In Vivo Evaluation of the Potential of Thyme and Lemon Hydrolates as Processing Aids to Reduce Norovirus Concentration during Oyster Depuration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Hydrolates
2.3. Detection and Quantification of Norovirus
2.3.1. Virus Extraction
2.3.2. Nucleic Acid Extraction
Norovirus Quantification
2.4. Sensory Evaluation
2.5. Statistical Data Analysis
3. Results
3.1. Chemical Composition of the Hydrolates
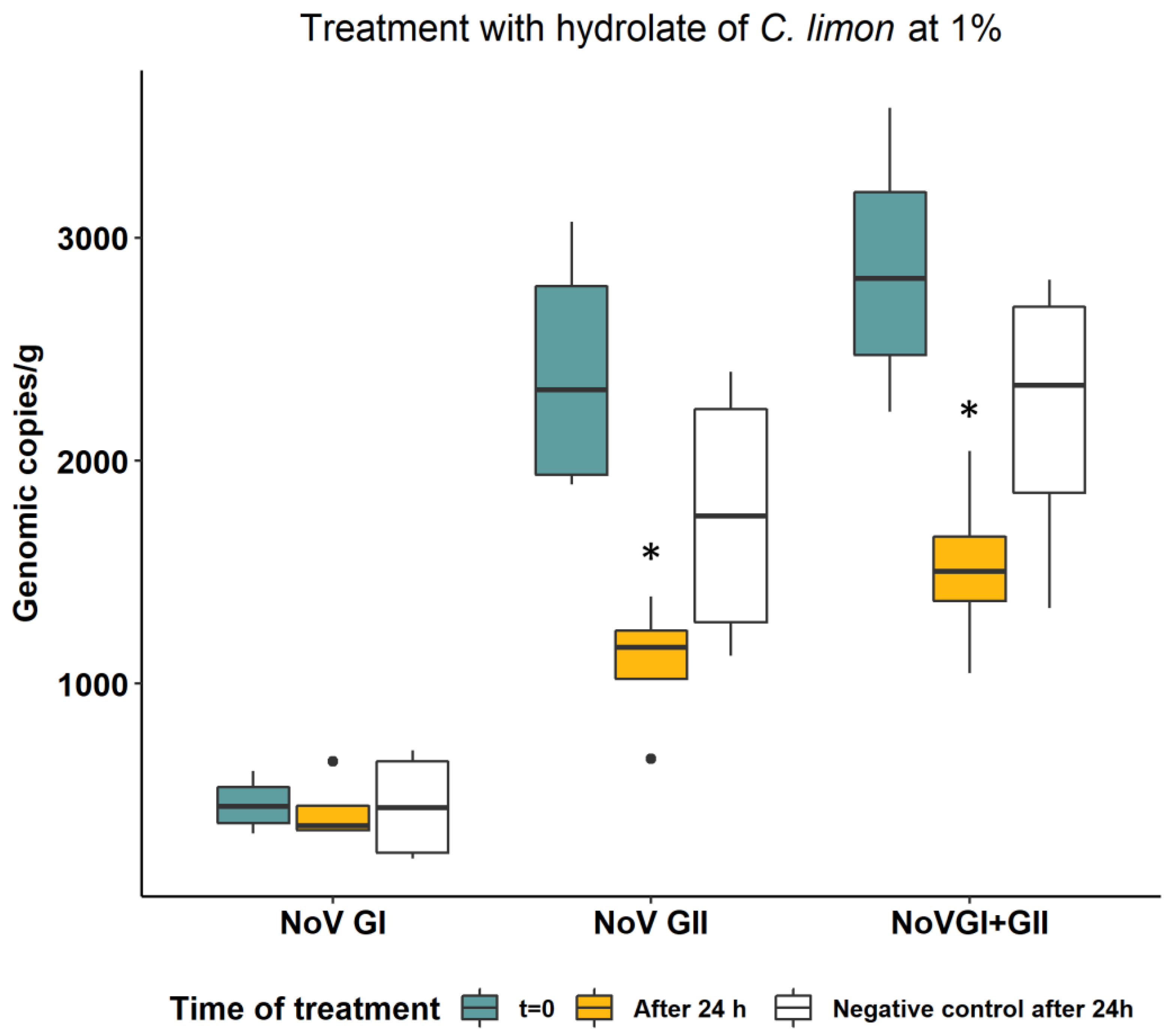
3.2. Norovirus Quantification in Treated and Untreated Oyster Samples
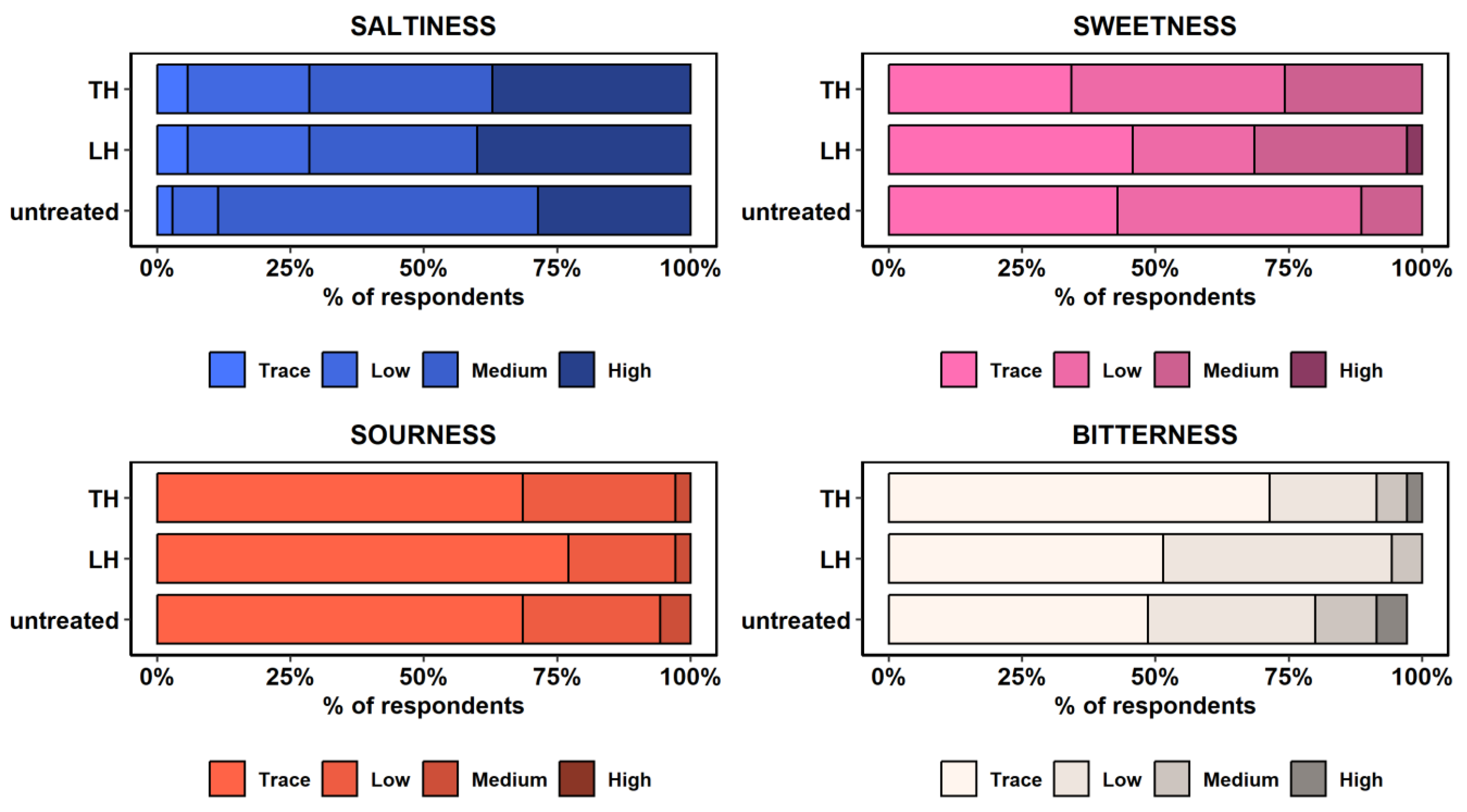
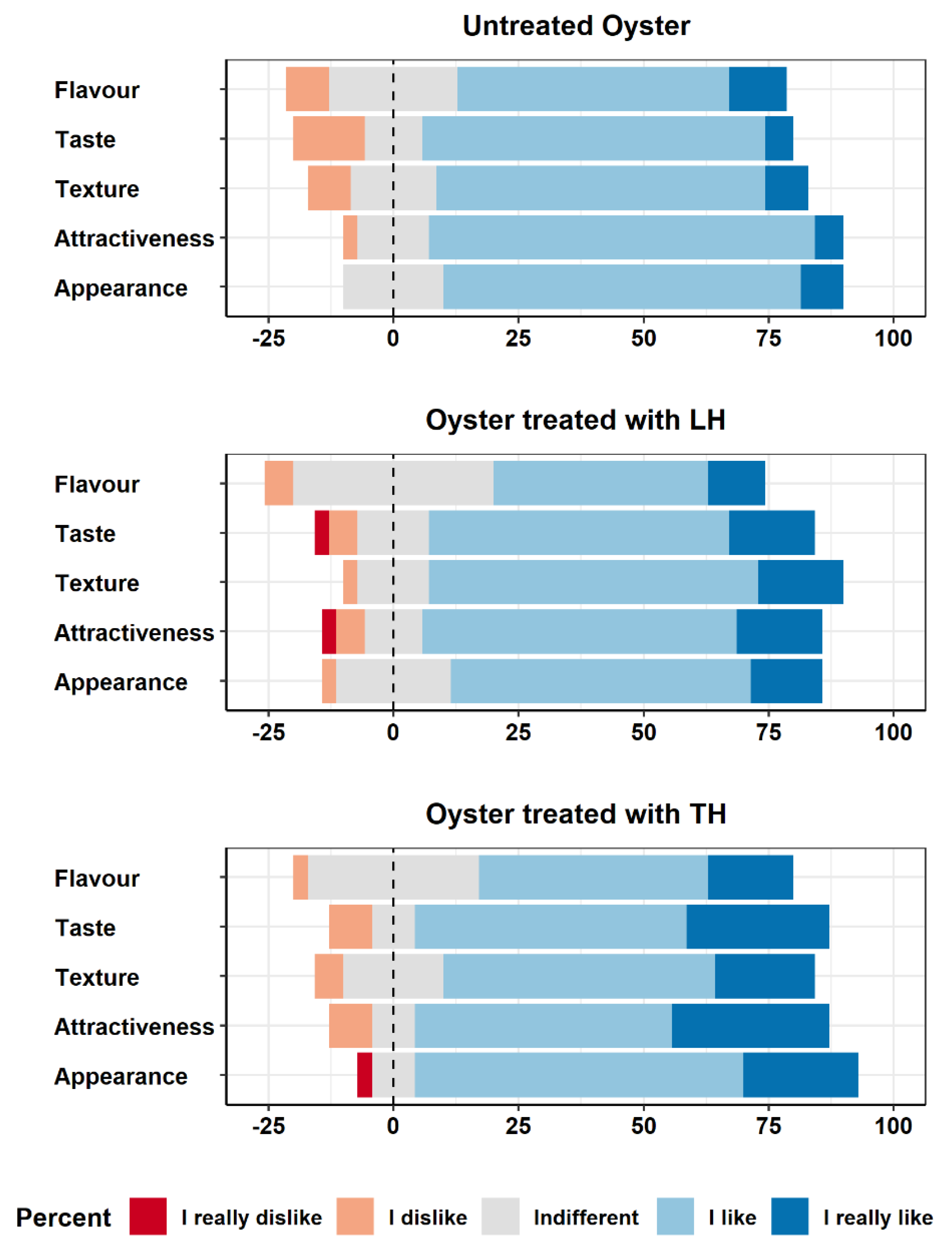
3.3. Sensory Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Botta, R.; Asche, F.; Borsum, J.S.; Camp, E.V. A Review of Global Oyster Aquaculture Production and Consumption. Mar. Policy 2020, 117, 103952. [Google Scholar] [CrossRef]
- McLeod, C.; Polo, D.; Le Saux, J.-C.; Le Guyader, F.S. Depuration and Relaying: A Review on Potential Removal of Norovirus from Oysters. Compr. Rev. Food Sci. Food Saf. 2017, 16, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, W.; Calci, K.R. Selective Accumulation May Account for Shellfish-Associated Viral Illness. Appl. Environ. Microbiol. 2000, 66, 1375–1378. [Google Scholar] [CrossRef] [PubMed]
- Bellou, M.; Kokkinos, P.; Vantarakis, A. Shellfish-Borne Viral Outbreaks: A Systematic Review. Food Environ. Virol. 2013, 5, 13–23. [Google Scholar] [CrossRef]
- Fouillet, A.; Fournet, N.; Forgeot, C.; Jones, G.; Septfons, A.; Franconeri, L.; Ambert-Balay, K.; Schmidt, J.; Guérin, P.; De Valk, H.; et al. Large Concomitant Outbreaks of Acute Gastroenteritis Emergency Visits in Adults and Food-Borne Events Suspected to Be Linked to Raw Shellfish, France, December 2019 to January 2020. Eurosurveillance 2020, 25, 2000060. [Google Scholar] [CrossRef]
- Li, Y.; Xue, L.; Gao, J.; Cai, W.; Zhang, Z.; Meng, L.; Miao, S.; Hong, X.; Xu, M.; Wu, Q.; et al. A Systematic Review and Meta-Analysis Indicates a Substantial Burden of Human Noroviruses in Shellfish Worldwide, with GII.4 and GII.2 Being the Predominant Genotypes. Food Microbiol. 2023, 109, 104140. [Google Scholar] [CrossRef]
- Karagiannis, I.; Detsis, M.; Gkolfinopoulou, K.; Pervanidou, D.; Panagiotopoulos, T.; Bonovas, S. An Outbreak of Gastroenteritis Linked to Seafood Consumption in a Remote Northern Aegean Island, February–March 2010. Rural Remote Health 2010, 10, 53–59. [Google Scholar] [CrossRef]
- Baker, K.; Morris, J.; McCarthy, N.; Saldana, L.; Lowther, J.; Collinson, A.; Young, M. An Outbreak of Norovirus Infection Linked to Oyster Consumption at a UK Restaurant, February 2010. J. Public Health 2011, 33, 205–211. [Google Scholar] [CrossRef]
- Alfano-Sobsey, E.; Sweat, D.; Hall, A.; Breedlove, F.; Rodriguez, R.; Greene, S.; Pierce, A.; Sobsey, M.; Davies, M.; Ledford, S.L. Norovirus Outbreak Associated with Undercooked Oysters and Secondary Household Transmission. Epidemiol. Infect. 2012, 140, 276–282. [Google Scholar] [CrossRef]
- Galmés Truyols, A.; Duran, J.G.; Riutort, A.N.; Cerdá, G.A.; Isabel, C.B.; Arbona, M.P.; Berga, J.V. Brote de norovirus en Mallorca asociado al consumo de ostras. Gac. Sanit. 2011, 25, 173–175. [Google Scholar] [CrossRef]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- Pilotto, M.R.; Souza, D.S.M.; Barardi, C.R.M. Viral Uptake and Stability in Crassostrea Gigas Oysters during Depuration, Storage and Steaming. Mar. Pollut. Bull. 2019, 149, 110524. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Yamaoka, Y.; Ito, A.; Kamaishi, T.; Sugiyama, R.; Estes, M.K.; Muramatsu, M.; Murakami, K. Evaluation of Heat Inactivation of Human Norovirus in Freshwater Clams Using Human Intestinal Enteroids. Viruses 2022, 14, 1014. [Google Scholar] [CrossRef] [PubMed]
- Sow, H.; Desbiens, M.; Morales-Rayas, R.; Ngazoa, S.E.; Jean, J. Heat Inactivation of Hepatitis A Virus and a Norovirus Surrogate in Soft-Shell Clams (Mya Arenaria). Foodborne Pathog. Dis. 2011, 8, 387–393. [Google Scholar] [CrossRef]
- Rowan, N.J. Current Decontamination Challenges and Potentially Complementary Solutions to Safeguard the Vulnerable Seafood Industry from Recalcitrant Human Norovirus in Live Shellfish: Quo Vadis? Sci. Total Environ. 2023, 874, 162380. [Google Scholar] [CrossRef]
- Han, S.; Jo, J.Y.; Park, S.R.; Choi, C.; Ha, S.-D. Impact of Chlorine Dioxide and Electron-Beam Irradiation for the Reduction of Murine Norovirus in Low-Salted “Jogaejeotgal”, a Traditional Korean Salted and Fermented Clam. Int. J. Food Microbiol. 2021, 342, 109073. [Google Scholar] [CrossRef]
- Kingsley, D.H.; Vincent, E.M.; Meade, G.K.; Watson, C.L.; Fan, X. Inactivation of Human Norovirus Using Chemical Sanitizers. Int. J. Food Microbiol. 2014, 171, 94–99. [Google Scholar] [CrossRef]
- Cook, N.; Knight, A.; Richards, G.P. Persistence and Elimination of Human Norovirus in Food and on Food Contact Surfaces: A Critical Review. J. Food Prot. 2016, 79, 1273–1294. [Google Scholar] [CrossRef]
- Govaris, A.; Pexara, A. Inactivation of Foodborne Viruses by High-Pressure Processing (HPP). Foods 2021, 10, 215. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Ezhilarasi, P.N.; Rajauria, G. Application of Cold Plasma on Food Matrices: A Review on Current and Future Prospects. J. Food Process. Preserv. 2021, 45, e15070. [Google Scholar] [CrossRef]
- Hirneisen, K.A.; Black, E.P.; Cascarino, J.L.; Fino, V.R.; Hoover, D.G.; Kniel, K.E. Viral Inactivation in Foods: A Review of Traditional and Novel Food-Processing Technologies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 3–20. [Google Scholar] [CrossRef]
- Feng, K.; Divers, E.; Ma, Y.; Li, J. Inactivation of a Human Norovirus Surrogate, Human Norovirus Virus-Like Particles, and Vesicular Stomatitis Virus by Gamma Irradiation. Appl. Environ. Microbiol. 2011, 77, 3507–3517. [Google Scholar] [CrossRef] [PubMed]
- Kulawik, P.; Rathod, N.B.; Ozogul, Y.; Ozogul, F.; Zhang, W. Recent Developments in the Use of Cold Plasma, High Hydrostatic Pressure, and Pulsed Electric Fields on Microorganisms and Viruses in Seafood. Crit. Rev. Food Sci. Nutr. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.S. Plant Extracts for the Control of Bacterial Growth: Efficacy, Stability and Safety Issues for Food Application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant Antimicrobial Polyphenols as Potential Natural Food Preservatives: Plant Polyphenols for Food Preservation. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef]
- Jamali, S.N.; Assadpour, E.; Feng, J.; Jafari, S.M. Natural Antimicrobial-Loaded Nanoemulsions for the Control of Food Spoilage/Pathogenic Microorganisms. Adv. Colloid Interface Sci. 2021, 295, 102504. [Google Scholar] [CrossRef]
- Li, Y.; Erhunmwunsee, F.; Liu, M.; Yang, K.; Zheng, W.; Tian, J. Antimicrobial Mechanisms of Spice Essential Oils and Application in Food Industry. Food Chem. 2022, 382, 132312. [Google Scholar] [CrossRef]
- Rao, J.; Chen, B.; McClements, D.J. Improving the Efficacy of Essential Oils as Antimicrobials in Foods: Mechanisms of Action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef]
- Huang, X.; Lao, Y.; Pan, Y.; Chen, Y.; Zhao, H.; Gong, L.; Xie, N.; Mo, C.-H. Synergistic Antimicrobial Effectiveness of Plant Essential Oil and Its Application in Seafood Preservation: A Review. Molecules 2021, 26, 307. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Tuchowska, A.; Janda-Milczarek, K. Plant Hydrolates—Antioxidant Properties, Chemical Composition and Potential Applications. Biomed. Pharmacother. 2021, 142, 112033. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, S.; Serio, A.; López, C.C.; Paparella, A. Hydrosols: Biological Activity and Potential as Antimicrobials for Food Applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
- Cozzi, L.; Vicenza, T.; Battistini, R.; Masotti, C.; Suffredini, E.; Di Pasquale, S.; Fauconnier, M.-L.; Ercolini, C.; Serracca, L. Effects of Essential Oils and Hydrolates on the Infectivity of Murine Norovirus. Viruses 2023, 15, 682. [Google Scholar] [CrossRef] [PubMed]
- Astani, A.; Schnitzler, P. Antiviral Activity of Monoterpenes Beta-Pinene and Limonene against Herpes Simplex Virus in Vitro. Iran. J. Microbiol. 2014, 6, 149–155. [Google Scholar]
- Gilling, D.H.; Kitajima, M.; Torrey, J.R.; Bright, K.R. Antiviral Efficacy and Mechanisms of Action of Oregano Essential Oil and Its Primary Component Carvacrol against Murine Norovirus. J. Appl. Microbiol. 2014, 116, 1149–1163. [Google Scholar] [CrossRef]
- Orhan, İ.E.; ÖzçeliK, B.; Kartal, M.; Kan, Y. Antimicrobial and Antiviral Effects of Essential Oils from Selected Umbelliferae and Labiatae Plants and Individual Essential Oil Components. Turk. J. Biol. 2012, 36, 239–246. [Google Scholar] [CrossRef]
- Pilau, M.R.; Alves, S.H.; Weiblen, R.; Arenhart, S.; Cueto, A.P.; Lovato, L.T. Antiviral Activity of the Lippia Graveolens (Mexican Oregano) Essential Oil and Its Main Compound Carvacrol against Human and Animal Viruses. Braz. J. Microbiol. 2011, 42, 1616–1624. [Google Scholar] [CrossRef]
- Purgatorio, C.; Serio, A.; Chaves-López, C.; Rossi, C.; Paparella, A. An Overview of the Natural Antimicrobial Alternatives for Sheep Meat Preservation. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4210–4250. [Google Scholar] [CrossRef]
- ISO 15216-1:2017; Amd 1:2021—Microbiology of Food and Animal Feed—Horizontal Method for Determination of Hepatitis A Virus and Norovirus in Food Using Real Time RT-PCR—Part1: Method for Quantification. ISO: Geneva, Switzerland, 2021.
- Maalouf, H.; Zakhour, M.; Le Pendu, J.; Le Saux, J.-C.; Atmar, R.L.; Le Guyader, F.S. Distribution in Tissue and Seasonal Variation of Norovirus Genogroup I and II Ligands in Oysters. Appl. Environ. Microbiol. 2010, 76, 5621–5630. [Google Scholar] [CrossRef]
- Le Guyader, F.S.; Loisy, F.; Atmar, R.L.; Hutson, A.M.; Estes, M.K.; Ruvoën-Clouet, N.; Pommepuy, M.; Le Pendu, J. Norwalk Virus–Specific Binding to Oyster Digestive Tissues. Emerg. Infect. Dis. 2006, 12, 931–936. [Google Scholar] [CrossRef]
- Le Guyader, F.S.; Atmar, R.L.; Le Pendu, J. Transmission of Viruses through Shellfish: When Specific Ligands Come into Play. Curr. Opin. Virol. 2012, 2, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Reichling, J.; Schneele, J.; Schnitzler, P. Inhibitory Effect of Essential Oils against Herpes Simplex Virus Type 2. Phytomedicine 2008, 15, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Deng, Z.; Shahidi, F. Natural Bioactive Substances for the Control of Food-Borne Viruses and Contaminants in Food. Food Prod. Process. Nutr. 2020, 2, 27. [Google Scholar] [CrossRef]
- Ma, L.; Yao, L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef]
- Gilling, D.H.; Kitajima, M.; Torrey, J.R.; Bright, K.R. Mechanisms of Antiviral Action of Plant Antimicrobials against Murine Norovirus. Appl. Environ. Microbiol. 2014, 80, 4898–4910. [Google Scholar] [CrossRef]
- Qiu, B.; Wei, F.; Su, J.; Hao, W.; Zhou, J.; Zhao, J.; Wang, Y.; Qu, Z. The Effects of β-Pinene, a Pine Needle Oil Monoterpene, on Adenovirus Type 3. Bull. Exp. Biol. Med. 2022, 172, 345–351. [Google Scholar] [CrossRef]
- Elizaquível, P.; Azizkhani, M.; Aznar, R.; Sánchez, G. The Effect of Essential Oils on Norovirus Surrogates. Food Control 2013, 32, 275–278. [Google Scholar] [CrossRef]
- Sánchez, G.; Aznar, R. Evaluation of Natural Compounds of Plant Origin for Inactivation of Enteric Viruses. Food Environ. Virol. 2015, 7, 183–187. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Recent Updates on the Chemistry, Bioactivities, Mode of Action, and Industrial Applications of Plant Essential Oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Bertrand, I.; Schijven, J.F.; Sánchez, G.; Wyn-Jones, P.; Ottoson, J.; Morin, T.; Muscillo, M.; Verani, M.; Nasser, A.; De Roda Husman, A.M.; et al. The Impact of Temperature on the Inactivation of Enteric Viruses in Food and Water: A Review: Virus Inactivation. J. Appl. Microbiol. 2012, 112, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.A.; Sousa, A.C.A.; Caramelo, D.; Delgado, F.; De Oliveira, A.P.; Pastorinho, M.R. Chemical Profile and Eco-Safety Evaluation of Essential Oils and Hydrolates from Cistus ladanifer, Helichrysum italicum, Ocimum basilicum and Thymbra capitata. Ind. Crops Prod. 2022, 175, 114232. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Rolo, J.; Gaspar, C.; Cavaleiro, C.; Salgueiro, L.; Palmeira-de-Oliveira, R.; Ferraz, C.; Coelho, S.; Pastorinho, M.R.; Sousa, A.C.; et al. Chemical Characterization and Bioactive Potential of Thymus × Citriodorus (Pers.) Schreb. Preparations for Anti-Acne Applications: Antimicrobial, Anti-Biofilm, Anti-Inflammatory and Safety Profiles. J. Ethnopharmacol. 2022, 287, 114935. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Citrus Fruit-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2021, 40, 5S–38S. [Google Scholar] [CrossRef] [PubMed]

| No. Peak | Compound | RI Cal. | RI Ref. | Content (%) 1 |
|---|---|---|---|---|
| 1 | Alpha-pinene | 937 | 939 | 1.25 |
| 2 | Camphene | 952 | 953 | 0.31 |
| 3 | Beta-pinene | 979 | 975 | 0.26 |
| 4 | Beta-myrcene | 992 | 991 | 2.02 |
| 5 | Alpha-phellandrene | 1005 | 1005 | 0.39 |
| 6 | Cymene | 1028 | 1026 | 11.23 |
| 7 | Gamma-terpinene | 1063 | 1062 | 6.04 |
| 8 | Linalool | 1103 | 1101 | 17.11 |
| 9 | Carvacrol | 1315 | 1317 | 58.67 |
| 10 | Caryophyllene oxide | 1593 | 1583 | 2.73 |
| No. Peak | Compound | RI Cal. | RI Ref. | Content (%) 1 |
|---|---|---|---|---|
| 1 | Alpha-pinene | 937 | 939 | 3.12 |
| 2 | Beta-pinene | 981 | 975 | 20.60 |
| 3 | Beta-myrcene | 992 | 991 | 2.72 |
| 4 | Limonene | 1039 | 1031 | 53.45 |
| 5 | Gamma-terpinene | 1065 | 1062 | 14.03 |
| 6 | Terpinolene | 1090 | 1089 | 0.70 |
| 7 | Linalool | 1100 | 1101 | 0.30 |
| 8 | Terpinen-4-ol | 1181 | 1178 | 0.10 |
| 9 | Alpha-terpineol | 1193 | 1189 | 0.35 |
| 10 | Neral | 1245 | 1242 | 1.28 |
| 11 | Geranial | 1274 | 1271 | 2.24 |
| 12 | Beta-bisabolene | 1512 | 1509 | 1.02 |
| Untreated Oyster | Oyster LH | Oyster TH | ||
|---|---|---|---|---|
| Physical characteristics | ||||
| Shade | ||||
| Whitish | 3 (8.6) | 1 (2.9) | 2 (5.7) | |
| Greenish | 12 (34.3) | 12 (34.3) | 9(25.7) | |
| Greyish | 20 (57.1) | 21 (60.0) | 24 (68.6) | |
| Opacity | ||||
| Clear | 16 (45.7) | 16 (45.7) | 15 (42.9) | |
| Opalescent | 16 (45.7) | 12 (34.3) | 13 (37.1) | |
| Cloudy | - | 3 (8.6) | 3 (8.6) | |
| Texture | Mushy | 1 (2.9) | 2 (5.7) | 2 (5.7) |
| Tender | 26 (74.3) | 20 (57.1) | 18 (51.4) | |
| Firm | 8 (22.9) | 11 (31.4) | 14 (40.0) | |
| Sensory characteristics | ||||
| Flavour (yes/no) | ||||
| Marine | 33 (94.3) * | 28 (80.0) | 26 (80.0) * | |
| Citrus | 4 (11.4) | 3 (8.6) | 3 (8.6) | |
| Garlic | 5 (14.3) | 5 (14.3) | 3 (8.6) | |
| Rosemary | 2 (5.71) | 5 (14.3) | 3 (8.6) | |
| Thyme | 3 (8.6) | 6 (17.1) | 5 (14.3) | |
| Taste (yes/no) | ||||
| Marine | 34 (97.1) | 33 (94.3) | 33 (94.3) | |
| Seaweed | 28 (80.0) | 23 (65.7) | 25 (71.4) | |
| Fruity | 1 (2.9) | 0 | 2 (5.71) | |
| Metallic | 19 (54.3) | 19 (54.3) | 13 (37.1) | |
| Thyme | 4 (11.4) | 4 (11.4) | 7 (20.0) | |
| Spinach | 7 (20.0) | 8 (22.9) | 7 (20.0) | |
| Citrus | 5 (14.3) | 7 (20.0) | 2 (5.71) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battistini, R.; Masotti, C.; Bianchi, D.M.; Decastelli, L.; Garcia-Vozmediano, A.; Maurella, C.; Fauconnier, M.-L.; Paparella, A.; Serracca, L. In Vivo Evaluation of the Potential of Thyme and Lemon Hydrolates as Processing Aids to Reduce Norovirus Concentration during Oyster Depuration. Foods 2023, 12, 3976. https://doi.org/10.3390/foods12213976
Battistini R, Masotti C, Bianchi DM, Decastelli L, Garcia-Vozmediano A, Maurella C, Fauconnier M-L, Paparella A, Serracca L. In Vivo Evaluation of the Potential of Thyme and Lemon Hydrolates as Processing Aids to Reduce Norovirus Concentration during Oyster Depuration. Foods. 2023; 12(21):3976. https://doi.org/10.3390/foods12213976
Chicago/Turabian StyleBattistini, Roberta, Chiara Masotti, Daniela Manila Bianchi, Lucia Decastelli, Aitor Garcia-Vozmediano, Cristiana Maurella, Marie-Laure Fauconnier, Antonello Paparella, and Laura Serracca. 2023. "In Vivo Evaluation of the Potential of Thyme and Lemon Hydrolates as Processing Aids to Reduce Norovirus Concentration during Oyster Depuration" Foods 12, no. 21: 3976. https://doi.org/10.3390/foods12213976
APA StyleBattistini, R., Masotti, C., Bianchi, D. M., Decastelli, L., Garcia-Vozmediano, A., Maurella, C., Fauconnier, M.-L., Paparella, A., & Serracca, L. (2023). In Vivo Evaluation of the Potential of Thyme and Lemon Hydrolates as Processing Aids to Reduce Norovirus Concentration during Oyster Depuration. Foods, 12(21), 3976. https://doi.org/10.3390/foods12213976







