Changes in Yield-Related Traits, Phytochemical Composition, and Antioxidant Activity of Pepper (Capsicum annuum) Depending on Its Variety, Fruit Position, and Ripening Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Processing
2.2. Agro-Morphological Characterization
2.3. Phytochemical Analysis
2.3.1. Capsaicin Determination
2.3.2. Total Phenolic Content
2.3.3. Total Flavonoid Content
2.3.4. DPPH-Scavenging Assay
2.3.5. Ferric Reducing Power Assay
2.4. Statistical Analysis
3. Results and Discussion
3.1. Variety Effect
3.2. Fruit Position Effect
3.3. Ripening Stage Effect
3.4. Interactions Effect
3.4.1. Variety × Fruit Position
3.4.2. Variety × Ripening Stage
3.4.3. Fruit Position × Ripening Stage
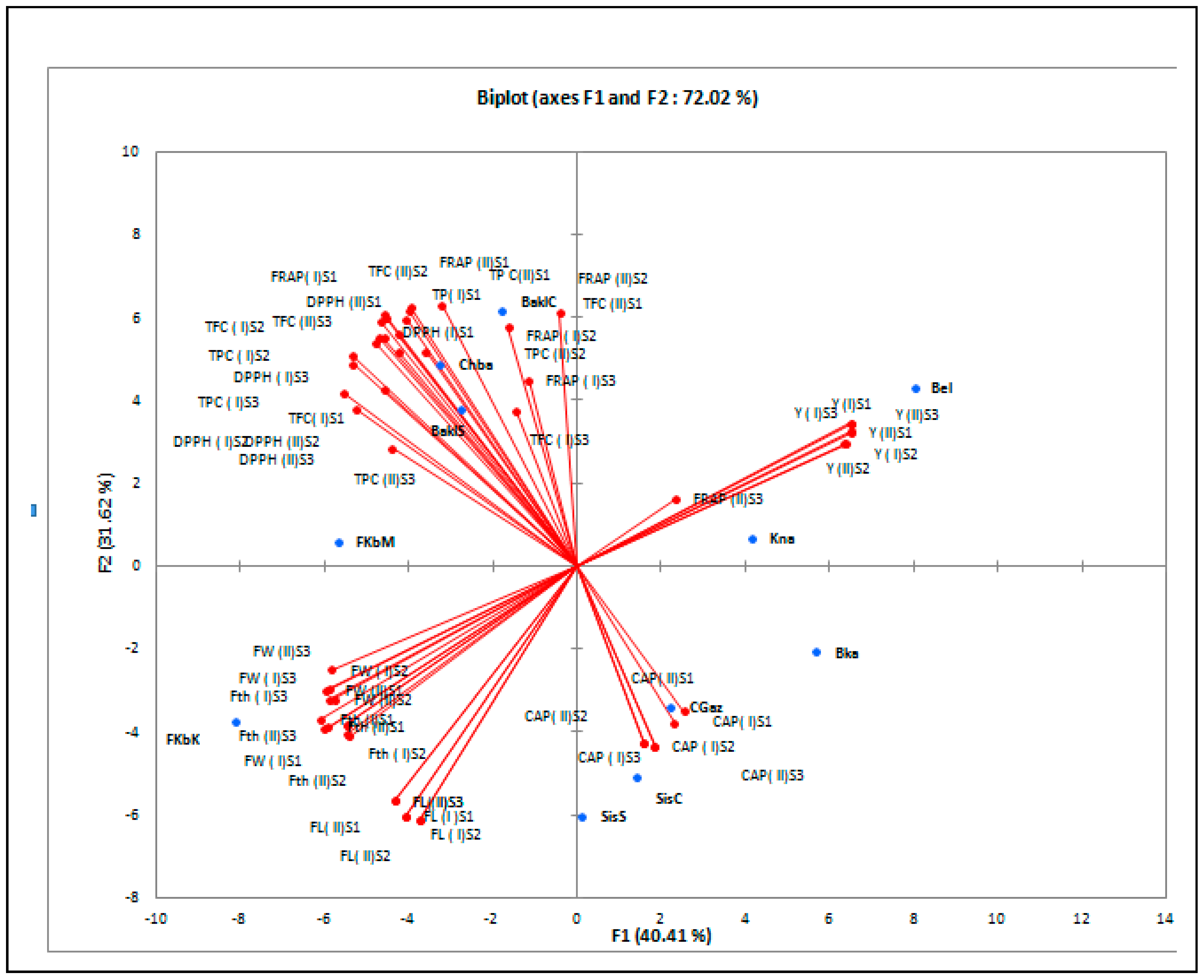
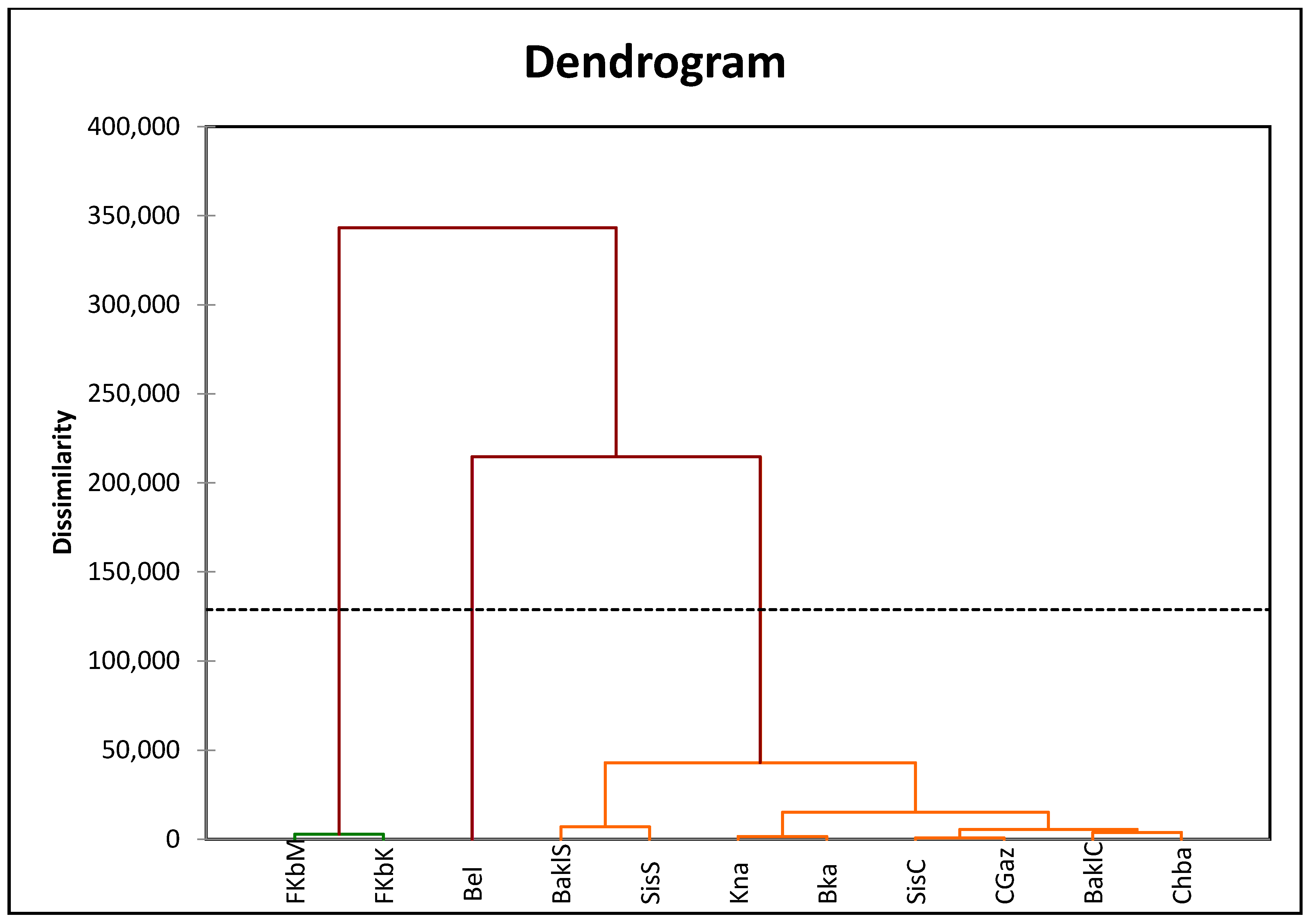
3.5. Correlations and Cluster Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aguilar-Meléndez, A.; Vásquez-Dávila, M.A.; Manzanero-Medina, G.I.; Katz, E.; Sánchez-Toledano, B.I.; Cuevas-Reyes, V.; Kallas, Z.; Zegbe, J.A. Chile (Capsicum spp.) as Food-Medicine Continuum in Multiethnic Mexico. Foods 2021, 10, 2502. [Google Scholar] [CrossRef]
- Sánchez-Toledano, B.I.; Cuevas-Reyes, V.; Kallas, Z.; Zegbe, J.A. Preferences in ‘Jalapeño’ Pepper Attributes: A Choice Study in Mexico. Foods 2021, 10, 3111. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kim, S.K.; Lee, H.J.; Lee, H.S.; Lee, J.H. Impact of Moderate and Extreme Climate Change Scenarios on Growth, Morphological Features, Photosynthesis, and Fruit Production of Hot Pepper. Ecol. Evol. 2018, 8, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, P.; Ficcadenti, N.; Rotino, G.L.; Festa, G.; Bertone, A.; Pepe, A.; Caramanico, R.; Migliori, C.A.; Spadafora, D.; Schiavi, M.; et al. Genotypic and Environmental Effects on the Agronomic, Health-Related Compounds and Antioxidant Properties of Chilli Peppers for Diverse Market Destinations. J. Sci. Food Agric. 2019, 99, 4550–4560. [Google Scholar] [CrossRef]
- Rodríguez-Calzada, T.; Qian, M.; Strid, Å.; Neugart, S.; Schreiner, M.; Torres-Pacheco, I.; Guevara-González, R.G. Effect of UV-B Radiation on Morphology, Phenolic Compound Production, Gene Expression, and Subsequent Drought Stress Responses in Chili Pepper (Capsicum annuum L.). Plant Physiol. Biochem. 2019, 134, 94–102. [Google Scholar] [CrossRef]
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in Phytochemical and Antioxidant Activity of Selected Pepper Cultivars (Capsicum Species) as Influenced by Maturity. J. Agric. Food Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Martínez, I.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Proal-Nájera, J.; Gallardo-Velázquez, T. Determination of Capsaicin, Ascorbic Acid, Total Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. var. Serrano by Mid Infrared Spectroscopy (Mid-FTIR) and Chemometric Analysis. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 133–142. [Google Scholar] [CrossRef]
- Mitic, V.D.; Cvetkovic, J.S.; Stankov-Jovanovic, V.P.; Dimitrijevic, M.V.; Stojanovic, G.S. Characterization of Pepper Genotypes from Serbia as a Function of Maturity by Antioxidant Activity with Chemometric Analysis. Anal. Lett. 2016, 49, 2234–2245. [Google Scholar] [CrossRef]
- Tripodi, P.; Cardi, T.; Bianchi, G.; Migliori, C.A.; Schiavi, M.; Rotino, G.L.; Lo Scalzo, R. Genetic and Environmental Factors Underlying Variation in Yield Performance and Bioactive Compound Content of Hot Pepper Varieties (Capsicum annuum) Cultivated in Two Contrasting Italian Locations. Eur. Food Res. Technol. 2018, 244, 1555–1567. [Google Scholar] [CrossRef]
- Estrada, B.; Bernal, M.A.; Díaz, J.; Pomar, F.; Merino, F. Capsaicinoids in Vegetative Organs of Capsicum annuum l. in Relation to Fruiting. J. Agric. Food Chem. 2002, 50, 1188–1191. [Google Scholar] [CrossRef]
- Coyago-Cruz, E.; Corell, M.; Moriana, A.; Hernanz, D.; Stinco, C.M.; Meléndez-Martínez, A.J. Effect of the Fruit Position on the Cluster on Fruit Quality, Carotenoids, Phenolics and Sugars in Cherry Tomatoes (Solanum lycopersicum L.). Food Res. Int. 2017, 100, 804–813. [Google Scholar] [CrossRef]
- Lombardo, S.; Restuccia, C.; Pandino, G.; Licciardello, F.; Muratore, G.; Mauromicale, G. Influence of an O3-Atmosphere Storage on Microbial Growth and Antioxidant Contents of Globe Artichoke as Affected by Genotype and Harvest Time. Innov. Food Sci. Emerg. Technol. 2015, 27, 121–128. [Google Scholar] [CrossRef]
- Drogoudi, P.D.; Pantelidis, G. Effects of Position on Canopy and Harvest Time on Fruit Physico-Chemical and Antioxidant Properties in Different Apple Cultivars. Sci. Hortic. 2011, 129, 752–760. [Google Scholar] [CrossRef]
- Grilo, F.; Caruso, T.; Wang, S.C. Influence of Fruit Canopy Position and Maturity on Yield Determinants and Chemical Composition of Virgin Olive Oil. J. Sci. Food Agric. 2019, 99, 4319–4330. [Google Scholar] [CrossRef]
- Nuncio-Jáuregui, N.; Calín-Sánchez, A.; Carbonell-Barrachina, A.; Hernández, F. Changes in Quality Parameters, Proline, Antioxidant Activity and Color of Pomegranate (Punica granatum L.) as Affected by Fruit Position within Tree, Cultivar and Ripening Stage. Sci. Hortic. 2014, 165, 181–189. [Google Scholar] [CrossRef]
- Pandino, G.; Meneghini, M.; Tavazza, R.; Lombardo, S.; Mauromicale, G. Phytochemicals Accumulation and Antioxidant Activity in Callus and Suspension Cultures of Cynara scolymus L. Plant Cell Tissue Organ Cult. 2017, 128, 223–230. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The Influence of Pre-Harvest Factors on the Quality of Globe Artichoke. Sci. Hortic. 2018, 233, 479–490. [Google Scholar] [CrossRef]
- Deepa, N.; Kaur, C.; George, B.; Singh, B.; Kapoor, H.C. Antioxidant Constituents in Some Sweet Pepper (Capsicum annuum L.) Genotypes during Maturity. LWT—Food Sci. Technol. 2007, 40, 121–129. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in Phenolic Compounds, Ascorbic Acid and Antioxidant Activity of Five Coloured Bell Pepper (Capsicum Annum) Fruits at Two Different Harvest Times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Mateos, R.M.; Jiménez, A.; Román, P.; Romojaro, F.; Bacarizo, S.; Leterrier, M.; Gómez, M.; Sevilla, F.; Del Río, L.A.; Corpas, F.J.; et al. Antioxidant Systems from Pepper (Capsicum annuum L.): Involvement in the Response to Temperature Changes in Ripe Fruits. Int. J. Mol. Sci. 2013, 14, 9556–9580. [Google Scholar] [CrossRef]
- Lahbib, K.; Dabbou, S.; Bnejdi, F.; Pandino, G.; Lombardo, S.; ElGazzeh, M.; ElBok, S. Agro-Morphological, Biochemical and Antioxidant Characterization of a Tunisian Chili Pepper Germplasm Collection. Agriculture 2021, 11, 1236. [Google Scholar] [CrossRef]
- Maurya, V.K.; Gothandam, K.M.; Ranjan, V.; Shakya, A.; Pareek, S. Effect of Drying Methods (Microwave Vacuum, Freeze, Hot Air and Sun Drying) on Physical, Chemical and Nutritional Attributes of Five Pepper (Capsicum annuum var. Annuum) Cultivars. J. Sci. Food Agric. 2018, 98, 3492–3500. [Google Scholar] [CrossRef]
- Jeeatid, N.; Techawongstien, S.; Suriharn, B.; Bosland, P.W.; Techawongstien, S. Light Intensity Affects Capsaicinoid Accumulation in Hot Pepper (Capsicum chinense Jacq.) Cultivars. Hortic. Environ. Biotechnol. 2017, 58, 103–110. [Google Scholar] [CrossRef]
- Bae, J.H.; Park, Y.J.; Namiesnik, J.; Gülçin, I.; Kim, T.C.; Kim, H.C.; Heo, B.G.; Gorinstein, S.; Ku, Y.G. Effects of Artificial Lighting on Bioactivity of Sweet Red Pepper (Capsicum annuum L.). Int. J. Food Sci. Technol. 2016, 51, 1378–1385. [Google Scholar] [CrossRef]
- Sadasivam, S.; Manikkam, A. Capsaicin. Biochem. Methods Agric. Sci. 1992, 193–194. [Google Scholar]
- Mocan, A.; Zengin, G.; Uysal, A.; Gunes, E.; Mollica, A.; Degirmenci, N.S.; Alpsoy, L.; Aktumsek, A. Biological and Chemical Insights of Morina persica L.: A Source of Bioactive Compounds with Multifunctional Properties. J. Funct. Foods 2016, 25, 94–109. [Google Scholar] [CrossRef]
- Um, H.-J.; Kim, G.-H. Studies on the Flavonoid Compositions of Elsholtzia spp. Korean J. Food Nutr. 2007, 2, 103–107. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 76, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gurung, T.; Techawongstien, S.; Suriharn, B.; Techawongstien, S. Impact of Environments on the Accumulation of Capsaicinoids in Capsicum spp. HortScience 2011, 46, 1576–1581. [Google Scholar] [CrossRef]
- Gurung, T.; Techawongstien, S.; Suriharn, B.; Techawongstien, S. Stability Analysis of Yield and Capsaicinoids Content in Chili (Capsicum spp.) Grown across Six Environments. Euphytica 2012, 187, 11–18. [Google Scholar] [CrossRef]
- Zewdie, Y.; Bosland, P.W. Evaluation of Genotype, Environment, and Genotype-by-Environment Interaction for Capsaicinoids in Capsicum annuum L. Euphytica 2000, 111, 185–190. [Google Scholar] [CrossRef]
- Materska, M.; Perucka, I. Antioxidant Activity of the Main Phenolic Compounds Isolated from Hot Pepper Fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Singh, V.; Upadhyay, G.; Pandey, A.K.; Prakash, D. Assessment of Phytochemical Composition and Antioxidant Potential in Some Indigenous Chilli Genotypes from North East India. Food Chem. 2015, 188, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Lahbib, K.; Dabbou, S.; Bok, S.E.L.; Pandino, G.; Lombardo, S.; Gazzah, M. EL Variation of Biochemical and Antioxidant Activity with Respect to the Part of Capsicum annuum Fruit from Tunisian Autochthonous Cultivars. Ind. Crops Prod. 2017, 104, 164–170. [Google Scholar] [CrossRef]
- de Jesús Ornelas-Paz, J.; Martínez-Burrola, J.M.; Ruiz-Cruz, S.; Santana-Rodríguez, V.; Ibarra-Junquera, V.; Olivas, G.I.; Pérez-Martínez, J.D. Effect of Cooking on the Capsaicinoids and Phenolics Contents of Mexican Peppers. Food Chem. 2010, 119, 1619–1625. [Google Scholar] [CrossRef]
- Medina-juárez, L.Á.; Molina-quijada, D.M.A.; Del Toro-sánchez, C.L.; González-Aguilar, G.A.; Gámez-Meza, N. Antioxidant Activity of Peppers (Capsicum annuum L.) Extracts and Characterization of Their Phenolic Constituents. Intersciencia 2012, 37, 588–593. [Google Scholar]
- Sim, K.H.; Sil, H.Y. Antioxidant Activities of Red Pepper (Capsicum annuum) Pericarp and Seed Extracts. Int. J. Food Sci. Technol. 2008, 43, 1813–1823. [Google Scholar] [CrossRef]
- Wubs, A.M.; Heuvelink, E.; Marcelis, L.F.M.; Hemerik, L. Quantifying Abortion Rates of Reproductive Organs and Effects of Contributing Factors Using Time-to-Event Analysis. Funct. Plant Biol. 2011, 38, 431–440. [Google Scholar] [CrossRef]
- Wubs, A.M.; Heuvelink, E.; Marcelis, L.F.M. Abortion of Reproductive Organs in Sweet Pepper (Capsicum annuum L.): A Review. J. Hortic. Sci. Biotechnol. 2009, 84, 467–475. [Google Scholar] [CrossRef]
- Kviklys, D.; Viškelis, J.; Liaudanskas, M.; Janulis, V.; Laužikė, K.; Samuolienė, G.; Uselis, N.; Lanauskas, J. Apple Fruit Growth and Quality Depend on the Position in Tree Canopy. Plants 2022, 11, 196. [Google Scholar] [CrossRef]
- Que, F.; Mao, L.; Fang, X.; Wu, T. Comparison of Hot Air-Drying and Freeze-Drying on the Physicochemical Properties and Antioxidant Activities of Pumpkin (Cucurbita moschata Duch.) Flours. Int. J. Food Sci. Technol. 2008, 43, 1195–1201. [Google Scholar] [CrossRef]
- Hamed, M.; Kalita, D.; Bartolo, M.E.; Jayanty, S.S. Capsaicinoids, Polyphenols and Antioxidant Activities of Capsicum annuum: Comparative Study of the Effect of Ripening Stage and Cooking Methods. Antioxidants 2019, 8, 364. [Google Scholar] [CrossRef]
- Ye, Z.; Shang, Z.; Li, M.; Zhang, X.; Ren, H.; Hu, X.; Yi, J. Effect of ripening and variety on the physiochemical quality and flavor of fermented Chinese chili pepper (Paojiao). Food Chem. 2022, 368, 130797. [Google Scholar] [CrossRef]
- Ananthan, R.; Subhash, K.; Longvah, T. Capsaicinoids, amino acid and fatty acid profiles in different fruit components of the world hottest Naga king chilli (Capsicum chinense Jacq). Food Chem. 2018, 238, 51–57. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Bashyal, U.; Lee, Y.-S. Variations in Proximate Nutrients, Phytochemicals, and Antioxidant Activity of Field-cultivated Red Pepper Fruits at Different Harvest Times. Hortic. Environ. Biotechnol. 2016, 57, 493–503. [Google Scholar] [CrossRef]
- Benelli, G.; Caruso, G.; Giunti, G.; Cuzzola, A.; Saba, A.; Raffaelli, A.; Gucci, R. Changes in Olive Oil Volatile Organic Compounds Induced by Water Status and Light Environment in Canopies of Olea europaea L. Trees. J. Sci. Food Agric. 2015, 95, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Suzuki, T.; Fujiwake, H.; Iwai, K. Formation and Accumulation of Pungent Principle of Hot Pepper Fruits, Capsaicin and Its Analogues, in Capsicum annuun var. Annuun cv. Karayatsubusa at Different Growth Stages after Flowering. Agric. Biol. Chem. 1979, 43, 2493–2498. [Google Scholar] [CrossRef]
- Palmer, J.W. The Effects of Row Orientation, Tree Height, Time of Year and Latitude on Light Interception and Distribution in Model Apple Hedgerow Canopies. J. Hortic. Sci. 1989, 64, 137–145. [Google Scholar] [CrossRef]
- Stewart, C.; Kang, B.C.; Liu, K.; Mazourek, M.; Moore, S.L.; Eun, Y.Y.; Kim, B.D.; Paran, I.; Jahn, M.M. The Pun1 Gene for Pungency in Pepper Encodes a Putative Acyltransferase. Plant J. 2005, 42, 675–688. [Google Scholar] [CrossRef]
- Giuffrida, D.; Dugo, P.; Torre, G.; Bignardi, C.; Cavazza, A.; Corradini, C.; Dugo, G. Characterization of 12 Capsicum Varieties by Evaluation of Their Carotenoid Profile and Pungency Determination. Food Chem. 2013, 140, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Meckelmann, S.W.; Riegel, D.W.; van Zonneveld, M.; Ríos, L.; Peña, K.; Mueller-Seitz, E.; Petz, M. Capsaicinoids, Flavonoids, Tocopherols, Antioxidant Capacity and Color Attributes in 23 Native Peruvian Chili Peppers (Capsicum spp.) Grown in Three Different Locations. Eur. Food Res. Technol. 2014, 240, 273–283. [Google Scholar] [CrossRef]
- Jeeatid, N.; Suriharn, B.; Techawongstien, S.; Chanthai, S.; Bosland, P.W.; Techawongstien, S. Evaluation of the Effect of Genotype-by-Environment Interaction on Capsaicinoid Production in Hot Pepper Hybrids (Capsicum chinense Jacq.) under Controlled Environment. Sci. Hortic. 2018, 235, 334–339. [Google Scholar] [CrossRef]
| Harvest Stage | Air Temperature at Harvest (°C) | Horticultural Maturity | Fruit Color |
|---|---|---|---|
| S1 (90 days after planting) | 31.4 | Ripen | green |
| S2 (125 days after planting) | 34.1 | Middle Ripen | orange |
| S3 (150 days after planting) | 36.2 | Fully Ripen | red |
| FW (g) | FL (cm) | Fth (mm) | Y (g plant−1) | CAP (mg−1 DW) | TPC (mg GAE g−1 DW) | TFC (mgNAEg−1 DW) | DPPH (%) | FRAP (mmol g−1) | |
|---|---|---|---|---|---|---|---|---|---|
| variety (A) | ** | ** | * | ** | ** | ** | ** | ** | ** |
| (78%) | (68.69%) | (3.30%) | (21.25%) | (52.6%) | (4.44%) | (13.24%) | (32.48%) | (8.62%) | |
| Fruit position (FP) | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| (17%) | (30.75%) | (92%) | (74.18%) | (45.5%) | (39.93%) | (14.28%) | (6.18%) | (17.09%) | |
| Ripening stage (RS) | ** | * | * | ns | ns | ** | ** | ** | ** |
| (1.31%) | (0.33%) | (0.07%) | (0.09%) | (0.39%) | (45.24%) | (63.41%) | (56.53%) | (71.09%) | |
| A × FP | ns | ns | ns | * | ns | * | ** | ** | * |
| (0.002%) | (0.00002%) | (0.0002%) | (0.21%) | (0.034%) | (0.12%) | (3.48%) | (0.33%) | (0.18%) | |
| A × RS | ns | ns | * | ns | ns | ** | * | ** | ** |
| (0.96%) | (0.21%) | (2.45%) | (0.15%) | (0.15%) | (0.27%) | (0.34%) | (2.01%) | (2.51%) | |
| FP × RS | ns | ns | ns | ns | ns | ** | ** | * | * |
| (0.06%) | (0.00001%) | (0.0001%) | (0.006%) | (0.01%) | (13.91%) | (5.22%) | (0.06%) | (0.35%) |
| FW (g) | FL (cm) | Fth (mm) | Y (g plant−1) | CAP (mg CAP g−1 DW) | TP (mg GAE g−1 DW) | TF (mg NAEg−1 DW) | DPPH (%) | FRAP (mmol g−1) | |
|---|---|---|---|---|---|---|---|---|---|
| Variety | |||||||||
| BaklC | 9.97 ± 0.60 e | 6.50 ± 0.55 d | 1.81 ± 0.06 b | 352.1 ± 3.56 h | 0.57 ± 0.06 e | 6.18 ± 0.35 d | 0.39 ± 0.04 d | 83.1 ± 1.56 f | 26.27 ± 0.95 f |
| Chba | 11.14 ± 0.52 g | 8.06 ± 0.45 e | 1.86 ± 0.04 d | 337.3 ± 4.78 g | 0.47 ± 0.02 d | 5.82 ± 0.78 d | 0.39 ± 0.05 d | 86.2 ± 1.05 g | 25.20 ± 1.77 e |
| BaklS | 9.45 ± 0.74 d | 9.68 ± 0.43 g | 1.97 ± 0.05 f | 292.0 ± 2.87 d | 0.67 ± 0.07 f | 5.89 ± 0.55 d | 0.38 ± 0.04 d | 86.3 ± 0.98 g | 26.32 ± 1.8 f |
| FKbM | 11.32 ± 0.59 g | 8.08 ± 0.65 f | 2.37 ± 0.03 i | 182.9 ± 2.87 b | 0.29 ± 0.05 a | 5.18 ± 0.66 c | 0.37 ± 0.05 d | 76.8 ± 0.88 e | 20.67 ± 0.91 bc |
| FKbK | 11.92 ± 0.45 h | 14.05 ± 0.34 j | 2.83 ± 0.04 j | 158.0 ± 3.9 a | 0.48 ± 0.07 d | 4.89 ± 0.43 c | 0.34 ± 0.02 c | 77.3 ± 1.45 e | 21.83 ± 0.98 d |
| Kna | 8.63 ± 0.63 b | 6.47 ± 0.36 c | 1.83 ± 0.05 c | 366.1 ± 4.87 i | 0.43 ± 0.04 c | 3.74 ± 0.23 a | 0.33 ± 0.06 c | 58.3 ± 1.64 b | 20.88 ± 102 cd |
| Bel | 7.52 ± 0.54 a | 2.53 ± 0.56 a | 1.67 ± 0.07 a | 532.5 ± 3.02 k | 0.41 ± 0.08 b | 4.07 ± 0.56 ab | 0.33 ± 0.02 c | 60.0 ± 0.14 c | 21.91 ± 0.88 d |
| SisS | 11.12 ± 0.78 g | 14.63 ± 0.69 k | 2.09 ± 0.09 g | 273.3 ± 4.76 c | 0.76 ± 0.08 gh | 3.90 ± 0.75 ab | 0.33 ± 0.02 c | 49.9 ± 0.22 a | 20.62 ± 0.85 bc |
| Bka | 9.09 ± 0.67 c | 5.75 ± 0.98 b | 1.83 ± 0.05 c | 388.0 ± 4.67 j | 0.77 ± 0.05 h | 4.28 ± 0.45 b | 0.26 ± 0.03 a | 57.2 ± 1.32 b | 19.60 ± 1.04 ab |
| SisC | 10.70 ± 0.47 f | 11.58 ± 0.45 h | 2.11 ± 0.04 h | 328.9 ± 3.89 e | 0.75 ± 0.02 g | 3.90 ± 0.32 ab | 0.28 ± 0.04 ab | 61.6 ± 1.68 d | 19.45 ± 0.76 a |
| CGaz | 9.89 ± 0.87 e | 11.62 ± 0.67 i | 1.88 ± 0.03 e | 334.5 ± 5.96 f | 0.68 ± 0.08 f | 4.03 ± 0.22 ab | 0.29 ± 0.02 b | 61.0 ± 0.94 cd | 20.03 ± 0.69 abc |
| Ripening stage | |||||||||
| S1 | 9.99 ± 0.56 a | 8.92 ± 0.44 b | 2.00 ± 0.03 a | 318.89 ± 4.88 a | 0.58 ± 0.08 b | 3.35 ± 0.87 a | 0.39 ± 0.06 c | 59.28 ± 0.88 a | 17.90 ± 0.40 a |
| S2 | 10.29 ± 0.86 b | 8.91 ± 0.67 a | 2.02 ± 0.02 b | 325.85 ± 3.9 c | 0.57 ± 0.04 b | 4.45 ± 0.66 b | 0.32 ± 0.08 b | 70.76 ± 1.05 b | 22.64 ± 0.55 b |
| S3 | 9.93 ± 0.77 a | 9.15 ± 0.55 c | 2.05 ± 0.04 c | 322.37 ± 2.4 b | 0.56 ± 0.05 a | 6.36 ± 0.76 c | 0.29 ± 0.03 a | 76.72 ± 1.76 c | 25.67 ± 0.76 c |
| Fruit position | |||||||||
| PII | 9.20 ± 0.56 a | 8.25 ± 0.48 a | 1.50 ± 0.04 a | 379.12 ± 3.43 b | 0.52 ± 0.04 a | 5.54 ± 0.98 b | 0.35 ± 0.04 b | 70.66 ±0.66 b | 23.18 ± 0.66 b |
| PI | 10.94 ± 0.66 b | 9.74 ± 0.59 b | 2.55 ±0.03 b | 265.62 ± 3.76 a | 0.62 ± 0.03 b | 3.89 ± 0.88 a | 0.32 ± 0.04 a | 67.19 ± 0.76 a | 20.96 ± 0.54 a |
| Ripening Stages | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAP | TPC | TFC | DPPH | FRAP | |||||||||||
| S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 | |
| BaklC | 0.77 ± 0.05 | 0.49 ± 0.07 | 0.76 ± 0.06 | 4.56 ± 0.42 | 5.81 ± 0.38 | 6.96 ± 0.31 | 0.45 ± 0.03 | 0.38 ± 0.04 | 0.35 ± 0.02 | 88.93 ± 3.05 | 89.78 ± 3.37 | 91.70 ± 3.36 | 25.94 ± 1.61 | 27.25 ± 1.34 | 27.99 ± 1.26 |
| Chba | 0.77 ± 0.06 | 0.77 ± 0.07 | 0.75 ± 0.04 | 4.53 ± 0.26 | 5.82 ± 0.29 | 7.11 ± 0.25 | 0.46 ± 0.04 | 0.37 ± 0.04 | 0.34 ± 0.02 | 88.93 ± 3.87 | 89.78 ± 3.80 | 92.77 ± 4.02 | 22.81 ± 1.08 | 25.97 ± 1.31 | 29.58 ± 1.38 |
| BaklS | 0.74 ± 0.04 | 0.76 ± 0.04 | 0.74 ± 0.04 | 4.58 ± 0.38 | 5.92 ± 0.33 | 7.17 ± 0.29 | 0.47 ± 0.04 | 0.35 ± 0.04 | 0.30 ± 0.03 | 89.77 ± 2.96 | 90.10 ± 3.62 | 93.62 ± 2.82 | 23.08 ± 0.87 | 27.76 ± 1.56 | 29.44 ± 0.82 |
| FKbM | 0.69 ± 0.07 | 0.66 ± 0.06 | 0.67 ± 0.05 | 3.51 ± 0.27 | 4.65 ± 0.24 | 7.19 ± 0.30 | 0.43 ± 0.03 | 0.35 ± 0.04 | 0.32 ± 0.05 | 70.24 ± 3.38 | 80.00 ± 4.89 | 91.51 ± 5.46 | 19.42 ± 1.72 | 19.35 ± 1.54 | 25.12 ± 1.30 |
| FKbK | 0.57 ± 0.07 | 0.56 ± 0.04 | 0.56 ± 0.06 | 3.48 ± 0.35 | 4.57 ± 0.33 | 6.64 ± 0.31 | 0.39 ± 0.03 | 0.34 ± 0.06 | 0.29 ± 0.03 | 68.25 ± 3.12 | 79.50 ± 4.52 | 92.73 ± 3.17 | 19.42 ± 1.57 | 21.52 ± 1.35 | 26.72 ± 1.40 |
| Kna | 0.48 ± 0.08 | 0.46 ± 0.04 | 0.47 ± 0.02 | 2.51 ± 0.03 | 3.38 ± 0.03 | 5.38 ± 0.33 | 0.39 ± 0.06 | 0.33 ± 0.04 | 0.28 ± 0.02 | 47.98 ± 2.78 | 57.62 ± 3.02 | 73.61 ± 2.64 | 15.25 ± 2.80 | 21.32 ± 1.03 | 24.97 ± 0.70 |
| Bel | 0.69 ± 0.05 | 0.67 ± 0.06 | 0.65 ± 0.04 | 2.29 ± 0.24 | 3.46 ± 0.34 | 6.03 ± 0.35 | 0.37 ± 0.04 | 0.33 ± 0.07 | 0.29 ± 0.03 | 50.08 ± 2.90 | 61.62 ± 3.03 | 73.18 ± 2.60 | 15.22 ± 1.09 | 21.27 ± 1.48 | 28.37 ± 1.30 |
| SisS | 0.33 ± 0.06 | 0.29 ± 0.04 | 0.27 ± 0.04 | 2.41 ± 0.27 | 3.81 ± 0.23 | 5.51 ± 0.25 | 0.36 ± 0.06 | 0.32 ± 0.02 | 0.30 ± 0.04 | 33.49 ± 3.13 | 57.43 ± 3.53 | 58.84 ± 3.67 | 14.19 ± 1.42 | 21.30 ± 1.14 | 26.42 ± 1.52 |
| Bka | 0.44 ± 0.06 | 0.50 ± 0.05 | 0.48 ± 0.05 | 2.92 ± 0.25 | 3.39 ± 0.23 | 6.09 ± 0.26 | 0.31 ± 0.06 | 0.25 ± 0.02 | 0.21 ± 0.04 | 45.46 ± 3.72 | 57.46 ± 3.07 | 68.90 ± 3.30 | 13.04 ± 1.18 | 21.64 ± 1.03 | 24.17 ± 0.90 |
| SisC | 0.45 ± 0.07 | 0.43 ± 0.06 | 0.41 ± 0.04 | 2.26 ± 0.04 | 3.43 ± 0.36 | 6.01 ± 0.37 | 0.34 ± 0.02 | 0.25 ± 0.05 | 0.24 ± 0.04 | 45.46 ± 3.59 | 69.52 ± 2.94 | 69.97 ± 2.50 | 12.64 ± 1.71 | 18.91 ± 1.35 | 26.80 ± 1.55 |
| CGaz | 0.43 ± 0.07 | 0.41 ± 0.04 | 0.37 ± 0.01 | 2.53 ± 0.30 | 3.74 ± 0.24 | 5.80 ± 0.27 | 0.34 ± 0.06 | 0.26 ± 0.05 | 0.25 ± 0.03 | 45.57 ± 2.67 | 68.56 ± 2.71 | 68.93 ± 3.79 | 15.97 ± 1.54 | 22.46 ± 1.17 | 21.66 ± 1.70 |
| CAP | TPC | TFC | DPPH | FRAP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 | |
| PI | 0.63 ± 0.07 | 0.63 ± 0.04 | 0.61 ± 0.04 | 2.93 ± 0.33 | 3.99 ± 0.26 | 4.56 ± 0.31 | 0.39 ± 0.04 | 0.29 ± 0.04 | 0.27 ± 0.03 | 58.37 ± 2.91 | 69.07 ± 2.6 | 74.77 ± 2.46 | 17.11 ± 1.7 | 23.66 ± 1.29 | 24.26 ± 1.8 |
| PII | 0.52 ± 0.05 | 0.52 ± 0.05 | 0.50 ± 0.03 | 3.53 ± 0.30 | 4.91 ± 0.26 | 8.14 ± 0.30 | 0.38 ± 0.06 | 0.35 ± 0.08 | 0.27 ± 0.03 | 64.21 ± 2.4 | 68.99 ± 2.9 | 84.46 ± 2.71 | 18.79 ± 0.93 | 23.66 ± 1.29 | 28.66 ± 1.31 |
| PI | PII | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FW | FL | Fth | Y | CAP | TPC | TFC | DPPH | FRAP | FW | FL | Fth | Y | CAP | TPC | TFC | DPPH | FRAP | |
| FW | - | - | ||||||||||||||||
| FL | 0.56 * | - | 0.51 * | - | ||||||||||||||
| FW | 0.68 * | 0.69 * | - | 0.69 * | 0.67 * | - | ||||||||||||
| Y | −0.62 * | −0.75 * | −0.69 * | - | −0.65 * | −0.71 * | −0.68 * | - | ||||||||||
| CAP | 0.56 * | 0.58 * | 0.65 * | −0.45 | - | 0.57 * | 0.57 * | 0.63 * | −0.47 | - | ||||||||
| TPC | 0.21 | 0.20 | 0.23 | −0.55 * | 0.73 * | - | 0.22 | 0.20 | 0.25 | −0.56 * | 0.74 * | - | ||||||
| TFC | 0.19 | 0.15 | 0.19 | −0.65 * | 0.67 * | 0.81 * | - | 0.18 | 0.17 | 0.19 | −0.67 * | 0.68 * | 0.82 * | - | ||||
| DPPH | 0.13 | 0.17 | 0.22 | −0.59 * | 0.73 * | 0.95 * | 0.81 * | - | 0.16 | 0.16 | 0.20 | −0.56 * | 0.75 * | 0.94 * | 0.84 * | - | ||
| FRAP | 0.18 | 0.15 | 0.23 | −0.65 * | 0.80 * | 0.94 * | 0.76 * | 0.94 * | - | 0.18 | 0.19 | 0.26 | −0.65 * | 0.82 * | 0.92 * | 0.75 * | 0.93 * | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahbib, K.; Bnejdi, F.; Pandino, G.; Lombardo, S.; El-Gazzah, M.; El-Bok, S.; Dabbou, S. Changes in Yield-Related Traits, Phytochemical Composition, and Antioxidant Activity of Pepper (Capsicum annuum) Depending on Its Variety, Fruit Position, and Ripening Stage. Foods 2023, 12, 3948. https://doi.org/10.3390/foods12213948
Lahbib K, Bnejdi F, Pandino G, Lombardo S, El-Gazzah M, El-Bok S, Dabbou S. Changes in Yield-Related Traits, Phytochemical Composition, and Antioxidant Activity of Pepper (Capsicum annuum) Depending on Its Variety, Fruit Position, and Ripening Stage. Foods. 2023; 12(21):3948. https://doi.org/10.3390/foods12213948
Chicago/Turabian StyleLahbib, Karima, Fethi Bnejdi, Gaetano Pandino, Sara Lombardo, Mohamed El-Gazzah, Safia El-Bok, and Samia Dabbou. 2023. "Changes in Yield-Related Traits, Phytochemical Composition, and Antioxidant Activity of Pepper (Capsicum annuum) Depending on Its Variety, Fruit Position, and Ripening Stage" Foods 12, no. 21: 3948. https://doi.org/10.3390/foods12213948
APA StyleLahbib, K., Bnejdi, F., Pandino, G., Lombardo, S., El-Gazzah, M., El-Bok, S., & Dabbou, S. (2023). Changes in Yield-Related Traits, Phytochemical Composition, and Antioxidant Activity of Pepper (Capsicum annuum) Depending on Its Variety, Fruit Position, and Ripening Stage. Foods, 12(21), 3948. https://doi.org/10.3390/foods12213948








