Extraction, Enzymatic Modification, and Anti-Cancer Potential of an Alternative Plant-Based Protein from Wolffia globosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical
2.2. Raw Material Preparation
2.3. Alkaline Extraction (ALK)
2.4. Ultrasonic-Assisted Extraction (UAE)
2.5. Amino Acid Profiles
2.6. Protein Pattern via Gel Electrophoresis
2.7. Monitoring of Protein Secondary Structure Changes
2.8. Protein Hydrolysate (PH) Preparation
2.9. Functional Property Determinations
2.9.1. Solubility
2.9.2. Emulsifying Properties
2.9.3. Foaming Properties
2.9.4. Oil-Binding Capacity
2.10. Cell Viability Assay
2.11. Statical Analysis
3. Results and Discussion
3.1. Model Fitting and Statistical Analysis
3.2. Influence of Independent Factors on the Protein Extraction Yield
3.3. Optimization and Validation
3.4. Amino Acid Profiles
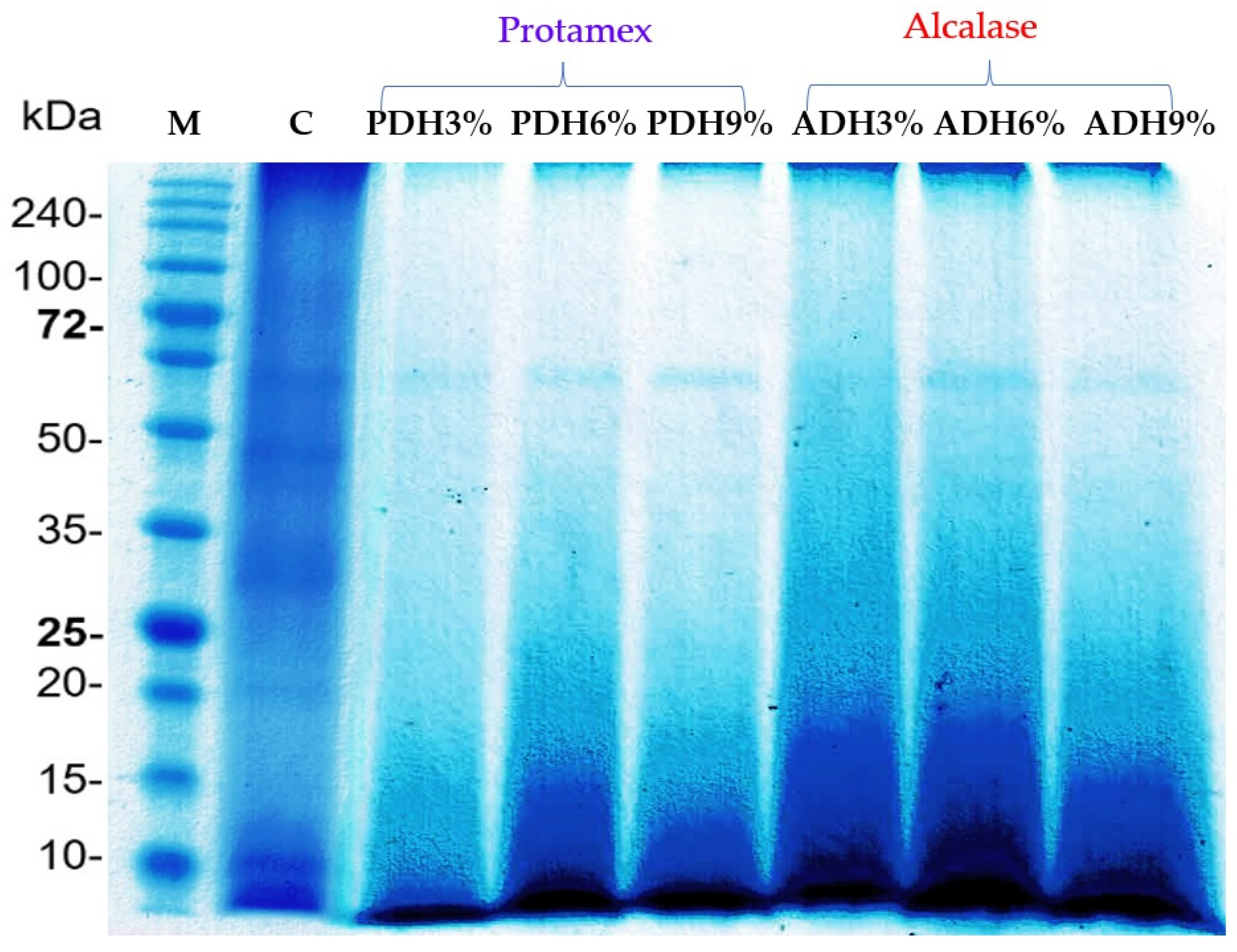
3.5. Protein Hydrolysis and Protein Patterns
3.6. Changes of Secondary Structures of Proteins
3.7. Functional Properties
3.7.1. Solubility
3.7.2. Foaming Properties
3.7.3. Emulsifying Properties
3.7.4. Oil-Binding Capacity
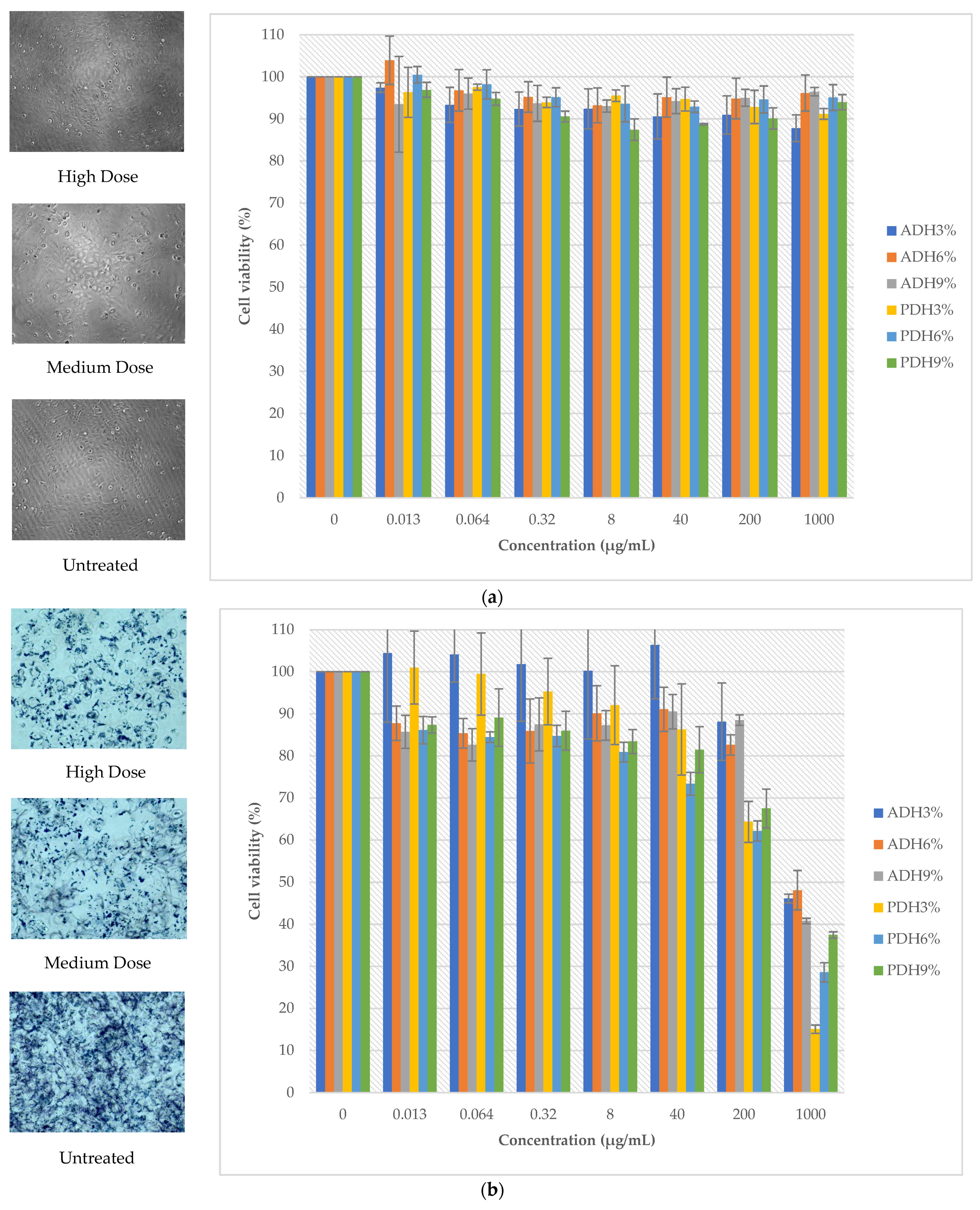
3.8. The Effect of Protein Hydrolysates on Cell Viability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pasiakos, S.; Agarwal, S.; Lieberman, H.; Fulgoni, V. Sources and Amounts of Animal, Dairy, and Plant Protein Intake of US Adults in 2007–2010. Nutrients 2015, 7, 7058–7069. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans 2020–2025, 9th ed.; US Government Publishing Office: Washington, DC, USA, 2020.
- World Resources Institute. 2019. Available online: https://www.wri.org/resources/data-/protein-scorecard (accessed on 13 October 2023).
- Parrini, S.; Aquilani, C.; Pugliese, C.; Bozzi, R.; Sirtori, F. Soybean Replacement by Alternative Protein Sources in Pig Nutrition and Its Effect on Meat Quality. Animals 2023, 13, 494. [Google Scholar] [CrossRef] [PubMed]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Mäkinen, S.; Eurola, M.; Jalava, T.; Pihlava, J.M.; Hellström, J.; Pihlanto, A. Nutritional value of commercial protein-rich plant products. Plant Foods Hum. Nutr. 2018, 73, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.N.; Galante, M.; Robson, M.; Boeris, V.; Spelzini, D. Amaranth, quinoa and chia protein isolates: Physicochemical and structural properties. Int. J. Biol. Macromol. 2018, 109, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Giacomino, S.; Peñas, E.; Ferreyra, V.; Pellegrino, N.; Fournier, M.; Apro, N.; Frías, J. Extruded flaxseed meal enhances the nutritional quality of cereal-based products. Plant Foods Hum. Nutr. 2013, 68, 131–136. [Google Scholar] [CrossRef]
- De Oliveira Sousa, A.G.; Fernandes, D.C.; Alves, A.M.; De Freitas, J.B.; Naves, M.M.V. Nutritional quality and protein value of exotic almonds and nut from the Brazilian Savanna compared to peanut. Food Res. Int. 2011, 44, 2319–2325. [Google Scholar] [CrossRef]
- Wanasundara, J.P.D.; Tan, S.; Alashi, A.M.; Pudel, F.; Blanchard, C. Proteins from canola/rapeseed: Current status. In Sustainable Protein Sources; Academic Press: Cambridge, MA, USA, 2017; pp. 285–304. [Google Scholar]
- Ruekaewma, N.; Piyatiratitivorakul, S.; Powtongsook, S. Culture system for Wolffia globosa L. (Lemnaceae) for hygiene human food. Songklanakarin J. Sci. Technol. 2015, 37, 575–580. [Google Scholar]
- Stomp, A.M. The duckweeds: A valuable plant for biomanufacturing. Biotechnol. Annu. Rev. 2005, 11, 69–99. [Google Scholar]
- Xu, J.; Shen, Y.; Zheng, Y.; Smith, G.; Sun, X.S.; Wang, D.; Zhao, Y.; Zhang, W.; Li, Y. Duckweed (Lemnaceae) for potentially nutritious human food: A review. Food Rev. Int. 2021, 39, 3620–3634. [Google Scholar] [CrossRef]
- Hromadkova, Z.; Ebringerová, A. Ultrasonic extraction of plant materials–investigationof hemicelluloserelease from buckwheat hulls. Ultrason. Sonochemistry 2003, 10, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Stanisavljević, I.T.; Lazić, M.L.; Veljković, V.B. Ultrasonic extraction of oil from tobacco (Nicotiana tabacum L.) seeds. Ultrason. Sonochemistry 2007, 14, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Olawuyi, I.F.; Kim, S.R.; Hahn, D.; Lee, W.Y. Influences of combined enzyme-ultrasonic extraction on the physicochemical characteristics and properties of okra polysaccharides. Food Hydrocoll. 2020, 100, 105396. [Google Scholar] [CrossRef]
- Lee, L.S.; Lee, N.; Kim, Y.H.; Lee, C.H.; Hong, S.P.; Jeon, Y.W.; Kim, Y.E. Optimization of ultrasonic extraction of phenolic antioxidants from green tea using response surface methodology. Molecules 2013, 18, 13530–13545. [Google Scholar] [CrossRef] [PubMed]
- Jamdar, S.N.; Rajalakshmi, V.; Pednekar, M.D.; Juan, F.; Yardi, V.; Sharma, V. Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem. 2010, 121, 178–184. [Google Scholar] [CrossRef]
- Arteaga, V.G.; Guardia, M.A.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innov. Food Sci. Emerg. Technol. 2020, 65, 102449. [Google Scholar] [CrossRef]
- Rao, P.S.; Bajaj, R.; Mann, B. Impact of sequential enzymatic hydrolysis on antioxidant activity and peptide profile of casein hydrolysate. J. Food Sci. Technol. 2020, 57, 4562–4575. [Google Scholar] [CrossRef] [PubMed]
- Pinuel, M.L.; Vilcacundo, E.; Boeri, P.A.; Barrio, D.A.; Morales, D.; Pinto, A.; Carrillo, W. Extraction of protein concentrate from red bean (Phaseolus vulgaris L.): Antioxidant activity and inhibition of lipid peroxidation. J. Appl. Pharm. Sci. 2019, 9, 45–58. [Google Scholar]
- Dang, Y.; Zhou, T.; Hao, L.; Cao, J.; Sun, Y.; Pan, D. In vitro and in vivo studies on the angiotensin-converting enzyme inhibitory activity peptides isolated from broccoli protein hydrolysate. J. Agric. Food Chem. 2019, 67, 6757–6764. [Google Scholar] [CrossRef]
- Hasani, K.; Ariaii, P.; Ahmadi, M. Antimicrobial, antioxidant and anti-cancer properties of protein hydrolysates from Indian mackerel (Rastrelliger kanagurta) waste prepared using commercial enzyme. Int. J. Pept. Res. Ther. 2022, 28, 86. [Google Scholar] [CrossRef]
- Kumar, D.; Chatli, M.K.; Singh, R.; Mehta, N.; Kumar, P. Antioxidant and antimicrobial activity of camel milk casein hydrolysates and its fractions. Small Rumin. Res. 2016, 139, 20–25. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Method of Analysis of the AOAC; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 2000. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, Y.; Jia, F.; Jin, H. Characterization of casein hydrolysates derived from enzymatic hydrolysis. Chem. Cent. J. 2013, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Phongthai, S.; Lim, S.T.; Rawdkuen, S. Optimization of microwave-assisted extraction of rice bran protein and its hydrolysates properties. J. Cereal Sci. 2016, 70, 146–154. [Google Scholar] [CrossRef]
- Xia, Y.; Bamdad, F.; Gänzle, M.; Chen, L. Fractionation and characterization of antioxidant peptides derived from barley glutelin by enzymatic hydrolysis. Food Chem. 2012, 134, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wen, H.; Li, C.; Gu, Z. Differences in functional properties and biochemical characteristics of congenetic rice proteins. J. Cereal Sci. 2009, 50, 184–189. [Google Scholar] [CrossRef]
- Paramee, S.; Sookkhee, S.; Sakonwasun, C.; Na Takuathung, M.; Mungkornasawakul, P.; Nimlamool, W.; Potikanond, S. Anti-cancer effects of Kaempferia parviflora on ovarian cancer SKOV3 cells. BMC Complement. Altern. Med. 2018, 18, 178. [Google Scholar] [CrossRef]
- Tang, D.S.; Tian, Y.J.; He, Y.Z.; Li, L.; Hu, S.Q.; Li, B. Optimization of ultrasonic-assisted protein extraction from brewer’s spent grain. Czech J. Food Sci. 2010, 28, 9–17. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, L.; Xiang, Y. Optimization of ultrasonic extraction condition for excess sludge protein using response surface methodology. Environ. Eng. Manag. J. EEMJ 2015, 14, 1151–1159. [Google Scholar] [CrossRef]
- Vedaraman, N.; Sandhya, K.V.; Charukesh, N.R.B.; Venkatakrishnan, B.; Haribabu, K.; Sridharan, M.R.; Nagarajan, R. Ultrasonic extraction of natural dye from Rubia Cordifolia, optimisation using response surface methodology (RSM) & comparison with artificial neural network (ANN) model and its dyeing properties on different substrates. Chem. Eng. Process. Process Intensif. 2017, 114, 46–54. [Google Scholar]
- Wouters, A.G.B.; Rombouts, I.; Fierens, E.; Brijs, K.; Delcour, J.A. Relevance of the Functional Properties of Enzymatic Plant Protein Hydrolysates in Food Systems. Compr. Rev. Food Sci. Food Saf. 2016, 15, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, B.R.; Valles, C.; Gilman, R.; Hardmeier, S.R.M.; Clark, D.; Garcia, H.H.; Gonzales, A.E.; Kohlstad, I.; Castro, M.; Valdivia, R.; et al. Amino Acid and Fatty Acid Profiles of the Inca Peanut (Plukenetia volubilis). Cereal Chem. 1992, 69, 461–463. [Google Scholar]
- Liang, X.; Yang, H.; Sun, J.; Cheng, J.; Luo, X.; Wang, Z.; Zheng, Y. Effects of enzymatic treatments on the hydrolysis and antigenicity reduction of natural cow milk. Food Sci. Nutr. 2021, 9, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Duangjarus, N.; Chaiworapuek, W.; Rachtanapun, C.; Ritthiruangdej, P.; Charoensiddhi, S. Antimicrobial and functional properties of duckweed (Wolffia globosa) protein and peptide extracts prepared by ultrasound-assisted extraction. Foods 2022, 11, 2348. [Google Scholar] [CrossRef] [PubMed]
- Fathollahy, I.; Farmani, J.; Kasaai, M.R.; Hamishehkar, H. Characteristics and functional properties of Persian lime (Citrus latifolia) seed protein isolate and enzymatic hydrolysates. Lebensm. Wiss. Technol. 2021, 140, 110765. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Li, Y.; Yang, Y.; Ju, X.; He, R. The preparation and physiochemical characterization of rapeseed protein hydrolysate-chitosan composite films. Food Chem. 2019, 272, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F.; Li, Y.Q.; Sun, G.J.; Wang, C.Y.; Liang, Y.; Zhao, X.Z.; Mo, H.Z. Influence of ultrasound treatment on the physicochemical and antioxidant properties of mung bean protein hydrolysate. Ultrason. Sonochemistry 2022, 84, 105964. [Google Scholar] [CrossRef] [PubMed]
- Akbari, N.; Milani Mohammadzadeh, J.; Biparva, P. Functional and conformational properties of proteolytic enzyme modified potato protein isolate. J. Sci. Food Agric. 2020, 100, 1320–1327. [Google Scholar] [CrossRef]
- Cui, Q.; Sun, Y.; Zhou, Z.; Cheng, J.; Guo, M. Effects of Enzymatic Hydrolysis on Physicochemical Properties and Solubility and Bitterness of Milk Protein Hydrolysates. Foods 2021, 10, 2462. [Google Scholar] [CrossRef]
- Chabanon, G.; Chevalot, I.; Framboisier, X.; Chenu, S.; Marc, I. Hydrolysis of rapeseed protein isolates: Kinetics, characterization and functional properties of hydrolysates. Process Biochem. 2007, 42, 1419–1428. [Google Scholar] [CrossRef]
- Barac, M.; Cabrilo, S.; Stanojevic, S.; Pesic, M.; Pavlicevic, M.; Zlatkovic, B.; Jankovic, M. Functional properties of protein hydrolysates from pea (Pisum sativum L.) seeds. Int. J. Food Sci. Technol. 2012, 47, 1457–1467. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, B.; Xiong, Y.L.; Xia, X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010, 118, 403–410. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, H.; Qian, H. Enzymatic preparation and functional properties of wheat gluten hydrolysates. Food Chem. 2007, 101, 615–620. [Google Scholar] [CrossRef]
- Bandyopadhyay, K.; Ghosh, S. Preparation and Characterization of Papain-Modified Sesame (Sesamum indicum L.) Protein Isolates. J. Agric. Food Chem. 2002, 50, 6854–6857. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Murphy, P.A.; Johnson, L.A. Physicochemical and Functional Properties of Soy Protein Substrates Modified by Low Levels of Protease Hydrolysis. J. Food Sci. 2005, 70, C180–C187. [Google Scholar] [CrossRef]
- Phongthai, S.; Singsaeng, N.; Nhoo-Ied, R.; Suwannatrai, T.; Schönlechner, R.; Unban, K.; Rawdkuen, S. Properties of peanut (KAC431) protein hydrolysates and their impact on the quality of gluten-free rice bread. Foods 2020, 9, 942. [Google Scholar] [CrossRef] [PubMed]
- Dinakarkumar, Y.; Krishnamoorthy, S.; Margavelu, G.; Ramakrishnan, G.; Chandran, M. Production and characterization of fish protein hydrolysate: Effective utilization of trawl by-catch. Food Chem. Adv. 2022, 1, 100138. [Google Scholar] [CrossRef]
- Guan, X.; Yao, H.; Chen, Z.; Shan, L.; Zhang, M. Some functional properties of oat bran protein concentrate modified by trypsin. Food Chem. 2007, 101, 163–170. [Google Scholar] [CrossRef]
- Elmalimadi, M.B.; Stefanovic, A.B.; Sekuljica, N.Z.; Zuza, M.G.; Lukovic, N.D.; Jovanovic, J.R.; Kneževic-Jugovic, Z.D. The synergistic effect of heat treatment on alcalase-assisted hydrolysis of wheat gluten proteins: Functional and antioxidant properties. J. Food Process. Preserv. 2017, 41, e13207. [Google Scholar] [CrossRef]
- Gashti, A.B.; Prakash, H. Characterization of antioxidant and antiproliferative activities of indian salmon (eleutheronema tetradactylum) protein hydrolysates. Int. J. Pharm. Pharm. Sci 2016, 8, 102–108. [Google Scholar]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef]
- Khongdetch, J.; Laohakunjit, N.; Kaprasob, R. King Boletus mushroom-derived bioactive protein hydrolysate: Characterisation, antioxidant, ACE inhibitory and cytotoxic activities. Int. J. Food Sci. Technol. 2022, 57, 1399–1410. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]

| Run | Factors | Response Value | Predict Value | ||
|---|---|---|---|---|---|
| Liquid–Solid Ratio (mL/g) | Time (min) | Amplitude (%) | |||
| 1 | 30 | 10 | 70 | 16.12 | 16.08 |
| 2 | 50 | 10 | 70 | 5.25 | 5.33 |
| 3 | 30 | 30 | 70 | 16.51 | 16.43 |
| 4 | 50 | 30 | 70 | 6.09 | 6.12 |
| 5 | 30 | 20 | 60 | 16.14 | 16.14 |
| 6 | 50 | 20 | 60 | 5.59 | 5.47 |
| 7 | 30 | 20 | 80 | 16.43 | 16.55 |
| 8 | 50 | 20 | 80 | 6.15 | 6.16 |
| 9 | 40 | 10 | 60 | 9.08 | 9.11 |
| 10 | 40 | 30 | 60 | 9.40 | 9.49 |
| 11 | 40 | 10 | 80 | 9.56 | 9.47 |
| 12 | 40 | 30 | 80 | 10.26 | 10.22 |
| 13 | 40 | 20 | 70 | 9.56 | 9.37 |
| 14 | 40 | 20 | 70 | 9.08 | 9.37 |
| 15 | 40 | 20 | 70 | 9.14 | 9.37 |
| 16 | 40 | 20 | 70 | 9.51 | 9.37 |
| 17 | 40 | 20 | 70 | 9.56 | 9.37 |
| Optimal | 30 | 25 | 78 | 14.13 | 15.70 |
| Alkaline extraction (pH 10, stirring for 30 min) | 6.52 | - | |||
| Source | Sum of Square | df | Mean of Square | F-Values | Prob > F |
|---|---|---|---|---|---|
| Model | 233.69 | 9 | 25.97 | 620.54 | 0.0001 |
| X1 | 221.75 | 1 | 221.75 | 5299.50 | 0.0001 |
| X2 | 0.63 | 1 | 0.63 | 15.17 | 0.0059 |
| X3 | 0.60 | 1 | 0.60 | 14.42 | 0.0067 |
| X12 | 10.28 | 1 | 10.28 | 245.75 | 0.0001 |
| X22 | 0.01 | 1 | 0.01 | 0.33 | 0.5862 |
| X32 | 0.09 | 1 | 0.09 | 2.15 | 0.1856 |
| X1X2 | 0.05 | 1 | 0.05 | 1.18 | 0.3142 |
| X1X3 | 0.02 | 1 | 0.02 | 0.42 | 0.5395 |
| X2X3 | 0.04 | 1 | 0.04 | 0.84 | 0.3896 |
| Lack of Fit | 0.7748 | ||||
| R2 | 0.9987 | ||||
| Adj R2 | 0.9971 |
| Essential Amino Acids | Wolffia globosa Protein Concentrate | Soybean Protein Concentrate | FAO/WHO |
|---|---|---|---|
| Histidine | 772.12 ± 13.02 | 1546 | 1600 |
| Isoleucine | 1357.53 ± 2.75 | 2402 | 1300 |
| Leucine | 3284.99 ± 5.11 | 3896 | 1900 |
| Lysine | 1584.08 ± 4.38 | 3306 | 1600 |
| Methionine | 464.01 ± 1.34 | 608 | 1700 |
| Phenylalanine | 1952.33 ± 6.93 | 2620 | 1900 |
| Threonine | 1205.05 ± 0.54 | 1910 | 900 |
| Valine | 2459.10 ± 9.82 | 2728 | 1300 |
| Total | 13,079.21 | 19,016 | 12,200 |
| Properties of Protein | Protein Concentrate | Alcalase | Protamex | ||||
|---|---|---|---|---|---|---|---|
| DH 3% | DH 6% | DH 9% | DH 3% | DH 6% | DH 9% | ||
| Functional Properties | |||||||
| Solubility | |||||||
| pH 3 | 1.60 ± 0.90 Dd | 34.47 ± 4.57 Cd | 56.30 ± 3.14 Ac | 56.97 ± 3.15 Ab | 50.72 ± 1.42 Ab | 44.07 ± 0.45 Bd | 62.81 ± 2.97 Ab |
| pH 5 | 25.76 ± 5.09 Cc | 57.76 ± 4.19 Bc | 67.92 ± 1.13 Ab | 70.08 ± 4.46 Ab | 61.61 ± 2.42 ABb | 63.01 ± 3.52 ABc | 64.81 ± 1.93 Ab |
| pH 7 | 57.07 ± 6.01 Bb | 63.01 ± 4.86 Bbc | 76.06 ± 2.13 Aab | 80.48 ± 6.24 Aa | 63.22 ± 4.86 Bb | 63.46 ± 1.56 Bc | 65.61 ± 3.62 Bb |
| pH 9 | 69.02 ± 8.22 Ba | 70.39 ± 5.26 ABab | 76.23 ± 4.10 ABab | 83.36 ± 7.72 Aa | 71.08 ± 2.98 ABa | 74.89 ± 2.23 ABb | 76.60 ± 2.12 ABa |
| pH 11 | 79.70 ± 5.29 ABa | 71.54 ± 2.71 Ba | 83.39 ± 3.11 Aa | 85.12 ± 4.14 Aa | 76.43 ± 3.86 ABa | 83.76 ± 4.13 Aa | 80.60 ± 4.26 ABa |
| Foaming activity (%) | 42.21 ± 1.12 cd | 55.00 ± 0.00 a | 46.69 ± 5.77 bc | 46.67 ± 5.77 bc | 50.00 ± 0.00 ab | 35.00 ± 5.00 e | 36.67 ± 2.89 de |
| Foam stability (%) | 29.68 ± 0.56 ab | 33.33 ± 1.86 a | 28.33 ± 2.89 b | 27.14 ± 4.95 bc | 26.67 ± 0.00 bc | 23.43 ± 1.42 cd | 21.96 ± 0.46 d |
| Emulsifying activity (%) | 96.00 ± 3.40 b | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| Emulsifying stability (%) | 80.57 ± 0.49 a | 63.89 ± 1.20 ab | 62.5 ± 0.00 cd | 62.5 ± 0.00 cd | 65.28 ± 1.20 b | 62.50 ± 0.00 cd | 61.81 ± 1.20 d |
| Oil-binding capacity (g/g) | 4.65 ± 0.05 a | 2.23 ± 0.01 c | 2.21 ± 0.01 c | 1.83 ± 0.01 d | 3.51 ± 0.07 b | 2.23 ± 0.07 c | 1.86 ± 0.06 d |
| Secondary Structure Portion (%) | |||||||
| α-helix | 26.55 | 29.71 | 40.26 | 37.64 | 36.70 | 34.05 | 29.56 |
| β-sheet | 18.45 | 13.93 | 13.64 | 13.63 | 12.79 | 13.25 | 14.03 |
| β-turn | 7.94 | 10.24 | 10.07 | 11.09 | 11.52 | 10.67 | 9.45 |
| Random coils | 47.06 | 46.13 | 36.03 | 37.64 | 38.98 | 42.02 | 46.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siriwat, W.; Ungwiwatkul, S.; Unban, K.; Laokuldilok, T.; Klunklin, W.; Tangjaidee, P.; Potikanond, S.; Kaur, L.; Phongthai, S. Extraction, Enzymatic Modification, and Anti-Cancer Potential of an Alternative Plant-Based Protein from Wolffia globosa. Foods 2023, 12, 3815. https://doi.org/10.3390/foods12203815
Siriwat W, Ungwiwatkul S, Unban K, Laokuldilok T, Klunklin W, Tangjaidee P, Potikanond S, Kaur L, Phongthai S. Extraction, Enzymatic Modification, and Anti-Cancer Potential of an Alternative Plant-Based Protein from Wolffia globosa. Foods. 2023; 12(20):3815. https://doi.org/10.3390/foods12203815
Chicago/Turabian StyleSiriwat, Warin, Sunisa Ungwiwatkul, Kridsada Unban, Thunnop Laokuldilok, Warinporn Klunklin, Pipat Tangjaidee, Saranyapin Potikanond, Lovedeep Kaur, and Suphat Phongthai. 2023. "Extraction, Enzymatic Modification, and Anti-Cancer Potential of an Alternative Plant-Based Protein from Wolffia globosa" Foods 12, no. 20: 3815. https://doi.org/10.3390/foods12203815
APA StyleSiriwat, W., Ungwiwatkul, S., Unban, K., Laokuldilok, T., Klunklin, W., Tangjaidee, P., Potikanond, S., Kaur, L., & Phongthai, S. (2023). Extraction, Enzymatic Modification, and Anti-Cancer Potential of an Alternative Plant-Based Protein from Wolffia globosa. Foods, 12(20), 3815. https://doi.org/10.3390/foods12203815







