Validation of UHPLC-ESI-MS/MS Method for Determining Steviol Glycoside and Its Derivatives in Foods and Beverages
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Standards
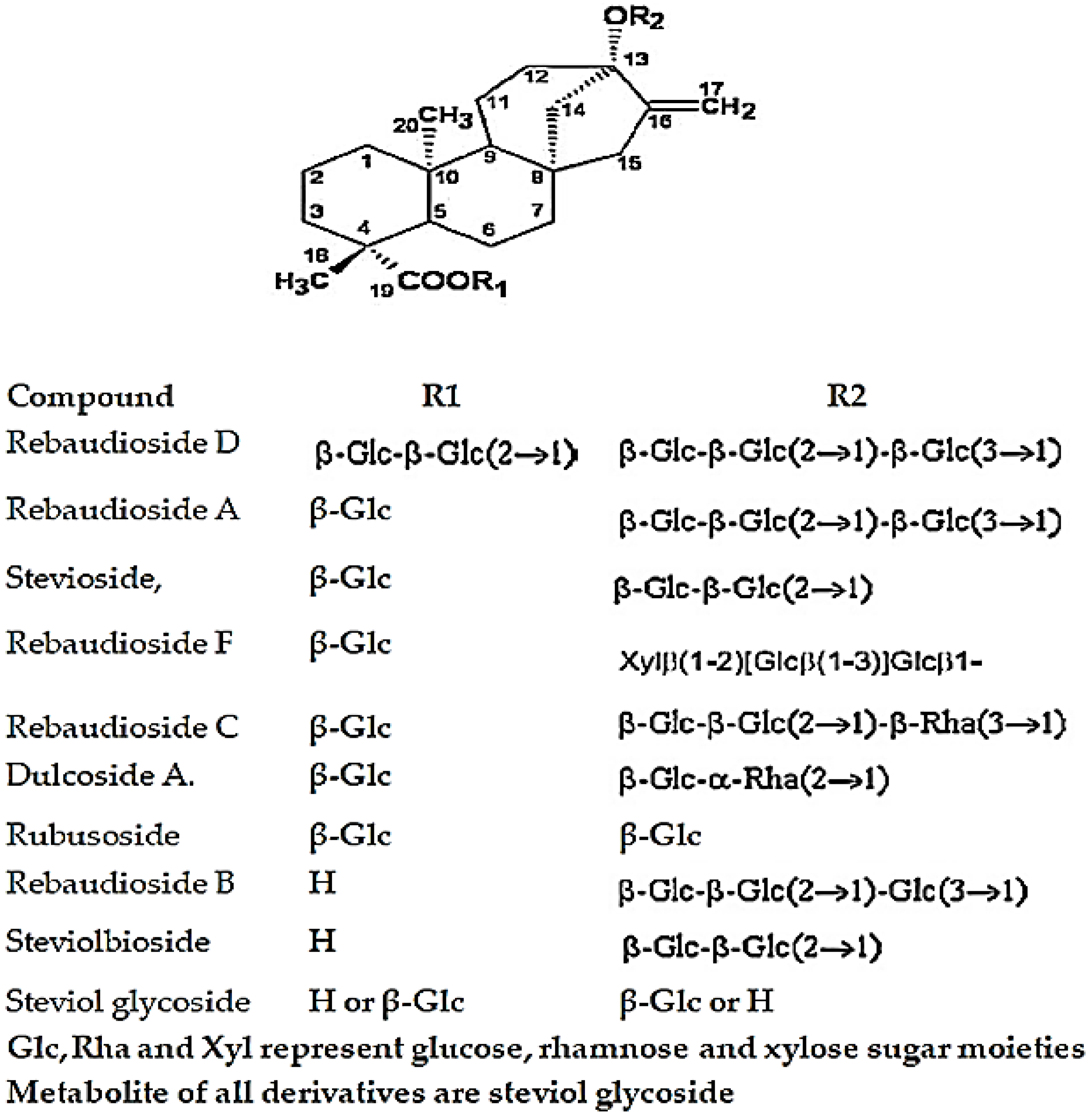
2.2. Standard Solution of Steviol Glycoside and Its Derivatives
2.3. Sample Preparation and Extraction
2.4. Sample Preparation for the Analysis of Steviol Glycoside and Its Derivatives
2.5. Condition and Instrument Optimization
2.6. Method Validation (MV) for Determination of Steviol Glycosides
2.6.1. Specificity
2.6.2. Linearity of Analysis Results for Steviol Glycoside and Its Derivatives
2.6.3. Matrix Effect (ME)
2.6.4. Precision and Accuracy
Precision
Accuracy
2.6.5. Limit of Detection (LOD) and Limitation of Quantity (LOQ)
2.7. Calculation Equation for Steviol Equivalents
2.8. Analysis of Steviol Glycoside and Its Derivatives in Foods and Beverages
3. Results and Discussion
3.1. Optimization of Instrument Conditions and Preparation of Sample
3.2. Method Validation
3.2.1. Selectivity and Specificity
3.2.2. Linearity
3.2.3. Matrix Effect (ME)
3.2.4. Precision and Accuracy
3.2.5. Limit of Detection (LOD) and Limit of Quantitation (LOQ)
3.3. The Analysis of Steviol Glycoside and Its Derivatives in Foods and Beverages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Guideline: Sugars Intake for Adults and Children; WHO: Geneva, Switzerland, 2015; Available online: https://www.who.int/nutrition/publications/guidelines/sugars_intake/en/ (accessed on 6 June 2023).
- Gardana, C.; Scaglianti, M.; Simonetti, P. Evaluation of steviol and its glycosides in Stevia rebaudiana leaves and commercial sweetener by ultra-high-performance liquid chromatography-mass spectrometry. J. Chromatogr. A 2010, 1217, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, E.J. Sweet and non-sweet constituents of Stevia rebaudiana (Bertoni) Bertoni. In Stevia, the Genus Stevia; Medicinal and Aromatic Plants—Industrial Profiles; Kinghorn, A.D., Ed.; Taylor and Francis: London, UK, 2002; pp. 68–85. [Google Scholar]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Zura-Bravo, L.; Kong, A.H. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 2012, 132, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, M.; Karhan, M. Characteristics of some beverages adjusted with stevia extract, and persistence of steviol glycosides in the mouth after consumption. Int. J. Gastron. Food Sci. 2021, 24, 100326. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.; Wan, Z.; Yang, X. Novel functional properties and applications of steviol glycosides in foods. Curr. Opin. Food Sci. 2022, 43, 91–98. [Google Scholar] [CrossRef]
- Carakostas, M.C.; Prakash, I.; Kinghorn, A.D.; Wu, C.D.; Soejarto, D.D. Steviol glycosides. In Alternative Sweeteners, 4th ed.; O’Brien-Nabors, L., Ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9780429109089. [Google Scholar]
- European Food Safety Authority (EFSA). Commission Regulation (EU) No 1131/2011 of 11 November 2011 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council with Regard to Steviol Glycosides. Off. J. Eur. Union 2011, 295, 205–211. Available online: http://data.europa.eu/eli/reg/2011/1131/oj (accessed on 6 June 2023).
- World Health Organization (WHO). Evaluation of Certain Food Additives: Sixty-Ninth Report of the Joint FAO/WHO Expert Committee on Food Additives. Available online: https://apps.who.int/iris/bitstream/handle/10665/44062/WHO_TRS_952_eng.pdf?sequence=1&isAllowed=y (accessed on 12 June 2023).
- Compendium of Food Additive Specifications. Joint FAO/WHO Expert Committee on Food Additives (JECFA), 84th Meeting 2017. FAO JECFA Monographs 20. Available online: https://www.fao.org/3/BU297en/bu297en.pdf (accessed on 12 June 2023).
- Notification of Ministry of Public Health. Notification of Ministry of Public Health (No 418) B.E. 2563 (2020). Available online: https://food.fda.moph.go.th/media.php?id=509429950466629632&name=P418.pdf (accessed on 20 June 2023). (In Thai).
- Kim, J.-M.; Koh, J.-H.; Park, J.-M. Validation of an HPLC Method for Pretreatment of Steviol Glycosides in Fermented Milk. Foods 2021, 10, 2445. [Google Scholar] [CrossRef]
- Bergs, D.; Burghoff, B.; Joehnck, M.; Martin, G.; Schembecker, G. Fast and isocratic HPLC-method for steviol glycosides analysis from Stevia rebaudiana leaves. J. Verbr. Leb. 2012, 7, 147–154. [Google Scholar] [CrossRef]
- Bayraktar, M.; Naziri, E.; Karabey, F.; Akgun, I.H.; Bedir, E.; Röck-Okuyucu, B.; Gürel, A. Enhancement of stevioside production by using biotechnological approach in in vitro culture of Stevia rebaudiana. Int. J. Sec. Metab. 2018, 5, 362–374. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J.; Tiwary, A.K. Optimization of novel method for the extraction of steviosides from Stevia rebaudiana leaves. Food Chem. 2012, 132, 1113–1120. [Google Scholar] [CrossRef]
- Chao, Y.Y.; Chen, Y.L.; Lin, H.Y.; Huang, Y.L. Rapid screening of basic colorants in processed vegetables through mass spectrometry using an interchangeable thermal desorption electrospray ionization source. Anal. Chim. Acta 2018, 1010, 44–53. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Di, D. Preparative separation and purification of Rebaudioside A from Stevia rebaudiana Bertoni crude extracts by mixed bed of macroporous adsorption resins. Food Chem. 2012, 132, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Di, D.; Bai, Q.; Li, J.; Chen, Z.; Lou, S.; Ye, H. Preparative separation and purification of rebaudioside a from steviol glycosides using mixed-mode macroporous adsorption resins. J. Agric. Food Chem. 2011, 59, 9629–9636. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, S.; Sharma, R.; Rajput, Y.S.; Mann, B.; Lata, K. Estimation of steviol glycosides in food matrices by high performance liquid chromatography. J. Food Sci. Technol. 2018, 55, 3325–3334. [Google Scholar] [CrossRef]
- Rodenburg, D.L.; Alves, K.; Perera, W.H.; Ramsaroop, T.; Carvalho, R.; McChesney, J.D. Development of HPLC analytical techniques for diterpene glycosides from Stevia rebaudiana (Bertoni) Bertoni: Strategies to scale-up. J. Braz. Chem. Soc. 2016, 27, 1406–1412. [Google Scholar] [CrossRef]
- Chen, B.; Li, R.; Chen, X.; Yang, S.; Li, S.; Yang, K.; Chen, G.; Ma, X. Purification and preparation of rebaudioside A from steviol glycosides using one-dimensional hydrophilic interaction chromatography. J. Chromatogr. Sci. 2016, 54, 1408–1414. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, H.; Dai, Z.; Jiang, D.; Sun, M.; Ke, Y.; Jin, Y.; Liang, X. A ternary eluent strategy to tune the peak shape of steviol glycosides in reversed-phase liquid chromatography. J. Chromatogr. B 2021, 1173, 122673. [Google Scholar] [CrossRef]
- Cacciola, F.; Delmonte, P.; Jaworska, K.; Dugo, P.; Mondello, L.; Rader, J.I. Employing ultra-high-pressure liquid chromatography as the second dimension in a comprehensive two-dimensional system for analysis of Stevia rebaudiana extracts. J. Chromatogr. A 2011, 1218, 2012–2018. [Google Scholar] [CrossRef]
- Shah, R.; de Jager, L.S. Recent analytical methods for the analysis of sweeteners in foods: A regulatory perspective. Food Drug Adm. Papers 2017, 5, 13–32. Available online: http://digitalcommons.unl.edu/usfda (accessed on 25 June 2023).
- FAO/WHO Expert Committee on Food Additives (JECFA). Steviol Glycosides from Stevia rebaudiana Bertoni. Available online: http://www.fao.org/3/BU297en/bu297en.pdf (accessed on 25 June 2023).
- Kolb, N.; Herrera, J.L.; Ferreyra, D.J.; Uliana, R.F. Analysis of sweet diterpene glycosides from Stevia rebaudiana: Improved HPLC method. J. Agric. Food Chem. 2001, 49, 4538–4541. [Google Scholar] [CrossRef]
- Zimmermann, B.F. Beaming steviol glycoside analysis into the next dimension. Food Chem. 2018, 241, 150–153. [Google Scholar] [CrossRef]
- AOAC. AOAC Guidelines for Single-Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals, Official Methods of Analysis, 19th ed.; AOAC, International: Gaithersburg, MD, USA, 2012; Appendix K. [Google Scholar]
- Guidance on Bioanalytical Method Validation, European Medicines Agency. 2011. Available online: https://www.ema.europa.eu/en/bioanalytical-method-validation-scientific-guideline (accessed on 5 July 2023).
- Magnusson, B.; Örnemark, U. EURACHEM Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; 2014; ISBN 9789187461590. Available online: https://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_EN.pdf (accessed on 12 July 2023).
- U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation; U.S. Department of Health and Human Services Food and Drug Administration: White Oak, MD, USA, 2001.
- ICH Harmonized Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology Q2(R1). In Proceedings of the International Conference of Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland, November 2005; Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 12 July 2023).
- IUPAC. Harmonized guidelines for single-laboratory validation of method of analyses (IUPAC technical report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- NordVal Protocol No. 2. Guide in Validation of Alternative Proprietary Chemical Methods. 2010. Available online: https://www.nmkl.org/nordval-international/apply-for-certificate/nordval-validation-protocol/ (accessed on 15 July 2023).
- SANCO/12571/2013, Guidance Document on Analytical Quality Control and Validation Procedures for Pesticide Residues Analysis in Food and Feed. 2013; ASEAN Guideline on Analytical Validation—Questions and Answers (version 1, 25 June 2012). Available online: https://www.fda.gov.ph/wp-content/uploads/2021/03/Analytical-Validation-QA-Version-25-June-2012.pdf (accessed on 15 July 2023).
- ASEAN. Technical Guidelines to ACTR on Quality: ASEAN Analytical Validation Guideline. 1996. Available online: https://asean.org/wp-content/uploads/2012/10/Asean-Analytical-Validation-gl.pdf (accessed on 20 July 2023).
- Kruve, A.; Rebane, R.; Kipper, K.; Oldekop, M.-L.; Evard, H.; Herodes, K.; Leito, I. Tutorial review on validation of liquid chromatography–mass spectrometry methods: Part I. Anal. Chim. Acta 2015, 870, 29–44. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Management of left-censored data in dietary exposure assessment of chemical substances. EFSA J. 2010, 8, 1557. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Addendum to the WHO Report ‘‘Reliable Evaluation of Low-Level Contamination of Food’’; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Wenzl, T.; Haedrich, J.; Schaechtele, A.; Piotr, R.; Stroka, J.; Eppe, G.; Scholl, G.; Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food. European Union Reference Laboratory. 2016, pp. 9–11. Available online: https://data.europa.eu/doi/10.2787/8931 (accessed on 20 July 2023).
- Nascimento, A.; Kogawa, A.C.; Salgado, H.R.N. Development and validation of an innovative and ecological analytical method using high performance liquid chromatography for quantification of cephalothin sodium in pharmaceutical dosage. J. Chromatogr. Sep. Tech. 2018, 9, 394–401. [Google Scholar] [CrossRef]
- Gardana, C.; Simonetti, P. Determination of steviol glycosides in commercial extracts of Stevia rebaudiana and sweeteners by ultra-high-performance liquid chromatography Orbitrap mass spectrometry. J. Chromatogr. A 2018, 1578, 8–14. [Google Scholar] [CrossRef]
- Geuns, J.; Ceunen, S.; Steurs, G. Synthesis of an IS and Steviol Glycoside Analysis by a Validated Internal Standard Method. Am. J. Anal. Chem. 2018, 9, 547–559. [Google Scholar] [CrossRef][Green Version]
- ASEAN Technical Guidelines to ACTR on Quality: ASEAN Analytical Validation Guideline. 2016. Available online: https://asean.org/wp-content/uploads/2017/03/67.-December-2016-ACTR.pdf (accessed on 23 July 2023).
- AOAC. Official Methods of Analysis. In Guidelines for Standard Method Performance Requirements Appendix F; AOAC: Arlington, VA, USA, 2016; pp. 1–18. [Google Scholar]
- Zarębska, M.; Hordyjewicz-Baran, Z.; Wasilewski, T.; Zajszły-Turko, E.; Stanek, N. A New LC-MS Method for Evaluating the Efficacy of Pesticide Residue Removal from Fruit Surfaces by Washing Agents. Processes 2022, 10, 793. [Google Scholar] [CrossRef]
- Steiner, D.; Krska, R.; Malachová, A.; Taschl, I.; Sulyok, M. Evaluation of matrix effects and extraction efficiencies of LC–MS/MS methods as the essential part for proper validation of multiclass contaminants in complex feed. J. Agric. Food Chem. 2020, 68, 3868–3880. [Google Scholar] [CrossRef]
- Kruve, A.; Künnapas, A.; Herodes, K.; Leito, I. Matrix effects in pesticide multi-residue analysis by liquid chromatography–mass spectrometry. J. Chromatogr. A 2008, 1187, 58–66. [Google Scholar] [CrossRef]
- Taylor, P.J. Matrix effects: The Achilles heel of quantitative high-performance liquid chromatography–electrospray–tandem mass spectrometry. Clin. Biochem. 2005, 38, 328–334. [Google Scholar] [CrossRef]
- Niessen, W.M.A.; Manini, P.; Andreoli, R. Matrix effects in quantitative pesticide analysis using liquid chromatography–mass spectrometry. Mass Spectrom. Rev. 2006, 25, 881–899. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Guidance Document on Pesticide Analytical Methods for Risk Assessment and Post-Approval Control and Monitoring Purposes. SANTE/2020/12830-rev. 1, 24 February 2021. Available online: https://food.ec.europa.eu/system/files/2021-03/pesticides_ppp_app-proc_guide_res_mrl-guidelines-2020-12830.pdf (accessed on 23 July 2023).
- Cuadros-Rodrıguez, L.; Garcıa-Campaña, A.M.; Almansa-López, E.; Egea-González, F.J.; Cano, M.L.C.; Frenich, A.G.; Martınez-Vidal, J.L. Correction function on biased results due to matrix effects: Application to the routine analysis of pesticide residues. Anal. Chim. Acta 2003, 478, 281–301. [Google Scholar] [CrossRef]
- Horwitz, W.; Albert, R. The Horwitz Ratio (HorRat): A Useful Index of Method Performance with Respect to Precision. J. AOAC Int. 2006, 89, 1095–1109. Available online: https://pubmed.ncbi.nlm.nih.gov/16915851/ (accessed on 25 July 2023). [CrossRef] [PubMed]
- European Commission. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed SANTE 11312/2021; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Australian Beverages Council Ltd. Application to Amend Schedule 15 of the Australia Newzealand Food Standards Code to Allow the Addition of Steviol Glycosides in Fruit Drinks. 2017. Available online: https://www.foodstandards.gov.au/code/applications/Documents/A1149%20Application_Redacted.pdf (accessed on 25 July 2023).
- JECFA. In joint FAO/WHO expert committee on food additives monograph 20. In Compendium of Food Additive Specifications from 84nd JECFA Meeting; JECFA: Rome, Italy, 2017. [Google Scholar]
- Perera, W.H.; Avula, B.; Khan, I.A.; McChesney, J.D. Assignment of sugar arrangement in branched steviol glycosides using electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Antignac, J.P.; Wash, F.; Monteau, H.D.; Brabander, F.A.; Bizet, B.L. The ion suppression phenomenon in liquid chromatography–mass spectrometry and its consequences in the field of residue analysis. Anal. Chim. Acta 2005, 529, 129–136. [Google Scholar] [CrossRef]
- Matuszewsky, B.K. Standard line slopes as a measure of a relative matrix effect in quantitative HPLC–MS bioanalysis. J. Chromatogr. B 2006, 830, 293–300. [Google Scholar] [CrossRef]
- Tonidandel, L.; Seraglia, R. Matrix effect signal suppression and enhancement in LC-ESI-MS. In Advances in LC–MS Instrumentation; Cappiello, A., Ed.; Elsevier Publications: Amsterdam, The Netherlands, 2007; pp. 193–210. [Google Scholar]
- Zhou, W.; Yang, S.; Wang, P.G. Matrix effects and application of matrix effect factor. Bioanalysis 2017, 9, 1839–1844. [Google Scholar] [CrossRef]
- Morlock, G.E.; Meyer, S.; Zimmermann, B.F.; Roussel, J.-M. High-performance thin-layer chromatography analysis of steviol glycosides in Stevia formulations and sugar-free food products, and benchmarking with (ultra) high-performance liquid chromatography. J. Chromatogr. A 2014, 1350, 102–111. [Google Scholar] [CrossRef]
- Oktavirina, V.; Prabawati, N.B.; Fathimah, R.N.; Palma, M.; Kurnia, K.A.; Darmawan, N.; Yulianto, B.; Setyaningsih, W. Analytical Methods for Determination of Non-Nutritive Sweeteners in Foodstuffs. Molecules 2021, 26, 3135. [Google Scholar] [CrossRef]
- Shah, R.; Farris, S.; De Jager, L.S.; Begley, T.H. A novel method for the simultaneous determination of 14 sweeteners of regulatory interest using UHPLC-MS/MS. Food Addit. Contam. Part A 2015, 32, 141–151. [Google Scholar] [CrossRef]
- Mortensen, A. Sweeteners permitted in the European Union, Safety aspects. Scandinavian J. Food Nutr. Res. 2006, 50, 104–116. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; De Jager, L.S.; Begley, T.H. Simultaneous determination of steviol and steviol glycosides by liquid chromatography-mass spectrometry. Food Addit. Contam. Part A 2012, 29, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Huvaere, K.; Vandevijvere, S.; Hasni, M.; Vinkx, C.; Van Loco, J. Dietary intake of artificial sweeteners by the Belgian population. Food Addit. Contam. Part A 2012, 29, 54–65. [Google Scholar] [CrossRef]
- Bartholomees, U.; Struyf, T.; Lauwers, O.; Ceunen, S.; Geuns, J.M.C. Validation of an HPLC method for direct measurement of steviol equivalents in foods. Food Chem. 2016, 190, 270–275. [Google Scholar] [CrossRef]
- Lim, H.S.; Choi, E.; Hwang, J.Y.; Lee, G.; Yun, S.S.; Kim, M. Improved method for the determination of 12 non-nutritive sweeteners and monitoring in various foods using liquid chromatography–tandem mass spectrometry. Food Addit. Contam. Part A 2018, 35, 1674–1688. [Google Scholar] [CrossRef]
- Park, J.-M.; Lee, J.-H.; Koh, J.-H.; Kim, J.-M. Pretreatment methods for analyzing steviol glycosides in diverse food samples. J. Food Sci. 2021, 86, 3075–3081. [Google Scholar] [CrossRef]
- Van Hout, M.W.; Hofland, C.M.; Niederländer, H.A.; De Jong, G.J. On-line coupling for solid-phase extraction with mass spectrometry for the analysis of biological samples II. Determination of clenbuterol in urine using multiple-stage mass spectrometry in an ion-trap mass spectrometer. Rapid Commun. Mass Spectrom. 2000, 14, 2103–2111. [Google Scholar] [CrossRef]
- Tachon, R.; Pichon, V.; Borgne, M.B.L.; Minet, J.-J. Comparison of solid-phase extraction sorbents for sample clean-up in the analysis of organic explosives. J. Chromatogr. A 2008, 1185, 1–8. [Google Scholar] [CrossRef]
- Mallet, C.R.; Lum, Z.; Mazzeo, J.R. A study of ion suppression effects in electrospray ionization from mobile phase additives and solid phase extracts. Rapid Commun. Mass Spectrom. 2004, 18, 49–58. [Google Scholar] [CrossRef]
- Han, C.; Li, X.; Jiao, H.; Gao, Y.; Zhang, Q. Accurate Determination, Matrix Effect Estimation, and Uncertainty Evaluation of Three Sulfonamides in Milk by Isotope Dilution Liquid Chromatography-Tandem Mass Spectrometry. J. Food Qual. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Preti, R. Core-Shell Columns in High-Performance Liquid Chromatography: Food Analysis Applications. Int. J. Anal. Chem. 2016, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fekete, S.; Fekete, J. Comparison of the performance of totally porous and core-shell particles. In UHPLC in Life Sciences; Guillarme, D., Veuthey, J.-L., Eds.; RSC Chromatography Monographs: Cambridge, UK, 2012; pp. 131–163, No. 16. [Google Scholar]
- Gritti, F.; Guiochon, G. Repeatability of the efficiency of columns packed with sub-3 μm core-shell particles: Part III. 2.7 μm Poroshell 120 EC-C18 particles in 4.6 mm and 2.1 mm x 100 mm column formats. J. Chromatogr. A 2012, 1252, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Avula, B.; Tang, W.; Wang, M.; Elsohly, M.A.; Khan, I.A. Ultra-HPLC method for quality and adulterant assessment of steviol glycosides sweeteners—Stevia rebaudiana and stevia products. Food Addit. Contam. Part A 2015, 32, 674–685. [Google Scholar]
- Fekete, S.; Olah, E.; Fekete, J. Fast liquid chromatography: The domination of core–shell and very fine particles. J. Chromatogr. A 2012, 1228, 57–71. [Google Scholar] [CrossRef]
- Gardner, C.; Wylie-Rosett, J.; Gidding, S.S.; Steffen, L.M.; Johnson, R.K.; Reader, D.; Lichtenstein, A.H. Nonnutritive sweeteners: Current use and health perspectives: A scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2012, 35, 1798–1808. [Google Scholar] [CrossRef]
- Sargent, M. (Ed.) Guide to Achieving Reliable Quantitative LC-MS Measurements; RSC Analytical Methods Committee: Teddington, UK, 2013; Volume 68, ISBN 978-0-948926-27-3. [Google Scholar]
- Commission Decision 2002/657/EC. Implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Commun. 2002, 221, 8–36. [Google Scholar]
- Aalizadeh, R.; Alygizakis, N.A.; Schymanski, E.L.; Krauss, M.; Schulze, T.; Ibáñez, M.; McEachran, A.D.; Chao, A.; Williams, A.J.; Gago-Ferrero, P.; et al. Development and Application of Liquid Chromatographic Retention Time Indices in HRMS-Based Suspect and Nontarget Screening. Anal. Chem. 2021, 93, 11601–11611. [Google Scholar] [CrossRef]
- Donato, R.; Cacciola, F.; Tranchida, P.Q.; Dugo, P.; Mondello, L. Mass spectrometry detection in comprehensive liquid chromatography: Basic concepts, instrumental aspects, applications and trends. Mass Spectrom. Rev. 2012, 31, 523–559. [Google Scholar] [CrossRef]
- Hollender, J.; Schymanski, E.L.; Singer, H.P.; Ferguson, P.L. Nontarget Screening with High Resolution Mass Spectrometry in the Environment: Ready to Go? Environ. Sci. Technol. 2017, 51, 11505–11512. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 333/2007 of 28 March 2007 laying down the methods of sampling and analysis for the official control of the levels of lead, cadmium, mercury, inorganic tin, 3-MCPD and benzo (a) pyrene in foodstuffs. Off. J. Eur. Union L 2007, 88, 29. [Google Scholar]
- Cappiello, A.; Famiglini, G.; Palma, P.; Pierini, E.; Termopoli, V.; Trufelli, H. Overcoming matrix effects in liquid chromatography-mass spectrometry. Anal. Chem. 2008, 80, 9343–9348. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Chen, D.; Wang, S. Drug confirmation by mass spectrometry: Identification criteria and complicating factors. Clin. Chim. Acta 2015, 438, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kojro, G.; Rudzki, P.J.; Pisklak, D.M.; Giebułtowicz, J. Matrix effect screening for cloud-point extraction combined withliquid chromatography coupled to mass spectrometry: Bioanalysis of pharmaceuticals. J. Chromatogr. A 2019, 1591, 44–54. [Google Scholar] [CrossRef]
- Fabregat-Cabello, N.; Zomer, P.; Sancho, J.V.; Roig-Navarro, A.F.; Mol, H.G.J. Comparison of approaches to deal with matrix effects in LC-MS/MS based determinations of mycotoxins in food and feed. World Mycotoxin J. 2016, 9, 149–161. [Google Scholar] [CrossRef]
- FAO/WHO. Chapter 6 Dietary Exposure Assessment for Chemicals in Food. In Principles and Methods for the Risk Assessment of Chemicals in Food; Environmental Health Criteria 240; FAO/WHO: Geneva, Switzerland, 2009; Revision 2020; Available online: https://www.who.int/publications/i/item/9789241572408 (accessed on 5 August 2023).
- WHO. Second workshop on reliable evaluation of low-level contamination of food. In Report on a Workshop in the Frame of GEMS/Food-EURO, GEMS/Food-EURO; World Health Organization Regional Office for Europe: Kulmbach, Rome, 1995. [Google Scholar]
- Codex Stan. General Standard for Food Additives Codex Stan 192-1995, Revision 2021. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B192-1995%252FCXS_192e.pdf (accessed on 10 August 2023).
- JECFA. 69th JECFA-Chemical and Technical Assessment (CTA), STEVIOL GLYCOSIDES Chemical and Technical Assessment. 2008. Available online: https://www.fao.org/3/at966e/at966e.pdf (accessed on 10 August 2023).

| Time (min) | % Mobile Phase A | % Mobile Phase B |
|---|---|---|
| 0–1.9 | 10 | 90 |
| 2.0–4.9 | 30 | 70 |
| 5.0–6.9 | 35 | 65 |
| 7.0–8.9 | 90 | 10 |
| 9.0–12.5 | 10 | 90 |
| Sweeteners | Formula | RT * (min) | Compound | |||||
|---|---|---|---|---|---|---|---|---|
| Precursor ion (m/z) | Product Ion | S-lens (RF Voltage) | ||||||
| Confirmatory Product Ions (m/z) | Collision Energy (eV) | Additional Product Ions (m/z) | Collision Energy (eV) | |||||
| Rebaudioside A | C44H70O23 | 4.856 | 965.5 | 803.45 | 27.87 | 641.36 317.29 | 55 55 | 280 |
| Rebaudioside B | C38H60O18 | 6.347 | 803.458 | 641.29 | 47.42 | 413.08 317.17 | 54.07 53.65 | 280 |
| Rebaudioside C | C44H70O22 | 5.214 | 949.508 | 787.38 | 32.2 | 641.29 479.24 | 55 55 | 280 |
| Rebaudioside D | C50H80O28 | 4.139 | 1127.558 | 803.35 | 51.93 | 641.32 623.30 | 55 55 | 280 |
| Rebaudioside F | C43H68O22 | 5.113 | 935.4 | 773.35 | 26.9 | 611.292 641.321 | 55 55 | 280 |
| Rubusoside | C32H50O13 | 5.693 | 641.348 | 478.92 | 14.14 | 521.45 317.15 | 23.07 46.95 | 226 |
| Dulcoside A | C38H60O17 | 5.303 | 787.458 | 625.45 | 18.1 | 479.29 317.25 | 55 55 | 272 |
| Stevioside | C38H60O18 | 4.895 | 803.458 | 641.39 | 19.66 | 479.22 317.34 | 55 55 | 257 |
| Steviolbioside | C38H50O13 | 6.478 | 641.448 | 479.17 | 42.32 | 461.29 317.37 | 48.39 42.49 | 280 |
| Analyte | RPD (%) | ||
|---|---|---|---|
| Beverage | Yogurt | Snack | |
| Reb A | 0.41 | 0.00 | 0.24 |
| Reb B | 1.10 | 0.18 | 0.18 |
| Reb C | 0.57 | 0.22 | 0.22 |
| Reb D | 0.41 | 0.29 | 0.29 |
| Reb F | 0.78 | 0.23 | 0.00 |
| Rub | 0.70 | 0.20 | 0.20 |
| Dul | 0.75 | 0.22 | 0.22 |
| Stevio | 0.41 | 0.24 | 0.24 |
| Stebio | 0.46 | 0.17 | 0.17 |
| Analyte | Beverage | Yogurt | Snack | |||
|---|---|---|---|---|---|---|
| R2 | Linear Regression | R2 | Linear Regression | R2 | Linear Regression | |
| Reb A | 0.9954 | y = 79,846x + 3076.40 | 0.9993 | y = 8 × 106x + 580,746 | 0.9995 | y = 8 × 106x − 253,645 |
| Reb B | 0.9911 | y = 23,115x + 1642.30 | 0.9991 | y = 2 × 106x + 22,497 | 0.9993 | y = 2 × 106x − 71,164 |
| Reb C | 0.9990 | y = 79,357x + 4082.30 | 0.9939 | y = 1 × 107x + 624,742 | 0.9988 | y = 1 × 107x − 555,332 |
| Reb D | 0.9931 | y = 26,518x + 1389.70 | 0.9994 | y = 4 × 106x + 129,063 | 0.9962 | y = 4 × 106x − 123,139 |
| Reb F | 0.9942 | y = 76,473x + 4149.00 | 0.9982 | y = 8 × 106x + 394,354 | 0.9990 | y = 7 × 106x − 308,079 |
| Rub | 0.9944 | y = 29,488x + 1149.00 | 0.9992 | y = 413,129x + 3182.2 | 0.9973 | y = 450,167x − 17,830 |
| Dul | 0.9950 | y = 22,672x + 2313.80 | 1.0000 | y = 4 × 106x + 95,758 | 0.9998 | y = 5 × 106x − 186,365 |
| Stevio | 0.9953 | y = 48,117x + 4517.90 | 0.9990 | y = 6 × 106x + 305,800 | 0.9996 | y = 6 × 106x − 215,946 |
| Stebio | 0.9961 | y = 32,307x + 3114.20 | 0.9990 | y = 5 × 106x + 111,242 | 0.9999 | y = 7 × 106x − 172,092 |
| Analyte | Percentage Absolute Matrix Effect (Mean ± SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Beverage | Yogurt | Snack | |||||||
| 0.2 mg L−1 | 0.5 mg L−1 | 1.0 mg L−1 | 0.2 mg L−1 | 0.5 mg L−1 | 1.0 mg L−1 | 0.2 mg L−1 | 0.5 mg L−1 | 1.0 mg L−1 | |
| Reb A | 94.43 ± 6.55 | 117.43 ± 4.65 | 103.00 ± 4.28 | 88.29 ± 0.76 | 92.29 ± 1.80 | 96.86 ± 1.77 | 125.14 ± 2.27 | 101.14 ± 2.67 | 100.43 ± 3.10 |
| Reb B | 89.14 ± 4.63 | 111.14 ± 3.18 | 101.14 ± 5.98 | 76.14 ± 2.61 | 79.43 ± 3.51 | 84.43 ± 2.70 | 92.71 ± 3.09 | 80.14 ± 1.86 | 79.00 ± 1.41 |
| Reb C | 98.86 ± 5.87 | 110.00 ± 3.06 | 101.86 ± 4.38 | 88.14 ± 3.72 | 89.43 ± 1.81 | 97.71 ± 4.07 | 114.71 ± 2.56 | 103.43 ± 2.64 | 98.43 ± 5.32 |
| Reb D | 96.71 ± 6.73 | 108.57 ± 2.23 | 105.43 ± 5.56 | 86.29 ± 3.95 | 92.29 ± 2.21 | 94.57 ± 3.87 | 108.29 ± 6.13 | 111.57 ± 4.65 | 106.29 ± 2.56 |
| Reb F | 98.00 ± 6.06 | 110.71 ± 3.99 | 100.71 ± 6.02 | 84.29 ± 4.79 | 93.14 ± 3.08 | 92.43 ± 5.03 | 109.71 ± 2.98 | 95.43 ± 3.10 | 94.14 ± 1.57 |
| Rub | 95.43 ± 4.20 | 110.86 ± 2.97 | 104.71 ± 5.22 | 87.71 ± 4.75 | 94.14 ± 3.39 | 94.57 ± 3.21 | 108.29 ± 3.40 | 92.43 ± 3.15 | 95.43 ± 2.07 |
| Dul | 97.86 ± 4.74 | 110.86 ± 2.73 | 93.71 ± 6.40 | 87.57 ± 3.51 | 94.86 ± 2.91 | 97.86 ± 4.60 | 121.43 ± 1.81 | 101.43 ± 3.31 | 99.71 ± 2.36 |
| Stebio | 87.57 ± 5.09 | 103.57 ± 3.21 | 96.71 ± 4.96 | 73.29 ± 3.25 | 75.29 ± 3.25 | 82.57 ± 2.57 | 82.43 ± 3.10 | 73.00 ± 1.53 | 71.43 ± 3.10 |
| Stevio | 93.14 ± 4.67 | 106.29 ± 3.59 | 97.86 ± 4.74 | 92.57 ± 4.20 | 90.14 ± 3.67 | 95.71 ± 4.92 | 120.00 ± 1.83 | 96.86 ± 1.77 | 96.57 ± 1.62 |
| Analyte | %RSD (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Beverage | Yogurt | Snack | |||||||
| 0.2 mg L−1 | 0.5 mg L−1 | 1.0 mg L−1 | 0.2 mg L−1 | 0.5 mg L−1 | 1.0 mg L−1 | 0.2 mg L−1 | 0.5 mg L−1 | 1.0 mg L−1 | |
| Reb A | 8.10 | 4.27 | 4.43 | 1.10 | 2.34 | 1.79 | 1.67 | 2.74 | 2.45 |
| Reb B | 7.39 | 3.39 | 6.30 | 3.59 | 4.55 | 3.49 | 2.84 | 1.96 | 1.77 |
| Reb C | 7.53 | 3.03 | 4.28 | 5.30 | 2.56 | 4.20 | 2.01 | 2.36 | 5.39 |
| Reb D | 8.95 | 2.51 | 5.52 | 5.69 | 2.42 | 4.30 | 2.60 | 3.73 | 2.15 |
| Reb F | 8.28 | 3.82 | 6.07 | 7.52 | 3.49 | 5.69 | 2.39 | 3.25 | 1.64 |
| Rub | 5.31 | 2.87 | 5.29 | 5.61 | 3.82 | 3.28 | 2.52 | 3.03 | 2.15 |
| Dul | 7.71 | 2.96 | 7.60 | 4.66 | 3.02 | 4.72 | 1.25 | 3.36 | 2.39 |
| Stevio | 7.80 | 3.91 | 5.35 | 5.75 | 4.29 | 5.13 | 1.41 | 1.28 | 1.69 |
| Stebio | 9.30 | 3.76 | 5.55 | 5.07 | 4.45 | 2.91 | 3.08 | 1.64 | 4.19 |
| Analyte | Precision (HorRat) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Beverage | Yogurt | Snack | |||||||
| 0.2 mg L−1 | 0.5 mg L−1 | 1.0 mg L−1 | 0.2 mg L−1 | 0.5 mg L−1 | 1.0 mg L−1 | 0.2 mg L−1 | 0.5 mg L−1 | 1.0 mg L−1 | |
| Reb A | 0.36 | 0.19 | 0.28 | 0.05 | 0.10 | 0.11 | 0.07 | 0.12 | 0.15 |
| Reb B | 0.33 | 0.15 | 0.39 | 0.16 | 0.20 | 0.22 | 0.13 | 0.09 | 0.11 |
| Reb C | 0.33 | 0.13 | 0.27 | 0.23 | 0.11 | 0.26 | 0.09 | 0.10 | 0.34 |
| Reb D | 0.40 | 0.11 | 0.34 | 0.25 | 0.11 | 0.27 | 0.12 | 0.16 | 0.13 |
| Reb F | 0.37 | 0.17 | 0.38 | 0.33 | 0.15 | 0.36 | 0.11 | 0.14 | 0.10 |
| Rub | 0.23 | 0.13 | 0.33 | 0.25 | 0.17 | 0.21 | 0.11 | 0.13 | 0.13 |
| Dul | 0.34 | 0.13 | 0.47 | 0.21 | 0.13 | 0.30 | 0.06 | 0.15 | 0.15 |
| Stevio | 0.34 | 0.17 | 0.33 | 0.25 | 0.19 | 0.32 | 0.06 | 0.06 | 0.11 |
| Stebio | 0.41 | 0.17 | 0.35 | 0.22 | 0.20 | 0.18 | 0.14 | 0.07 | 0.26 |
| Analyte | Mean of % Recovery (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Beverage | Yogurt | Snack | |||||||
| 0.2 mg L−1 | 0.5 mg L−1 | 1.0 mg L−1 | 0.2 mg L−1 | 0.5 mg L−1 | 1.0 mg L−1 | 0.2 mg L−1 | 0.5 mg L−1 | 1.0 mg L−1 | |
| Reb A | 100.53 | 114.67 | 104.53 | 82.71 | 92.72 | 97.25 | 119.14 | 103.33 | 100.04 |
| Reb B | 79.19 | 103.03 | 100.84 | 75.73 | 78.70 | 83.42 | 94.65 | 80.97 | 80.52 |
| Reb C | 93.75 | 111.60 | 108.06 | 79.89 | 89.97 | 94.60 | 116.15 | 100.39 | 99.71 |
| Reb D | 87.90 | 107.39 | 103.36 | 82.85 | 93.60 | 94.82 | 118.09 | 115.45 | 108.85 |
| Reb F | 88.50 | 108.82 | 98.68 | 77.34 | 92.93 | 92.31 | 113.84 | 94.07 | 94.20 |
| Rub | 96.15 | 112.10 | 102.78 | 87.77 | 92.49 | 93.68 | 103.58 | 92.69 | 93.72 |
| Dul | 86.24 | 110.49 | 94.57 | 87.39 | 94.04 | 97.93 | 118.38 | 100.84 | 100.04 |
| Stevio | 80.12 | 103.92 | 97.59 | 89.42 | 91.76 | 95.83 | 116.82 | 97.88 | 97.01 |
| Stebio | 74.93 | 102.12 | 94.50 | 70.00 | 75.36 | 81.18 | 84.49 | 73.92 | 72.43 |
| Analyte | LOD (mg kg−1) | LOQ (mg kg−1) | ||||
|---|---|---|---|---|---|---|
| Beverage | Yogurt | Snack | Beverage | Yogurt | Snack | |
| Rebaudioside A | 0.060 | 0.003 | 0.015 | 0.201 | 0.011 | 0.049 |
| Rebaudioside B | 0.053 | 0.012 | 0.024 | 0.175 | 0.041 | 0.081 |
| Rebaudioside C | 0.052 | 0.016 | 0.017 | 0.175 | 0.053 | 0.058 |
| Rebaudioside D | 0.050 | 0.015 | 0.019 | 0.165 | 0.050 | 0.065 |
| Rebaudioside F | 0.056 | 0.022 | 0.021 | 0.187 | 0.075 | 0.069 |
| Rubusoside | 0.057 | 0.028 | 0.029 | 0.191 | 0.093 | 0.098 |
| Dulcoside A | 0.060 | 0.018 | 0.013 | 0.199 | 0.061 | 0.045 |
| Stevioside | 0.056 | 0.023 | 0.015 | 0.187 | 0.078 | 0.049 |
| Steviolbioside | 0.078 | 0.020 | 0.029 | 0.261 | 0.066 | 0.098 |
| (1) | ||||||||||
| Sample | Analysis | RebA (n = 20) | RebB (n = 3) | RebC (n = 2) | RebD (n = 0) | RebF (n = 0) | Rub (n = 0) | Dul (n = 0) | Stevio (n = 5) | Stebio (n = 0) |
| Beverage (n = 20) | Mean ± SD (mg kg−1) (Min-Max) | 84.60 ± 208.52 (0.96–886.16) | 6.49 ± 6.07 (2.63–13.48) | 2.08 (1.83–2.32) | ND | ND | ND | ND | 4.03 ± 4.27 (1.32–11.52) | ND |
| Precision (%RSD) | 0.93–10.41 | 1.43–3.41 | - | - | - | - | - | 0.64–4.10 | - | |
| Accuracy (%Recovery) | 79.39–113.45 | 65.83–105.21 | 70.89–102.00 | 70.36–114.52 | 84.83–105.23 | 83.47–106.38 | 77.17–123.62 | 80.00–96.78 | 73.33–104.95 | |
| (2) | ||||||||||
| Sample | Analysis | RebA (n = 10) | RebB (n = 1) | RebC (n = 0) | RebD (n = 0) | RebF (n = 0) | Rub (n = 0) | Dul (n = 0) | Stevio (n = 7) | Stebio (n = 0) |
| Yogurt (n = 10) | Mean ± SD (mg kg−1) (Min-Max) | 13.00 ± 6.63 (2.02–23.85) | 0.42 (0.42) | ND | ND | ND | ND | ND | 5.33 ± 4.86 (1.19–11.17) | ND |
| Precision (%RSD) | 0.85–13.55 | - | - | - | - | - | - | 0.67–13.15 | - | |
| Accuracy (%Recovery) | 100.85–113.45 | 65.83–88.37 | 86.33–97.28 | 100.83–106.22 | 84.83–105.23 | 83.47–96.13 | 77.17–101.52 | 80.00–90.35 | 70.00–90.35 | |
| (3) | ||||||||||
| Sample | Analysis | RebA (n = 8) | RebB (n = 5) | RebC (n = 3) | RebD (n = 4) | RebF (n = 3) | Rub (n = 0) | Dul (n = 3) | Stevio (n = 5) | Stebio (n = 2) |
| Snack (n = 8) | Mean ± SD (mg kg−1) (Min-Max) | 67.49 ± 72.48 (1.18–168.67) | 5.58 ± 4.63 (1.30–12.48) | 19.69 ± 20.90 (7.30–43.83) | 1.49 ± 1.70 (0.62–4.04) | 4.67 ± 4.88 (1.81–10.30) | ND | 18.40 ± 19.12 (6.85–40.48) | 54.11 ± 91.73 (1.02–215.59) | 8.80 (1.64–15.95) |
| Precision (%RSD) | 0.50–12.45 | 2.91–9.63 | 4.04–6.62 | 2.85–13.97 | 4.18–9.53 | - | 4.57–10.59 | 0.76–8.08 | - | |
| Accuracy (%Recovery) | 80.85–114.09 | 89.56–113.75 | 115.04–120.00 | 115.12–117.74 | 104.90–108.89 | 106.81–115.71 | 107.57–109.60 | 89.86–103.23 | 75.34–93.32 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phungsiangdee, Y.; Chaothong, P.; Karnpanit, W.; Tanaviyutpakdee, P. Validation of UHPLC-ESI-MS/MS Method for Determining Steviol Glycoside and Its Derivatives in Foods and Beverages. Foods 2023, 12, 3941. https://doi.org/10.3390/foods12213941
Phungsiangdee Y, Chaothong P, Karnpanit W, Tanaviyutpakdee P. Validation of UHPLC-ESI-MS/MS Method for Determining Steviol Glycoside and Its Derivatives in Foods and Beverages. Foods. 2023; 12(21):3941. https://doi.org/10.3390/foods12213941
Chicago/Turabian StylePhungsiangdee, Yollada, Pimpuk Chaothong, Weeraya Karnpanit, and Pharrunrat Tanaviyutpakdee. 2023. "Validation of UHPLC-ESI-MS/MS Method for Determining Steviol Glycoside and Its Derivatives in Foods and Beverages" Foods 12, no. 21: 3941. https://doi.org/10.3390/foods12213941
APA StylePhungsiangdee, Y., Chaothong, P., Karnpanit, W., & Tanaviyutpakdee, P. (2023). Validation of UHPLC-ESI-MS/MS Method for Determining Steviol Glycoside and Its Derivatives in Foods and Beverages. Foods, 12(21), 3941. https://doi.org/10.3390/foods12213941






