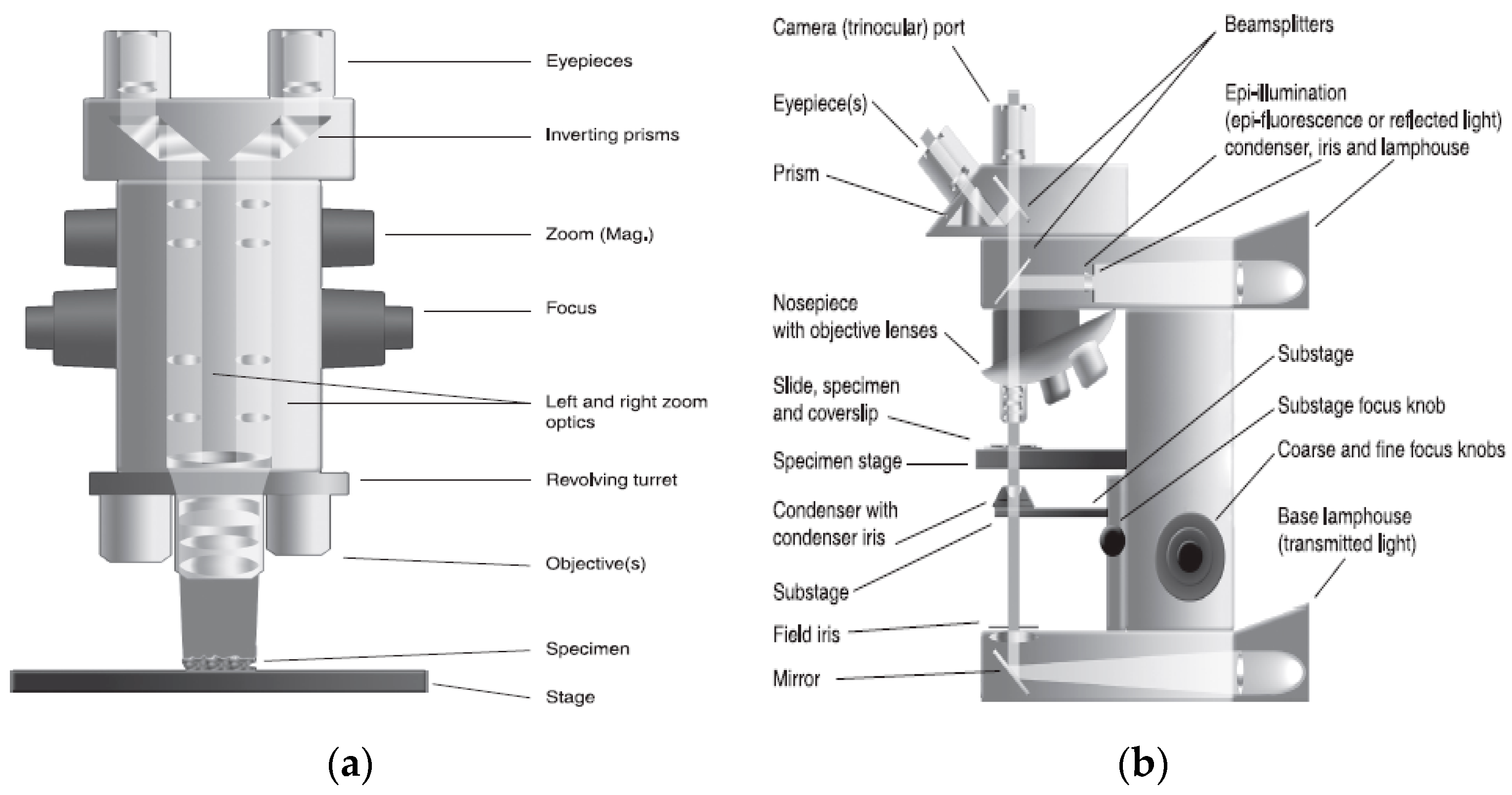
3.1. Light Microscopy (LM) and Cryo-Light Microscopy (Cryo-LM)
3.2. Application of LM and Cryo-LM to Visualizate Ice Crystals in Frozen Foods
4. Electron Microscopy Applied to Frozen Foods
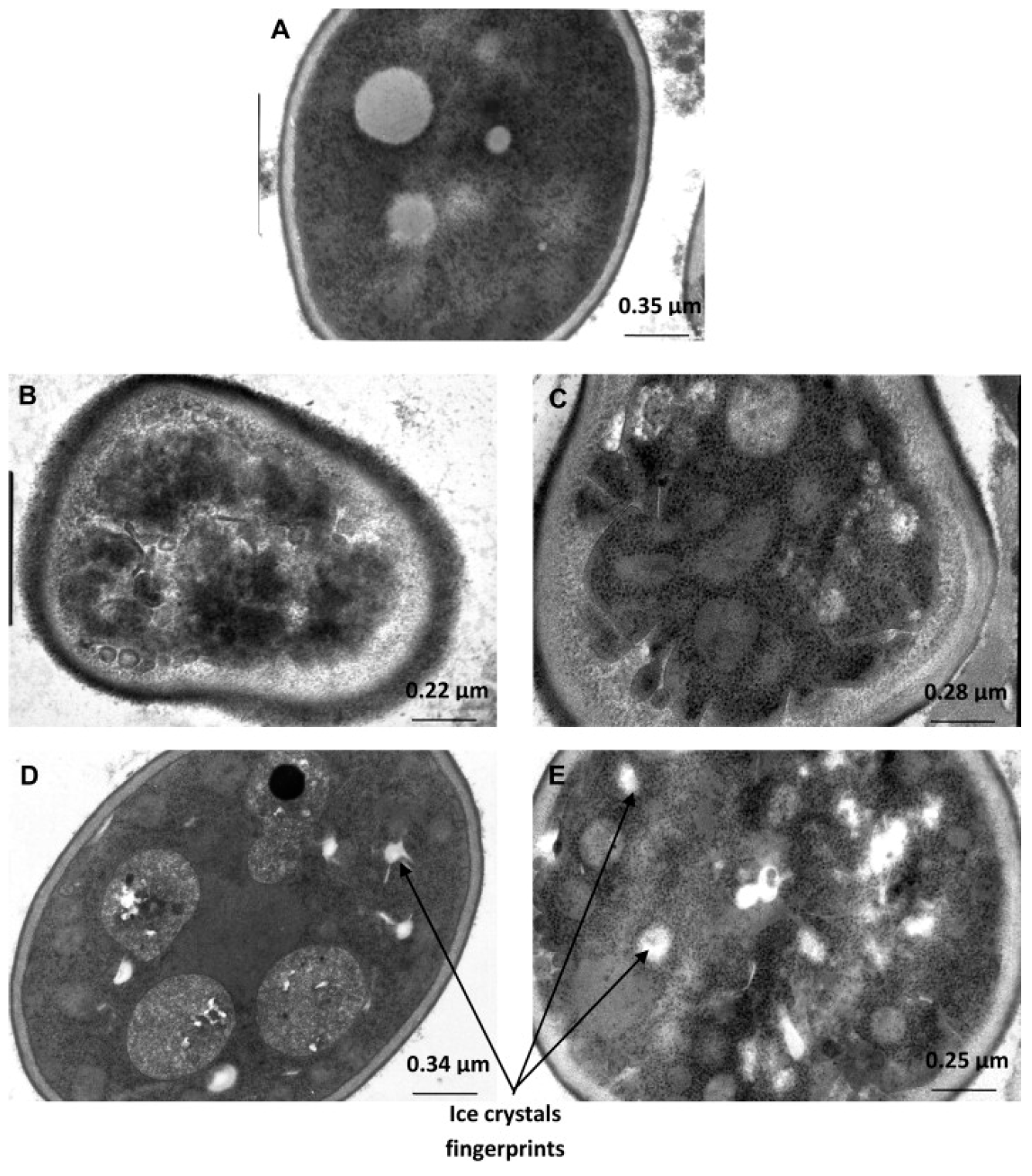
4.1. TEM
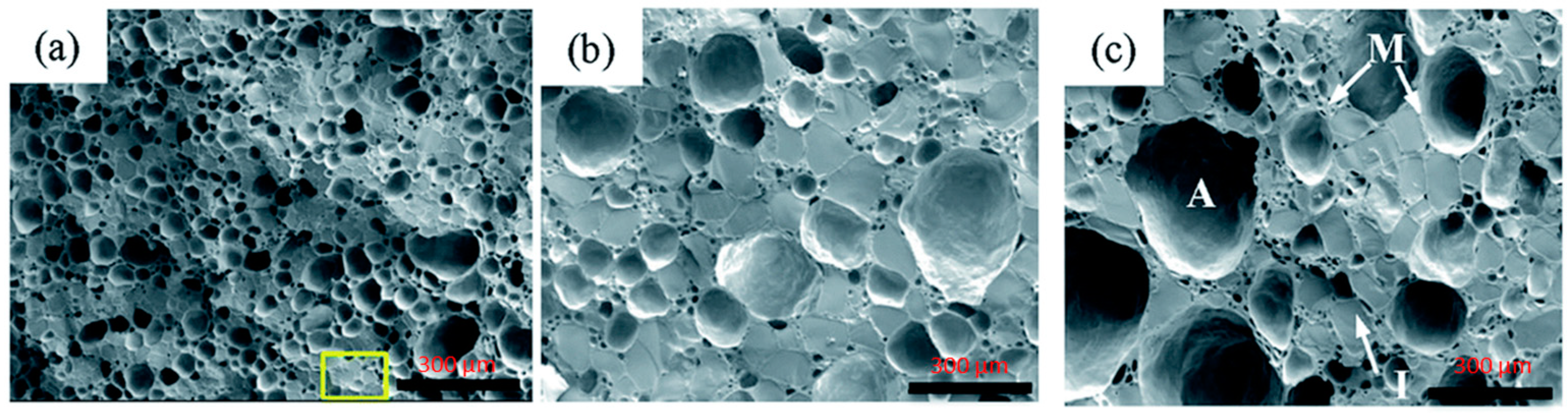
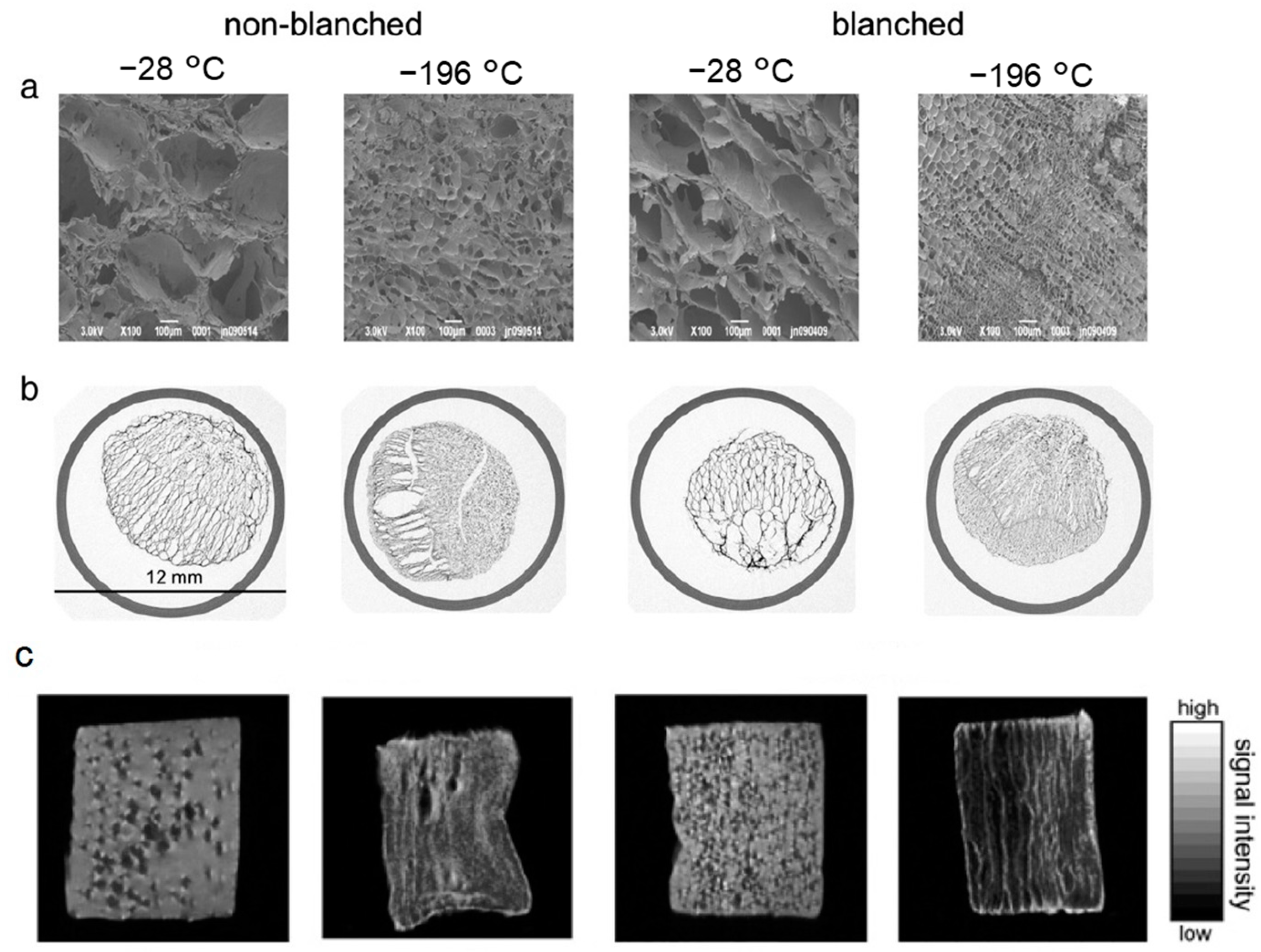
4.2. SEM
Cryo-SEM
4.3. ESEM
5. Other Microscopy Techniques
Application in Frozen Foods
6. Conclusions and Future Challenges
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Zhang, M.; Mujumdar, A.S. Technological innovations or advancement in detecting frozen and thawed meat quality: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1483–1499. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; He, X.; Nirasawa, S.; Tatsumi, E.; Liu, H.J.; Liu, H. Effects of high voltage electrostatic field on the freezing behavior and quality of pork tenderloin. J. Food Eng. 2017, 204, 18–26. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Z.; Sun, D.W. Effects of freezing on cell structure of fresh cellular food materials: A review. Trends Food Sci. Technol. 2018, 75, 46–55. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, M.; Liu, W.; Mujumdar, A.S.; Bai, B. Novel synergistic freezing methods and technologies for enhanced food product quality: A critical review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1979–2001. [Google Scholar] [CrossRef]
- Wu, C.H.; Yuan, C.H.; Ye, X.Q.; Hu, Y.Q.; Chen, S.G.; Liu, D.H. A critical review on superchilling preservation technology in aquatic product. J. Integr. Agric. 2014, 13, 2788–2806. [Google Scholar] [CrossRef]
- Jia, G.; Chen, Y.; Sun, A.; Orlien, V. Control of ice crystal nucleation and growth during the food freezing process. Crit. Rev. Food Sci. Nutr. 2022, 21, 2433–2454. [Google Scholar] [CrossRef]
- You, Y.; Kang, T.; Jun, S. Control of ice nucleation for subzero food preservation. Food Eng. Rev. 2021, 13, 15–35. [Google Scholar] [CrossRef]
- Petzold, G.; Aguilera, J.M. Ice morphology: Fundamentals and technological applications in foods. Food Biophys. 2009, 4, 378–396. [Google Scholar] [CrossRef]
- Kiani, H.; Sun, D.W. Water crystallization and its importance to freezing of foods: A review. Trends Food Sci. Technol. 2011, 22, 407–426. [Google Scholar] [CrossRef]
- Kumar, P.K.; Rasco, B.A.; Tang, J.; Sablani, S.S. State/phase transitions, ice recrystallization, and quality changes in frozen foods subjected to temperature fluctuations. Food Eng. Rev. 2020, 12, 421–451. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, Q.; Sun, D.W. Measuring and controlling ice crystallization in frozen foods: A review of recent developments. Trends Food Sci. Technol. 2019, 90, 13–25. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Tobar-Bolaños, G.; Casas-Forero, N.; Zúñiga, R.N.; Petzold, G. Quality attributes of cryoconcentrated calafate (Berberis microphylla) juice during refrigerated storage. Foods 2020, 9, 1314. [Google Scholar] [CrossRef] [PubMed]
- Marcellini, M.; Noirjean, C.; Dedovets, D.; Maria, J.; Deville, S. Time-lapse, in situ imaging of ice crystal growth using confocal microscopy. ACS Omega 2016, 1, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liu, Z.; Li, Y.; Li, Y.; Wang, Z. Experimental study of water drop freezing process on cryogenic cold surface. Int. J. Refrig. 2023, 150, 265–274. [Google Scholar] [CrossRef]
- Chasnitsky, M.; Cohen, S.R.; Rudich, Y.; Braslavsky, I. Atomic force microscopy imaging of ice crystal surfaces formed in aqueous solutions containing ice-binding proteins. J. Cryst. Growth 2023, 601, 126961. [Google Scholar] [CrossRef]
- Sazaki, G.; Inomata, M.; Asakawa, H.; Yokoyama, E.; Nakatsubo, S.; Murata, K.; Nagashima, K.; Furukawa, Y. In-situ optical microscopy observation of elementary steps on ice crystals grown in vapor and their growth kinetics. Prog. Cryst. Growth Charact. Mater. 2021, 67, 100550. [Google Scholar] [CrossRef]
- Tai, K.; Liu, Y.; Dillon, S.J. In situ cryogenic transmission electron microscopy for characterizing the evolution of solidifying water ice in colloidal systems. Microsc. Microanal. 2014, 20, 330–337. [Google Scholar] [CrossRef]
- Ong, L.; Dagastine, R.R.; Kentish, S.E.; Gras, S.L. Microstructure of milk gel and cheese curd observed using cryo scanning electron microscopy and confocal microscopy. LWT-Food Sci. Technol. 2011, 44, 1291–1302. [Google Scholar] [CrossRef]
- Bai, N.; Guo, X.N.; Xing, J.J.; Zhu, K.X. Effect of freeze-thaw cycles on the physicochemical properties and frying performance of frozen Youtiao dough. Food Chem. 2022, 386, 132854. [Google Scholar] [CrossRef]
- Voda, A.; Homan, N.; Witek, M.; Duijster, A.; van Dalen, G.; Van der Sman, R.; Nijsse, J.; Van Vliet, L.; Van As, H.; van Duynhoven, J. The impact of freeze-drying on microstructure and rehydration properties of carrot. Food Res. Int. 2012, 49, 687–693. [Google Scholar] [CrossRef]
- Kono, S.; Kon, M.; Araki, T.; Sagara, Y. Effects of relationships among freezing rate, ice crystal size and color on surface color of frozen salmon fillet. J. Food Eng. 2017, 214, 158–165. [Google Scholar] [CrossRef]
- Pu, Y.E.; Ma, L.; Dear, B.; Zhu, A.; Li, J.; Zhang, S.; Shi, W. Understanding the impact of microstructures on reconstitution and drying kinetics of lyophilized cake using X-ray Microscopy and image-based simulation. J. Pharm. Sci. 2023, 112, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Peng, Y.; Yang, D.; Wang, Y.; Zhao, R.; Chao, K.; Guo, Q. Detection of frozen pork freshness by fluorescence hyperspectral image. J. Food Eng. 2022, 316, 110840. [Google Scholar] [CrossRef]
- Reid, D.S. Overview of phisycal/chemical aspects of freezing. In Quality in Frozen Food, 1st ed.; Erickson, M.C., Hung, Y.-C., Eds.; Chapman and Hall: New York, NY, USA, 1997; pp. 10–28. [Google Scholar]
- Nesvadba, P. Thermal properties and ice crystal development in frozen foods. In Frozen Food Science and Technology, 1st ed.; Evans, J.A., Ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008; pp. 1–25. [Google Scholar]
- Echlin, P. Low-Temperature Microscopy and Analysis, 1st ed.; Plenum Publishing Press Corporation: New York, NY, USA, 1992; pp. 265–498. [Google Scholar]
- Gunning, P.A. Light microscopy: Principles and applications to food microstructures. In Food Microstructures, 1st ed.; Morris, V.J., Groves, K., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 62–95. [Google Scholar]
- Ninagawa, T.; Eguchi, A.; Kawamura, Y.; Konishi, T.; Narumi, A. A study on ice crystal formation behavior at intracellular freezing of plant cells using a high-speed camera. Cryobiology 2016, 73, 20–29. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Jha, P.K.; Tavakoli, J.; Daraei-Garmakhany, A.; Xanthakis, E.; Le-Bail, A. Review on identification, underlying mechanisms and evaluation of freezing damage. J. Food Eng. 2019, 255, 50–60. [Google Scholar] [CrossRef]
- Moreno, F.L.; Quintanilla-Carvajal, M.X.; Sotelo, L.I.; Osorio, C.; Raventós, M.; Hernández, E.; Ruiz, Y. Volatile compounds, sensory quality and ice morphology in falling-film and block freeze concentration of coffee extract. J. Food Eng. 2015, 166, 64–71. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Guerra-Valle, M.; Gianelli, M.P.; Petzold, G. Evaluation of freeze crystallization on pomegranate juice quality in comparison with conventional thermal processing. Food Biosci. 2021, 41, 101106. [Google Scholar] [CrossRef]
- Wang, Y.; Miyazaki, R.; Saitou, S.; Hirasaka, K.; Takeshita, S.; Tachibana, K.; Taniyama, S. The effect of ice crystals formations on the flesh quality of frozen horse mackerel (Trachurus japonicus). J. Texture Stud. 2018, 49, 485–491. [Google Scholar] [CrossRef]
- Du, X.; Li, H.; Dong, C.; Ren, Y.; Pan, N.; Kong, B.; Liu, H.; Xia, X. Effect of ice structuring protein on the microstructure and myofibrillar protein structure of mirror carp (Cyprinus carpio L.) induced by freeze-thaw processes. LWT-Food Sci. Technol. 2021, 139, 110570. [Google Scholar] [CrossRef]
- Li, M.; Reeder, M.W.; Wu, T. Depletion interaction may reduce ice recrystallization inhibition activity of cellulose nanocrystals (CNCs) at high concentrations. Food Hydrocoll. 2023, 139, 108576. [Google Scholar] [CrossRef]
- Tan, M.; Ye, J.; Chu, Y.; Xie, J. The effects of ice crystal on water properties and protein stability of large yellow croaker (Pseudosciaena crocea). Int. J. Refrig. 2021, 130, 242–252. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, X.; Zhang, Z.; Long, G.; Lyu, F.; Cai, Y.; Liu, J.; Ding, Y. Effects of immersion freezing on ice crystal formation and the protein properties of snakehead (Channa argus). Foods 2020, 9, 411. [Google Scholar] [CrossRef]
- Gutiérrez, M.; De Araújo, L.; Silveira Júnior, V. An empirical model to estimate the growth of ice crystals for storage of Tilapia at variable temperature conditions. Food Sci. Technol. 2019, 39, 567–572. [Google Scholar] [CrossRef]
- Bin, L.; Jianfei, S.; Zhaodn, Y.; Rachid, B. Effects of magnetic field on the phase change cells and the formation of ice crystals in biomaterials: Carrot case. J. Therm. Sci. Eng. Appl. 2017, 9, 031005. [Google Scholar] [CrossRef]
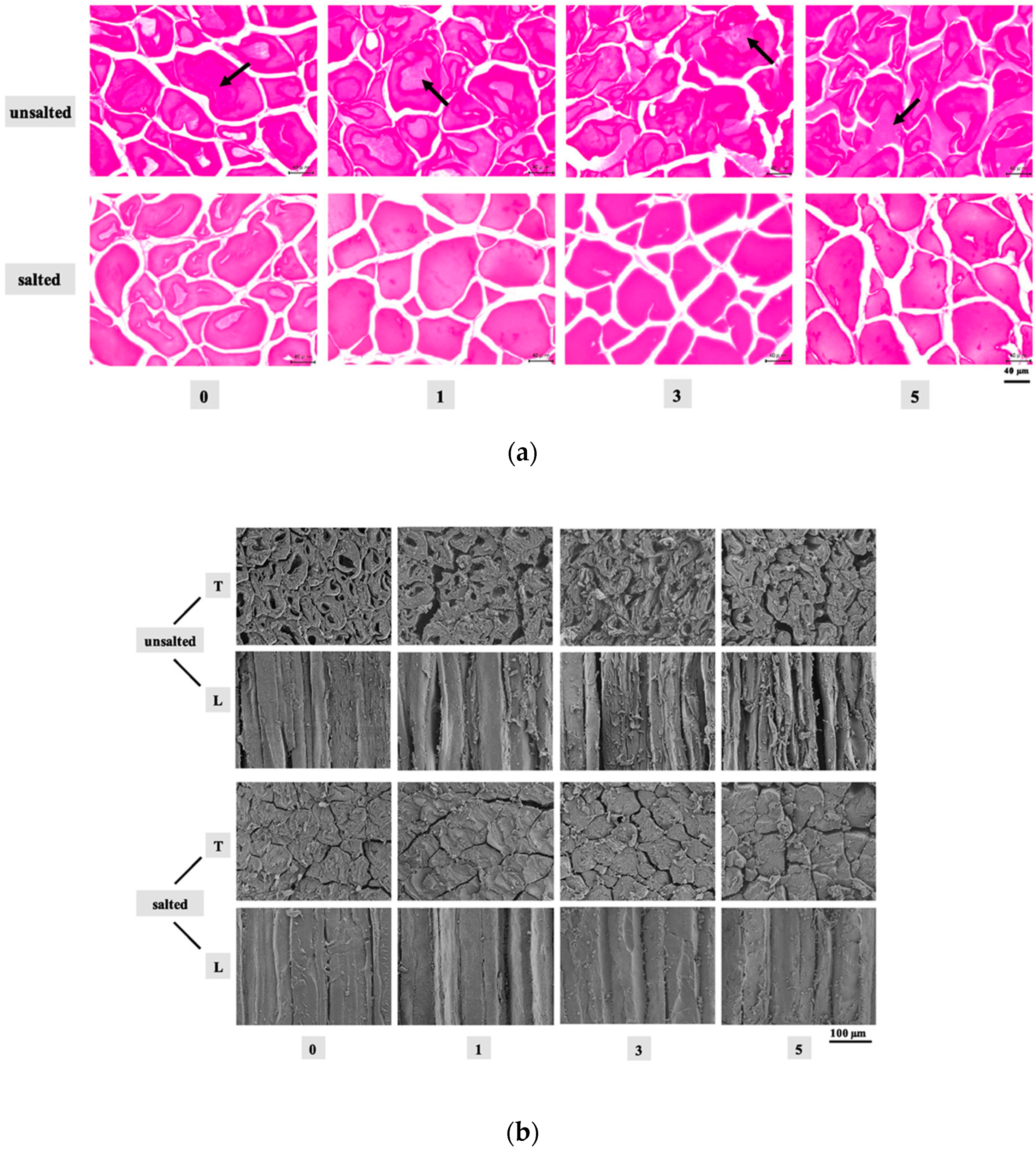
- Jiang, Q.; Jia, R.; Nakazawa, N.; Hu, Y.; Osako, K.; Okazaki, E. Changes in protein properties and tissue histology of tuna meat as affected by salting and subsequent freezing. Food Chem. 2019, 271, 550–560. [Google Scholar] [CrossRef]
- Jiang, Q.; Yin, T.; Yang, F.; Yu, D.; Xu, Y.; Tie, H.; Gao, P.; Wang, B.; Xia, W. Effect of freezing methods on quality changes of grass carp during frozen storage. J. Food Eng. 2020, 43, e13539. [Google Scholar] [CrossRef]
- Robles, C.M.; Quintanilla-Carvajal, M.X.; Moreno, F.L.; Hernández, E.; Raventós, M.; Ruiz, Y. Ice morphology modification and solute recovery improvement by heating and annealing during block freeze-concentration of coffee extracts. J. Food Eng. 2016, 189, 72–81. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, Q.; Xia, X.; Kong, B.; Diao, X. Effects of ultrasound-assisted freezing at different power levels on the structure and thermal stability of common carp (Cyprinus carpio) proteins. Ultrason. Sonochem. 2019, 54, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Chernobai, N.; Shevchenko, N.; Vozovyk, K.; Kadnikova, N.; Rozanov, L. Cryo-Light Microscopy to study the freezing behavior of microalgae cells. Cryobiology 2021, 103, 196. [Google Scholar] [CrossRef]
- Leng, D.; Zhang, H.; Tian, C.; Li, P.; Kong, F.; Zhan, B. Static magnetic field assisted freezing of four kinds of fruits and vegetables: Micro and macro effects. Int. J. Refrig. 2023, 146, 118–125. [Google Scholar] [CrossRef]
- Islam, M.N.; Zhang, M.; Fang, Z.; Sun, J. Direct contact ultrasound assisted freezing of mushroom (Agaricus bisporus): Growth and size distribution of ice crystals. Int. J. Refrig. 2015, 57, 46–53. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Kot, A.; Jakubczyk, E.; Buniowska-Olejnik, M.; Nowacka, M. Effect of ultrasound-assisted freezing on the crystal structure of mango sorbet. Crystals 2023, 13, 396. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, M.; Mujumdar, A.S.; Hu, R. Combination strategy of CO2 pressurization and ultrasound: To improve the freezing quality of fresh-cut honeydew melon. Food Chem. 2022, 383, 132327. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, M.; Mujumdar, A.S.; Chen, B. Effects of electric and magnetic field on freezing characteristics of gel model food. Food Res. Int. 2023, 166, 112566. [Google Scholar] [CrossRef]
- Aguilera, J.M. Rational food design and food microstructure. Trends Food Sci. Technol. 2022, 122, 256–264. [Google Scholar] [CrossRef]
- Franken, L.E.; Grünewald, K.; Boekema, E.J.; Stuart, M.C. A technical introduction to transmission electron microscopy for soft-matter: Imaging, possibilities, choices, and technical developments. Small 2022, 16, 1906198. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci. 2017, 133, 10–18. [Google Scholar] [CrossRef]
- Meziani, S.; Ioannou, I.; Jasniewski, J.; Belhaj, N.; Muller, J.M.; Ghoul, M.; Desobry, S. Effects of freezing treatments on the fermentative activity and gluten network integrity of sweet dough. LWT-Food Sci. Technol. 2012, 46, 118–126. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, S.; Duffy, B.; Jaiswal, A.K. Nanostructured materials for food applications: Spectroscopy, microscopy and physical properties. Bioengineering 2019, 6, 26. [Google Scholar] [CrossRef]
- Ban, C.; Yoon, S.; Han, J.; Kim, S.O.; Han, J.S.; Lim, S.; Choi, Y.J. Effects of freezing rate and terminal freezing temperature on frozen croissant dough quality. LWT-Food Sci. Technol. 2016, 73, 219–225. [Google Scholar] [CrossRef]
- Jiang, Q.; Nakazawa, N.; Hu, Y.; Osako, K.; Okazaki, E. Changes in quality properties and tissue histology of lightly salted tuna meat subjected to multiple freeze-thaw cycles. Food Chem. 2019, 293, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Nishino, Y.; Miyazaki, K.; Kaise, M.; Miyazawa, A. Fine cryo-SEM observation of the microstructure of emulsions frozen via high-pressure freezing. Microscopy 2022, 71, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P.; Thirawong, N.; Cheewatanakornkool, K.; Burapapadh, K.; Sae-Ngow, W. Cryo-scanning electron microscopy (cryo-SEM) as a tool for studying the ultrastructure during bead formation by ionotropic gelation of calcium pectinate. Int. J. Pharm. 2008, 352, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Wang, L.; Wu, T.; Chen, S.; Ding, T.; Hu, Y. A study of ice crystal development in hairtail samples during different freezing processes by cryosectioning versus cryosubstitution method. Int. J. Refrig. 2018, 87, 39–46. [Google Scholar] [CrossRef]
- Kong, C.H.Z.; Hamid, N.; Ma, Q.; Lu, J.; Wang, B.G.; Sarojini, V. Antifreeze peptide pretreatment minimizes freeze-thaw damage to cherries: An in-depth investigation. LWT-Food Sci. Technol. 2017, 84, 441–448. [Google Scholar] [CrossRef]
- Guo, E.; Zeng, G.; Kazantsev, D.; Rockett, P.; Bent, J.; Kirkland, M.; van Dalen, G.; Eastwood, D.S.; StJohn, D.; Lee, P.D. Synchrotron X-ray tomographic quantification of microstructural evolution in ice cream—A multi-phase soft solid. RSC Adv. 2017, 7, 15561–15573. [Google Scholar] [CrossRef]
- Pach, E.; Verdaguer, A. Studying ice with environmental scanning electron microscopy. Molecules 2022, 27, 258. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Zhu, X.; Fei, L.; Huang, H.; Wang, Y. Applications of ESEM on materials science: Recent updates and a look forward. Small Methods 2020, 4, 1900588. [Google Scholar] [CrossRef]
- Hajji, W.; Gliguem, H.; Bellagha, S.; Allaf, K. Structural and textural improvements of strawberry fruits by partial water removal prior to conventional freezing process. J. Food Meas. Charact. 2022, 16, 3344–3353. [Google Scholar] [CrossRef]
- Phothiset, S.; Charoenrein, S. Effects of freezing and thawing on texture, microstructure and cell wall composition changes in papaya tissues. J. Sci. Food Agric. 2014, 94, 189–196. [Google Scholar] [CrossRef]
- Dawson, P.; Al-Jeddawi, W.; Rieck, J. The Effect of different freezing rates and long-term storage temperatures on the stability of sliced peaches. Int. J. Food Sci. 2020, 2020, 9178583. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chotiko, A.; Kyereh, E.; Zhang, J.; Liu, C.; Reyes, V.V.; Bankston, D.; Sathivel, S. Development of a combined osmotic dehydration and cryogenic freezing process for minimizing quality changes during freezing with application to fruits and vegetables. J. Food Process. Preserv. 2017, 41, e12926. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, M.; Bhandari, B.; Cheng, X.; Sun, J. Effect of ultrasound immersion freezing on the quality attributes and water distributions of wrapped red radish. Food Bioprocess Technol. 2015, 8, 1366–1376. [Google Scholar] [CrossRef]
- Egelandsdal, B.; Abie, S.M.; Bjarnadottir, S.; Zhu, H.; Kolstad, H.; Bjerke, F.; Martinsen, Ø.G.; Mason, A.; Münch, D. Detectability of the degree of freeze damage in meat depends on analytic-tool selection. Meat Sci. 2019, 152, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Masselot, V.; Bosc, V.; Benkhelif, H. Influence of stabilizers on the microstructure of fresh sorbets: X-ray micro-computed tomography, cryo-SEM, and focused beam reflectance measurement analyses. J. Food Eng. 2021, 300, 110522. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Sinrod, A.J.G.; Dao, L.; Takeoka, G.; Williams, T.; Wood, D.; Chiou, B.S.; Bridges, D.F.; Wu, V.C.H.; Lyu, C.; et al. Preservation of grape tomato by isochoric freezing. Food Res. Int. 2021, 143, 110228. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.H.; Ding, Y.; Xiao, H.W.; Sablani, S.S.; Nie, Y.; Wu, S.J.; Tang, X.M. Changes in the vitamin C content of mango with water state and ice crystals under state/phase transitions during frozen storage. J. Food Eng. 2018, 222, 49–53. [Google Scholar] [CrossRef]
- Zielinska, M.; Markowski, M.; Zielinska, D. The effect of freezing on the hot air and microwave vacuum drying kinetics and texture of whole cranberries. Dry. Technol. 2019, 37, 1714–1730. [Google Scholar] [CrossRef]
- Kahraman, O.; Malvandi, A.; Vargas, L.; Feng, H. Drying characteristics and quality attributes of apple slices dried by a non-thermal ultrasonic contact drying method. Ultrason. Sonochem. 2021, 73, 105510. [Google Scholar] [CrossRef]
- Schoeman, L.; Williams, P.; Du Plessis, A.; Manley, M. X-ray micro-computed tomography (µCT) for non-destructive characterization of food microstructure. Trends Food Sci. Technol. 2016, 47, 10–24. [Google Scholar] [CrossRef]
- Reis, M.; Martínez, E.; Saitua, E.; Rodríguez, R.; Perez, I.; Olabarrieta, I. Non-invasive differentiation between fresh and frozen/thawed tuna fillets using near infrared spectroscopy (Vis-NIRS). LWT-Food Sci. Technol. 2017, 78, 129–137. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.W.; Pu, H.; Wei, Q. Principles and applications of spectroscopic techniques for evaluating food protein conformational changes: A review. Trends Food Sci. Technol. 2017, 67, 207–219. [Google Scholar] [CrossRef]
- Orlando, A.; Franceschini, F.; Muscas, C.; Pidkova, S.; Bartoli, M.; Rovere, M.; Tagliaferro, A. A comprehensive review on Raman spectroscopy applications. Chemosensors 2021, 9, 262. [Google Scholar] [CrossRef]
- Kirtil, E.; Oztop, M.H. 1H nuclear magnetic resonance relaxometry and magnetic resonance imaging and applications in food science and processing. Food Eng. Rev. 2016, 8, 1–22. [Google Scholar] [CrossRef]
- Hatzakis, E. Nuclear magnetic resonance (NMR) spectroscopy in food science: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 189–220. [Google Scholar] [CrossRef]
- Abasi, S.; Minaei, S.; Jamshidi, B.; Fathi, D. Dedicated non-destructive devices for food quality measurement: A review. Trends Food Sci. Technol. 2018, 78, 197–205. [Google Scholar] [CrossRef]
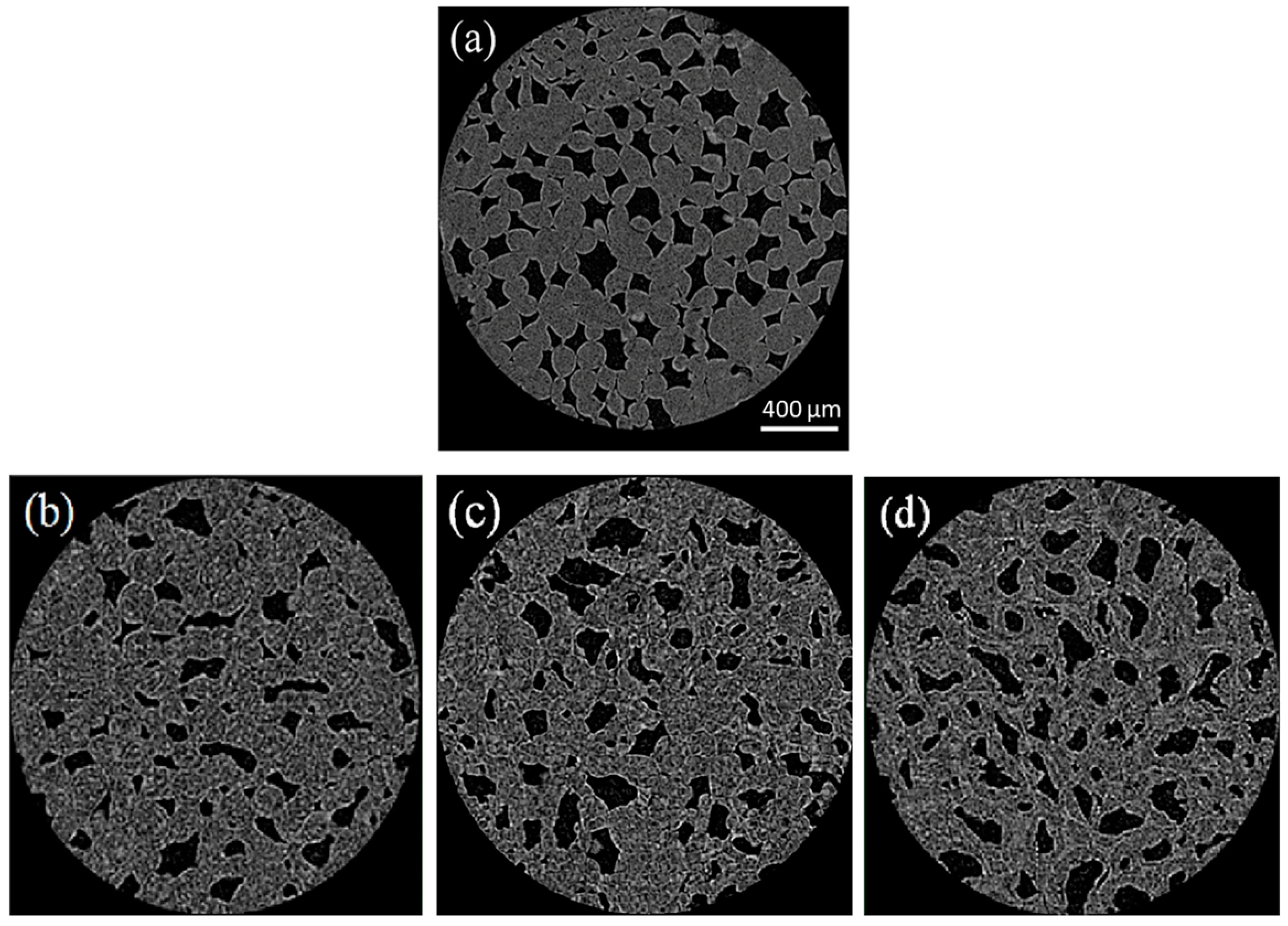
- Cantre, D.; Herremans, E.; Verboven, P.; Ampofo-Asiama, J.; Nicolaï, B. Characterization of the 3-D microstructure of mango (Mangifera indica L. cv. Carabao) during ripening using X-ray computed microtomography. Innov. Food Sci. Emerg. Technol. 2014, 24, 28–39. [Google Scholar] [CrossRef]
- Vicent, V.; Ndoye, F.T.; Verboven, P.; Nicolaï, B.; Alvarez, G. Modeling ice recrystallization in frozen carrot tissue during storage under dynamic temperature conditions. J. Food Eng. 2020, 278, 109911. [Google Scholar] [CrossRef]
- Kobayashi, R.; Kimizuka, N.; Watanabe, M.; Suzuki, T. The effect of supercooling on ice structure in tuna meat observed by using X-ray computed tomography. Int. J. Refrig. 2015, 60, 270–277. [Google Scholar] [CrossRef]
- Zennoune, A.; Latil, P.; Flin, F.; Perrin, J.; Weitkamp, T.; Scheel, M.; Geindreau, C.; Benkhelifa, H.; Ndoye, F.T. Investigating the influence of freezing rate and frozen storage conditions on a model sponge cake using synchrotron X-rays micro-computed tomography. Food Res. Int. 2022, 162, 112116. [Google Scholar] [CrossRef]
- Vicent, V.; Verboven, P.; Ndoye, F.T.; Alvarez, G.; Nicolaï, B. A new method developed to characterize the 3D-microstructure of frozen apple using X-ray micro-CT. J. Food Eng. 2017, 212, 154–164. [Google Scholar] [CrossRef]
- Zhao, Y.; Takhar, P. Micro X-ray computed tomography and image analysis of frozen potatoes subjected to freeze-thaw cycles. LWT-Food Sci. Technol. 2017, 79, 278–286. [Google Scholar] [CrossRef]
- Li, F.; Du, X.; Ren, Y.; Kong, B.; Wang, B.; Xia, X.; Bao, Y. Impact of ice structuring protein on myofibrillar protein aggregation behaviour and structural property of quick-frozen patty during frozen storage. Int. J. Biol. Macromol. 2021, 178, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, H.; Yang, D.; Liu, J.; Wu, J.; Ge, Y. Effect of pectin oligosaccharide on quality control of quick-frozen pumpkin puree. Int. J. Food Sci. Technol. 2022, 57, 1061–1073. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wang, H.; Oladejo, A.O.; Zhang, H.; Liu, X. Effects of ultrasound-assisted freezing on the water migration of dough and the structural characteristics of gluten components. J. Cereal Sci. 2020, 94, 102893. [Google Scholar] [CrossRef]
- Saki, N.; Ghaffari, M.; Nikoo, M. Effect of active ice nucleation bacteria on freezing and the properties of surimi during frozen storage. LWT-Food Sci. Technol. 2023, 176, 114548. [Google Scholar] [CrossRef]
- Hu, C.; Xie, J. The effect of multiple freeze–thaw cycles on protein oxidation and quality of Trachurus murphyi. J. Food Process. Preserv. 2021, 45, e15998. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Z.; Liu, M.; Qu, Z.; Liu, H.; Song, J.; Zhu, M.; Zhang, X.; He, B.; Wang, J. Effect of curdlan on the aggregation behavior and structure of gluten in frozen-cooked noodles during frozen storage. Int. J. Biol. Macromol. 2022, 205, 274–282. [Google Scholar] [CrossRef]
- Vicent, V.; Fatou-Touti, N.; Verboven, P.; Nicolaï, B.; Alvarez, G. Effect of dynamic storage temperatures on the microstructure of frozen carrot imaged using X-ray micro-CT. J. Food Eng. 2019, 246, 232–241. [Google Scholar] [CrossRef]
- Mulot, V.; Fatou-Toutie, N.; Benkhelifa, H.; Pathier, D.; Flick, D. Investigating the effect of freezing operating conditions on microstructure of frozen minced beef using an innovative X-ray micro-computed tomography method. J. Food Eng. 2019, 262, 13–21. [Google Scholar] [CrossRef]
- Andoa, Y.; Hagiwara, S.; Nabetani, H.; Okunishi, T.; Okadome, H. Impact of ice crystal development on electrical impedance characteristics and mechanical property of green asparagus stems. J. Food Eng. 2019, 256, 46–52. [Google Scholar] [CrossRef]
- Kobayashi, R.; Suzuki, T. Effect of supercooling accompanying the freezing process on ice crystals and the quality of frozen strawberry tissue. Int. J. Refrig. 2019, 99, 94–100. [Google Scholar] [CrossRef]
- Iskandar, C.D.; Zainuddin, Z.; Munawar, A.A.; Barus, R.A. Rapid assessment of frozen beef quality using near infrared technology. J. Kedokt. Hewan-Indones. J. Vet. Sci. 2023, 17, 42–47. [Google Scholar] [CrossRef]
- Nieto-Ortega, S.; Melado-Herreros, Á.; Foti, G.; Olabarrieta, I.; Ramilo-Fernández, G.; Gonzalez Sotelo, C.; Teixeira, B.; Velasco, A.; Mendes, R. Rapid differentiation of unfrozen and frozen-thawed tuna with non-destructive methods and classification models: Bioelectrical impedance analysis (BIA), near-infrared spectroscopy (NIR) and time domain reflectometry (TDR). Foods 2022, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Sun, D.; Pu, H.; Wei, Q. Heterospectral two-dimensional correlation analysis with near-infrared hyperspectral imaging for monitoring oxidative damage of pork myofibrils during frozen storage. Food Chem. 2018, 248, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Chen, W.H.; Tian, H.Y.; Liu, Y. Detection of frozen-thawed cycles for frozen tilapia (Oreochromis) fillets using near infrared spectroscopy. J. Aquat. Food Prod. Technol. 2018, 27, 609–618. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W.; Xie, Y.; Ozaki, Y. Non-destructive prediction of texture of frozen/thaw raw beef by Raman spectroscopy. J. Food Eng. 2020, 266, 109693. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Wang, H.; Liu, X.; Zhang, Y.; Zhang, H. In-situ analysis of the water distribution and protein structure of dough during ultrasonic-assisted freezing based on miniature Raman spectroscopy. Ultrason. Sonochem. 2020, 67, 105149. [Google Scholar] [CrossRef]
- Yu, G.; Li, R.; Hubel, A. Interfacial interactions of sucrose during cryopreservation detected by Raman spectroscopy. Langmuir 2019, 35, 7388–7395. [Google Scholar] [CrossRef]
- Sun, X.; Guo, R.; Kou, Y.; Song, H.; Zhan, T.; Wu, J.; Song, L.; Zhang, H.; Xie, F.; Wang, J.; et al. Inhibition of ice recrystallization by tamarind (Tamarindus indica L.) seed polysaccharide and molecular weight effects. Carbohydr. Polym. 2023, 301, 120358. [Google Scholar] [CrossRef]
- Li, T.; Kuang, S.; Xiao, T.; Hu, L.; Nie, P.; Ramaswamy, H.S.; Yu, Y. The effect of pressure–shift freezing versus air freezing and liquid immersion on the quality of frozen fish during storage. Foods 2022, 11, 1842. [Google Scholar] [CrossRef] [PubMed]
- Anderssen, K.E.; Syed, S.; Stormo, S.K. Quantification and mapping of tissue damage from freezing in cod by magnetic resonance imaging. Food Control 2021, 123, 107734. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, M.; Fang, Z.; Fu, J. Effect of static magnetic field assisted freezing on the product quality of Korla fragrant pear. J. Food Process. Eng. 2023, in press. [CrossRef]
- Hu, R.; Zhang, M.; Mujumdar, A.S. Novel assistive technologies for efficient freezing of pork based on high voltage electric field and static magnetic field: A comparative study. Innov. Food Sci. Emerg. Technol. 2022, 80, 103087. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, X.; Zhu, K. Effects of frozen storage on the quality characteristics of frozen cooked noodles. Food Chem. 2019, 283, 522–529. [Google Scholar] [CrossRef]
| Food Product | Microscopic Technique | Objectives | Reference |
|---|---|---|---|
| Large yellow croaker (Pseudosciaena crocea) | LM | Effects of ice crystals obtained through refrigerator (−20 °C), spiral freezer, and liquid nitrogen freezer, on water properties and protein stability | [35] |
| Snakehead (Channa argus) | LM | Effects of immersion freezing (−20, −30, and −40 °C) on ice crystal formation and protein properties in comparison to conventional air freezing (−20 °C) | [36] |
| Tilapia | LM | Obtention of equivalent diameter data of ice crystals to develop models for the prediction of ice crystal growth during recrystallization in frozen samples | [37] |
| Carrot | LM | Effect of direct current and alternating current magnetic field on the biological phase transformation and ice crystal formation | [38] |
| Tuna meat | LM | Effects of salting and subsequent freezing on the physicochemical and histological properties (diameter and area ratio of ice crystals) | [39] |
| Grass carp | LM | Effects of different freezing methods on ice crystals, distribution of water, and freshness properties during frozen storage | [40] |
| Coffee extract | LM | Effects of the application of annealing on the ice morphology, and the solute yield on block freeze concentrations | [41] |
| Common carp (Cyprinus carpio) | LM | Observation of ice crystals after ultrasound-assisted immersion freezing application | [42] |
| Microalgae Cells | Cryo-LM | Determine the most important parameters for the safety of cells in the process of cryopreservation: temperature parameters of phase transitions, the presence of extra- and intracellular ice, its structure, crystal size, and their growth rate | [43] |
| Fruits and vegetables (apple, peach, cucumber and Indian jujube) | Cryo-LM | Effects of static magnetic field (intensities between 0 to 45 mT) on micro and macroscales, comparing ice crystal size, time through −1 °C to −5 °C, drip loss and texture | [44] |
| Mushroom (Agaricus bisporus) | Cryo-LM | Observe the ice crystal morphology after the application of contact ultrasound (300 W, 20 kHz) during freezing and frozen storage | [45] |
| Mango Sorbet | Cryo-LM | Effect of ultrasound-assisted immersion freezing with respect to traditional freezing technologies | [46] |
| Honeydew melon | Cryo-LM | Observe the microstructure of samples exposed to CO2 pre-injection at different pressures combined with ultrasound-assistance (0.15 W/cm2 at 20 kHz) for quick freezing | [47] |
| Gel model food | Cryo-LM | Effects of freezing process assisted by electrostatic field, by static magnetic field, and by electrostatic field combined with static magnetic field on nucleation temperature of ice crystals | [48] |
| Food Product | Microscopic Technique | Objectives | Reference |
|---|---|---|---|
| Common carp (Cyprinus carpio) | TEM | Changes in primary, secondary, and tertiary structures of myofibril protein of frozen samples at different ultrasonic power level | [42] |
| Porcine longissimus muscle | TEM | Influence of multiple freeze-thaw cycles on (micro)structure damage, and myofibrillar proteins’ structure changes | [51] |
| Papaya tissues | TEM | Investigate the effect of freezing and thawing on texture, microstructure, and cell wall composition changes | [64] |
| Sliced Peaches | SEM | Determine if freezing rates and holding temperatures influence quality during short- and long-term frozen storage | [65] |
| Tomatoes | SEM | Effect of a combined osmo-dehydro-cryogenic-freezing process on quality characteristics of fruits and vegetables (lycopene content, color, and cell structures) | [66] |
| Red Radish | SEM | Effect of wrapped and ultrasonic treatment on freezing time, drip loss, texture, and sensory evaluation of red radish cylinders frozen by immersion freezing | [67] |
| Cherries | Cryo-SEM | Effects of the application of synthetic AFPs as a pretreatment in cherries before freezing to evaluate their potential to minimize freeze-thaw damage in frozen samples | [59] |
| Pork meet | Cryo-SEM | Identification of the degree of freeze damage in meat on analytic tool selection | [68] |
| Fresh sorbets | Cryo-SEM | Determine the effect of several stabilizers on the establishment of the microstructure | [69] |
| Grape tomato | Cryo-SEM | Evaluate the effects of isochoric freezing on microstructural changes after 4 weeks of preservation | [70] |
| Strawberry fruits | ESEM | Investigate the impacts of initial water content on the freezing and thawing profiles, the structural cell changes and textural characteristics, in fresh and partially dehydrated samples | [63] |
| Mango | ESEM | Relationship of vitamin C content with water state and ice crystals under different state/phase transitions (temperature fluctuations) during frozen storage | [71] |
| Cranberries | ESEM | Evaluate the effects of freezing and drying on the structure | [72] |
| Apple slices | ESEM | Evaluation of microstructures of the samples dried with novel non-thermal ultrasound contact drying method | [73] |
| Food Product | Microscopic Technique | Objectives | Reference |
|---|---|---|---|
| Pork patties | AFM | Explore the cryoprotective effect of ice structuring protein applied to myofibrillar protein during frozen storage | [87] |
| Pumpkin puree | AFM | Explored the mechanisms underlying the effects of pectin oligosaccharide on the quality control compared to trehalose and the changes in structure and properties during frozen storage | [88] |
| Dough | AFM | Effects of ultrasound-assisted freezing on the freezing time and water migration and the structural characteristics of gluten components | [89] |
| Surimi from Talang queenfish (Scomberoides commersonnianus) | AFM | Effects of active ice nucleation bacteria (Pseudomonas syringae) on freezing, ice crystal formation, aggregation, and oxidation of during frozen storage | [90] |
| Fish (Trachurus murphyi) | AFM | Investigate how freeze–thaw processing and storage affects myofibrillar protein | [91] |
| Noodles | AFM | Comprehensive theories for the strengthening effect of curdlan on quality from the perspective of gluten structure | [92] |
| Carrot | X-ray μCT | Visualization of 3D ice crystals during months with changes in temperature | [93] |
| Minced beef | X-ray μCT | Quantify 3D ice crystals in frozen samples according to the integral cooling rate (for mechanical and cryogenic freezing operating conditions) | [94] |
| Green asparagus | X-ray μCT | Influence of the various characteristics of ice crystals formed at different freezing rates on cell membrane damage and mechanical property | [95] |
| Strawberry | X-ray μCT | Effect of supercooled freezing on quality and the characteristics of ice crystals in frozen tissues prepared by supercooled freezing | [96] |
| Beef | NIR | Determine and assess quality parameters of meat product in frozen samples | [97] |
| Tuna | NIR | Discriminate between unfrozen and frozen-thawed samples | [98] |
| Pork | NIR | Monitoring the oxidative damage of myofibrils during frozen storage | [99] |
| Tilapia (Oreochromis) | NIR | Detect the frozen-thawed cycles in frozen fillets | [100] |
| Raw beef | Raman Spectroscopy | Predict the texture of different frozen/thaw samples from continuous freezing and repeated freeze-thaw treatments | [101] |
| Dough | Raman Spectroscopy | Effects of ultrasonic-assisted freezing on the water distribution and protein molecular structure | [102] |
| Sucrose solution | Raman Spectroscopy | Study the interactions between sucrose and water during freezing and explore the biophysical environment at interfaces between cells and nonfrozen sucrose solution, between cells and extracellular ice, and between nonfrozen sucrose solution and ice | [103] |
| Tamarind | Raman Spectroscopy | Evaluation of the interaction of seed polysaccharide with ice crystals and water molecules with a view to gaining a greater understanding of the action mechanism. | [104] |
| Largemouth bass fish (Micropterus salmoides) | Raman Spectroscopy | Study the quality during freezing with pressure changes in storage at −30 °C in comparison to two freezing techniques | [105] |
| Cod (Gadus morhua) | MRI | Map and quantify tissue damage from freezing | [106] |
| Korla fragrant pear | NMR MRI | Explore the influence of different static magnetic field on the product quality | [107] |
| Pork tenderloin | NMR | Effect of different freezing methods on the moisture state and moisture distribution of thawed pork tenderloin | [108] |
| Noodles | NMR MRI | Determine the water distribution and migration during frozen storage for 12 weeks | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Bermúdez, I.; Castillo-Suero, A.; Cortés-Inostroza, A.; Jeldrez, C.; Dantas, A.; Hernández, E.; Orellana-Palma, P.; Petzold, G. Observation and Measurement of Ice Morphology in Foods: A Review. Foods 2023, 12, 3987. https://doi.org/10.3390/foods12213987
Pérez-Bermúdez I, Castillo-Suero A, Cortés-Inostroza A, Jeldrez C, Dantas A, Hernández E, Orellana-Palma P, Petzold G. Observation and Measurement of Ice Morphology in Foods: A Review. Foods. 2023; 12(21):3987. https://doi.org/10.3390/foods12213987
Chicago/Turabian StylePérez-Bermúdez, Indira, Alison Castillo-Suero, Anielka Cortés-Inostroza, Cristóbal Jeldrez, Adriana Dantas, Eduardo Hernández, Patricio Orellana-Palma, and Guillermo Petzold. 2023. "Observation and Measurement of Ice Morphology in Foods: A Review" Foods 12, no. 21: 3987. https://doi.org/10.3390/foods12213987
APA StylePérez-Bermúdez, I., Castillo-Suero, A., Cortés-Inostroza, A., Jeldrez, C., Dantas, A., Hernández, E., Orellana-Palma, P., & Petzold, G. (2023). Observation and Measurement of Ice Morphology in Foods: A Review. Foods, 12(21), 3987. https://doi.org/10.3390/foods12213987








