Dietary Supplementation with Pomegranate and Onion Affects Lipid and Protein Oxidation in the Breast Meat, Thigh, and Liver, Cellular Stress Protein Responses, and Gene Expression of Liver Enzymes Involved in Protein Synthesis in Broilers
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experimentation and Sampling
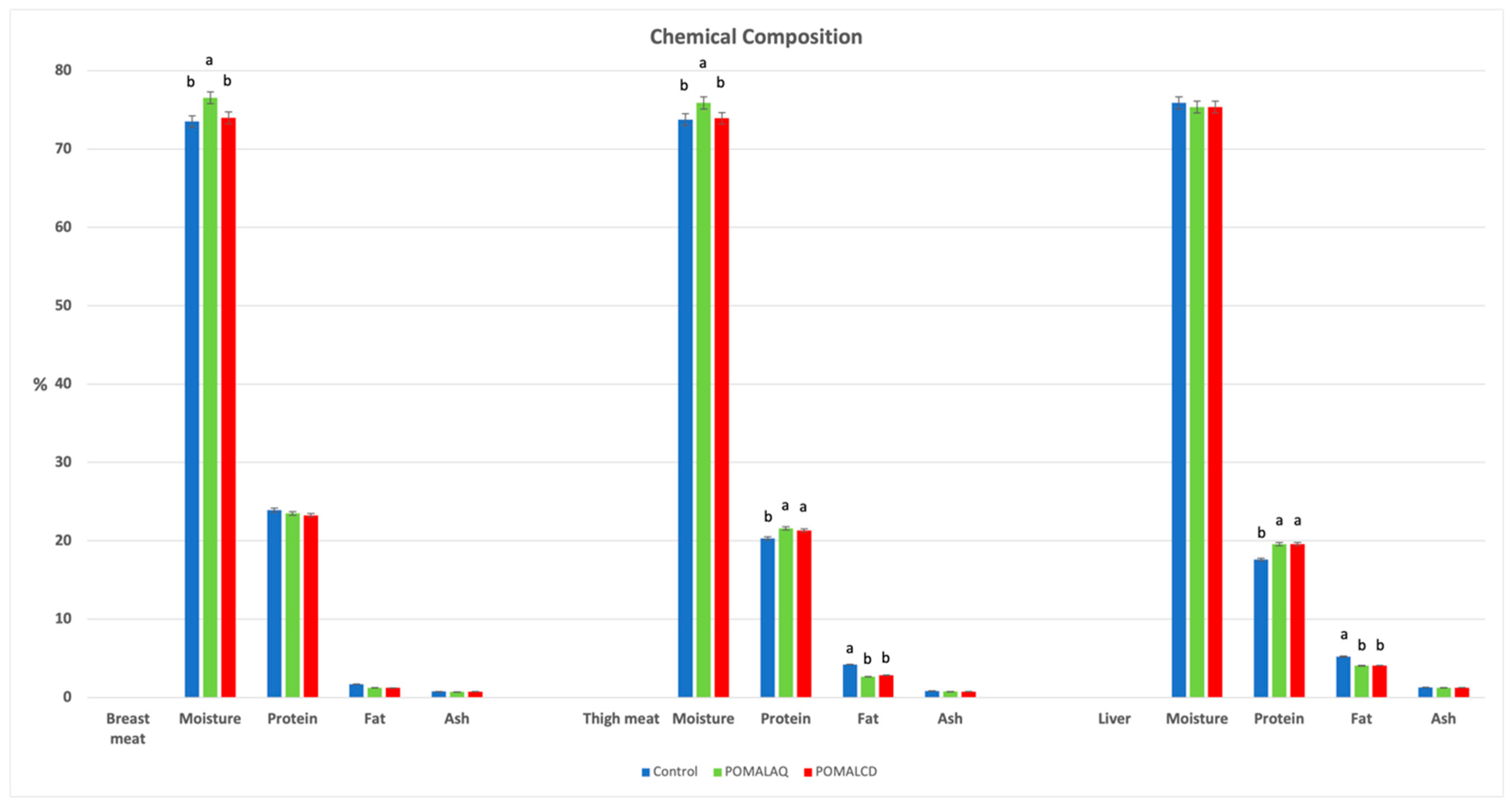
2.2. Breast Meat, Thigh Meat, and Liver Composition, Lipid and Protein Oxidation Status, and Total Antioxidant Capacity
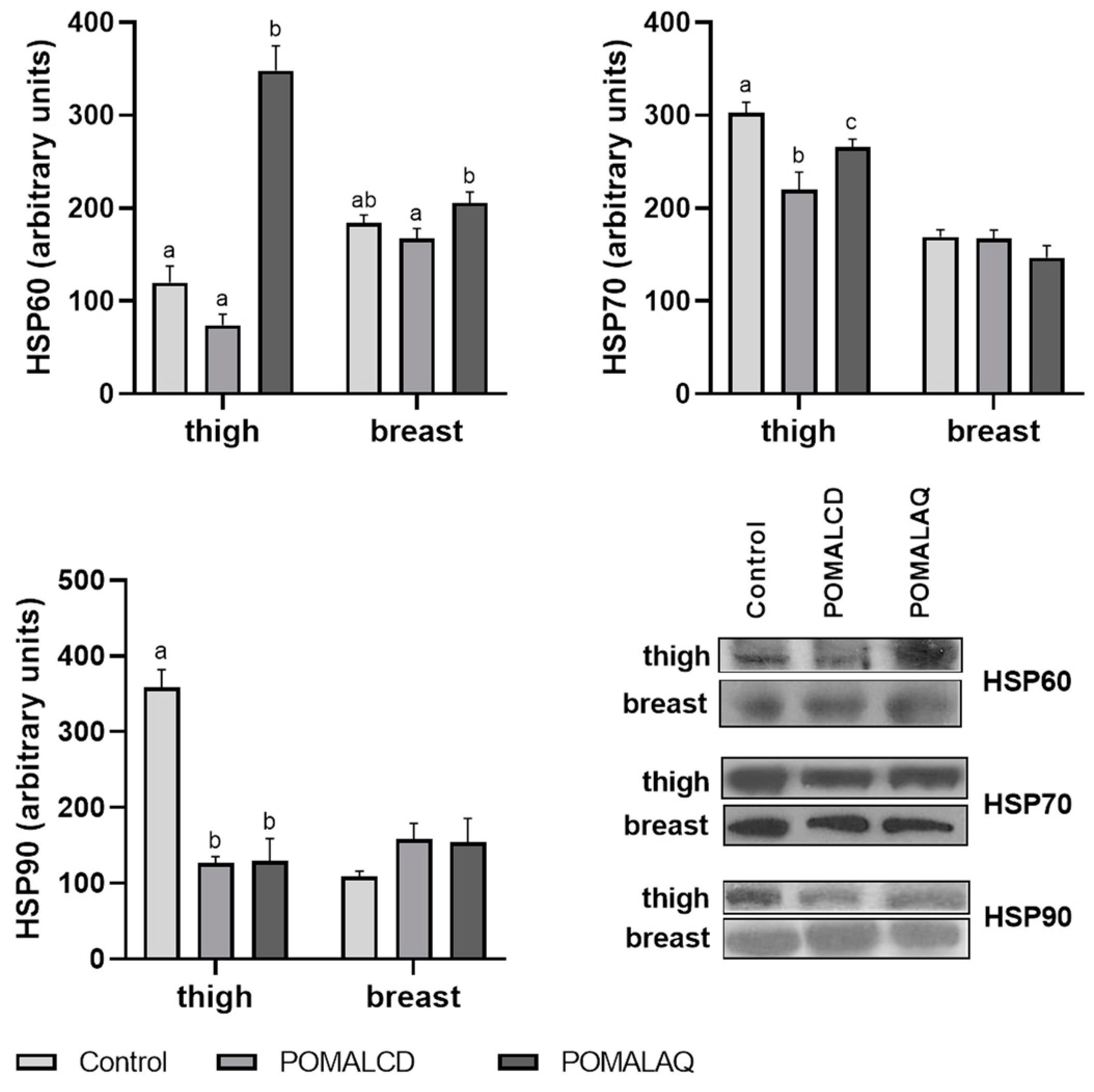
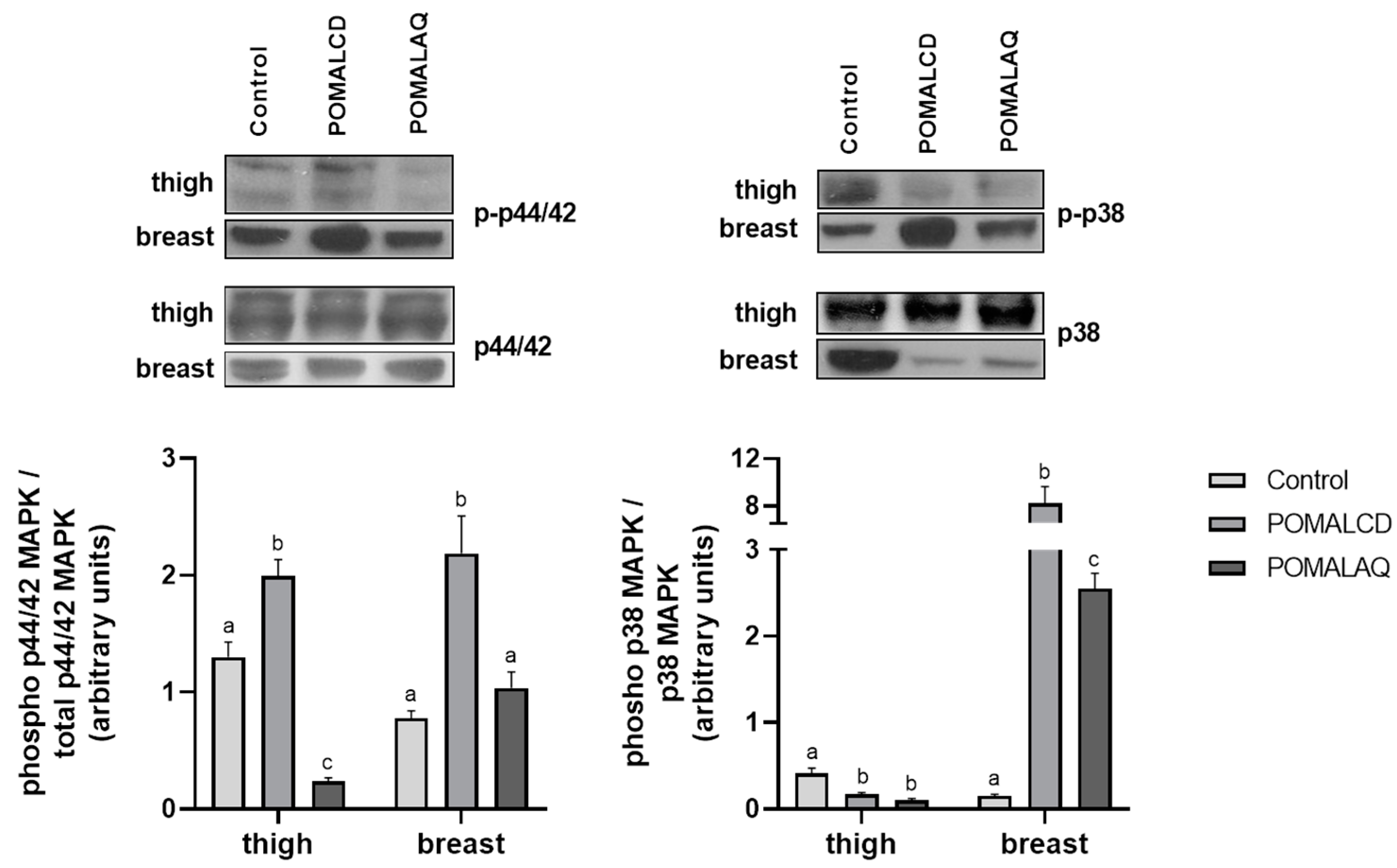
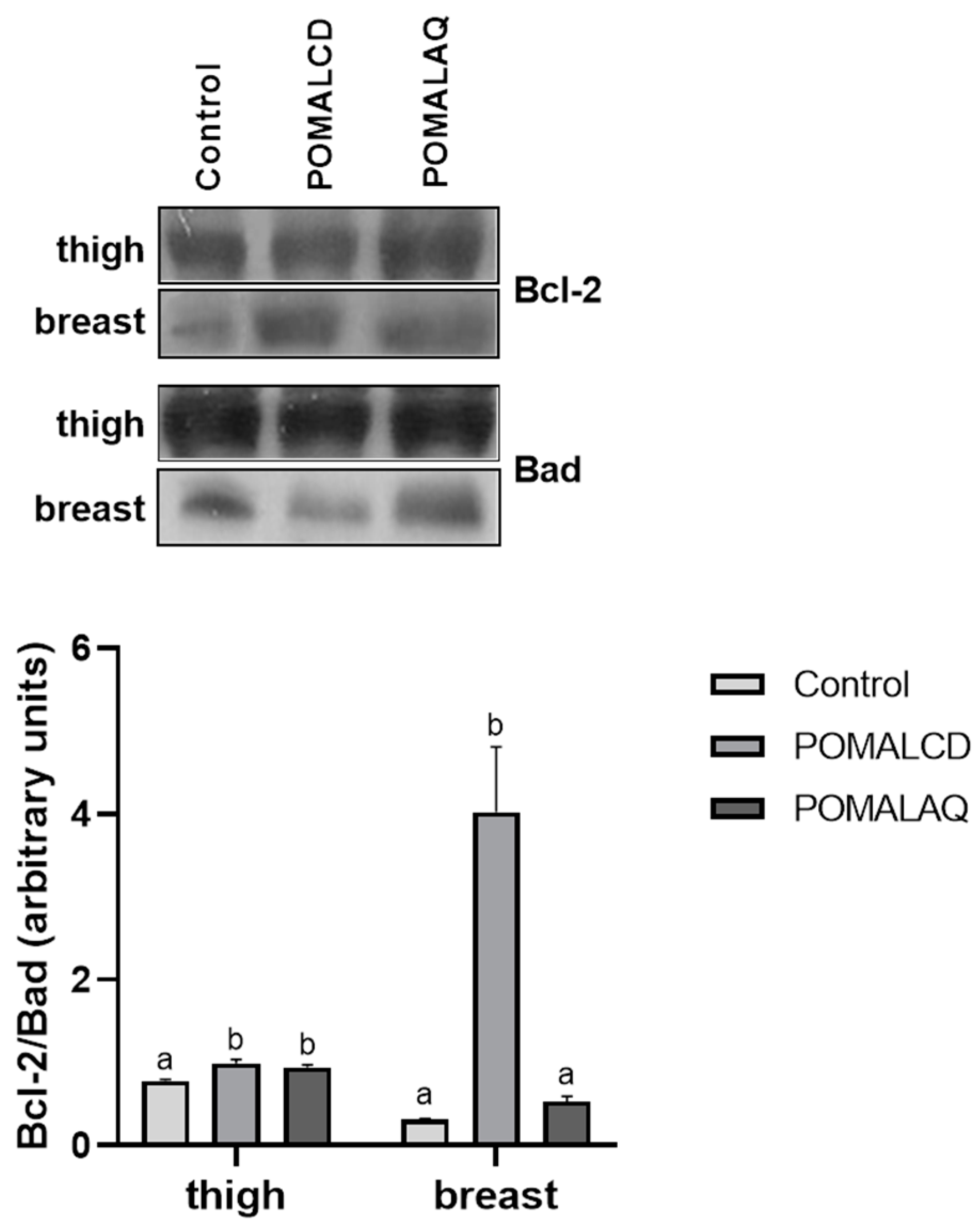
2.3. Protein Apoptosis
SDS–PAGE/Immunoblot Analysis
2.4. Enzyme Gene Expression of Amino Acids in Liver Samples
RNA Isolation, RT-PCR Analysis, and Quantitative Real-Time PCR Analysis
2.5. Statistical Analysis of Meat Oxidation, Liver Gene Expression, and Protein Apoptosis Activation
3. Results
3.1. Breast, Thigh, and Liver Composition and Oxidative Status
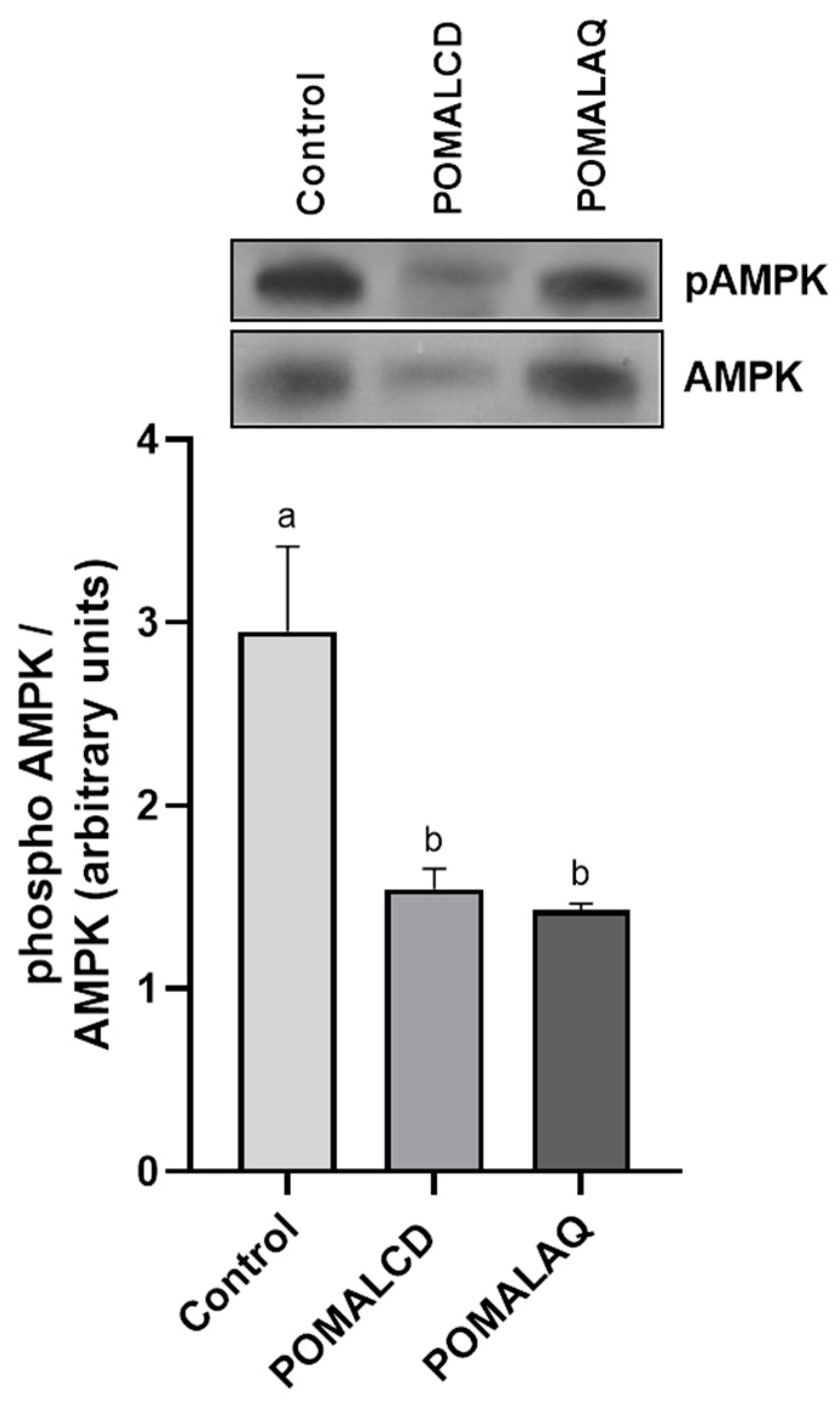
3.2. Cellular Stress Response (Heat Shock Response, MAPK Pathways, and Apoptosis)
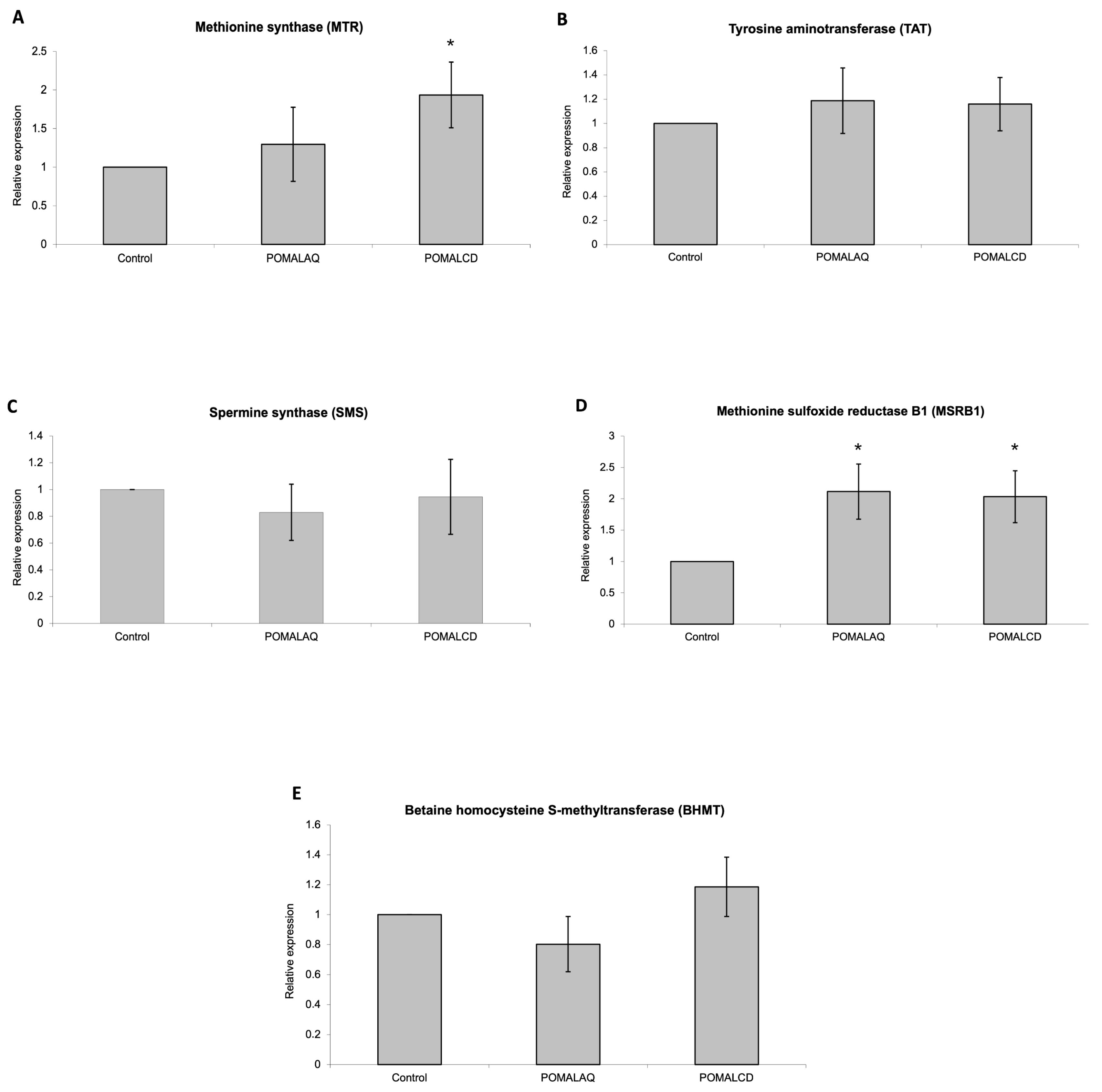
3.3. Changes in Gene Expression
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- OECD/FAO. OECD-FAO Agricultural Outlook 2021–2030; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Kim, M.; Voy, B.H. Fighting Fat with Fat: N-3 Polyunsaturated Fatty Acids and Adipose Deposition in Broiler Chickens. Front. Physiol. 2021, 12, 755317. [Google Scholar] [CrossRef] [PubMed]
- Kishawy, A.T.; Amer, S.A.; Abd El-Hack, M.E.; Saadeldin, I.M.; Swelum, A.A. The impact of dietary linseed oil and pomegranate peel extract on broiler growth, carcass traits, serum lipid profile, and meat fatty acid, phenol, and flavonoid contents. Asian-Australas. J. Anim. Sci. 2019, 32, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Akuru, E.A.; Oyeagu, C.E.; Mpendulo, T.C.; Rautenbach, F.; Oguntibeju, O.O. Effect of pomegranate (Punica granatum L) peel powder meal dietary supplementation on antioxidant status and quality of breast meat in broilers. Heliyon 2020, 6, e05709. [Google Scholar] [CrossRef] [PubMed]
- Akuru, E.A.; Mpendulo, C.T.; Oyeagu, C.E.; Nantapo, C.W.T. Pomegranate (Punica granatum L.) peel powder meal supplementation in broilers: Effect on growth performance, digestibility, carcase and organ weights, serum and some meat antioxidant enzyme biomarkers. Ital. J. Anim. Sci. 2021, 20, 119–131. [Google Scholar] [CrossRef]
- Ahmadipour, B.; Pat, S.; Abaszadeh, S.; Hassanpour, H.; Khajali, F. Pomegranate peel as a phytogenic in broiler chickens: Influence upon antioxidant, lipogenesis and hypotensive response. Vet. Med. Sci. 2021, 7, 1907–1913. [Google Scholar] [CrossRef]
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review. Foods 2021, 10, 657. [Google Scholar] [CrossRef]
- Kaderides, K.; Mourtzinos, I.; Goula, A.M. Stability of pomegranate peel polyphenols encapsulated in orange juice industry by-product and their incorporation in cookies. Food Chem. 2020, 310, 125849. [Google Scholar] [CrossRef]
- Saki, A.A.; Rabet, M.; Zamani, P.; Yousefi, A. The Effects of Different Levels of Pomegranate Seed Pulp with Multi-Enzyme on Performance, Egg Quality and Serum Antioxidant in Laying Hens. Iran. J. Appl. Anim. Sci. 2014, 4, 803–808. [Google Scholar]
- Boroushaki, M.T.; Mollazadeh, H.; Afshari, A.R. Pomegranate seed oil: A comprehensive review on its therapeutic effects. Int. J. Pharm. Sci. Res. 2016, 7, 430. [Google Scholar]
- Białek, M.; Białek, A.; Lepionka, T.; Paśko, P.; Galanty, A.; Tokarz, A.; Czauderna, M. Punica granatum (Pomegranate) Seed Oil and Momordica charantia (Bitter Melon) Extract Affect the Lipid’s Profile and Oxidative Stability of Femoral Muscles of Rats. Eur. J. Lipid Sci. Technol. 2019, 121, 1800420. [Google Scholar] [CrossRef]
- Białek, P.D.A.; Białek, P.M.; Lepionka, P.T.; Tober, E.; Czauderna, P.M. The Quality Determination of Selected Commercial Online Purchased Edible Pomegranate Seed Oils with New Argentometric Liquid Chromatography Method. J. Diet. Suppl. 2021, 18, 351–371. [Google Scholar] [CrossRef] [PubMed]
- El-Hamamsy, S.M.A.; El-khamissi, H.A.Z. Phytochemicals, Antioxidant Activity and Identification of Phenolic Compounds by HPLC of Pomegranate (Punica granatum L.) Peel Extracts. J. Agric. Chem. Biotechnol. 2020, 11, 79–84. [Google Scholar] [CrossRef]
- Khan, S.; Patel, A.; Bhise, K. Antioxidant activity of pomegranate peel powder. J. Drug Deliv. Ther. 2017, 7, 81–84. [Google Scholar] [CrossRef]
- Khoddami, A.; Man, Y.B.C.; Roberts, T.H. Physico-chemical properties and fatty acid profile of seed oils from pomegranate (Punica granatum L.) extracted by cold pressing. Eur. J. Lipid Sci. Technol. 2014, 116, 553–562. [Google Scholar] [CrossRef]
- Munir, M.T. Effect of garlic on the health and performance of broilers. Veterinaria 2015, 3, 32–39. [Google Scholar]
- Malematja, E.; Ng’Ambi, J.W.; Chitura, T.; Nemauluma, M.F.D.; Kolobe, S.D.; Manyelo, T.G. Onion meal and onion extracts (Allium cepa L.) as natural growth promoters for use in poultry production: A Review. Appl. Ecol. Environ. Res. 2022, 20, 383–396. [Google Scholar] [CrossRef]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Cools, K.; Terry, L.A.; Esteban, R.M. Characterization of industrial onion wastes (Allium cepa L.): Dietary fibre and bioactive compounds. Plant Foods Hum. Nutr. 2011, 66, 48–57. [Google Scholar] [CrossRef]
- Osojnik Črnivec, I.G.; Skrt, M.; Šeremet, D.; Sterniša, M.; Farčnik, D.; Štrumbelj, E.; Poljanšek, A.; Cebin, N.; Pogačnik, L.; Smole Možina, S.; et al. Waste streams in onion production: Bioactive compounds, quercetin and use of antimicrobial and antioxidative properties. Waste Manag. 2021, 126, 476–486. [Google Scholar] [CrossRef]
- Ifesan, B.O. Chemical composition of onion peel (Allium cepa) and its ability to serve as a preservative in cooked beef. Int. J. Sci. Res. Methodol. 2017, 7, 1–10. [Google Scholar]
- Walag, A.M.P.; Ahmed, O.; Jeevanandam, J.; Akram, M.; Ephraim-Emmanuel, B.C.; Egbuna, C.; Semwal, P.; Iqbal, M.; Hassan, S.; Uba, J.O. Health Benefits of Organosulfur Compounds. In Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations; Egbuna, C., Dable Tupas, G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 445–472. [Google Scholar] [CrossRef]
- Saleh, H.; Golian, A.; Kermanshahi, H.; Mirakzehi, M.T. Effects of dietary α-tocopherol acetate, pomegranate peel, and pomegranate peel extract on phenolic content, fatty acid composition, and meat quality of broiler chickens. J. Appl. Anim. Res. 2017, 45, 629–636. [Google Scholar] [CrossRef]
- Rezvani, M.R.; Rahimi, S.; Dadpasand, M. Effect of adding pomegranate peel powder to fat-containing diets on performance of broilers. Anim. Prod. 2016, 18, 335–346. [Google Scholar] [CrossRef]
- der Poel, A.F.B.v.; Abdollahi, M.R.; Cheng, H.; Colovic, R.; den Hartog, L.A.; Miladinovic, D.; Page, G.; Sijssens, K.; Smillie, J.F.; Thomas, M.; et al. Future directions of animal feed technology research to meet the challenges of a changing world. Anim. Feed Sci. Technol. 2020, 270, 114692. [Google Scholar] [CrossRef]
- Abraha, B.; Admassu, H.; Mahmud, A.; Tsighe, N.; Shui, X.W.; Fang, Y. Effect of processing methods on nutritional and physico-chemical composition of fish: A review. MOJ Food Process. Technol. 2018, 6, 376–382. [Google Scholar] [CrossRef]
- Akande, Κ.Ε.; Fabiyi, E.F. Effect of processing methods on some antinutritional factors in legume seeds for poultry feeding. Int. J. Poult. Sci. 2010, 9, 996–1001. [Google Scholar] [CrossRef]
- Latshaw, J.D. Daily Energy Intake of Broiler Chickens is Altered by Proximate Nutrient Content and Form of the Diet. Poult. Sci. 2008, 87, 89–95. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Li, Z.; Miao, Z.; Ding, L.; Teng, X.; Bao, J. Energy metabolism disorder mediated ammonia gas-induced autophagy via AMPK/mTOR/ULK1-Beclin1 pathway in chicken livers. Ecotoxicol. Environ. Saf. 2021, 217, 112219. [Google Scholar] [CrossRef]
- Towler, M.C.; Hardie, D.G. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 2007, 100, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.S.; Lollo, P.C.B.; Morato, P.N.; Amaya-Farfan, J. Dietary Nutrients and Bioactive Substances Modulate Heat Shock Protein (HSP) Expression: A Review. Nutrients 2018, 10, 683. [Google Scholar] [CrossRef]
- Jäättelä, M. Heat shock proteins as cellular lifeguards. Ann. Med. 1999, 31, 261–271. [Google Scholar] [CrossRef]
- Welch, W.J. The role of heat-shock proteins as molecular chaperones. Curr. Opin. Cell Biol. 1991, 3, 1033–1038. [Google Scholar] [CrossRef]
- Antonopoulou, E.; Chouri, E.; Feidantsis, K.; Lazou, A.; Chatzifotis, S. Effects of partial dietary supplementation of fish meal with soymeal on the stress and apoptosis response in the digestive system of common dentex (Dentex dentex). J. Biol. Res. Thessalon. 2017, 24, 14. [Google Scholar] [CrossRef] [PubMed]
- Dhibi, M.; Brahmi, F.; Mnari, A.; Houas, Z.; Chargui, I.; Bchir, L.; Gazzah, N.; Alsaif, M.A.; Hammami, M. The intake of high fat diet with different trans fatty acid levels differentially induces oxidative stress and non alcoholic fatty liver disease (NAFLD) in rats. Nutr. Metab. 2011, 8, 65. [Google Scholar] [CrossRef]
- Feidantsis, K.; Soumalevris, A.; Panteli, N.; Chatzifotis, S.; Antonopoulou, E. Synergistic effect of long-term feed deprivation and temperature on the cellular physiology of meagre (Argyrosomus regius). J. Therm. Biol. 2022, 105, 103207. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, H.; Liu, X.; Peng, X.; Deng, J.; Zuo, Z.; Cui, W.; Deng, Y.; Wang, K. Dietary high vanadium causes oxidative damage-induced renal and hepatic toxicity in broilers. Biol. Trace Elem. Res. 2012, 145, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ni, B.; Zhang, F.; Hu, Y.; Zhao, J. High Glucose-Induced Oxidative Stress Mediates Apoptosis and Extracellular Matrix Metabolic Imbalances Possibly via p38 MAPK Activation in Rat Nucleus Pulposus Cells. J. Diabetes Res. 2016, 2016, 3765173. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Sinha-Hikim, A.P.; Shen, R.; Kim, H.; French, S.W.; Vaziri, N.D.; Crum, A.; Rajavashisth, T.B.; Norris, K.C. A novel cystine based antioxidant attenuates oxidative stress and hepatic steatosis in diet-induced obese mice. Exp. Mol. Pathol. 2011, 91, 419–428. [Google Scholar] [CrossRef][Green Version]
- Dokou, S.; Vasilopoulou, K.; Bonos, E.; Grigoriadou, K.; Savvidou, S.; Stefanakis, M.K.; Christaki, S.; Kyriakoudi, A.; Mourtzinos, I.; Tzora, A.; et al. Effects of dietary supplementation with phytobiotic encapsulated plant extracts on broilers’ performance parameters, welfare traits and meat characteristics. Ann. Anim. Sci. ahead of print. 2023. [Google Scholar] [CrossRef]
- Krens, S.F.G.; Spaink, H.P.; Snaar-Jagalska, B.E. Functions of the MAPK family in vertebrate-development. FEBS Lett. 2006, 580, 4984–4990. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian Mitogen-Activated Protein Kinase Signal Transduction Pathways Activated by Stress and Inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef]
- Panteli, N.; Demertzioglou, M.; Feidantsis, K.; Karapanagiotis, S.; Tsele, N.; Tsakoniti, K.; Gkagkavouzis, K.; Mylonas, C.C.; Kormas, K.A.; Mente, E.; et al. Advances in understanding the mitogenic, metabolic, and cell death signaling in teleost development: The case of greater amberjack (Seriola dumerili, Risso 1810). Fish Physiol. Biochem. 2022, 48, 1665–1684. [Google Scholar] [CrossRef] [PubMed]
- Henson, P.M.; Hume, D.A. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006, 27, 244–250. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Alves, N.L.; Derks, I.A.M.; Reedquist, K.A.; Eldering, E. Apoptosis induced by overall metabolic stress converges on the Bcl-2 family proteins Noxa and Mcl-1. Apoptosis 2011, 16, 708–721. [Google Scholar] [CrossRef]
- Prakash, A.; Saxena, V.K.; Kumar, R.; Tomar, S.; Singh, M.K.; Singh, G. Differential gene expression in liver of colored broiler chicken divergently selected for residual feed intake. Trop. Anim. Health Prod. 2021, 53, 403. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; Vasilopoulos, S.; Dokou, S.; Papagrigoriou, T.; Ganguly, B.; Savvidou, S.; Symeon, G.; Michailidis, G.; Lazari, D. Effects of dietary supplementation with herbal extract as methionine replacer on growth performance, meat composition, oxidative stability and liver gene expression in broiler chickens. Arch. Zootech. 2022, 25, 39–62. [Google Scholar] [CrossRef]
- Vasilopoulos, S.; Dokou, S.; Papadopoulos, G.A.; Savvidou, S.; Christaki, S.; Kyriakoudi, A.; Dotas, V.; Tsiouris, V.; Bonos, E.; Skoufos, I.; et al. Dietary Supplementation with Pomegranate and Onion Aqueous and Cyclodextrin Encapsulated Extracts Affects Broiler Performance Parameters, Welfare and Meat Characteristics. Poultry 2022, 1, 74–93. [Google Scholar] [CrossRef]
- Kornbrust, D.J.; Mavis, R.D. Relative susceptibility of microsomes from lung, heart, liver, kidney, brain and testes to lipid peroxidation: Correlation with vitamin E content. Lipids 1980, 15, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.U.; Nam, K.C. Effects of ascorbic acid and antioxidants on color, lipid oxidation and volatiles of irradiated ground beef. Radiat. Phys. Chem. 2004, 71, 151–156. [Google Scholar] [CrossRef]
- Patsoukis, N.; Zervoudakis, G.; Panagopoulos, N.T.; Georgiou, C.D.; Angelatou, F.; Matsokis, N.A. Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazol-induced epileptic seizure. Neurosci. Lett. 2004, 357, 83–86. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Antonopoulou, E.; Chatzigiannidou, I.; Feidantsis, K.; Kounna, C.; Chatzifotis, S. Effect of water temperature on cellular stress responses in meagre (Argyrosomus regius). Fish Physiol. Biochem. 2020, 46, 1075–1091. [Google Scholar] [CrossRef]
- Pfaffl, M. Quantification Strategies in Real-Time PCR. In AZ of Quantitative PCR; International University Line: La Jolla, CA, USA, 2004; pp. 87–112. Available online: https://www.gene-quantification.de/chapter-3-pfaffl.pdf (accessed on 1 August 2023).
- Michailidis, G.; Anastasiadou, M.; Guibert, E.; Froment, P. Activation of innate immune system in response to lipopolysaccharide in chicken Sertoli cells. Reproduction 2014, 148, 259–270. [Google Scholar] [CrossRef]
- Ghosh, S.; Chatterjee, P.N.; Maity, A.; Mukherjee, J.; Batabyal, S.; Chatterjee, J.K. Effect of supplementing pomegranate peel infusion on body growth, feed efficiency, biochemical metabolites and antioxidant status of broiler chicken. Trop. Anim. Health Prod. 2020, 52, 3899–3905. [Google Scholar] [CrossRef]
- Nikfarjam, M.; Rashki Ghaleno, L.; Shahverdi, A.H.; Mirshahvalad, S.H.; Ghoreishi, S.M.; Alizadeh, A.R. Effects of Dietary Pomegranate Peel on Antioxidant Gene Expression and DJ-1 Protein Abundance in Ram Testes. Int. J. Fertil. Steril. 2021, 15, 258–262. [Google Scholar] [CrossRef]
- Aditya, S.; Ahammed, M.; Jang, S.H.; Ohh, S.J. Effects of dietary onion (Allium cepa) extract supplementation on performance, apparent total tract retention of nutrients, blood profile and meat quality of broiler chicks. Asian-Australas. J. Anim. Sci. 2017, 30, 229–235. [Google Scholar] [CrossRef]
- Adhami, V.M.; Mukhtar, H. Polyphenols from green tea and pomegranate for prevention of prostate cancer. Free Radic. Res. 2006, 40, 1095–1104. [Google Scholar] [CrossRef]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 143, 397–405. [Google Scholar] [CrossRef]
- Hamad, K.M.; Kareem, K.Y. Growth performance, carcass quality, and economics of production of Japanese quails fed with pomegranate peel powder. Int. Trans. J. Eng. Manag. Appl. Sci. Technol. 2019, 10, 2228–9860. [Google Scholar] [CrossRef]
- Bishayee, A.; Thoppil, R.J.; Darvesh, A.S.; Ohanyan, V.; Meszaros, J.G.; Bhatia, D. Pomegranate phytoconstituents blunt the inflammatory cascade in a chemically induced rodent model of hepatocellular carcinogenesis. J. Nutr. Biochem. 2013, 24, 178–187. [Google Scholar] [CrossRef]
- Chen, B.; Tuuli, M.G.; Longtine, M.S.; Shin, J.S.; Lawrence, R.; Inder, T.; Nelson, D.M. Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1142–E1152. [Google Scholar] [CrossRef]
- Bastaki, S.M.A.; Ojha, S.; Kalasz, H.; Adeghate, E. Chemical constituents and medicinal properties of Allium species. Mol. Cell. Biochem. 2021, 476, 4301–4321. [Google Scholar] [CrossRef]
- Kyung, K.H. Antimicrobial properties of allium species. Curr. Opin. Biotechnol. 2012, 23, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Enoka, V.I.L.; Kikuvi, G.M.; Ndung’u, P.W. Effect of garlic (Allium sativum) and onion (Allium cepa L.) extract chitosan nanoparticles on antioxidant enzymes activities and relative weight of visceral organs of rainbow rooster chicken. Int. J. Livest. Prod. 2020, 11, 188–199. [Google Scholar] [CrossRef]
- Sunu, P.; Sunarti, D.; Mahfudz, L.D.; Yunianto, V.D. Effect of synbiotic from Allium sativum and Lactobacillus acidophilus on hematological indices, antioxidative status and intestinal ecology of broiler chicken. J. Saudi Soc. Agric. Sci. 2021, 20, 103–110. [Google Scholar] [CrossRef]
- Liu, C.P.; Fu, J.; Xu, F.P.; Wang, X.S.; Li, S. The role of heat shock proteins in oxidative stress damage induced by Se deficiency in chicken livers. BioMetals 2015, 28, 163–173. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Jin Park, H. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Wei, X.; Li, S.; Li, T.; Liu, L.; Liu, Y.; Wang, H.; Zhou, Y.; Liang, F.; Yu, X.; Zang, W.; et al. Pomegranate peel extract ameliorates liver fibrosis induced by carbon tetrachloride in rats through suppressing p38MAPK/Nrf2 pathway. J. Funct. Foods 2020, 65, 103712. [Google Scholar] [CrossRef]
- Khan, N.; Syed, D.N.; Pal, H.C.; Mukhtar, H.; Afaq, F. Pomegranate Fruit Extract Inhibits UVB-induced Inflammation and Proliferation by Modulating NF-κB and MAPK Signaling Pathways in Mouse Skin†. Photochem. Photobiol. 2012, 88, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, Y.; Aisa, H.A. Anti-inflammatory effect of pomegranate flower in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages. Pharm. Biol. 2017, 55, 2095–2101. [Google Scholar] [CrossRef]
- Lee, B.K.; Jung, Y.-S. Allium cepa Extract and Quercetin Protect Neuronal Cells from Oxidative Stress via PKC-ε Inactivation/ERK1/2 Activation. Oxidative Med. Cell. Longev. 2016, 2016, 2495624. [Google Scholar] [CrossRef]
- Hui, C.; Jun, W.; Ya, L.N.; Ming, X. Effect of Allium sativum (garlic) diallyl disulfide (DADS) on human non-small cell lung carcinoma H1299 cells. Trop. Biomed. 2008, 25, 37–45. [Google Scholar]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Yu, C.; Minemoto, Y.; Zhang, J.; Liu, J.; Tang, F.; Bui, T.N.; Xiang, J.; Lin, A. JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol. Cell 2004, 13, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Shaoul, R.; Moati, D.; Schwartz, B.; Pollak, Y.; Sukhotnik, I. Effect of Pomegranate Juice on Intestinal Recovery Following Methotrexate-Induced Intestinal Damage in a Rat Model. J. Am. Coll. Nutr. 2018, 37, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Takahashi, H.; Hosono, K.; Endo, H.; Kato, S.; Yoneda, K.; Nozaki, Y.; Fujita, K.; Yoneda, M.; Wada, K.; et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int. J. Oncol. 2009, 34, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.N.; Lansky, E.P.; Varani, J. Pomegranate as a cosmeceutical source: Pomegranate fractions promote proliferation and procollagen synthesis and inhibit matrix metalloproteinase-1 production in human skin cells. J. Ethnopharmacol. 2006, 103, 311–318. [Google Scholar] [CrossRef]
- Viollet, B.; Foretz, M.; Guigas, B.; Horman, S.; Dentin, R.; Bertrand, L.; Hue, L.; Andreelli, F. Activation of AMP-activated protein kinase in the liver: A new strategy for the management of metabolic hepatic disorders. J. Physiol. 2006, 574, 41–53. [Google Scholar] [CrossRef]
- Zhang, N. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef]
- Tesseraud, S.; Everaert, N.; Boussaid-Om Ezzine, S.; Collin, A.; Métayer-Coustard, S.; Berri, C.M. Manipulating tissue metabolism by amino acids. World’s Poult. Sci. J. 2011, 67, 243–252. [Google Scholar] [CrossRef]
- Fagundes, N.S.; Milfort, M.C.; Williams, S.M.; Da Costa, M.J.; Fuller, A.L.; Menten, J.F.; Rekaya, R.; Aggrey, S.E. Dietary methionine level alters growth, digestibility, and gene expression of amino acid transporters in meat-type chickens. Poult. Sci. 2020, 99, 67–75. [Google Scholar] [CrossRef]
- Sharifian, M.; Hosseini-Vashan, S.J.; Fathi Nasri, M.H.; Perai, A.H. Pomegranate peel extract for broiler chickens under heat stress: Its influence on growth performance, carcass traits, blood metabolites, immunity, jejunal morphology, and meat quality. Livest. Sci. 2019, 227, 22–28. [Google Scholar] [CrossRef]
- Abdel Baset, S.; Ashour, E.A.; Abd El-Hack, M.E.; El-Mekkawy, M.M. Effect of different levels of pomegranate peel powder and probiotic supplementation on growth, carcass traits, blood serum metabolites, antioxidant status and meat quality of broilers. Anim. Biotechnol. 2022, 33, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Eid, Y.; Kirrella, A.A.; Tolba, A.; El-Deeb, M.; Sayed, S.; El-Sawy, H.B.; Shukry, M.; Dawood, M.A.O. Dietary Pomegranate By-Product Alleviated the Oxidative Stress Induced by Dexamethasone in Laying Hens in the Pre-Peak Period. Animals 2021, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, F.; Khajali, F. Flavonoid antioxidants in chicken meat production: Potential application and future trends. World’s Poult. Sci. J. 2021, 77, 347–361. [Google Scholar] [CrossRef]
- Adamiec, J.; Kalemba, D. Analysis of Microencapsulation Ability of Essential Oils during Spray Drying. Dry. Technol. 2006, 24, 1127–1132. [Google Scholar] [CrossRef]
- Gungor, E.; Altop, A.; Erener, G.; Coskun, I. Effect of raw and fermented pomegranate pomace on performance, antioxidant activity, intestinal microbiota and morphology in broiler chickens. Arch. Anim. Nutr. 2021, 75, 137–152. [Google Scholar] [CrossRef]
| Gene | Primer Pair Sequence | Amplicon Size | GenBank |
|---|---|---|---|
| MTR | 5′-tatgctgctgtcaggtctgg-3′ 5′-tggctacagtcagggcttct-3′ | 146 bp | NM_001031104.1 |
| TAT | 5′-gctggagccatgtacctgat-3′ 5′-accacacggaagaagtttgg-3′ | 152 bp | XM_414240.5 |
| SMS | 5′-ctgcggttgattcttgacct-3′ 5′-atgtaggagggaacgcacac-3′ | 173 bp | NM_001030803.1 |
| MSRB1 | 5′-gaggcgaagtgttcaaggac-3′ 5′-acttgccacaggacaccttt-3′ | 192 bp | NM_001135558.2 |
| BHMT | 5′-ggtgcttccattgttggagt-3′ 5′-caggtgggctttcagcttag-3′ | 108 bp | XM_414685.5 |
| β-actin | 5′-ctccctgatggtcaggtcat-3′ 5′-atgccagggtacattgtggt-3′ | 203 bp | L08165 |
| Control 3 | POMALAQ 3 | POMALCD 3 | SEM 4 | p | |
|---|---|---|---|---|---|
| Breast meat TBARS after iron oxidation (ng/g) | |||||
| Minute 30 | 4.95 a | 2.75 b | 1.45 c | 0.06 | <0.001 |
| Minute 60 | 12.8 a | 5.21 b | 4.95 b | 3.18 | <0.001 |
| Thigh meat TBARS after iron oxidation (ng/g) | |||||
| Minute 30 | 7.27 a | 5.77 b | 4.12 c | 0.12 | <0.001 |
| Minute 60 | 28.6 a | 16.5 b | 11.4 c | 0.18 | <0.001 |
| Liver TBARS after iron oxidation (ng/g) | |||||
| Minute 30 | 7.27 a | 5.77 b | 4.12 c | 0.12 | <0.001 |
| Minute 60 | 28.6 a | 16.5 b | 11.4 c | 0.18 | <0.001 |
| Protein carbonyls in breast meat after iron oxidation (ng/g) | 8.33 a | 1.47 c | 2.99 b | 0.21 | <0.001 |
| Protein carbonyls in thigh meat after iron oxidation (ng/g) | 9.87 a | 2.06 b | 3.75 b | 0.14 | <0.001 |
| Protein carbonyls in liver after iron oxidation (ng/g) | 10.71 a | 3.31 b | 5.19 b | 0.28 | <0.001 |
| Breast meat TAC | 14.1 c | 31.4 a | 22.9 b | 1.66 | <0.001 |
| Thigh meat TAC | 25.7 c | 42.1 a | 33.5 b | 2.12 | <0.001 |
| Liver TAC | 28.1 c | 52.6 a | 41.3 b | 3.31 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savvidou, S.; Panteli, N.; Dotas, V.; Symeon, G.; Galamatis, D.; Panitsidis, I.; Voutsinou, E.; Tatidou, C.; Kumar, P.; Antonopoulou, E.; et al. Dietary Supplementation with Pomegranate and Onion Affects Lipid and Protein Oxidation in the Breast Meat, Thigh, and Liver, Cellular Stress Protein Responses, and Gene Expression of Liver Enzymes Involved in Protein Synthesis in Broilers. Foods 2023, 12, 3870. https://doi.org/10.3390/foods12203870
Savvidou S, Panteli N, Dotas V, Symeon G, Galamatis D, Panitsidis I, Voutsinou E, Tatidou C, Kumar P, Antonopoulou E, et al. Dietary Supplementation with Pomegranate and Onion Affects Lipid and Protein Oxidation in the Breast Meat, Thigh, and Liver, Cellular Stress Protein Responses, and Gene Expression of Liver Enzymes Involved in Protein Synthesis in Broilers. Foods. 2023; 12(20):3870. https://doi.org/10.3390/foods12203870
Chicago/Turabian StyleSavvidou, Soumela, Nikolas Panteli, Vassilios Dotas, George Symeon, Dimitrios Galamatis, Ioannis Panitsidis, Eirini Voutsinou, Christina Tatidou, Prafulla Kumar, Efthimia Antonopoulou, and et al. 2023. "Dietary Supplementation with Pomegranate and Onion Affects Lipid and Protein Oxidation in the Breast Meat, Thigh, and Liver, Cellular Stress Protein Responses, and Gene Expression of Liver Enzymes Involved in Protein Synthesis in Broilers" Foods 12, no. 20: 3870. https://doi.org/10.3390/foods12203870
APA StyleSavvidou, S., Panteli, N., Dotas, V., Symeon, G., Galamatis, D., Panitsidis, I., Voutsinou, E., Tatidou, C., Kumar, P., Antonopoulou, E., Michailidis, G., & Giannenas, I. (2023). Dietary Supplementation with Pomegranate and Onion Affects Lipid and Protein Oxidation in the Breast Meat, Thigh, and Liver, Cellular Stress Protein Responses, and Gene Expression of Liver Enzymes Involved in Protein Synthesis in Broilers. Foods, 12(20), 3870. https://doi.org/10.3390/foods12203870









