Evaluation of Quality Parameters and Functional Activity of Ottobratica Extra Virgin Olive Oil Enriched with Zingiber officinale (Ginger) by Two Different Enrichment Processes during One-Year Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Analytical Methods
2.2.1. Ginger Powder, EVOO and FVOO Extraction Procedure
2.2.2. Total Phenol Content and Total Carotenoid Content in Ginger Powder
2.2.3. Free Acidity, Peroxide Value and Spectrophotometric Indices in EVOO and FVOOs
2.2.4. Total Phenol of EVOO and FVOOs
2.2.5. Colour in EVOO and FVOOs
2.2.6. Chlorophyll and Carotenoid in EVOO and FVOOs
2.2.7. α-Tocopherol Content in EVOO and FVOOs
2.2.8. EVOO and FVOOs Phenolic Profile
2.2.9. ABTS and DPPH Tests
2.2.10. β-Carotene Bleaching Test
2.2.11. FRAP
2.2.12. Carbohydrate Hydrolysing Enzyme Inhibitory Effect
2.2.13. Pancreatic Lipase Inhibitory Effect
2.2.14. Sensory Analysis
2.3. Statistical Analysis
3. Results and Discussion
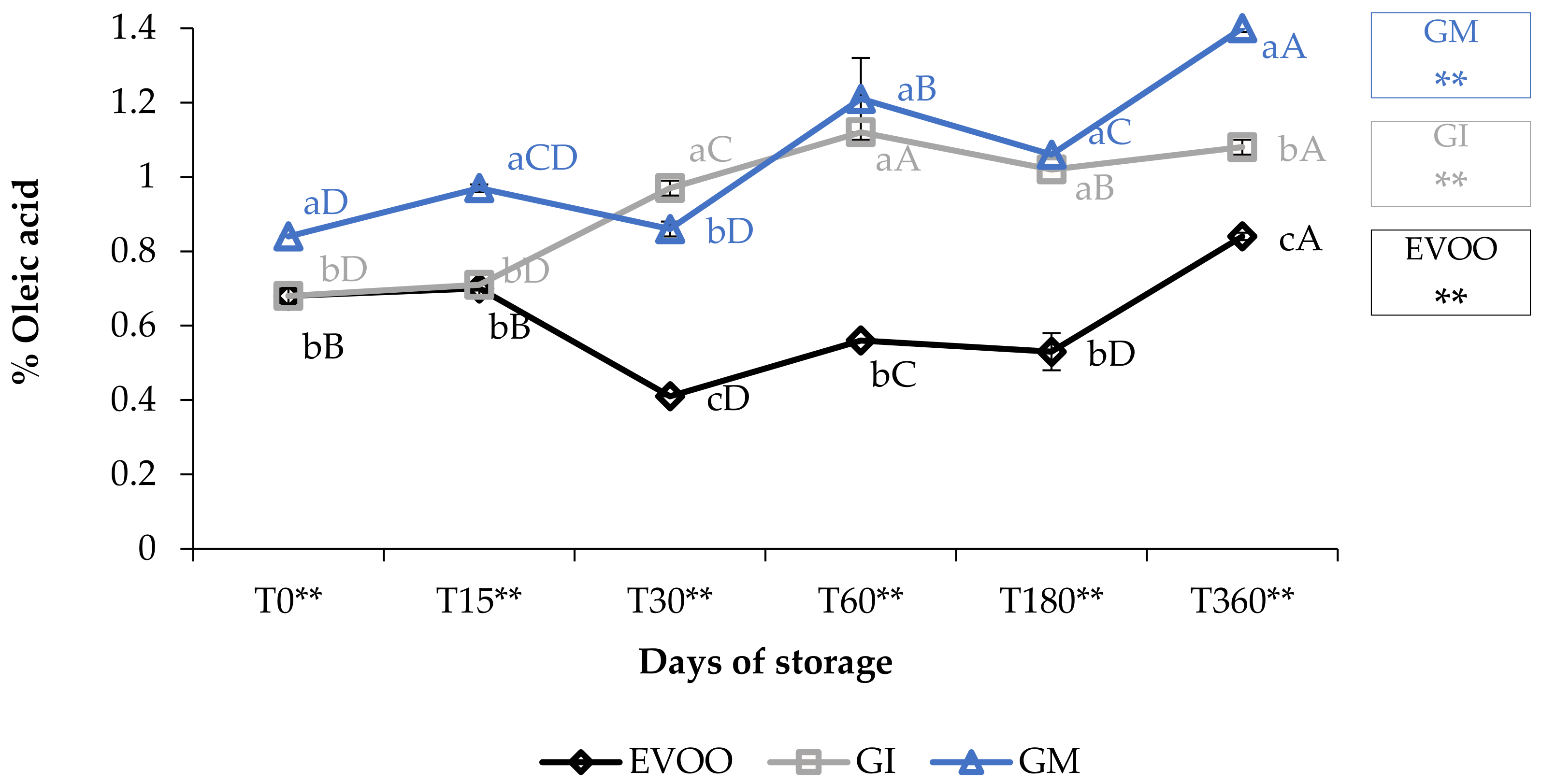
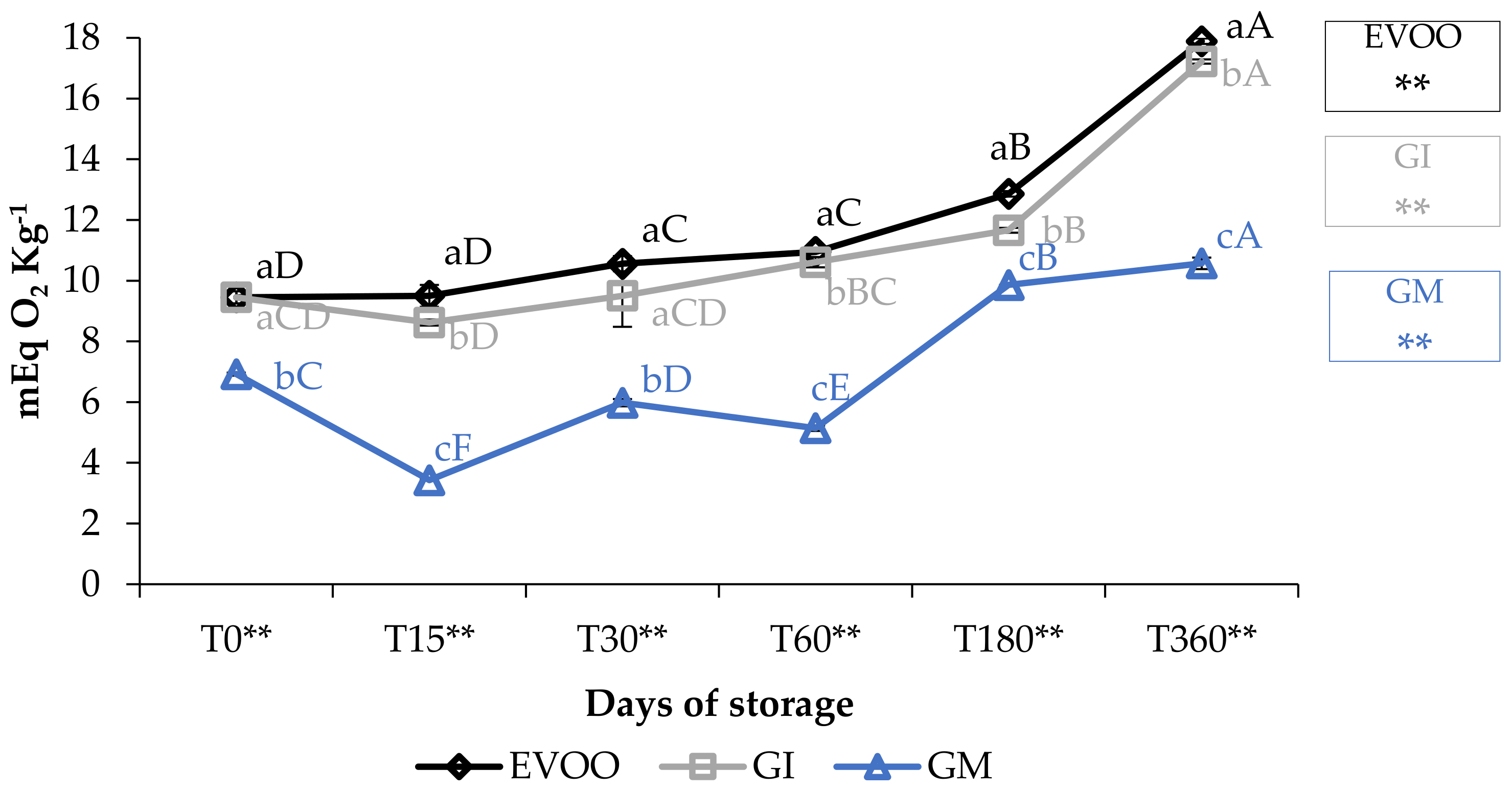
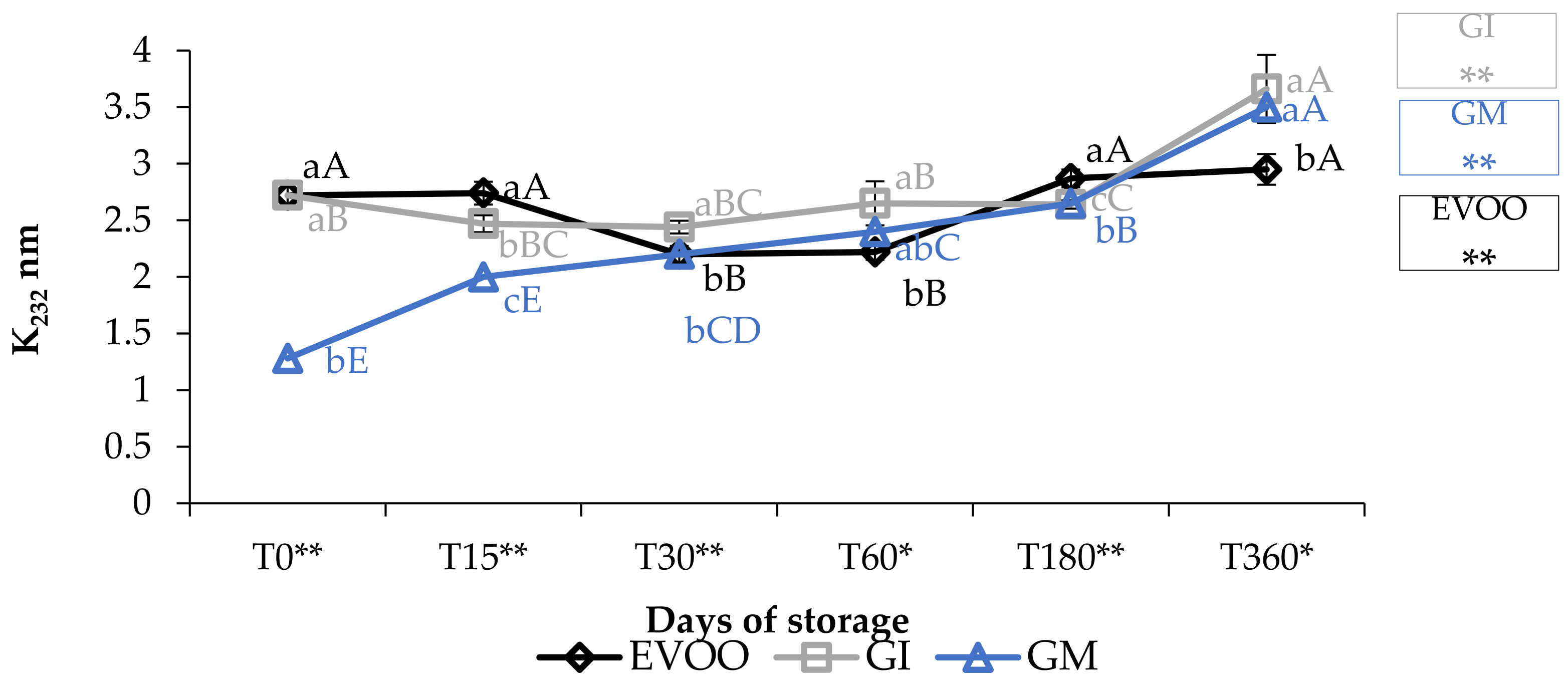
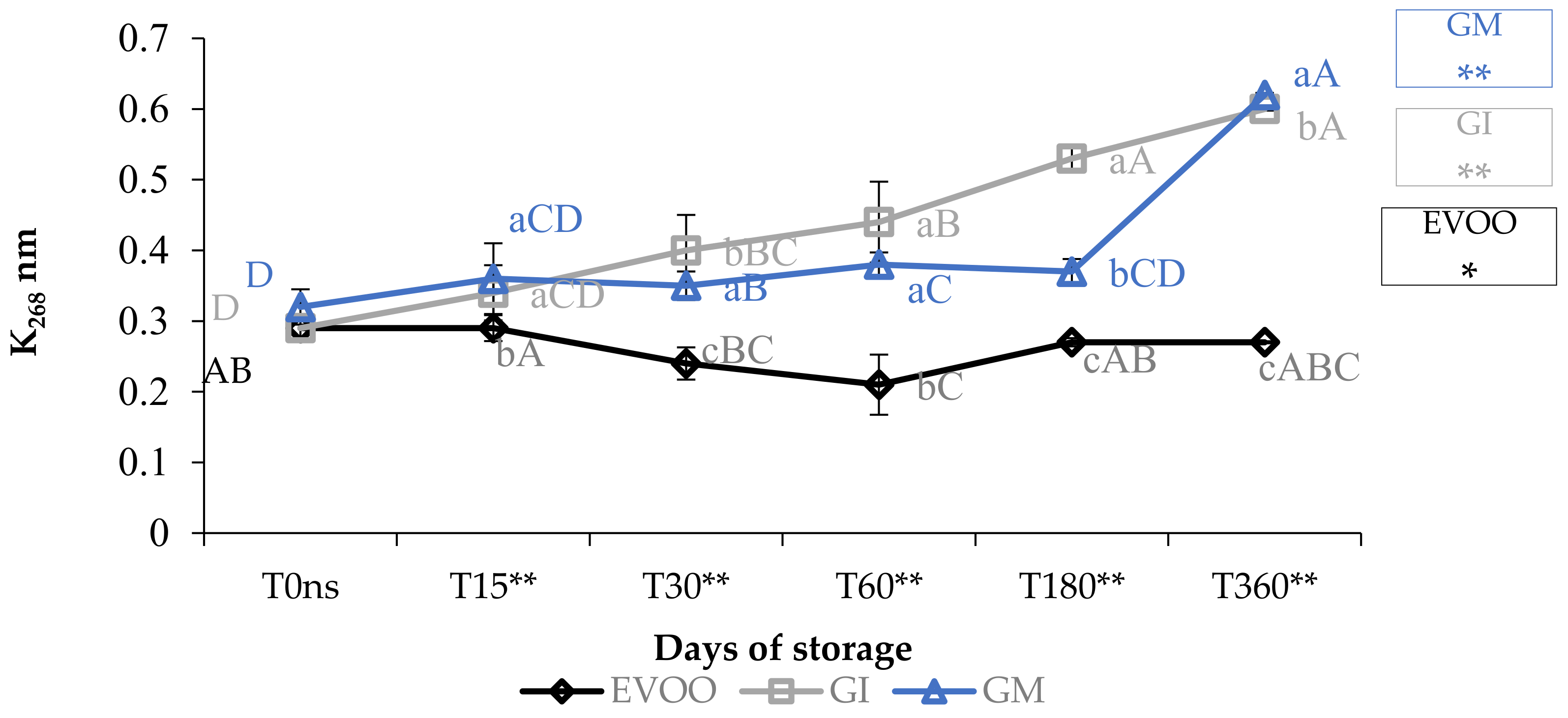
3.1. Free Acidity, Peroxide Value and Spectrophotometric Indices
3.2. Colour, Chlorophyll and Carotenoid
3.3. TPC, α-Tocopherol Content and Individual Phenols by UHPLC
3.4. Antioxidant Activity
3.5. Inhibitory Activity against Key Enzymes Linked to Type 2 Diabetes and Obesity
3.6. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| ABTS | 2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid |
| BHT | Butylated hydroxytoluene |
| DPPH | 1,1-Diphenyl-2-picryl-hydrazil |
| EVOO | Control |
| FA | Free Acidity |
| FRAP | Ferric Reducing Antioxidant Power |
| GI | Ginger flavoured olive oil by infusion |
| GM | Ginger flavoured olive oil by malaxation |
| PV | Peroxide Value |
| TPC | Total Polyphenols Content |
| TChlC | Total Chlorophyll Content |
| TCC | Total Carotenoid Content. |
References
- European Union Commission. Regulation CE 432/2012. OJEU, L136/1; European Union Commission: Brussels, Belgium, 2012. [Google Scholar]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A. Simal-Gandara, Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Dipalmo, T.; Crupi, P.; Durante, V.; Pesce, V.; Maiellaro, I.; Lovece, A.; Mercurio, A.; Laghezza, A.; Corbo, F.; et al. Comparison between different flavored olive oil production techniques: Healthy value and process efficiency. Plant Food Hum. Nutr. 2016, 71, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Durante, V.; Varva, G.; Silletti, R.; Previtali, M.A.; Viggiani, I.; Squeo, G.; Summo, C.; Pasqualone, A.; Gomes, T.; et al. Effect of infusion of spices into the oil vs. combined malaxation of olive paste and spices on quality of naturally flavored virgin olive oils. Food Chem. 2016, 202, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, A.M.; Sicari, V.; Poiana, M.; Cilona, V.; Cannizzaro, A.; Scolaro, F. Characteristics of virgin olive oils extracted from Ottobratica and Roggianella varieties cultivated in the Italian province of Reggio Calabria. Ind. Aliment. 2007, 46, 1139–1145. [Google Scholar]
- Piscopo, A.; De Bruno, A.; Zappia, A.; Ventre, C.; Poiana, M. Characterization of monovarietal olive oils obtained from mills of Calabria region (Southern Italy). Food Chem. 2016, 213, 313–318. [Google Scholar] [CrossRef]
- Sicari, V.; Leporini, M.; Giuffrè, A.M.; Aiello, F.; Falco, T.; Pagliuso, M.T.; Ruffolo, A.; Reitano, A.; Romeo, R.; Tundis, R.; et al. Quality parameters, chemical compositions and antioxidant activities of Calabrian (Italy) monovarietal extra virgin olive oils from autochthonous (Ottobratica) and allochthonous (Coratina, Leccino, and Nocellara Del Belice) varieties. J. Food Meas. Charact. 2021, 15, 363–375. [Google Scholar] [CrossRef]
- Mele, M.A.; Islam, M.Z.; Kang, H.M.; Giuffrè, A.M. Pre-and post-harvest factors and their impact on oil composition and quality of olive fruit. Emir. J. Food Agric. 2018, 30, 592–603. [Google Scholar] [CrossRef]
- Giuffrè, A.M. The evolution of free acidity and oxidation related parameters in olive oil during olive ripening from cultivars grown in the region of Calabria, South Italy. Emir. J. Food Agric. 2018, 30, 539–548. [Google Scholar]
- Giuffrè, A.M. Biometric evaluation of twelve olive cultivars under rainfed conditions in the region of Calabria, South Italy. Emir. J. Food Agric. 2017, 29, 696–709. [Google Scholar] [CrossRef]
- Srinivasan, K. Ginger rhizomes (Zingiber officinale): A spice with multiple health beneficial potentials. PharmaNutrition 2017, 5, 18–28. [Google Scholar] [CrossRef]
- Hamam, M.; Di Vita, G.; Zanchini, R.; Spina, D.; Raimondo, M.; Pilato, M.; D’Amico, M. Consumers’Attitudes and Purchase Intention for a Vitamin-Enriched Extra Virgin Olive Oil. Nutrients 2022, 14, 1658. [Google Scholar] [CrossRef] [PubMed]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef]
- Tundis, R.; Conidi, C.; Loizzo, M.R.; Sicari, V.; Cassano, A. Olive Mill Wastewater Polyphenol-Enriches Fractions by Integrated Membrane Process: A Promising Source of Antioxidant, Hypolipidemic and Hypoglycaemic Compounds. Antioxidants 2020, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, C.; Segura-Cerretero, A.; Contreras, M. Phenolic compounds as natural and multifuncional anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef]
- Mannina, L.; D’Imperio, M.; Gobbino, M.; D’Amico, I.; Casini, A.; Emanuele, M.C.; Sobolev, A.P. Nuclear magnetic resonance study of flavoured olive oils. Flavour. Fragr. J. 2012, 27, 250–259. [Google Scholar] [CrossRef]
- Kamandloo, F.; Rezaei, K.; Aghakhani, A. Effects of herb (mint, ginger, and cinnamon) addition on the formation of 3-monochloropropanediol esters in the refined olive oil. J. Food Process. Preserv. 2022, 46, 16974. [Google Scholar] [CrossRef]
- Contreras-López, E.; Castañeda-Ovando, A.; Jaimez-Ordaz, J.; Cruz-Cansino, N.; González-Olivares, L.G.; Rodríguez-Martínez, J.S.; Ramírez-Godínez, J. Release of Antioxidant Compounds of Zingiber officinale by Ultrasound-Assisted Aqueous Extraction and Evaluation of Their In Vitro Bioaccessibility. Appl. Sci. 2020, 10, 4987. [Google Scholar] [CrossRef]
- Montedoro, G.F.; Servili, M.; Baldioli, M.; Miniati, E. Simple and hydrolyzable phenolic compounds in virgin olive oil. Their extraction, separation and quantitative compounds and semiquantitative evaluation by HPLC. J. Agric. Food Chem. 1992, 40, 571–1576. [Google Scholar] [CrossRef]
- Sepahpour, S.; Salemat, J.; Abdul Manup, M.Y.; Khatib, A.; Abdull Razis, A.F. Comparative Analysis of Chemical Composition, Antioxidant Activity and Quantitative Characterization of Some Phenolic Compounds in Selected Herbs and Spices in Different Solvent Extraction Systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef]
- Silva de Rocha, A.; Rocha, E.K.; Alves, M.L.; Amaral de Moraes, B.; Carvalho de Castro, T.; Albarello, N.; Simões-Gurgel, C. Production and optimization through elicitation of carotenoid pigments in the in vitro cultures of Cleome rosea Vahl (Cleomaceae). J. Plant Biochem. Biotechnol. 2013, 24, 105–113. [Google Scholar] [CrossRef]
- European Union Commission. Consolidated Text on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis; Regulation 01991R2568-IT-04.12.2016-031.005; European Union Commission: Brussels, Belgium, 2016. [Google Scholar]
- Baiano, A.; Gambacorta, G.; Terracone, C.; Previtali, M.A.; Lamacchia, C.; La Notte, E. Changes in phenolic content and antioxidant activity of Italian extra-virgin live oils during storage. J. Food Sci. 2009, 74, 177–183. [Google Scholar] [CrossRef]
- Minguez-Mosquera, M.I.; Rejano-Navarro, L.; Gandui-Rojas, B.; Sànchez-Gomez, A.H.; Garrido-Fernando, J. Color-Pigment correlation in Virgin Olive Oil. J. Am. Oil Chem. Soc. 1991, 68, 332–336. [Google Scholar] [CrossRef]
- De Bruno, A.; Romeo, R.; Piscopo, A.; Poiana, M. Antioxidant quantification in different portions obtained during olive oil extraction process in an olive oil press mill. J. Sci. Food Agric. 2021, 101, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Romeo, R.; De Bruno, A.; Imeneo, V.; Piscopo, A.; Poiana, M. Evaluation of enrichment with antioxidants from olive oil mill wastes in hydrophilic model system. J. Food Process. Pres. 2019, 43, 14211. [Google Scholar] [CrossRef]
- Leporini, M.; Loizzo, M.R.; Tenuta, M.C.; Falco, T.; Sicari, V.; Pellicanò, T.M.; Tundis, R. Calabrian extra-virgin olive oil from Frantoio cultivar: Chemical composition and health properties. Emir. J. Food Agric. 2018, 30, 631–637. [Google Scholar] [CrossRef]
- Plastina, P.; Tundis, R.; La Torre, C.; Sicari, V.; Giuffrè, A.M.; Neri, A.; Bonesi, M.; Leporini, M.; Fazio, A.; Falco, T.; et al. The addition of Capsicum baccatum to Calabrian monovarietal extra virgin olive oils leads to flavored olive oils with enhanced oxidative stability. Ital. J. Food Sci. 2021, 33, 61–72. [Google Scholar] [CrossRef]
- Tundis, R.; Conidi, C.; Loizzo, M.R.; Sicari, V.; Romeo, R.; Cassano, A. Concentration of bioactive phenolic compounds in olive mill wastewater by direct contact membrane distillation. Molecules 2021, 26, 1808. [Google Scholar] [CrossRef] [PubMed]
- Lamas, S.; Rodrigues, N.; Perese, A.M.; Pereira, J.A. Flavoured and forti ed olive oils—Pros and cons. Trends Food Sci. Tech. 2022, 124, 108–127. [Google Scholar] [CrossRef]
- Ayadi, M.A.; Grati-Kamoun, N.; Attia, H. Physico-chemical change and heat stability of extra virgin olive oils flavoured by selected Tunisian aromatic plants. Food Chem. Toxicol. 2009, 47, 2613–2619. [Google Scholar] [CrossRef]
- Sousa, A.; Casal, S.; Malheiro, R.; Lamas, H.; Bento, A.; Pereira, J.A. Aromatized olive oils: Influence of flavouring in quality, composition, stability, antioxidants, and antiradical potential. LWT Food Sci. Technol. 2015, 60, 22–28. [Google Scholar] [CrossRef]
- Piscopo, A.; De Bruno, A.; Zappia, A.; Gioffrè, G.; Grillone, N.; Mafrica, R.; Poiana, M. Effect of olive storage temperature on the quality of Carolea and Ottobratica oils. Emir. J. Food Agric. 2018, 30, 563–572. [Google Scholar] [CrossRef]
- Sicari, V. Antioxidant potential of extra virgin olive oils extracted from three different varieties cultivated in the Italian province of Reggio Calabria. J. Appl. Bot. Food Qual. 2017, 90, 76–82. [Google Scholar] [CrossRef]
- Moustakime, J.; Hazzoumi, Z.; Joutei, K.A. Aromatization of virgin olive oil by seeds of Pimpinella anisum using three different methods: Physico-chemical change and thermal stability of flavored oils. Grain Oil Sci. Technol. 2021, 4, 108–124. [Google Scholar] [CrossRef]
- Díaz-Montaña, E.J.; Aparicio-Ruiz, R.; Morales, M.T. Effect of Flavorization on Virgin Olive Oil Oxidation and Volatile Profile. Antioxidants 2023, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Issaoui, M.; Flamini, G.; Souid, S.; Bendini, A.; Barbieri, S.; Gharbi, I.; Toschi, T.G.; Cioni, P.L.; Hammami, M. How the Addition of Spices and Herbs to Virgin Olive Oil to Produce Flavored Oils Affects Consumer Acceptance. Nat. Prod. Commun. 2016, 11, 6. [Google Scholar] [CrossRef]
- Mafrica, R.; Piscopo, A.; De Bruno, A.; Poiana, M. Effects of Climate on Fruit Growth and Development on Olive Oil Quality in Cultivar Carolea. Agriculture 2021, 11, 147. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Jerkovic, P.; Maldini, M.; Serreli, G. Phenolic Compounds, Antioxidant Activity, and Other Characteristics of Extra Virgin Olive Oils from Italian Autochthonous Varieties Tonda di Villacidro, Tonda di Cagliari, Semidana, and Bosana. J. Chem. 2016, 2016, 8462741. [Google Scholar] [CrossRef]
- Piscopo, A.; Zappia, A.; De Bruno, A.; Poiana, M. Effect of the Harvesting Time on the Quality of Olive Oils Produced in Calabria. Eur. J. Lipid Sci. Technol. 2018, 120, 1700304. [Google Scholar] [CrossRef]
- Sacchi, R.; Della Medaglia, D.; Paduano, A.; Caporaso, N.; Genovese, A. Characterisation of lemon-flavoured olive oils. LWT Food Sci. Technol. 2017, 79, 326–332. [Google Scholar] [CrossRef]
- Montesano, D.; Rocchetti, G.; Cossignani, L.; Senizza, B.; Pollini, L.; Lucini, L.; Blasi, F. Untargeted Metabolomics to Evaluate the Stability of Extra-Virgin Olive Oil with Added Lycium barbarum Carotenoid during Storage. Foods 2019, 8, 179. [Google Scholar] [CrossRef]
- Sicari, G.; Giuffrè, A.M.; Louadj, L.; Poiana, M. Evolution of phenolic compounds of virgin olive oil during 12 months storage. Riv. Ital. Delle Sostanze Grasse 2010, 87, 109–116. [Google Scholar]
- Jiao, W.; Sang, Y.; Wang, X.; Wang, S. Effects of 6-Shogaol on Glucose Uptake and Intestinal Barrier Integrity in Caco-2 Cells. Foods 2023, 12, 503. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Bonesi, M.; Falco, T.; Leporini, M.; Pagliuso, M.; Sicari, V.; Tundis, R. Carolea olive oil enriched with an infusion of Capsicuum annuum and C. chinense dried pepper powders to produce an added value flavoured olive oils. J. Food Process. Preserv. 2021, 45, 15776. [Google Scholar] [CrossRef]
- Baiano, A.; Previtali, M.A.; Viggiani, I.; Varva, G.; Squeo, G.; Paradiso, V.M.; Summo, C.; Gomes, T.; Caponio, F. As oil blending affects physical, chemical, and sensory characteristics of avoured olive oils. Eur. Food Res. Technol. 2016, 242, 1693–1708. [Google Scholar] [CrossRef]
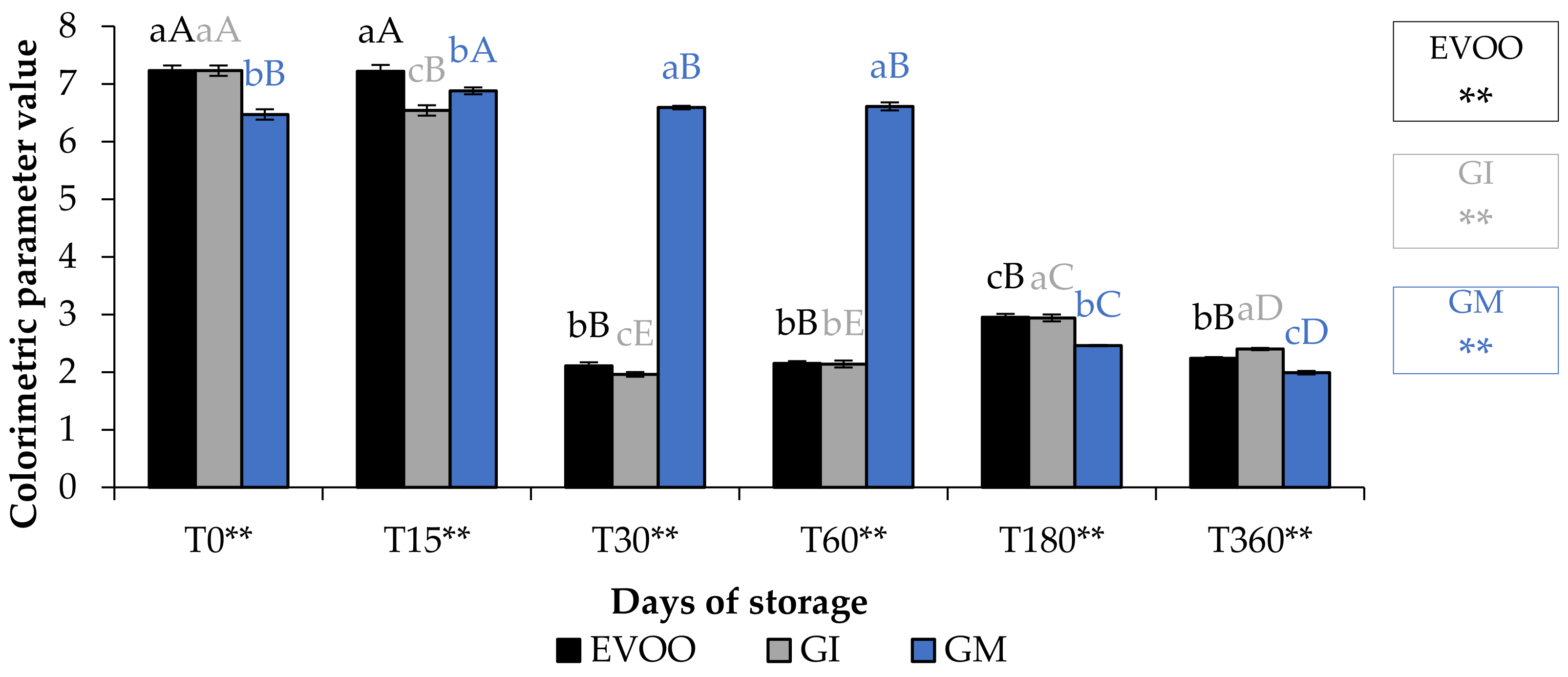
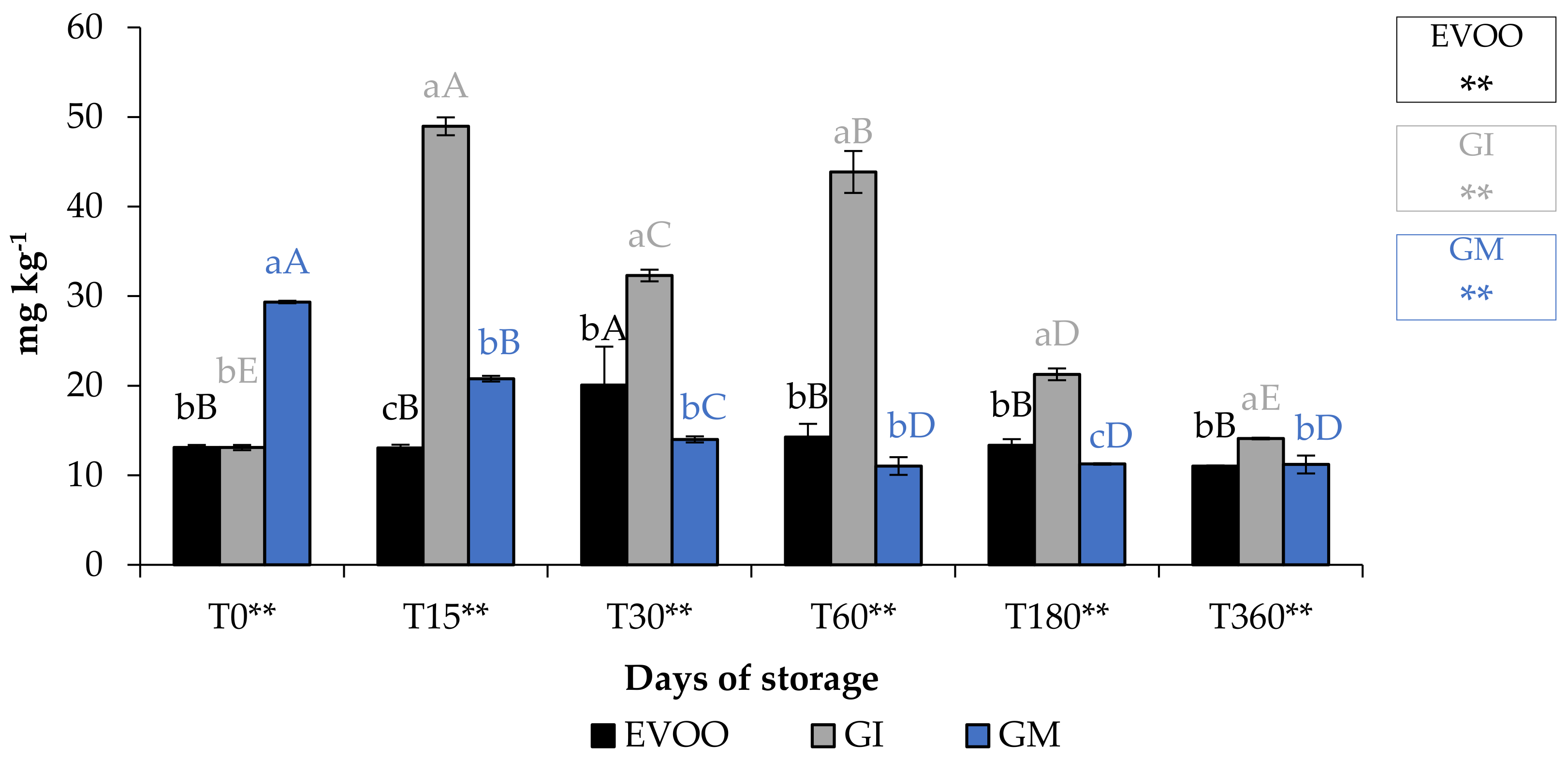


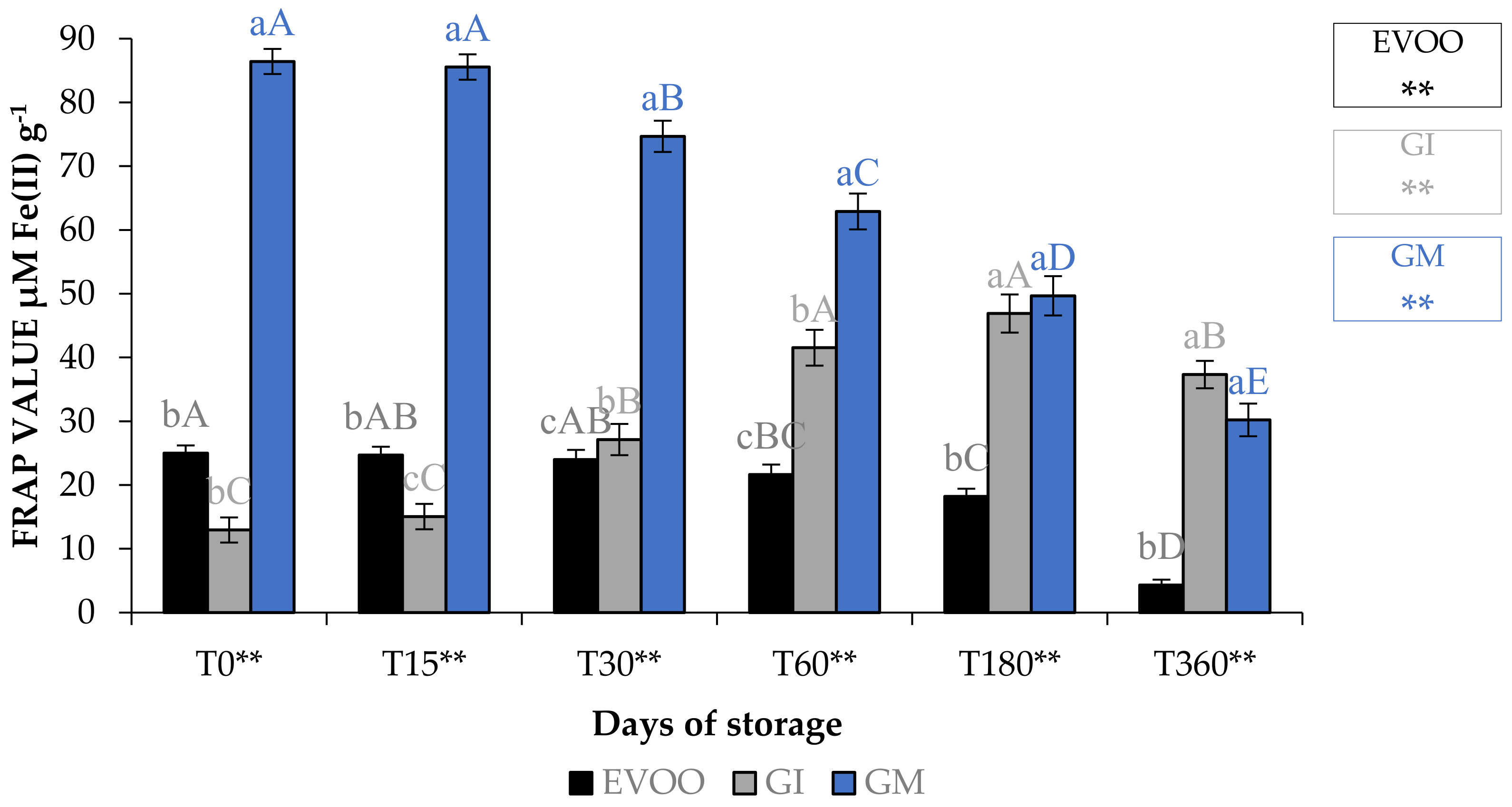
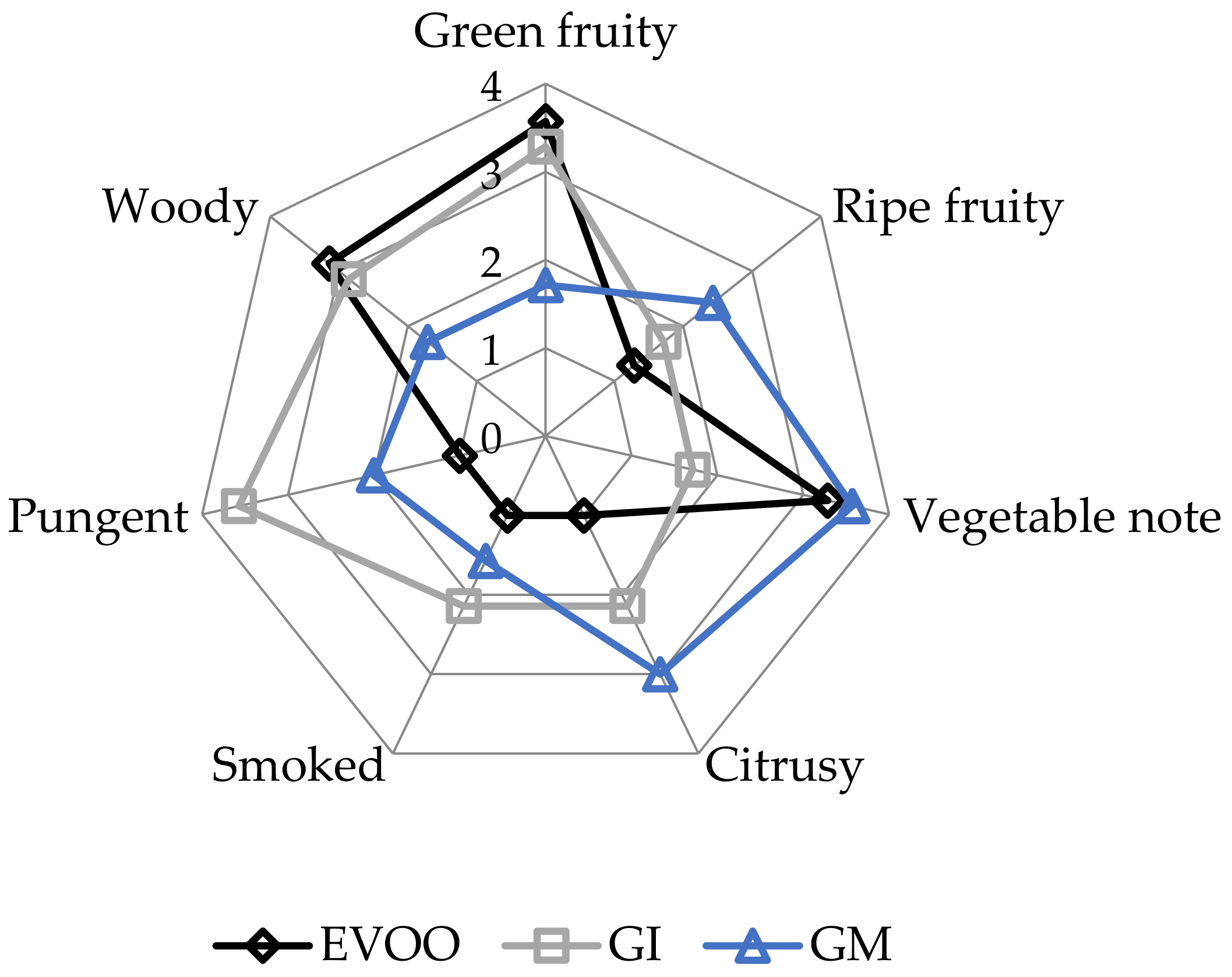

| T0 | T15 | T30 | T60 | T180 | T360 | Sign | |
|---|---|---|---|---|---|---|---|
| EVOO | −0.003 ± 0.00 bBC | −0.003 ± 0.00 C | −0.003 ± 0.00 bBC | −0.003 ± 0.00 BC | −0.001 ± 0.00 AB | 0.000 ± 0.00 bA | ** |
| GI | −0.003 ± 0.00 bAB | −0.004 ± 0.00 B | −0.004 ± 0.00 bB | −0.003 ± 0.00 AB | −0.001 ± 0.00 A | −0.004 ± 0.00 cAB | * |
| GM | 0.001 ± 0.00 a | −0.003 ± 0.00 | 0.000 ± 0.00 a | 0.003 ± 0.00 | 0.000 ± 0.00 | 0.006 ± 0.00 a | ns |
| Sign | * | ns | ** | ns | ns | ** |
| TPC 1 | TCC 2 | DPPH 3 | ABTS 3 | β-carotene 3 | FRAP 4 | α-Amylase 3 | α-Glucosidase 3 | Lipase 3 |
|---|---|---|---|---|---|---|---|---|
| 15.03 ± 1.23 | 19.33 ± 0.77 | 32.15 ± 2.15 | 5.32 ± 0.21 | 19.61 ± 2.79 | 46.16 ± 3.82 | 62.21 ± 3.26 | 71.46 ± 3.82 | 115.27 ± 4.76 |
| T0 | T15 | T30 | T60 | T180 | T360 | Sign | |
|---|---|---|---|---|---|---|---|
| EVOO | 354.63 ± 19.36 aA | 261.63 ± 45.96 B | 234.22 ± 64.72 aB | 223.72 ± 38.15 B | 246.61 ± 25.72 B | 79.53 ± 1.41 bC | ** |
| GI | 351.20 ± 15.01 aA | 286.68 ± 23.61 B | 224.11 ± 23.01 bC | 222.70 ± 27.14 BC | 240.91 ± 9.26 C | 85.48 ± 1.06 abD | ** |
| GM | 317.81 ± 9.52 bA | 304.49 ± 29.23 AB | 278.49 ± 6.80 aA | 271.58 ± 2.98 B | 210.62 ± 24.05 C | 101.16 ± 3.24 aD | ** |
| Sign | * | ns | ** | ns | ns | ** |
| Compounds | Amount |
|---|---|
| 3,4-Dihydroxybenzoic acid | 192.41 ± 2.23 |
| Vanillic acid | 4.39 ± 0.23 |
| Caffeic acid | 14.13 ± 1.56 |
| p-Coumaric acid | 7.68 ± 1.20 |
| Ferulic acid | 8.40 ± 0.87 |
| Rutin | 106.65 ± 2.65 |
| Quercetin | 69.42 ± 1.08 |
| Apigenin | 272.70 ± 4.03 |
| Naringenin | 26.95 ± 0.95 |
| Kaempferol | 59.28 ± 0,66 |
| Isoramnetin | 96.16 ± 1.32 |
| 6-Gingerol | 2058.43 ± 4.65 |
| 6-Shogaol | 5.28 ± 1.11 |
| Apigenin 7-O-Glucoside | 263.22 ± 0.99 |
| Gallic acid | 40.12 ± 2.43 |
| Chlorogenic acid | 24.65 ± 2.09 |
| Syringic acid | 4.50 ± 0.16 |
| Luteolin 7-O-Glucoside | 45.06 ± 1.43 |
| Compounds | T0 | T15 | T30 | T60 | T180 | T360 | Sign |
|---|---|---|---|---|---|---|---|
| Hydroxytyrosol | 16.15 ± 1.54 cd | 15.28 ± 0.27 cd | 15.17 ± 0.50 d | 19.46 ± 0.01 bc | 27.08 ± 0.95 a | 25.01 ± 2.50 ab | ** |
| Tyrosol | 15.61 ± 2.03 bc | 15.11 ± 0.30 bc | 14.39 ± 0.93 bc | 18.19 ± 0.17 ab | 11.58 ± 1.51 c | 21.09 ± 0.93 a | ** |
| Vanillic acid | 1.47 ± 0.02 a | 0.39 ± 0.09 d | 1.24 ± 0.08 c | 1.38 ± 0.12 b | 0.00 e | 0.00 e | ** |
| Homovanillic acid | 1.92 ± 0.03 e | 2.03 ± 0.05 d | 3.57 ± 1.03 a | 2.44 ± 0.14 b | 2.35 ± 0.16 c | 1.94 ± 0.14 de | ** |
| Chlorogenic acid | 1.92 ± 0.19 a | 1.85 ± 0.06 b | 1.83 ± 0.21 ab | 1.71 ± 0.25 ab | 1.65 ± 0.10 c | 1.60 ± 0.17 ab | ** |
| Quercetin 3,4′-Diglucoside | 0.91 ± 0.07 b | 1.39 ± 0.16 a | 1.20 ± 0.16 ab | 1.05 ± 0.07 ab | 0.00 c | 0.00 c | ** |
| p-Coumaric acid | 3.45 ± 0.65 a | 3.34 ± 0.51 a | 2.89 ± 0.04 b | 1.14 ± 0.01 e | 1.44 ± 0.17 d | 1.65 ± 0.20 c | ** |
| Luteolin-7-O-Glucoside | 3.07 ± 0.91 cd | 2.41 ± 0.06 e | 7.42 ± 0.10 a | 3.39 ± 0.01 b | 3.05 ± 0.59 c | 2.99 ± 0.03 d | ** |
| Cinnamin acid | 0.91 ± 0.36 c | 0.98 ± 0.14 bc | 2.73 ± 1.07 a | 1.08 ± 0.26 b | 0.54 ± 0.09 d | 0.61 ± 0.02 d | ** |
| Oleuropein | 0.48 ± 0.08 b | 0.48 ± 0.05 b | 0.86 ± 0.37 a | 0.46 ± 0.02 b | 0.43 ± 0.06 b | 0.10 ± 0.01 c | ** |
| Pinoresinol | 43.38 ± 0.36 b | 42.11 ± 3.86 b | 55.75 ± 3.46 a | 44.58 ± 1.76 b | 41.67 ± 1.87 b | 44.07 ± 1.10 ab | ** |
| Quercetin | 12.94 ± 0.55 c | 13.00 ± 1.14 c | 17.17 ± 5.06 a | 12.26 ± 0.92 c | 14.73 ± 0.62 b | 12.93 ± 4.48 c | ** |
| Apigenin | 58.98 ± 11.81 a | 50.64 ± 3.58 cb | 55.35 ± 5.42 b | 53.41 ± 1.94 bc | 49.53 ± 0.55 b | 53.21 ± 0.98 d | ** |
| Isoramnetin 3-O-Glucoside | 0.12 ± 0.02 bc | 0.12 ± 0.03 c | 0.31 ± 0.15 a | 0.14 ± 0.02 b | 0.00 d | 0.00 d | ** |
| Apigenin 7-O-Glucoside | 1.80 ± 0.30 b | 1.78 ± 0.12 b | 4.20 ± 2.06 a | 1.29 ± 0.19 c | 0.77 ± 0.11 d | 0.66 ± 0.09 d | ** |
| Compounds | T0 | T15 | T30 | T60 | T180 | T360 | Sign |
|---|---|---|---|---|---|---|---|
| Hydroxytyrosol | 10.44 ± 0.44 b | 7.23 ± 0.11 c | 5.98 ± 0.20 c | 7.32 ± 1.50 c | 8.84 ± 0.54 c | 14.42 ± 0.80 a | ** |
| Tyrosol | 9.45 ± 0.11 c | 12.65 ± 0.20 b | 11.04 ± 0.63 b | 11.46 ± 0.66 b | 9.24 ± 0.24 c | 18.67 ± 1.48 a | ** |
| 4-Hydroxyphenyl acetate | 1.08 ± 0.08 a | 0.94 ± 0.01 b | 0.53 ± 0.09 c | 0.82 ± 0.06 b | 0.00 c | 0.00 c | ** |
| Caffeic acid | 2.56 ± 0.14 a | 2.12 ± 0.26 b | 1.76 ± 0.08 bc | 1.78 ± 0.07 bc | 0.90 ± 0.05 d | 1.17 ± 0.15 c | ** |
| Vanillic acid | 3.32 ± 0.15 a | 1.03 ± 0.05 b | 0.92 ± 0.24 b | 0.87 ± 0.04 b | 0.00 c | 0.00 c | ** |
| Homovanillic acid | 3.22 ± 0.06 a | 2.38 ± 0.03 b | 2.22 ± 0.22 b | 2.19 ± 0.09 b | 0.00 c | 0.00 c | ** |
| Vanillin | 2.77 ± 0.08 a | 2.46 ± 0.02 b | 2.20 ± 0.05 b | 2.31 ± 0.16 b | 0.39 ± 0.13 c | 1.07 ± 0.07 bc | * |
| Chlorogenic acid | 3.66 ± 0.21 a | 2.83 ± 0.12 b | 2.38 ± 0.09 b | 2.66 ± 0.14 b | 2.55 ± 0.27 b | 2.25 ± 0.23 b | ** |
| Quercetin 3,4′-Diglucoside | 4.45 ± 0.19 a | 3.51 ± 0.03 c | 3.25 ± 0.20 c | 3.85 ± 0.11 b | 0.00 d | 0.00 d | ** |
| p-Coumaric acid | 2.32 ± 0.01 a | 1.05 ± 0.05 bc | 0.93 ± 0.16 c | 1.02 ± 0.03 b | 0.00 d | 0.00 d | ** |
| Ferulic acid | 1.88 ± 0.04 a | 1.56 ± 0.13 b | 1.44 ± 0.06 b | 1.36 ± 0.18 b | 0.83 ± 0.03 c | 1.00 ± 0.07 bc | ** |
| Rutin | 5.23 ± 0.22 a | 4.99 ± 0.46 b | 4.00 ± 0.10 e | 4.45 ± 0.53 c | 4.25 ± 0.57 d | 3.83 ± 0.11 f | ** |
| Luteolin 7-O-Glucoside | 3.78 ± 0.10 c | 2.95 ± 0.09 d | 2.62 ± 0.04 d | 2.60 ± 0.13 d | 4.86 ± 0.64 b | 6.75 ± 0.50 a | ** |
| Oleuropein | 0.08 ± 0.00 b | 0.03 ± 0.00 c | 0.03 ± 0.00 c | 0.03 ± 0.00 c | 0.08 ± 0.00 b | 0.17 ± 0.00 a | ** |
| Cinnamic acid | 0.65 ± 0.05 a | 0.38 ± 0.04 b | 0.00 c | 0.00 c | 0.00 c | 0.00 c | ** |
| Pinoresinol | 51.34 ± 1.51 a | 47.66 ± 1.24 b | 46.38 ± 1.95 b | 46.51 ± 1.78 b | 52.29 ± 0.70 a | 47.69 ± 0.76 b | ** |
| Quercetin | 2.32 ± 0.14 b | 1.57 ± 0.02 b | 1.68 ± 0.08 b | 3.22 ± 0.11 a | 2.46 ± 0.07 a | 3.35 ± 0.11 a | ** |
| Apigenin | 55.56 ± 2.47 b | 51.21 ± 2.07 c | 42.96 ± 1.64 e | 35.19 ± 12.54 f | 58.43 ± 5.17 a | 44.63 ± 2.50 d | ** |
| Isoramnetin 3-O-Glucoside | 0.83 ± 0.09 a | 0.47 ± 0.05 d | 0.62 ± 0.14 bc | 0.68 ± 0.00 b | 0.59 ± 0.08 cd | 0.55 ± 0.03 d | ** |
| Apigenin 7-O-Glucoside | 1.34 ± 0.07 a | 1.01 ± 0.06 c | 1.21 ± 0.11 b | 0.95 ± 0.18 c | 0.85 ± 0.05 d | 0.13 ± 0.01 e | ** |
| Kaempferol | 5.08 ± 0.24 a | 4.09 ± 0.04 b | 3.57 ± 0.00 c | 3.49 ± 0.10 c | 2.86 ± 0.05 d | 2.81 ± 0.06 d | ** |
| Isoramnetin | 2.55 ± 0.11 c | 1.02 ± 0.02 d | 1.13 ± 0.09 d | 2.66 ± 0.14 c | 6.88 ± 0.81 b | 16.00 ± 3.24 a | ** |
| 6-Gingerol | 22.56 ± 0.13 a | 21.00 ± 0.27 b | 19.97 ± 0.76 b | 20.79 ± 0.83 b | 18.40 ± 0.18 c | 23.31 ± 0.26 a | ** |
| 6-Shogaol | 0.43 ± 0.06 a | 0.26 ± 0.15 c | 0.21 ± 0.09 c | 0.34 ± 0.00 ab | 0.12 ± 0.05 d | 0.27 ± 0.02 bc | ** |
| Compounds | T0 | T15 | T30 | T60 | T180 | T360 | Sign |
|---|---|---|---|---|---|---|---|
| Hydroxytyrosol | 26.90 ± 0.90 a | 7.21 ± 0.19 b | 6.33 ± 0.86 b | 8.37 ± 0.93 b | 6.59 ± 0.09 b | 9.57 ± 0.03 b | ** |
| Tyrosol | 20.66 ± 0.16 c | 41.07 ± 2.13 b | 39.13 ± 3.48 b | 41.45 ± 2.65 b | 17.56 ± 2.31 c | 61.19 ± 4.63 a | ** |
| 3,4-Dihydroxybenzoic acid | 0.28 ± 0.03 a | 0.00 b | 0.00 b | 0.00 b | 0.00 b | 0.00 b | ** |
| 4-Hydroxyphenyl acetate | 3.33 ± 0.10 a | 3.29 ± 0.31 a | 3.04 ± 0.23 a | 3.49 ± 0.47 a | 1.00 ± 0.10 c | 2.50 ± 0.28 b | ** |
| Caffeic acid | 2.21 ± 0.16 a | 0.00 b | 0.00 b | 0.00 b | 0.00 b | 0.00 b | ** |
| Vanillic acid | 6.34 ± 0.34 a | 2.56 ± 0.29 b | 1.32 ± 0.18 c | 0.57 ± 0.02 c | 0.66 ± 0.10 c | 0.71 ± 0.08 c | ** |
| Homovanillic acid | 3.33 ± 0.41 a | 2.06 ± 0.09 b | 2.36 ± 0.10 b | 3.37 ± 0.10 a | 0.00 c | 0.00 c | ** |
| Vanillin | 1.59 ± 0.12 bc | 3.18 ± 0.04 a | 1.96 ± 0.36 ab | 2.11 ± 0.10 ab | 0.00 d | 0.00 d | ** |
| Chlorogenic acid | 10.03 ± 0.90 a | 10.06 ± 0.74 a | 10.73 ± 0.74 a | 10.96 ± 0.30 a | 4.04 ± 0.00 b | 3.16 ± 0.32 b | ** |
| Quercetin 3,4′-Diglucoside | 1.93 ± 0.10 b | 1.36 ± 0.06 b | 4.21 ± 0.50 a | 4.61 ± 0.43 a | 1.00 ± 0.24 b | 0.95 ± 0.04 b | ** |
| p-Coumaric acid | 0.21 ± 0.03 c | 0.39 ± 0.02 b | 0.46 ± 0.00 a | 0.38 ± 0.08 b | 0.00 d | 0.00 d | ** |
| Ferulic Acid | 0.63 ± 0.04 b | 0.39 ± 0.03 d | 0.63 ± 0.00 b | 0.71 ± 0.10 a | 0.52 ± 0.08 c | 0.00 d | ** |
| Rutin | 1.12 ± 0.12 bc | 1.57 ± 0.09 b | 2.18 ± 0.21 a | 2.06 ± 0.30 a | 0.84 ± 0.08 c | 0.00 d | ** |
| o-Coumaric acid | 0.46 ± 0.06 c | 0.73 ± 0.04 abc | 0.59 ± 0.07 bc | 0.62 ± 0.04 c | 0.08 ± 0.01 a | 0.80 ± 0.04 ab | ** |
| Luteolin 7-O-Glucoside | 0.21 ± 0.01 e | 5.17 ± 0.38 b | 6.04 ± 0.00 a | 2.83 ± 0.03 d | 3.09 ± 0.04 c | 0.00 f | ** |
| Oleuropein | 0.13 ± 0.02 a | 0.09 ± 0.00 b | 0.06 ± 0.00 c | 0.06 ± 0.00 c | 0.04 ± 0.00 c | 0.00 d | ** |
| Cinnamic acid | 1.07 ± 0.07 a | 0.74 ± 0.04 b | 0.01 ± 00 c | 0.00 d | 0.00 d | 0.00 d | ** |
| Pinoresinol | 9.22 ± 0.31 c | 28.54 ± 2.86 b | 25.40 ± 1.59 b | 26.34 ± 1.17 b | 24.41 ± 0.54 b | 71.34 ± 5.35 a | ** |
| Luteolin | 2.18 ± 0.25 a | 0.16 ± 0.05 b | 0.11 ± 0.03 b | 0.00 c | 0.00 c | 0.00 c | ** |
| Quercetin | 1.59 ± 0.13 b | 2.70 ± 0.21 b | 0.73 ± 0.01 c | 0.74 ± 0.00 c | 2.38 ± 0.41 b | 7.41 ± 0.30 a | ** |
| Apigenin | 35.06 ± 0.77 a | 4.69 ± 0.44 c | 5.10 ± 2.03 c | 6.60 ± 0.67 c | 8.77 ± 0.91 c | 17.12 ± 1.97 b | ** |
| Isoramnetin 3-O-Glucoside | 0.86 ± 0.05 d | 2.53 ± 0.18 c | 2.12 ± 0.51 c | 2.66 ± 0.32 c | 4.10 ± 1.27 a | 3.03 ± 0.73 b | ** |
| Naringenin | 3.19 ± 0.13 a | 1.07 ± 0.05 c | 0.54 ± 0.19 c | 1.77 ± 0.74 b | 0.00 d | 0.00 d | ** |
| Kaempferol | 3.09 ± 0.08 d | 9.26 ± 0.23 c | 11.06 ± 0.51 b | 11.89 ± 0.40 b | 9.00 ± 2.20 c | 22.51 ± 0.24 a | ** |
| Isoramnetin | 3.04 ± 0.06 c | 6.45 ± 0.22 b | 9.49 ± 0.10 a | 9.97 ± 0.44 a | 9.07 ± 0.57 a | 0.22 ± 0.03 d | ** |
| 6-Gingerol | 5.07 ± 6.03 d | 52.93 ± 3.51 bc | 55.35 ± 3.89 b | 59.14 ± 3.72 b | 48.41 ± 0.75 c | 128.98 ± 1.78 a | ** |
| 6-Shogaol | 1.57 ± 0.32 c | 2.27 ± 0.24 b | 2.87 ± 0.21 a | 3.13 ± 0.44 a | 1.76 ± 0.03 c | 1.74 ± 0.04 c | ** |
| Apigenin 7-O-Glucoside | 0.80 ± 0.14 b | 0.95 ± 0.07 a | 0.07 ± 0.04 d | 0.13 ± 0.03 c | 0.00 e | 0.00 e | ** |
| T0 | T15 | T30 | T60 | T180 | T360 | Sign | |
|---|---|---|---|---|---|---|---|
| DPPH | |||||||
| EVOO | 12.33 ± 3.45 bC | 14.09 ± 3.21 bC | 15.72 ± 2.87 bBC | 20.77 ± 2.82 bBC | 19.61 ± 3.09 bB | 29.54 ± 3.77 bA | ** |
| GI | 17.42 ± 2.27 bB | 19.33 ± 2.81 abB | 21.05 ± 2.76 bB | 37.23 ± 2.08 aA | 39.67 ± 2.20 aA | 44.21 ± 2.36 aA | ** |
| GM | 17.42 ± 2.27 aC | 18.8 ±2.32 aC | 19.48 ± 2.76 abC | 22.22 ± 2.08 bCB | 27.63 ± 2.89 bB | 35.35 ± 2.94 bA | ** |
| Sign | ** | * | * | ** | ** | ** | |
| ABTS | |||||||
| EVOO | 3.43 ± 0.25 bB | 4.98 ± 0.77 aB | 5.16 ± 0.93 bB | 7.39 ± 0.91 B | 11.43 ± 0.86 bB | 15.21 ± 1.19 bA | ** |
| GI | 4.75 ± 0.24 bC | 4.89 ± 0.45 aC | 5.86 ± 0.27 abC | 7.51 ± 0.14 B | 8.32 ± 0.67 bB | 11.31 ± 1.09 bA | ** |
| GM | 4.75 ± 0.24 aC | 5.61 ± 0.28 aC | 6.07 ± 0.34 aC | 7.62 ± 0.57 C | 18.28 ± 1.13 aB | 26.31 ± 1.47 aA | ** |
| Sign | * | * | * | ns | ** | ** | |
| T0 | T15 | T30 | T60 | T180 | T360 | Sign | |
|---|---|---|---|---|---|---|---|
| EVOO | 48.72 ± 3.45 aD | 52.21 ± 3.89 aD | 59.8 3 ± 4.40 aC | 77.05 ± 4.42 aB | >100 aA | >100 aA | ** |
| GI | 18.68 ± 2.59 aD | 19.98 ± 2.71 bD | 23.41 ± 2.19 bCD | 27.72 ± 2.08 bBC | 32.09 ± 2.11 cB | 46.10 ± 2.80 cA | ** |
| GM | 18.68 ± 2.59 bE | 20.09 ± 2.69 bE | 27.92 ± 2.75 bD | 33.12 ± 3.08 bC | 50.96 ± 3.88 bB | 77.67 ± 4.09 bA | ** |
| Sign | ** | ** | ** | ** | ** | ** |
| T0 | T15 | T30 | T60 | T180 | T360 | Sign | |
|---|---|---|---|---|---|---|---|
| α-amylase | |||||||
| EVOO | 269.02 ± 3.77 aE | 275.21 ± 3.85 aD | 303.38 ± 3.92 aB | 345.31 ± 4.05 aA | 240.29 ± 3.87 bF | 289.32 ± 4.90 cC | ** |
| GI | 126.95 ± 3.56 aD | 131.23 ± 3.87 bD | 170.47 ± 3.44 bC | 256.93 ± 3.35 bB | 263.22 ± 3.77 aB | 305.11 ± 4.09 bA | ** |
| GM | 126.93 ± 3.56 bE | 131.09 ± 3.68 bE | 155.89 ± 3.44 cD | 175.06 ± 3.35 cC | 220.17 ± 2.22 cB | 328.10 ± 3.55 aA | ** |
| Sign | ** | ** | ** | ** | ** | ** | |
| α-glucosidase | |||||||
| EVOO | 137.34 ± 3.73 bF | 145.18 ± 3.79 bE | 198.81 ± 3.82 D | 337.56 ± 3.90 aC | 587.49 ± 3.56 aB | 778.23 ± 4.67 aA | ** |
| GI | 181.67 ± 3.45 bD | 184.12 ± 3.87 aDC | 193.46 ± 3.09 C | 208.11 ± 3.01 bB | 219.36 ± 3.20 cB | 269.71 ± 3.85 cA | ** |
| GM | 181.67 ± 3.45 aE | 185.90 ± 3.67 aDE | 196.74 ± 3.89 D | 210.71 ± 4.01 bC | 235.54 ± 4.89 bB | 407.89 ± 5.08 bA | ** |
| Sign | ** | ** | ns | ** | ** | ** | |
| T0 | T15 | T30 | T60 | T180 | T360 | Sign | |
|---|---|---|---|---|---|---|---|
| EVOO | 143.46 ± 4.85 aF | 155.52 ± 4.87 aE | 173.43 ± 4.91 aD | 206.54 ± 5.01 aC | 253.81 ± 4.81 aB | 312.97 ± 5.44 aA | ** |
| GI | 63.45 ± 4.09 aD | 65.07 ± 4.26 bD | 107.93 ± 4.22 bC | 167.82 ± 4.02 cB | 169.56 ± 4.14 bB | 195.96 ± 4.77 bA | ** |
| GM | 63.45 ± 1.09 bE | 65.48 ± 1.15 bE | 79.36 ± 1.22 cD | 91.94 ± 1.02 bC | 110.95 ± 2.46 cB | 309.21 ± 2.87 aA | ** |
| Sign | ** | ** | ** | ** | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Custureri, I.M.G.; Sicari, V.; Loizzo, M.R.; Tundis, R.; Soria, A.C.; Giuffrè, A.M. Evaluation of Quality Parameters and Functional Activity of Ottobratica Extra Virgin Olive Oil Enriched with Zingiber officinale (Ginger) by Two Different Enrichment Processes during One-Year Storage. Foods 2023, 12, 3822. https://doi.org/10.3390/foods12203822
Custureri IMG, Sicari V, Loizzo MR, Tundis R, Soria AC, Giuffrè AM. Evaluation of Quality Parameters and Functional Activity of Ottobratica Extra Virgin Olive Oil Enriched with Zingiber officinale (Ginger) by Two Different Enrichment Processes during One-Year Storage. Foods. 2023; 12(20):3822. https://doi.org/10.3390/foods12203822
Chicago/Turabian StyleCustureri, Irene Maria Grazia, Vincenzo Sicari, Monica Rosa Loizzo, Rosa Tundis, Ana Cristina Soria, and Angelo Maria Giuffrè. 2023. "Evaluation of Quality Parameters and Functional Activity of Ottobratica Extra Virgin Olive Oil Enriched with Zingiber officinale (Ginger) by Two Different Enrichment Processes during One-Year Storage" Foods 12, no. 20: 3822. https://doi.org/10.3390/foods12203822
APA StyleCustureri, I. M. G., Sicari, V., Loizzo, M. R., Tundis, R., Soria, A. C., & Giuffrè, A. M. (2023). Evaluation of Quality Parameters and Functional Activity of Ottobratica Extra Virgin Olive Oil Enriched with Zingiber officinale (Ginger) by Two Different Enrichment Processes during One-Year Storage. Foods, 12(20), 3822. https://doi.org/10.3390/foods12203822










