Abstract
Food safety concerns regarding foodborne pathogen contamination have gained global attention due to its significant implications. In this study, we developed a detection system utilizing a PCR array combined with an automated magnetic bead-based system and CE technology to enable the detection of three foodborne pathogens, namely Salmonella enterica, Listeria monocytogenes, and Staphylococcus aureus. The results showed that our developed method could detect these pathogens at concentrations as low as 7.3 × 101, 6.7 × 102, and 6.9 × 102 cfu/mL, respectively, in the broth samples. In chicken samples, the limit of detection for these pathogens was 3.1 × 104, 3.5 × 103, and 3.9 × 102 cfu/g, respectively. The detection of these pathogens was accomplished without the necessity for sample enrichment, and the entire protocols, from sample preparation to amplicon analysis, were completed in approximately 3.5 h. Regarding the impact of the extraction method on detection capability, our study observed that an automated DNA extraction system based on the magnetic bead method demonstrated a 10-fold improvement or, at the very least, yielded similar results compared to the column-based method. These findings demonstrated that our developed model is effective in detecting low levels of these pathogens in the samples analyzed in this study. The PCR-CE method developed in this study may help monitor food safety in the future. It may also be extended to identify other foodborne pathogens across a wide range of food samples.
1. Introduction
Bacterial food-borne diseases present a substantial global public health challenge, especially in developing nations. The World Health Organization (WHO) reports that food-borne or waterborne diarrhea causes approximately 2.2 million deaths worldwide each year [1]. The primary implicated microorganisms behind foodborne outbreaks are typically Salmonella, Listeria, and Staphylococcus. For instance, Dewey-Mattia et al. [2] reported that Salmonella spp., L. monocytogenes, and S. aureus caused 896, 191, and 35 foodborne outbreaks, respectively, in the United States from 2009 to 2015, which resulted in 23,662, 2378, and 1255 illnesses, respectively. The authors also reported that most of the infections were linked to the consumption of seafood and meat. In European countries, these pathogens caused 10,954, 90, and 1084 foodborne outbreaks, respectively, from 2016 to 2021, resulting in 72,954, 798, and 8948 illnesses, respectively [3]. In China, Chen et al. [4] reported that Salmonella and S. aureus caused 81 and 24 foodborne outbreaks, respectively, in Zhejiang Province from 2015 to 2020, which resulted in 1032 and 288 illnesses, respectively.
Poultry meat, including chicken meat, is known for being a rich source of essential nutrients, including protein, vitamins, minerals, niacin, amino acids, and fats. However, it can also serve as a potential carrier for foodborne pathogens like Salmonella spp., L. monocytogenes, and S. aureus [5,6,7,8,9,10,11]. In a recent study, Gonçalves-Tenório et al. [5] focused on analyzing the prevalence of pathogens in poultry meat across European countries using a meta-analysis approach. Their analysis, based on 78 published studies conducted in 21 European countries, revealed that the prevalence of L. monocytogenes and Salmonella spp. in poultry meat was 19.3% (95% CI: 14.4–25.3%) and 7.10% (95% CI: 4.6–10.8%), respectively [5]. Siriken et al. [6] collected 150 chicken samples from various supermarkets in Samsun, Turkey, and found that 42.7% of these samples contained Salmonella spp. Another study by Thung et al. [7], involving 120 chicken samples collected from wet markets and hypermarkets in Selangor, Malaysia, found that Salmonella Enteritidis and Salmonella Typhimurium were present in 6.7% and 2.5% of the samples, respectively, with the estimated quantities ranging from <3 to 15 MPN/g. Furthermore, Rortana et al. [8] collected 156 chicken meat samples from traditional markets in Cambodia, reporting that 40.4% and 46.2% of these samples contained Salmonella spp. and S. aureus, respectively. Additionally, in a set of 18 chicken meat samples collected from supermarkets, they did not detect any S. aureus, but 16.7% of them contained Salmonella spp. In terms of L. monocytogenes, recent studies have revealed its presence in chicken samples at rates of 45.0%, 46.7%, and 56.0% in Iran [9], Turkey [10], and Spain [11], respectively.
To enhance the control of microbial contamination in food products and reduce the occurrence of foodborne illnesses, rapid and accurate bacterial detection methods are, therefore, warranted. Although conventional culture-based methods have long been considered the “gold standard” for identifying pathogenic bacteria, they come with several drawbacks [12,13]. Typically, culture-based methods are time-consuming, labor-intensive, and less sensitive. The use of culture-based methods could also lead to potential false negative results, particularly when dealing with viable but non-culturable (VBNC) pathogens [12,13]. The utilization of polymerase chain reaction (PCR)-based methods has revolutionized pathogen detection by enabling faster, more sensitive, and more specific detection of pathogens in food samples [12,13].
However, the accuracy of PCR results relies, in part, on the quality and quantity of the sample. The analysis via PCR-based methods may produce false negative or false positive results [14,15]. False negative results may occur because of a failure of nucleic acid amplification caused by an insufficient quantity of bacterial cells and the presence of PCR inhibitors [14,15]. Relying on long enrichment times to produce a high number of bacterial cells in a particular sample can be disadvantageous since it fails to prevent food contamination from undesired bacteria. Several studies have reported that certain food and culture media components inhibit PCR [16,17]. False positive results may occur due to contamination during sample preparation, PCR amplification, or amplicon analysis [18]. Therefore, there is a high demand for a simple and selective food sample preparation method that involves the selective separation and concentration of the target bacteria from the food matrix, limits the amplification of DNA from viable cells, and minimizes the occurrence of contamination during the analysis.
Recently, there has been a rise in the production and commercialization of automated devices that use magnetic beads to minimize contamination during DNA extraction [19,20,21]. These devices can capture target bacteria selectively from different sample matrices. The magnetism of the beads can then be used to separate and concentrate the bacteria from large sample volumes using an external magnetic field, which could make the sample enrichment unnecessary as well as help eliminate PCR inhibitors [16,22]. After the amplification of nucleic acid, several researchers have used capillary electrophoresis (CE) [23,24] to improve the amplicon analysis as well as minimize the occurrence of post-PCR contamination. In this study, we aimed to develop a rapid detection method for detecting and identifying S. enterica, L. monocytogenes, and S. aureus in broth and chicken meats using a PCR array combined with an automated magnetic bead-based system and CE assay. While previous studies have explored the individual effectiveness of magnetic beads or CE in improving PCR performance for detecting foodborne pathogens in food samples [25,26,27,28,29,30], limited information is available regarding the overall effectiveness of integrating these technologies into the entire process of foodborne pathogen detection in poultry meat samples [31]. Chicken meat was used as the consumption of this food was expected to grow more quickly than any other significant meat [32,33].
2. Materials and Methods
2.1. Bacterial Strains, Media, and Culture Conditions
The bacterial species used in this study are purchased from the Bioresource Collection and Research Center (BCRC) Taiwan, including S. enterica BCRC 10747, L. monocytogenes BCRC 14845, and S. aureus BCRC 11863. S. enterica was cultured in tryptic soy broth (TSB) (HiMedia, Mumbai, India) and xylose lysine deoxycholate agar (Neogen, Lansing, MI, USA). L. monocytogenes was cultured in TSB with 0.6% yeast extract and nutrient agar (HiMedia). S. aureus was cultured in brain heart infusion (BHI) (HiMedia) broth and BHI agar (HiMedia). Each bacteria species was cultured in 10 mL broth or spread on an agar plate medium and incubated at 37 °C overnight. The stock for each bacteria species was preserved in aliquots of each culture with 25% glycerol and stored in a freezer at a temperature of −80 °C.
Each of the overnight bacteria cultures was centrifuged (3000× g, 15 min, 4 °C) and washed in phosphate-buffered saline (PBS). The bacteria pellets were then resuspended in PBS, and the optical density (OD) was adjusted to 1.0 to obtain 7.3 × 108 of S. enterica, 6.7 × 108 of L. monocytogenes, and 6.9 × 107 cfu/mL of S. aureus. The number of bacterial cells was enumerated using the plate count method. Next, each bacteria strain was prepared in equal concentration (i.e., ~107 cfu/mL). Thereafter, the mixture of bacteria strains was prepared by mixing the 3 target pathogens in equal volumes. The mixture of bacterial strains was serially diluted in volumes ranging from 100 to 107 CFU/mL separately.
2.2. Inoculation of Chicken Meat Samples
The chicken meat was purchased from a local slaughterhouse and subsequently divided into several parts, with each part weighing 10 g in a sterile plastic bag with a lateral filter (3M™, Saint Paul, MN, USA) and stored at −20 °C for overnight. To eliminate any background bacteria, the samples were sterilized using 6 kGy of gamma irradiation. The irradiation of samples was conducted at the laboratory of the National Atomic Research Institute located in Taoyuan, Taiwan. Under the guidelines developed by the Codex Alimentarius Commission, gamma ray irradiation of food at doses up to 10 kGy is considered safe for human consumption [34]. Regulations in the US and China permit the irradiation of frozen food up to 7 kGy and 8 kGy, respectively [35,36]. For our study, we used the dose of 6 kGy of gamma irradiation, as previous studies have shown that this level of irradiation can reduce pathogen levels in meat samples by more than six orders of magnitude [37,38,39]. Post-irradiation, all samples were stored in a freezer at −20 °C. To inoculate the samples, 100 µL of a mixture of bacterial suspensions with concentrations of 107, 106, 105, 104, 103, and 102 CFU/mL were applied to the surface of 10 g of chicken sample, resulting in bacterial concentrations of approximately 105, 104, 103, 102, 101, and 100 CFU/g. Control samples were also prepared without inoculation. Post-inoculation, the surface of each sample was gently massaged and air-dried in a laminar hood for 5 min. Thereafter, 90 mL of PBS was added to each sample and stomached at the highest speed for 1.5 min using a stomacher (BagMixer Interscience, Saint-Nom-la-Breteche, France) to obtain 1:10 homogenate. Subsequently, each homogenate was serially diluted in PBS, and the number of bacterial cells was determined using the plate count method. The suspension was plated on xylose lysine deoxycholate agar (HiMedia) for S. enterica, Listeria selective agar (HiMedia) for L. monocytogenes and mannitol salt agar (HiMedia) for S. aureus.
2.3. Genomic DNA Extraction
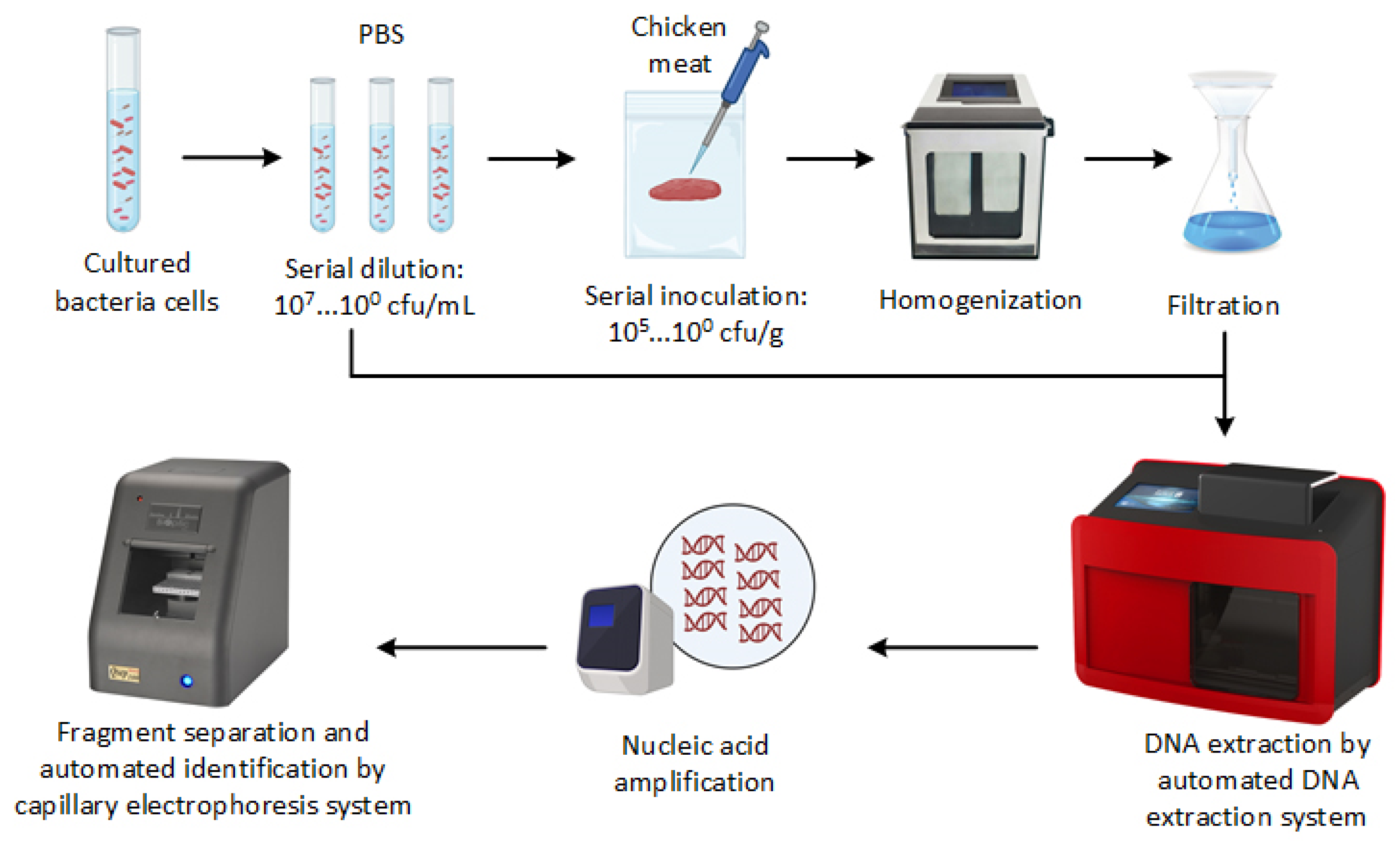
Figure 1 presents the general overview of PCR-CE developed in this study. To increase the concentration of bacterial cells, 1 mL of each bacterial suspension or stomached sample aliquot was centrifuged at 21,000× g for 1 min, and the supernatant was discarded. This process was repeated three times for each sample. Next, genomic DNA was extracted using an automated nucleic acid extractor with a nano-MB nucleic acid extraction kit (TANbead, Taipei, Taiwan). For comparison, we also extracted the DNA from stomached chicken samples using the spin column-based DNA extraction method. The DNA extraction via the spin column-based method was carried out using the PrestoTM Mini gDNA Bacteria Kit (Geneaid Biotech Ltd., New Taipei, Taiwan) according to the manufacturer’s instructions. The extracted DNA was dissolved in 100 µL Tris-EDTA buffer and then stored at −20 °C or subjected to PCR-CE assay immediately.
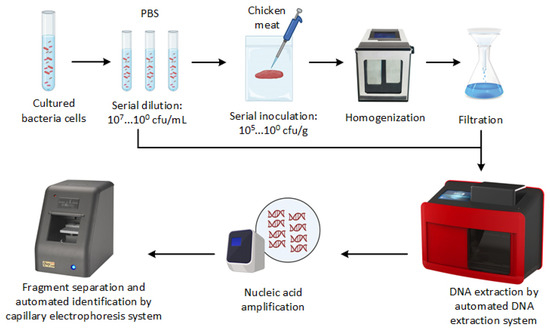
Figure 1.
Schematic overview of the development of PCR-CE system for detecting pathogens.
2.4. Optimization of the PCR-CE
Table 1 presents the sequences of the primers used in this study, which were obtained from published studies [40,41,42,43]. Simplex PCR was performed using Thermocycling (Biometra Tone, Analytik Jena, Jena, Germany). All oligonucleotides used in this research were synthesized using Genomics (New Taipei City, Taiwan). The annealing temperature and the PCR reagents were optimized to obtain high amplification efficiency for each gene in the PCR assay. PCR reagents were obtained from 6 different companies available in Taiwan, including 1 type of premix mixture and 5 types of HotStart Taq DNA polymerase (Table 2). DNA amplification was performed in a total volume of 25 µL of PCR mixture containing 2.5 µL of the DNA sample, 0.5 µL of forward primer, 0.5 µL of reverse primer, and various concentrations of master mix (Table 2). The concentration of each master mix was prepared based on the company protocols. Next, the amplified products were analyzed via capillary electrophoresis using Qsep100 Advance coupled with S2 Standard Cartridge (BiOptic Inc., New Taipei City, Taiwan). This CE system is equipped with a fluorescence detector and a separate voltage of 1~15 kV.

Table 1.
Oligonucleotide primers and probes used in the polymerase chain reaction assays.

Table 2.
PCR mixture using various polymerase enzymes.
3. Results
3.1. Identification of Pathogens via the PCR-CE
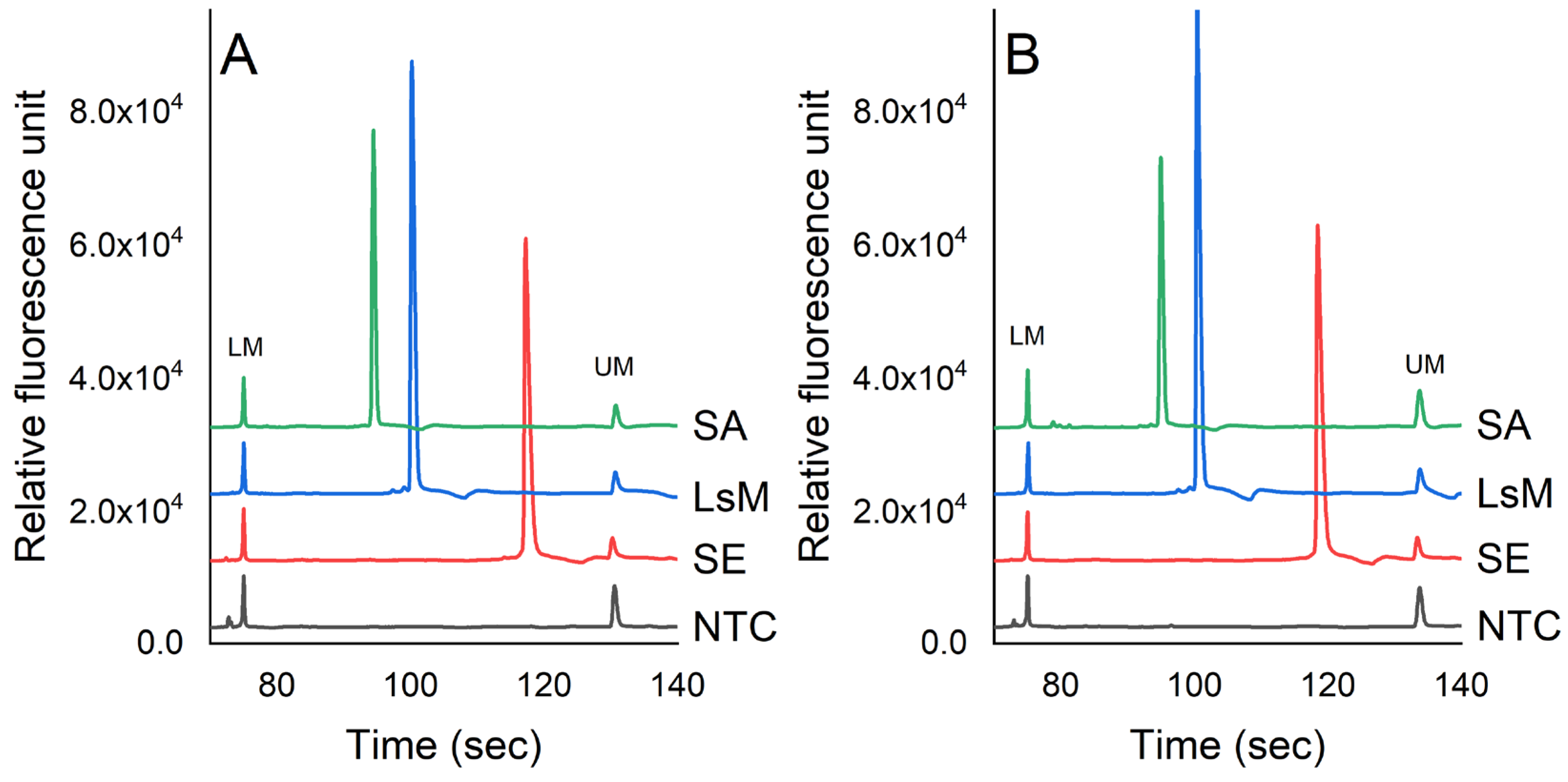
Figure 2 presents the detection of S. enterica, L. monocytogenes, and S. aureus via the PCR-CE system in pure culture and chicken samples. The molecular sizes of the PCR products corresponding to the target pathogens were determined by analyzing the peak profiles of a 1000 bp DNA ladder. The concentrations of S. enterica, L. monocytogenes, and S. aureus were 7.3 × 105, 6.7 × 105, and 6.9 × 105 cfu/mL, respectively, in PBS. In the chicken samples, the concentrations of these pathogens were 5.6 × 105, 7.0 × 105, and 6.3 × 105 cfu/g, respectively. The optimum temperature cycle for the PCR assay was established as follows: initial denaturation at 95 °C for 10 min, denaturation at 95 °C for 30 s, annealing temperature at 57 °C for 30 s, extension at 72 °C for 50 s, and final extension at 72 °C for 5 min. The results showed that there was no amplification observed in the negative control (NTC) and non-target bacteria, indicating that the PCR-CE method developed in this study exhibited good specificity. The PCR products for L. monocytogenes (164 bp), S. aureus (210 bp), and S. enterica (392 bp) matched the expected sizes compared to the DNA-1000 ladder.
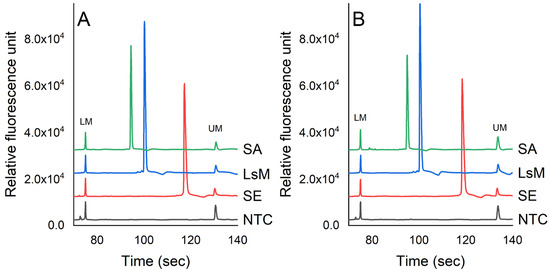
Figure 2.
Detection of pathogens in (A) PBS and (B) chicken samples using PCR-CE system. The concentrations of S. enterica (SE), L. monocytogenes (LsM), and S. aureus (SA) were 7.3 × 105, 6.7 × 105, and 6.9 × 105 cfu/mL, respectively, in PBS. In chicken samples, the concentrations of these pathogens were 5.6 × 105, 7.0 × 105, and 6.3 × 105 cfu/g, respectively (LM = lower bound marker, UM = upper bound marker).
3.2. Sensitivity Evaluation of PCR-CE System in Pure Culture of Bacteria
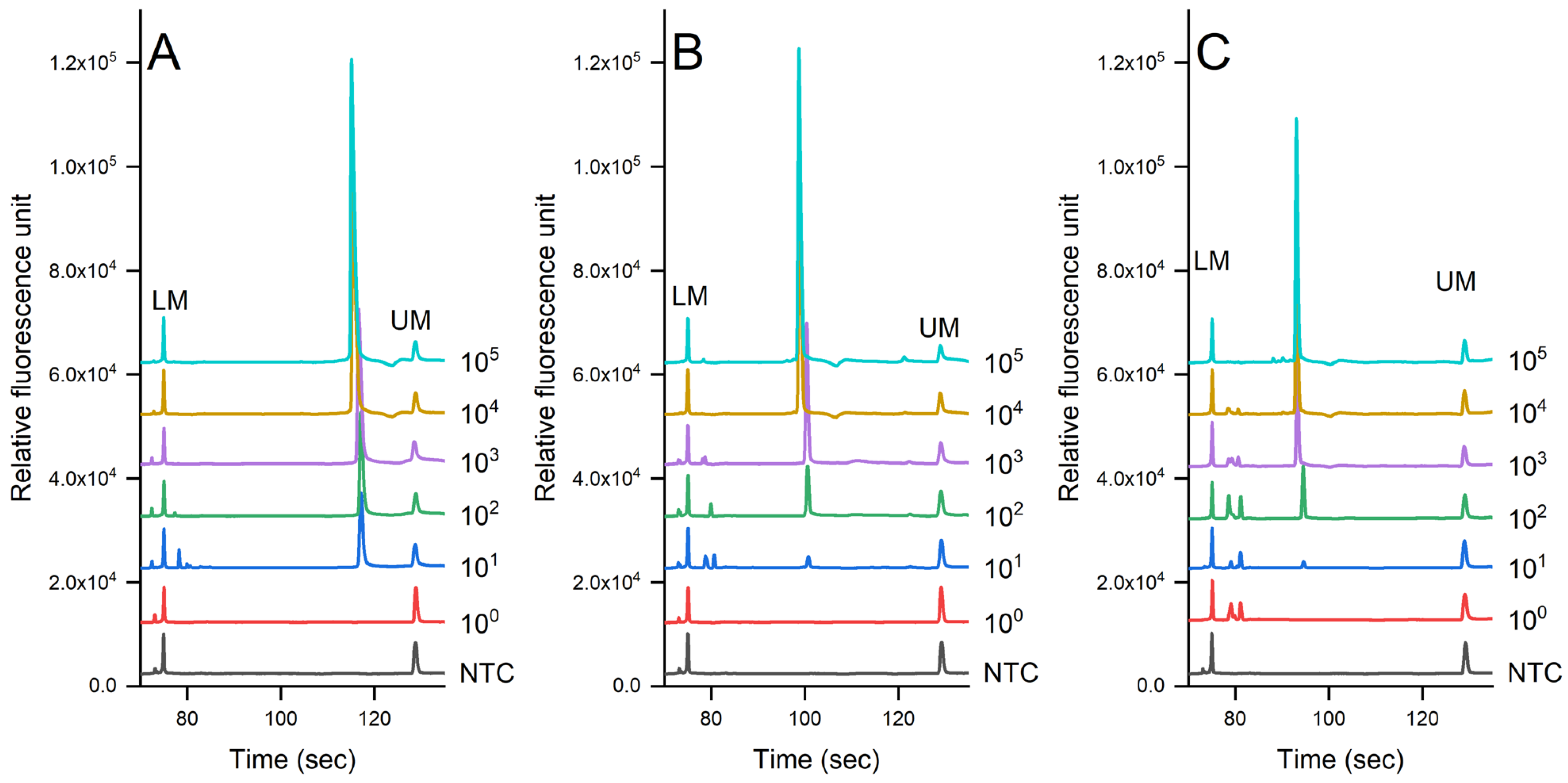
Figure 3 shows the evaluation of the sensitivity of the PCR-CE system on the detection of pathogens in pure culture using the genomic DNA extracted via the auto-extractor system (magnetic bead-based method). A serial dilution was then prepared from 105 to 100 cfu/mL. The results showed that the limit of detection (LOD) was 7.3 × 101 cfu/mL for S. enterica, 6.7 × 102 cfu/mL for L. monocytogenes, and 6.9 × 102 cfu/mL for S. aureus. We noted that there were some slight shifts in the signal due to the changes in the CE cartridge.
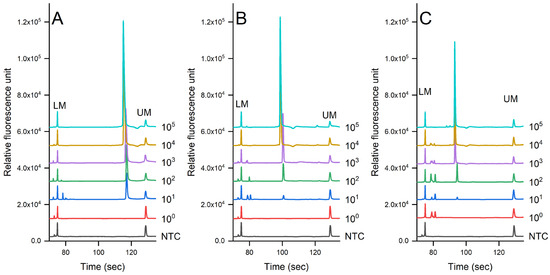
Figure 3.
Sensitivity evaluation of (A) S. enterica, (B) L. monocytogenes, and (C) S. aureus in pure culture detected via PCR-CE systems (Serial dilution of bacterial concentration was prepared from 100 to 105 cfu/mL, NTC = non-template control).
3.3. Evaluation of the Sensitivity of the PCR-CE System in Artificially Contaminated Samples
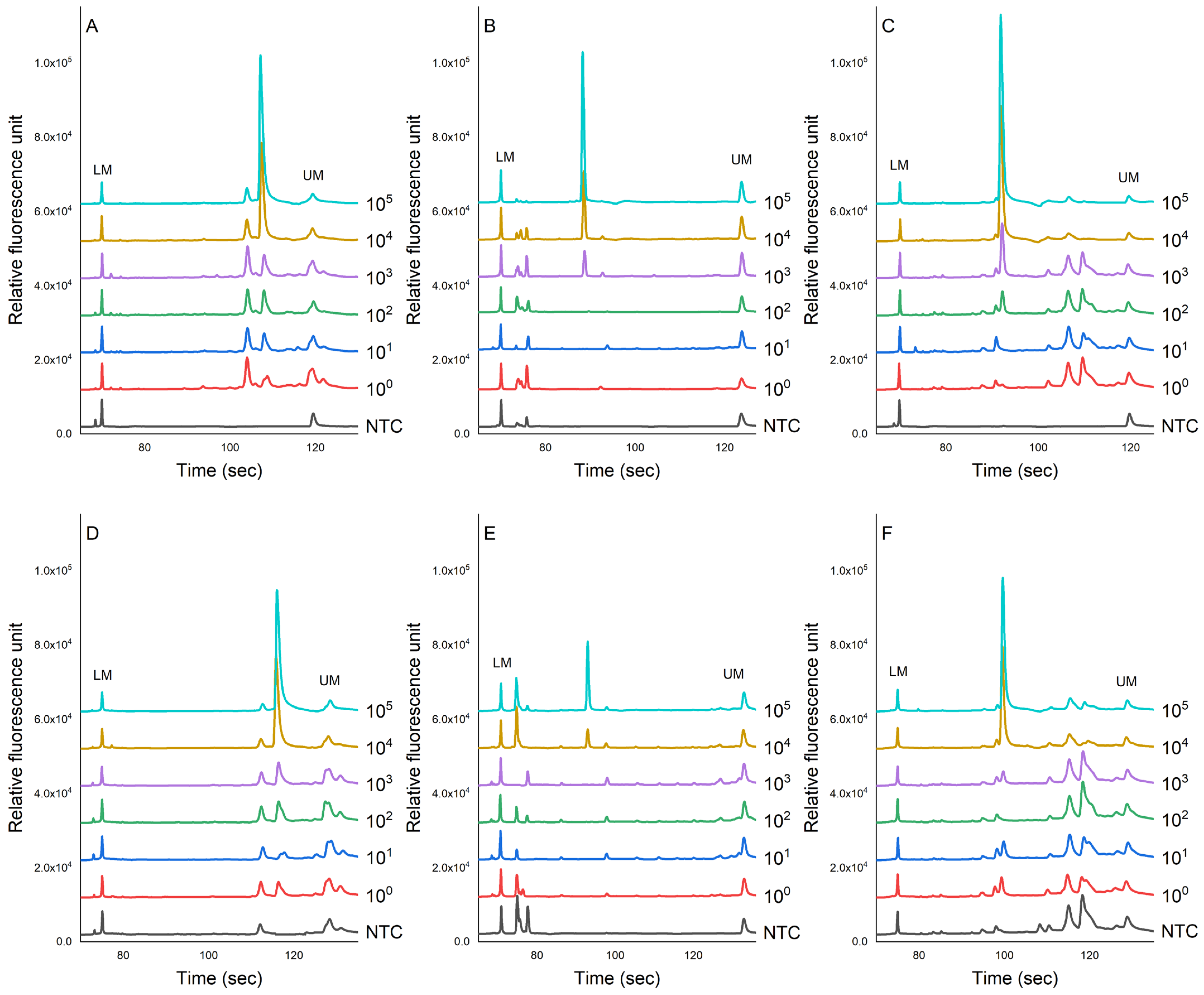
Figure 4 shows the evaluation of the sensitivity of the PCR-CE system on the detection of pathogens inoculated in chicken samples. The genomic DNA was extracted using magnetic bead-based and column-based methods for comparisons. The results showed that the peak value of each PCR product showed a downward trend as the concentration of bacteria decreased. This study found that the LOD for the magnetic bead-based method for S. enterica, L. monocytogenes, and S. aureus was 3.1 × 104, 3.5 × 103, and 3.9 × 102 cfu/g, respectively (Figure 4A–C). No specific amplification peaks were observed in the NTC. For the column-based method, we found that these pathogens in chicken meats could be detected as low as 3.1 × 104, 5.3 × 104, and 6.1 × 104 cfu/g, respectively.
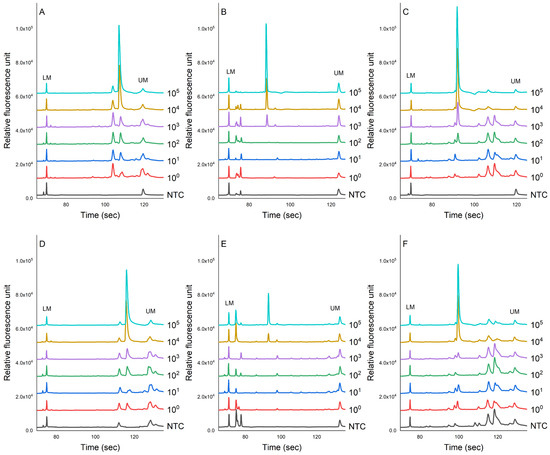
Figure 4.
The sensitivity evaluation of the PCR-CE system for detecting pathogens, namely (A) L. monocytogenes, (B) S. enterica, and (C) S. aureus, inoculated in chicken meat samples. Two different extraction methods were employed: the magnetic bead-based method (A–C) and the column-based method (D–F). NTC = non-template control).
3.4. Evaluation of PCR-CE Using Multiple Enzymes
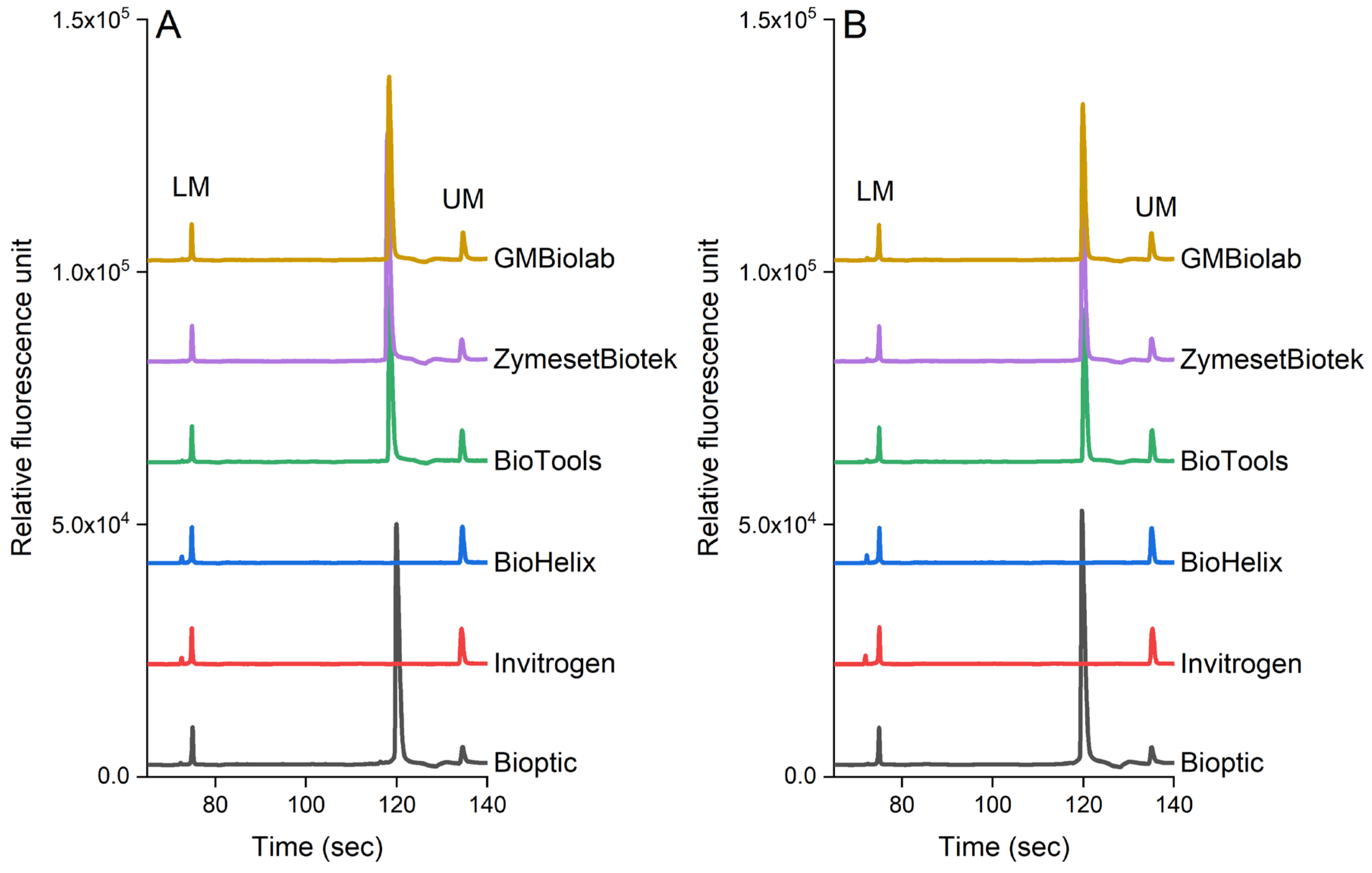
Figure 5 shows the effect of PCR reagents on the detection of pathogens in pure culture and chicken samples. In our study, we selected six commercial kits available in Taiwan. S. enterica was used in the pure culture and inoculated chicken samples as this pathogen is frequently found in poultry products (the genomic DNA was extracted from 105 cfu/mL each sample). Our results indicated that the analytical sensitivity was also influenced by the brand of the PCR reagents. PCR reagents from BiOptic, BioTools, ZymesetBiotek, and GMbiolab produced the expected peak for S. enterica (invA gene, 392 bp), while other brands (Invitrogen and BioHelix) did not. As shown in Figure 5, these PCR reagents have similar effects on the detection of pathogens in both the pure culture (Figure 5A) and chicken samples (Figure 5B).
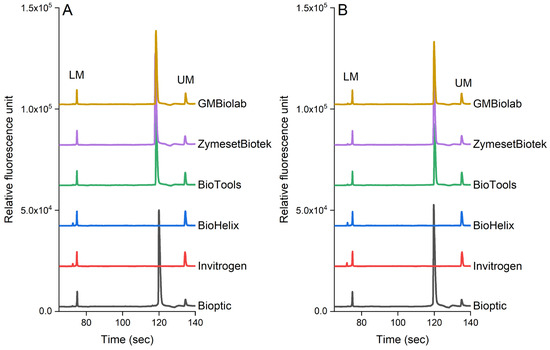
Figure 5.
The performance of the PCR-CE system using various commercial enzymes in (A) pure culture and (B) chicken samples. The genomic DNA was extracted using a magnetic bead-based method from 7.3 × 105 cfu/mL and 5.6 × 105 of S. enterica in pure culture and chicken samples, respectively.
4. Discussion
In recent years, the development and diversification of food supply chains have raised concerns about foodborne illnesses caused by pathogens, posing a significant threat to food safety. Fresh food from different markets, coupled with complex food processing techniques, increases the likelihood of multiple pathogen contamination. Common foodborne pathogens, such as S. enterica, S. aureus, and L. monocytogenes, are responsible for a range of foodborne diseases. In Taiwan, Lai et al. [44] reported that these pathogens caused annual cases of 185,977, 432, and 17 foodborne illnesses based on surveillance data from 2012 to 2015. In another study, Yu et al. [45] revealed that Salmonella and S. aureus accounted for 14% and 19% of foodborne diseases in Taiwan based on surveillance data from 2014 to 2018. Hence, the development of rapid and accurate detection and identification of these pathogens in food is, therefore, crucial to effectively control food poisoning and prevent its further dissemination.
Several techniques have been developed to identify pathogenic bacteria in food and water. While culture-based methods have been commonly employed, they suffer from limitations such as time-consuming processes, potential false negative results, and labor-intensive procedures [12]. Serotyping is another widely used method for detecting and distinguishing pathogens; however, this approach has its drawbacks, including the inability to serotype a small percentage (between 5% and 8%) of isolates and potential errors in typing due to the loss of surface antigens [46,47]. Recent advancements in technology have prompted researchers to explore molecular biology methods and novel nanomaterials for pathogen detection [12,48]. Examples of these molecular methods include PCR-based methods, loop-mediated isothermal amplification (LAMP), recombinase polymerase amplification (RPA), rolling circle amplification (RCA), and DNA microarray analysis, among others [12,48]. In our study, the PCR technique was employed to amplify nucleic acid due to its reputation as one of the most reliable and accurate methods for pathogen detection. We acknowledge that various PCR-based detection methods have seen advancements over the last decade, with qPCR remaining the most commonly used approach [14]. However, as outlined in our prior review [14], each method has its own set of advantages and disadvantages. In our present study, we propose an alternative solution for PCR-based detection. Our current PCR-CE system not only offers relative cost-effectiveness compared to other PCR-based techniques but also offers the potential for portability [49]. This feature makes it a valuable tool for on-site pathogen detection. Additionally, our research demonstrated that the integration of an automated magnetic bead-based DNA extraction system with a PCR array and CE assay led to a rapid pathogen detection process, reduced contamination risks, and high-resolution analysis results.
Before the amplification process, genomic DNA from the target pathogens is typically extracted using a column-based DNA extraction method [50]. However, this method has its limitations. Firstly, it can be both labor-intensive and time-consuming due to its multi-step nature, involving binding, washing, and elution. These numerous steps contribute to extended handling time, rendering it less suitable for high-throughput scenarios [50]. Furthermore, the column-based approach may lead to lower DNA yield and purity, particularly when dealing with samples containing minimal DNA content or complex matrices [50,51]. In response to these challenges, researchers have devised an innovative alternative for isolating genomic DNA from samples—the magnetic bead-based method [51]. This technique offers several distinct advantages. Notably, it significantly enhances the speed and simplicity of the extraction process. By reducing the number of procedural steps, magnetic bead-based extraction expedites DNA isolation. Moreover, its compatibility with automation makes it ideal for processing a considerable number of samples simultaneously, making it particularly attractive for high-throughput laboratory settings. Additionally, the magnetic bead-based method often yields DNA samples of higher purity and improved recovery rates. In our study, the DNA from the bacterial cells was extracted using magnetic beads in an automated DNA extractor system. This extractor system only required 20–30 min of sample pre-treatment and approximately 1 h of DNA extraction. Compared to the column-based extraction method, the application of this automated machine based on magnetic bead-based separation exhibits a similar result and sometimes an improvement in detection capability by at least 10 times (Figure 4). Prior research has indicated that coating magnetic beads with antibodies could enhance the efficiency of DNA extraction [52,53,54]. However, the costs associated with acquiring or developing the necessary antibodies impede the widespread adoption of this technique [14]. Nevertheless, in our study, the peak signals generated from DNA extracted using the magnetic bead-based method (Figure 4A–C) were generally stronger than those produced using the column-based method (Figure 4D–F). This signifies that the automated DNA extractor system based on magnetic bead separation facilitates more efficient DNA extraction while concurrently reducing the presence of inhibitors, thereby optimizing the nucleic acid amplification process in the PCR.
Table 3 presents the detection limits of the PCR array combined with CE technology, comparing the results from previous studies with those from this present study. Zhou et al. [55] used multiplex PCR combined with a CE system to detect Salmonella, E. coli O157:H7, L. monocytogenes, S. aureus, Shigella spp., and C. jejuni in broths and found that their system could detect these pathogens at concentrations of 4.2 × 102, 9.3 × 101, 3.1 × 102, 2.7 × 102, 8.5 × 101, and 6.6 × 101 cfu/mL, respectively, without the pre-enrichment of the samples. In their protocol, the entire pathogen detection process could be completed within 4 h, including 1 h for DNA extraction, 2 h for PCR amplification, 45 min/row for CE separation, and 10 min for interpretation [55]. In another study, Ruan et al. [56] adopted a combination of duplex PCR and the CE system to identify Cronobacter spp. in broth, and their approach successfully detected this pathogen at a concentration of 1.6 × 101 cfu/mL without requiring sample pre-enrichment. Regarding the detection of pathogens in food samples, Alarcón et al. [27] demonstrated that the CE system, in conjunction with a multiplex PCR array, could detect S. aureus, L. monocytogenes, and Salmonella spp. in raw beef at concentrations as low as 2.6 × 103, 5.7 × 102, and 7.9 × 102 cfu/mL, respectively, without the need for enrichment. In their system, amplicon analysis via the CE system completed the analysis within 25 min. In terms of food safety management, allowing the detection of pathogens without requiring sample enrichment would provide early information to food managers so that necessary preventive action can be taken in a timely manner. In terms of protecting public health, such early information would help food safety authorities in the decision-making process to prevent the occurrence of food poisoning.

Table 3.
Comparison of employing PCR coupled with CE array in detecting foodborne pathogens in broth and food samples.
In our system, we used a simplex PCR to amplify the nucleic acid from the extracted DNA of S. aureus, L. monocytogenes, and Salmonella cultured in broth and inoculated in chicken meat. The inoculation of these pathogens in chicken meat was aimed to mimic the real situation. The detection of these pathogens using the CE system was completed within 4 min/row (Table 4). The PCR-CE system developed in our lab could detect these pathogens as low as 7.3 × 101 for S. enterica, 6.7 × 102 for L. monocytogenes, and 6.9 × 102 for S. aureus, without the need for enrichment (Figure 3). In chicken meat samples, these pathogens could be detected as low as 7.3 × 104, 6.7 × 103, and 6.9 × 102 cfu/mL, respectively, without the need for enrichment (Figure 4A–C). The less sensitive detection of these pathogens in chicken meat samples may be attributed to the presence of inhibitors in the chicken meat suspension [57]. These inhibitors could have potentially reduced the effectiveness of magnetic beads in capturing bacterial cells during DNA extraction or inhibited the amplification of target pathogens during the PCR process [58]. Magnetic beads may not efficiently capture bacteria in food samples that contain complex matrices with various components, including fats, proteins, and other particles, as they can get entangled or adsorb non-target substances, reducing the effectiveness of bacterial isolation [57,58]. A previous study has mentioned that the presence of fat and protein can inhibit nucleic acid amplification during the PCR process [57]. Additionally, other compounds used in DNA extraction, such as detergents, lysozyme, NaOH, and alcohols, can also have inhibitory effects [57]. We noticed that several studies have attempted to separate bacterial cells from food matrices by applying the principle of buoyant density gradient centrifugation [59,60,61]. This method involves the utilization of substances such as Percoll to effectively isolate bacterial cells from food matrices. However, the practical implementation of this technique is hindered by the requirement to prepare solutions with varying density gradients and the need for specialized tools and high-speed centrifugation equipment. Furthermore, the application of this method would be time-consuming and raise the risk of cross-contamination during the transfer of samples from one tube to another. Therefore, future studies must focus on reducing or eliminating the impact of these inhibitors to enhance the performance of nucleic acid amplification.

Table 4.
Comparison of the time spent for pathogen identification between the culture-based method and PCR-CE method developed in this study.
Furthermore, the adoption of the PCR-CE system in our study eliminates the requirement for an electrophoresis gel, a common component in traditional PCR methods. CE proves highly effective in segregating DNA fragments based on their size and charge [24,62,63]. In CE, a narrow capillary tube is filled with a conductive buffer solution. The application of an electric field across the capillary prompts the migration of the negatively charged DNA fragments [24,62,63]. Smaller fragments migrate swiftly, covering more distance within the capillary, whereas larger ones lag behind [24,62,63]. A detector records their migration time and peak intensity, facilitating the precise analysis and quantification of PCR products. Additionally, this system minimizes cross-contamination during amplicon analysis, a crucial consideration due to its potential to yield false positive results [13,14,15]. Our approach eliminates the need to transfer the amplicon from the PCR tube, allowing direct application in the CE system. This streamlined process significantly reduces the risk of cross-contamination and enhances analysis reliability. Despite the advantages of CE in DNA fragment separation, there is a need for the full integration of PCR arrays and CE technology, along with the refinement of software tailored to the PCR-CE system, to enhance pathogen detection efficiency.
We observed that as the bacterial concentration decreased, there was a corresponding increase in the occurrence of non-specific signals. These signals did not appear in samples with higher bacterial concentrations (i.e., 105 cfu per mL or g, Figure 2). Actually, the occurrence of non-specific signals is a common issue encountered when working with PCR products [64,65,66,67]. This phenomenon may arise due to the amplification of unintended DNA fragments. Previous studies have suggested the use of organic molecules such as dimethyl sulfoxide (DMSO), glycerol, polyethylene glycol, formamide, and betaine to prevent the amplification of unintended DNA fragments [64,66,67,68]. However, in our study, the application of these organic molecules did not improve PCR amplification. Nevertheless, the occurrence of non-specific signals became less significant when a capillary electrophoresis system was employed, as presented in this study (Figure 3 and Figure 4). Capillary electrophoresis offered high-resolution detection, making it easier to identify non-specific signals, thereby reducing their impact on the analysis and ensuring the accuracy and reliability of the obtained results.
In terms of the formulation of PCR reagents from different brands, our study confirmed that it can affect the amplification of nucleic acid and thus affect the amplicon analysis. A previous study conducted by Lin et al. [49] observed that the formulation of PCR reagents obtained from various manufacturers had an impact on amplicon analysis. It is worth noting that Lin et al.’s study focused on detecting viruses in shrimp samples [49]. They discovered that PCR reagents manufactured by ThermoFisher, Biotools, and BiOptic enabled the detection of the target viruses in their samples, whereas the reagent from Takara (Takara Bio Inc., Tokyo, Japan) did not yield successful results. In this study, the effectiveness of the PCR-CE system was also dependent on the choice of PCR reagent. Our observation showed that two out of six types of reagents tested failed to enable the amplification of the nucleic acid during the PCR process. These findings suggest that caution should be exercised when using the PCR-CE system developed in this study, as different formulations of PCR reagents can impact the PCR process. Manufacturers may formulate their reagents according to their technologies, which may not work well when combined with technologies developed by other manufacturers.
Finally, the PCR-CE system that we have developed in this study demonstrated the capability to detect and identify the target pathogens within approximately 3.5 h, thereby improving upon the 4–5 h required in conventional PCR methods and the even lengthier 94 h necessitated in culture-based techniques (Table 4). This acceleration in the processing time can be attributed to the elimination of the requirement for sample enrichment and the use of CE, which facilitates the rapid separation of DNA fragments. The rapid detection of foodborne pathogens is crucial for effective food safety management [13,14]. It enables timely responses, minimizes the impact of outbreaks, and ultimately protects public health by ensuring that consumers can confidently enjoy the food they consume.
One limitation of this study is the inability of PCR-CE to differentiate between signals originating from viable cells and DNA released from dead cells. A positive signal could originate from non-viable cells, potentially resulting in false positive results. Such outcomes could have financial and legal implications for food business operators. One potential solution to address this concern is the use of RNA as an indicator [69]. However, RNA is generally less stable than DNA and can degrade quickly when exposed to environmental factors, such as temperature, pH, and enzymatic activity [70]. Another approach involves the application of viability dyes, such as propidium monoazide (PMA), DyeTox13, or thiazole orange monoazide (TOMO) [14,71]. Previous studies have indicated that these dyes could inhibit signals arising from dead cells [72,73]. However, the effectiveness of these dyes may be influenced by the food matrices and bacterial species [71]. Thus, future studies are suggested to explore the effectiveness of these dyes in combination with the PCR-CE assay for detecting live foodborne pathogens in various food samples.
5. Conclusions
In conclusion, the utilization of an automatic DNA extractor in conjunction with a PCR-CE assay, developed in this study, presents a faster and more cost-effective approach to detecting S. enterica, L. monocytogenes, and S. aureus in the pure culture and chicken meat. The entire process, encompassing automatic DNA extraction, PCR, and CE, can be accomplished in under 4 h. By directly integrating the automated magnetic bead-based DNA extraction system with the PCR array and CE technology, the risk of contamination is minimized. These combined technologies hold the potential to enhance food safety monitoring and offer a practical and indispensable application to ensure food safety in the future. Additionally, the developed method not only proves effective in detecting low levels of these pathogens in the samples analyzed in this study but can also be extended to identify other foodborne pathogens across a wide range of food samples.
Author Contributions
Conceptualization, N.N.; Experimental operation, N.N.; Writing—Original Draft Preparation, N.N.; Writing—Review and Editing, N.N., H.-Y.L., S.-K.T., H.-I.H. and H.-J.L.; Funding Acquisition, H.-Y.L. and H.-J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science and Technology Council (Taipei, Taiwan), grant numbers NSTC110-2313-B-019-003-MY3 and NSTC111-2311-B-019-003-MY3. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Data Availability Statement
Data are available on request from the authors.
Acknowledgments
The automated genomic DNA extractor was kindly provided by Taiwan Advanced Nanotech Inc. (TANBead, Taipei, Taiwan). We also would like to thank BiOptic Inc. (New Taipei, Taiwan) for providing the capillary electrophoresis system.
Conflicts of Interest
S.-K.T. is employed by the company BiOptic Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
References
- WHO. WHO’s First Ever Global Estimates of Foodborne Diseases Find Children under 5 Account for almost One Third of Deaths. Available online: https://www.who.int/news/item/03-12-2015-who-s-first-ever-global-estimates-of-foodborne-diseases-find-children-under-5-account-for-almost-one-third-of-deaths (accessed on 6 September 2023).
- Dewey-Mattia, D.; Manikonda, K.; Hall, A.J.; Wise, M.E.; Crowe, S.J. Surveillance for Foodborne Disease Outbreaks—United States, 2009–2015. MMWR Surveill. Summ. 2018, 67, 1–11. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. European Food Safety Authority Foodborne Outbreaks Report; The European Food Safety Authority: Parma, Italy, 2020.
- Chen, L.; Sun, L.; Zhang, R.; Liao, N.; Qi, X.; Chen, J. Surveillance for Foodborne Disease Outbreaks in Zhejiang Province, China, 2015–2020. BMC Public Health 2022, 22, 135. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Tenório, A.; Silva, B.; Rodrigues, V.; Cadavez, V.; Gonzales-Barron, U. Prevalence of Pathogens in Poultry Meat: A Meta-Analysis of European Published Surveys. Foods 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Siriken, B.; Türk, H.; Yildirim, T.; Durupinar, B.; Erol, I. Prevalence and Characterization of Salmonella Isolated from Chicken Meat in Turkey. J. Food Sci. 2015, 80, M1044–M1050. [Google Scholar] [CrossRef] [PubMed]
- Thung, T.Y.; Mahyudin, N.A.; Basri, D.F.; Wan Mohamed Radzi, C.W.J.; Nakaguchi, Y.; Nishibuchi, M.; Radu, S. Prevalence and Antibiotic Resistance of Salmonella Enteritidis and Salmonella Typhimurium in Raw Chicken Meat at Retail Markets in Malaysia. Poult. Sci. 2016, 95, 1888–1893. [Google Scholar] [CrossRef]
- Rortana, C.; Nguyen-Viet, H.; Tum, S.; Unger, F.; Boqvist, S.; Dang-Xuan, S.; Koam, S.; Grace, D.; Osbjer, K.; Heng, T.; et al. Prevalence of Salmonella spp. and Staphylococcus aureus in Chicken Meat and Pork from Cambodian Markets. Pathogens 2021, 10, 556. [Google Scholar] [CrossRef] [PubMed]
- Farhoumand, P.; Hassanzadazar, H.; Soltanpour, M.S.; Aminzare, M.; Abbasi, Z. Prevalence, Genotyping and Antibiotic Resistance of Listeria monocytogenes and Escherichia coli in Fresh Beef and Chicken Meats Marketed in Zanjan, Iran. Iran. J. Microbiol. 2020, 12, 537. [Google Scholar] [CrossRef]
- Elmali, M.; Can, H.Y.; Yaman, H. Prevalence of Listeria monocytogenes in Poultry Meat. Food Sci. Technol. 2015, 35, 672–675. [Google Scholar] [CrossRef]
- Panera-Martínez, S.; Rodríguez-Melcón, C.; Serrano-Galán, V.; Alonso-Calleja, C.; Capita, R. Prevalence, Quantification and Antibiotic Resistance of Listeria monocytogenes in Poultry Preparations. Food Control 2022, 135, 108608. [Google Scholar] [CrossRef]
- Mangal, M.; Bansal, S.; Sharma, S.K.; Gupta, R.K. Molecular Detection of Foodborne Pathogens: A Rapid and Accurate Answer to Food Safety. Crit. Rev. Food Sci. Nutr. 2016, 56, 1568–1584. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, J.; Shi, Z.; Cao, A.; Fang, W.; Yan, D.; Wang, Q.; Li, Y. Molecular Methods for Identification and Quantification of Foodborne Pathogens. Molecules 2022, 27, 8262. [Google Scholar] [CrossRef] [PubMed]
- Ndraha, N.; Lin, H.-Y.; Wang, C.-Y.; Hsiao, H.-I.; Lin, H.-J. Rapid Detection Methods for Foodborne Pathogens Based on Nucleic Acid Amplification: Recent Advances, Remaining Challenges, and Possible Opportunities. Food Chem. Mol. Sci. 2023, 7, 100183. [Google Scholar] [CrossRef]
- Panwar, S.; Duggirala, K.S.; Yadav, P.; Debnath, N.; Yadav, A.K.; Kumar, A. Advanced Diagnostic Methods for Identification of Bacterial Foodborne Pathogens: Contemporary and Upcoming Challenges. Crit. Rev. Biotechnol. 2022, 43, 1–19. [Google Scholar] [CrossRef]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR Inhibitors—Occurrence, Properties and Removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef]
- Sidstedt, M.; Rådström, P.; Hedman, J. PCR Inhibition in QPCR, DPCR and MPS—Mechanisms and Solutions. Anal. Bioanal. Chem. 2020, 412, 2009–2023. [Google Scholar] [CrossRef]
- Maurer, J.J. Rapid Detection and Limitations of Molecular Techniques. Annu. Rev. Food Sci. Technol. 2011, 2, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, Y.; Huang, L.; Guo, J.; Wang, A.; Ma, C.; Shi, C. Direct Capture and Amplification of Nucleic Acids Using a Universal, Elution-Free Magnetic Bead-Based Method for Rapid Pathogen Detection in Multiple Types of Biological Samples. Anal. Bioanal. Chem. 2023, 415, 427–438. [Google Scholar] [CrossRef]
- He, Z.; Tang, C.; Chen, X.; Liu, H.; Yang, G.; Xiao, Z.; Li, W.; Deng, Y.; Jin, L.; Chen, H.; et al. Based on Magnetic Beads to Develop the Kit for Extraction of High-Quality Cell-Free DNA from Blood of Breast Cancer Patients. Mater. Express 2019, 9, 956–961. [Google Scholar] [CrossRef]
- Jin, B.; Ma, B.; Li, J.; Hong, Y.; Zhang, M. Simultaneous Detection of Five Foodborne Pathogens Using a Mini Automatic Nucleic Acid Extractor Combined with Recombinase Polymerase Amplification and Lateral Flow Immunoassay. Microorganisms 2022, 10, 1352. [Google Scholar] [CrossRef]
- Hedman, J.; Rådström, P. Overcoming Inhibition in Real-Time Diagnostic PCR. In PCR Detection of Microbial Pathogens; Wilks, M., Ed.; Humana: Totowa, NJ, USA, 2013; pp. 17–48. [Google Scholar]
- Butler, J.M. Separation of DNA Restriction Fragments and PCR Products. In Analysis of Nucleic Acids by Capillary Electrophoresis; Heller, C., Ed.; Chromatographia CE Series; Vieweg + Teubner Verlag: Wiesbaden, Germany, 1997; Volume 1, pp. 195–212. ISBN 978-3-322-91017-2. [Google Scholar]
- Lian, D.-S.; Zeng, H.-S. Capillary Electrophoresis Based on Nucleic Acid Detection as Used in Food Analysis. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1281–1295. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, L.; Zhang, Y.; He, P.; Wang, Q. Simultaneous Detection of Three Foodborne Pathogenic Bacteria in Food Samples by Microchip Capillary Electrophoresis in Combination with Polymerase Chain Reaction. J. Chromatogr. A 2018, 1555, 100–105. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zheng, B.; Qu, L.; Li, C. Determination of Foodborne Pathogenic Bacteria by Multiplex PCR-Microchip Capillary Electrophoresis with Genetic Algorithm-Support Vector Regression Optimization. Anal. Chim. Acta 2009, 643, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, B.; García-Cañas, V.; Cifuentes, A.; González, R.; Aznar, R.; Alarcon, B.; Garcia-Canas, V.; Cifuentes, A.; Gonzalez, R.; Aznar, R. Simultaneous and Sensitive Detection of Three Foodborne Pathogens by Multiplex PCR, Capillary Gel Electrophoresis, and Laser-Induced Fluorescence. J. Agric. Food Chem. 2004, 52, 7180–7186. [Google Scholar] [CrossRef]
- Denis, E.; Bielińska, K.; Wieczorek, K.; Osek, J. Multiplex Real-Time PCRs for Detection of Salmonella, Listeria monocytogenes, and Verotoxigenic Escherichia coli in Carcasses of Slaughtered Animals. J. Vet. Res. 2016, 60, 287–292. [Google Scholar] [CrossRef]
- Leader, B.T.; Frye, J.G.; Hu, J.; Fedorka-Cray, P.J.; Boyle, D.S. High-Throughput Molecular Determination of Salmonella enterica Serovars by Use of Multiplex PCR and Capillary Electrophoresis Analysis. J. Clin. Microbiol. 2009, 47, 1290–1299. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Y.; Peng, X.; Ni, Y.; Wang, F.; Dou, X. Rapid Analysis for Staphylococcus aureus via Microchip Capillary Electrophoresis. Sensors 2021, 21, 1334. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Huang, Y.; Ma, Y.; Yu, H.; Pang, B.; Liu, X.; Yin, C.; Wang, X.; Wei, Y.; Tian, Y.; et al. Detection of Four Foodborne Pathogens Based on Magnetic Separation Multiplex PCR and Capillary Electrophoresis. Biotechnol. J. 2022, 17, 2100335. [Google Scholar] [CrossRef]
- Research and Markets Global Chicken Market, Size, Forecast 2023–2028, Industry Trends, Growth, Share, Outlook, Impact of Inflation, Opportunity Company Analysis. Available online: https://www.researchandmarkets.com/report/chicken (accessed on 7 September 2023).
- Miller, M.; Gerval, A.; Hansen, J.; Grossen, G. Poultry Expected to Continue Leading Global Meat Imports as Demand Rises. Available online: https://www.ers.usda.gov/amber-waves/2022/august/poultry-expected-to-continue-leading-global-meat-imports-as-demand-rises/ (accessed on 7 September 2023).
- Joint FAO/WHO Codex Alimentarius Commission. General Standard for Irradiated Foods—CODEX STAN 106–1983, REV. 1–2003; Joint FAO/WHO Codex Alimentarius Commission: Rome, Italy, 2003; pp. 1–3. [Google Scholar]
- Wang, K.; Pang, X.; Zeng, Z.; Xiong, H.; Du, J.; Li, G.; Baidoo, I.K. Research on Irradiated Food Status and Consumer Acceptance: A Chinese Perspective. Food Sci. Nutr. 2023, 11, 4964–4974. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Irradiation in The Production, Processing and Handling of Food; 21 C.F.R. § 179; The Code of Federal Regulations: New York, NY, USA, 2023; pp. 460–466.
- Suklim, K.; Flick, G.J.; Vichitphan, K. Effects of Gamma Irradiation on the Physical and Sensory Quality and Inactivation of Listeria monocytogenes in Blue Swimming Crab Meat (Portunas pelagicus). Radiat. Phys. Chem. 2014, 103, 22–26. [Google Scholar] [CrossRef]
- Spoto, M.H.F.; Gallo, C.R.; Alcarde, A.R.; Gurgel, M.S.d.A.; Blumer, L.; Walder, J.M.M.; Domarco, R.E. Gamma Irradiation in the Control of Pathogenic Bacteria in Refrigerated Ground Chicken Meat. Sci. Agric. 2000, 57, 389–394. [Google Scholar] [CrossRef]
- Gezgin, Z.; Gunes, G. Influence of Gamma Irradiation on Growth and Survival of Escherichia coli O157:H7 and Quality of Cig Kofte, a Traditional Raw Meat Product. Int. J. Food Sci. Technol. 2007, 42, 1067–1072. [Google Scholar] [CrossRef]
- Lee, N.; Kwon, K.Y.; Oh, S.K.; Chang, H.-J.; Chun, H.S.; Choi, S.-W. A Multiplex PCR Assay for Simultaneous Detection of Escherichia coli O157:H7, Bacillus cereus, Vibrio parahaemolyticus, Salmonella spp., Listeria monocytogenes, and Staphylococcus aureus in Korean Ready-to-Eat Foo. Foodborne Pathog. Dis. 2014, 11, 574–580. [Google Scholar] [CrossRef]
- Molina, F.; Lopez-Acedo, E.; Tabla, R.; Roa, I.; Gomez, A.; Rebollo, J. Improved Detection of Escherichia coli and Coliform Bacteria by Multiplex PCR. BMC Biotechnol. 2015, 15, 48. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X.; Wang, Q. Sensitive and Specific Detection of E. coli, Listeria monocytogenes, and Salmonella enterica Serovar Typhimurium in Milk by Microchip Electrophoresis Combined with Multiplex PCR Amplification. Microchem. J. 2020, 157, 104876. [Google Scholar] [CrossRef]
- Chin, W.H.; Sun, Y.; Høgberg, J.; Quyen, T.L.; Engelsmann, P.; Wolff, A.; Bang, D.D. Direct PCR—A Rapid Method for Multiplexed Detection of Different Serotypes of Salmonella in Enriched Pork Meat Samples. Mol. Cell Probes 2017, 32, 24–32. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Chung, Y.-A.; Wu, Y.-C.; Fang, C.-T.; Chen, P.-J. Disease Burden from Foodborne Illnesses in Taiwan, 2012–2015. J. Formos. Med. Assoc. 2020, 119, 1372–1381. [Google Scholar] [CrossRef]
- Yu, C.-P.; Chou, Y.-C.; Wu, D.-C.; Cheng, C.-G.; Cheng, C.-A. Surveillance of Foodborne Diseases in Taiwan. Medicine 2021, 100, e24424. [Google Scholar] [CrossRef]
- Salazar, J.K.; Wang, Y.; Yu, S.; Wang, H.; Zhang, W. Polymerase Chain Reaction-Based Serotyping of Pathogenic Bacteria in Food. J. Microbiol. Methods 2015, 110, 18–26. [Google Scholar] [CrossRef]
- Lukinmaa, S.; Nakari, U.-M.; Eklund, M.; Siitonen, A. Application of Molecular Genetic Methods in Diagnostics and Epidemiology of Food-Borne Bacterial Pathogens. APMIS 2004, 112, 908–929. [Google Scholar] [CrossRef]
- Priyanka, B.; Patil, R.; Dwarakanath, S. A Review on Detection Methods Used for Foodborne Pathogens. Indian J. Med. Res. 2016, 144, 327. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Yen, S.-C.; Tsai, S.-K.; Shen, F.; Lin, J.H.-Y.; Lin, H.-J. Combining Direct PCR Technology and Capillary Electrophoresis for an Easy-to-Operate and Highly Sensitive Infectious Disease Detection System for Shrimp. Life 2022, 12, 276. [Google Scholar] [CrossRef]
- Emaus, M.N.; Varona, M.; Eitzmann, D.R.; Hsieh, S.-A.; Zeger, V.R.; Anderson, J.L. Nucleic Acid Extraction: Fundamentals of Sample Preparation Methodologies, Current Advancements, and Future Endeavors. TrAC Trends Anal. Chem. 2020, 130, 115985. [Google Scholar] [CrossRef]
- Barbosa, C.; Nogueira, S.; Gadanho, M.; Chaves, S. DNA Extraction: Finding the Most Suitable Method. In Molecular Microbial Diagnostic Methods: Pathways to Implementation for the Food and Water Industries; Cook, N., D’Agostino, M., Thompson, K.C., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 135–154. ISBN 9780124171701. [Google Scholar]
- Gheorghiu, E. Detection of Pathogenic Bacteria by Magneto-Immunoassays: A Review. J. Biomed. Res. 2021, 35, 277. [Google Scholar] [CrossRef]
- Herzig, G.P.D.; Aydin, M.; Dunigan, S.; Shah, P.; Jeong, K.C.; Park, S.H.; Ricke, S.C.; Ahn, S. Magnetic Bead-based Immunoassay Coupled with Tyramide Signal Amplification for Detection of Salmonella in Foods. J. Food Saf. 2016, 36, 383–391. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Chen, J.; Hua, D.; Li, Y.; Deng, H.; Li, Y.; Liang, Z.; Huang, J. Rapid Detection of Food-Borne Salmonella Contamination Using IMBs-QPCR Method Based on PagC Gene. Braz. J. Microbiol. 2018, 49, 320–328. [Google Scholar] [CrossRef]
- Zhou, B.; Xiao, J.; Liu, S.; Yang, J.; Wang, Y.; Nie, F.; Zhou, Q.; Li, Y.; Zhao, G. Simultaneous Detection of Six Food-Borne Pathogens by Multiplex PCR with a GeXP Analyzer. Food Control 2013, 32, 198–204. [Google Scholar] [CrossRef]
- Ruan, J.; Li, M.; Liu, Y.-P.; Li, Y.-Q.; Li, Y.-X. Rapid and Sensitive Detection of Cronobacter spp. (Previously Enterobacter sakazakii) in Food by Duplex PCR Combined with Capillary Electrophoresis-Laser-Induced Fluorescence Detector. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 921, 15–20. [Google Scholar] [CrossRef]
- Rossen, L.; Nørskov, P.; Holmstrøm, K.; Rasmussen, O.F. Inhibition of PCR by Components of Food Samples, Microbial Diagnostic Assays and DNA-Extraction Solutions. Int. J. Food Microbiol. 1992, 17, 37–45. [Google Scholar] [CrossRef]
- Bicart-See, A.; Rottman, M.; Cartwright, M.; Seiler, B.; Gamini, N.; Rodas, M.; Penary, M.; Giordano, G.; Oswald, E.; Super, M.; et al. Rapid Isolation of Staphylococcus aureus Pathogens from Infected Clinical Samples Using Magnetic Beads Coated with Fc-Mannose Binding Lectin. PLoS ONE 2016, 11, e0156287. [Google Scholar] [CrossRef]
- Fukushima, H.; Katsube, K.; Hata, Y.; Kishi, R.; Fujiwara, S. Rapid Separation and Concentration of Food-Borne Pathogens in Food Samples Prior to Quantification by Viable-Cell Counting and Real-Time PCR. Appl. Environ. Microbiol. 2007, 73, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Matsuda, Y.; Yamazaki, Y. Separation of Viable Lactic Acid Bacteria from Fermented Milk. Heliyon 2018, 4, e00597. [Google Scholar] [CrossRef]
- Fukushima, H.; Shimizu, S.; Inatsu, Y. Yersinia enterocolitica and Yersinia pseudotuberculosis Detection in Foods. J. Pathog. 2011, 2011, 735308. [Google Scholar] [CrossRef]
- Wang, M.; Gong, Q.; Liu, W.; Tan, S.; Xiao, J.; Chen, C. Applications of Capillary Electrophoresis in the Fields of Environmental, Pharmaceutical, Clinical, and Food Analysis (2019–2021). J. Sep. Sci. 2022, 45, 1918–1941. [Google Scholar] [CrossRef]
- Buszewski, B.; Maślak, E.; Złoch, M.; Railean-Plugaru, V.; Kłodzińska, E.; Pomastowski, P. A New Approach to Identifying Pathogens, with Particular Regard to Viruses, Based on Capillary Electrophoresis and Other Analytical Techniques. TrAC Trends Anal. Chem. 2021, 139, 116250. [Google Scholar] [CrossRef]
- Musso, M.; Bocciardi, R.; Parodi, S.; Ravazzolo, R.; Ceccherini, I. Betaine, Dimethyl Sulfoxide, and 7-Deaza-DGTP, a Powerful Mixture for Amplification of GC-Rich DNA Sequences. J. Mol. Diagn. 2006, 8, 544–550. [Google Scholar] [CrossRef]
- Grunenwald, H. Optimization of Polymerase Chain Reactions. In PCR Protocols; Bartlett, J.M.S., Stirling, D., Eds.; Humana Press: Totowa, NJ, USA, 2003; pp. 89–100. [Google Scholar]
- Jensen, M.A.; Fukushima, M.; Davis, R.W. DMSO and Betaine Greatly Improve Amplification of GC-Rich Constructs in de Novo Synthesis. PLoS ONE 2010, 5, e11024. [Google Scholar] [CrossRef]
- Strien, J.; Sanft, J.; Mall, G. Enhancement of PCR Amplification of Moderate GC-Containing and Highly GC-Rich DNA Sequences. Mol. Biotechnol. 2013, 54, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Karunanathie, H.; Kee, P.S.; Ng, S.F.; Kennedy, M.A.; Chua, E.W. PCR Enhancers: Types, Mechanisms, and Applications in Long-Range PCR. Biochimie 2022, 197, 130–143. [Google Scholar] [CrossRef]
- Foddai, A.C.G.; Grant, I.R. Methods for Detection of Viable Foodborne Pathogens: Current State-of-Art and Future Prospects. Appl. Microbiol. Biotechnol. 2020, 104, 4281–4288. [Google Scholar] [CrossRef]
- Becskei, A.; Rahaman, S. The Life and Death of RNA across Temperatures. Comput. Struct. Biotechnol. J. 2022, 20, 4325–4336. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Li, J.; Hu, J.; Lin, G.; Tan, B.K.; Lin, S. Current Perspectives on Viable but Non-Culturable Foodborne Pathogenic Bacteria: A Review. Foods 2023, 12, 1179. [Google Scholar] [CrossRef]
- Wang, A.; Liu, L.; Zhang, S.; Ye, W.; Zheng, T.; Xie, J.; Wu, S.; Wu, Z.; Feng, Q.; Dong, H.; et al. Development of a Duplex Real-Time Reverse Transcription-Polymerase Chain Reaction Assay for the Simultaneous Detection of Goose Astrovirus Genotypes 1 and 2. J. Virol. Methods 2022, 310, 114612. [Google Scholar] [CrossRef]
- Chen, M.; Lan, X.; Zhu, L.; Ru, P.; Xu, W.; Liu, H. PCR Mediated Nucleic Acid Molecular Recognition Technology for Detection of Viable and Dead Foodborne Pathogens. Foods 2022, 11, 2675. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).