Influence of Actinidin-Induced Hydrolysis on the Functional Properties of Milk Protein and Whey Protein Concentrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Materials
2.2. Sample Preparation and Enzymatic Hydrolysis of Milk Protein Systems
2.3. Particle Size and Zeta Potential Measurement
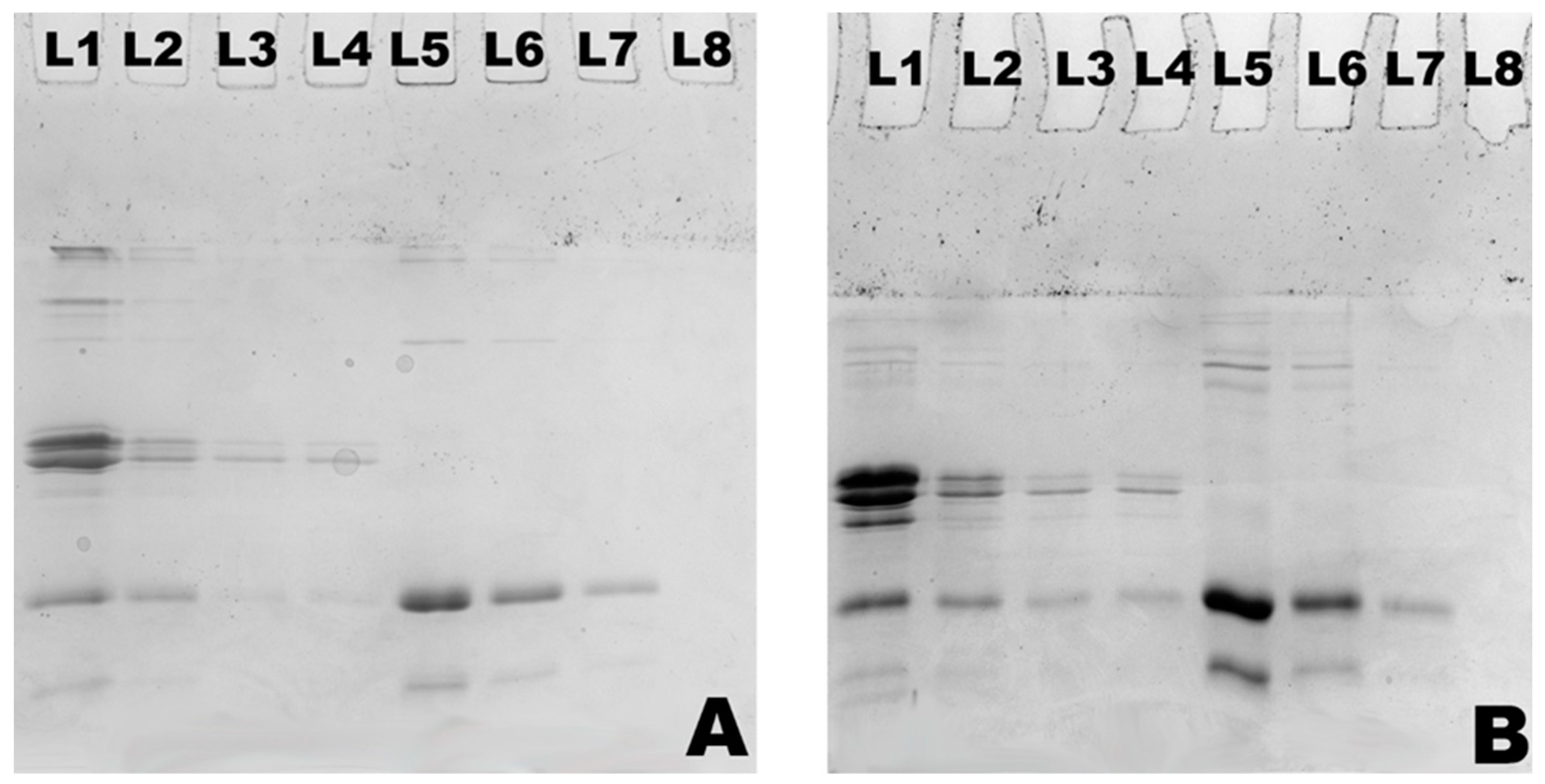
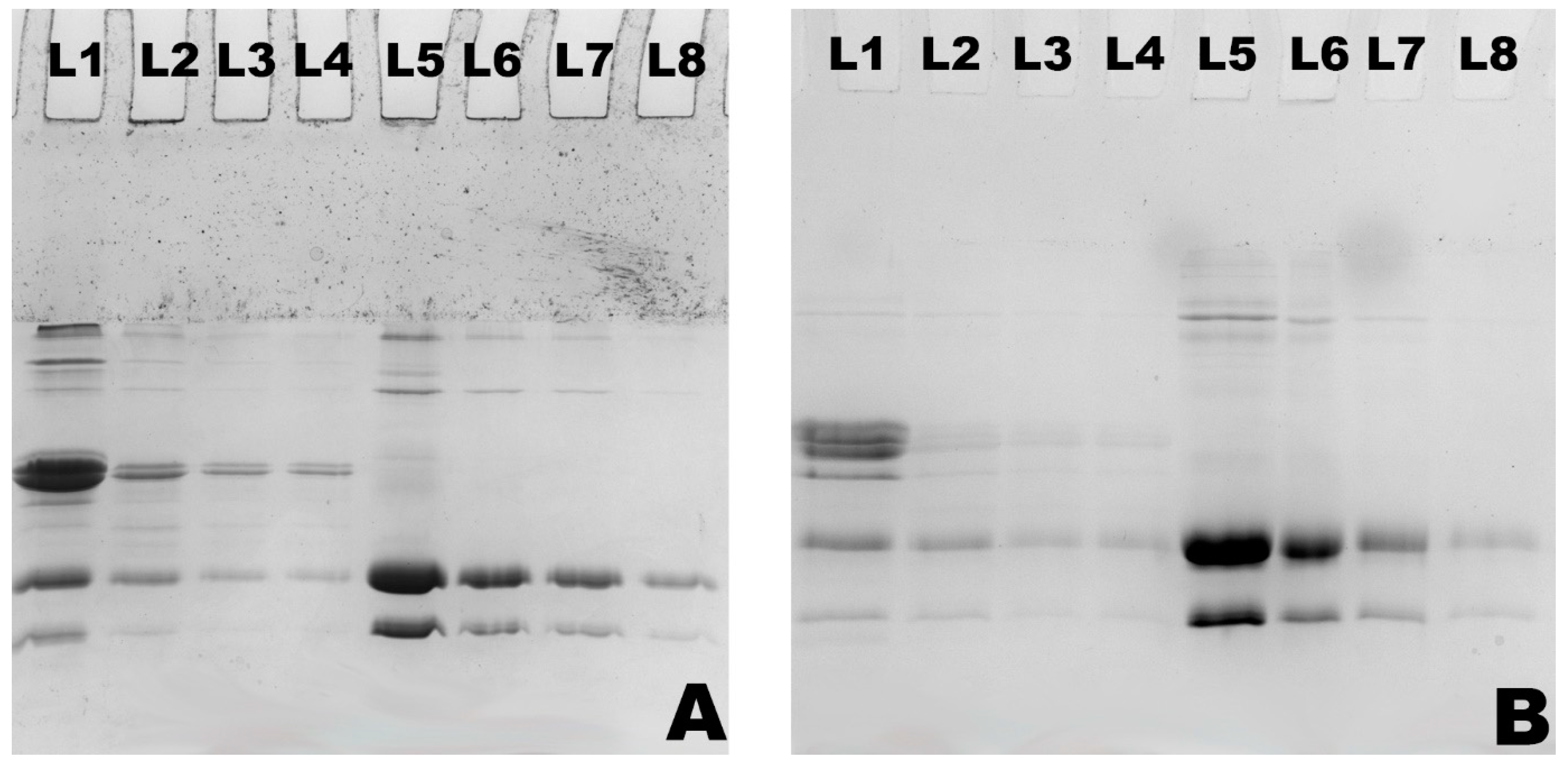
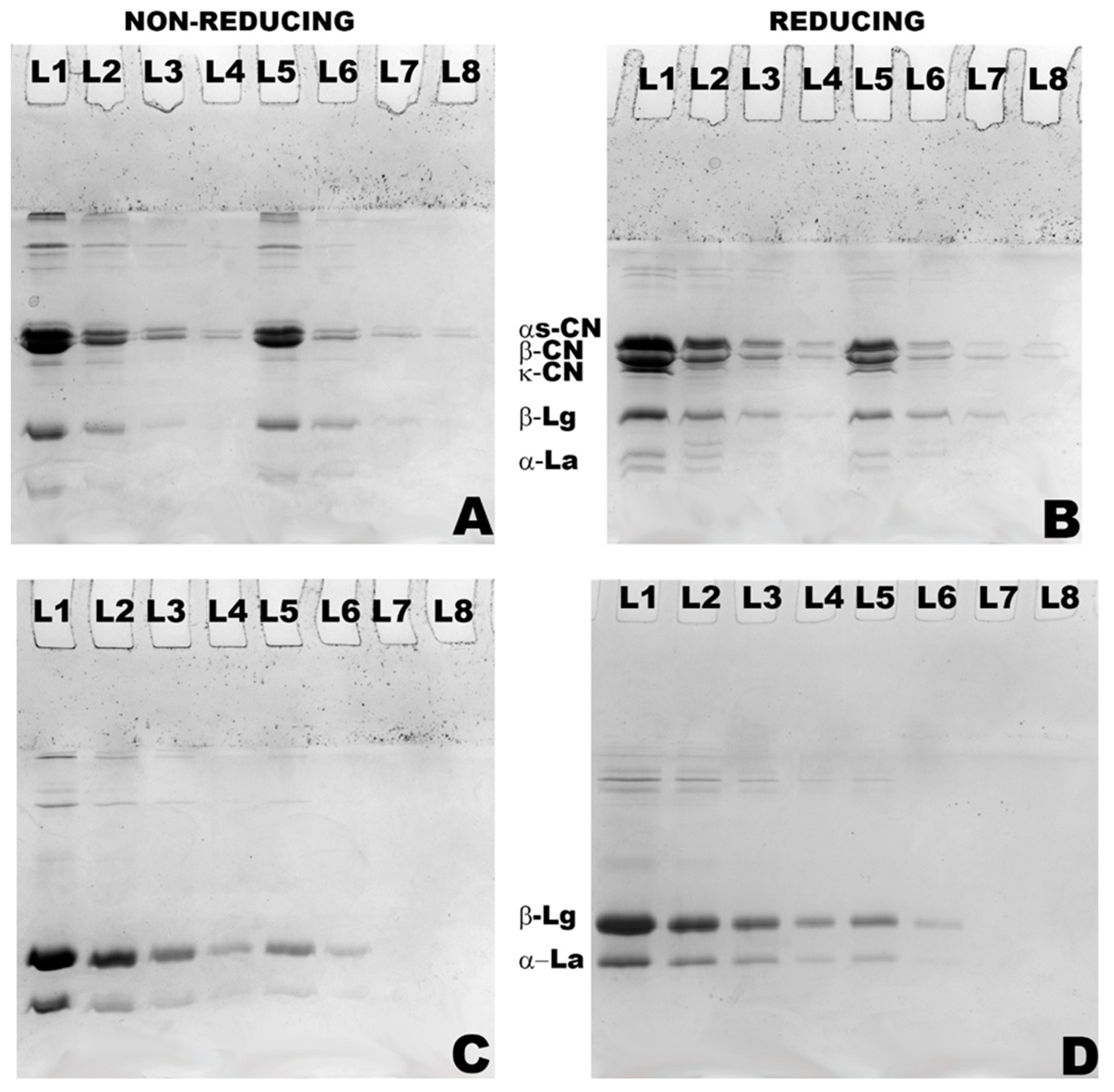
2.4. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Functional Properties of MPs
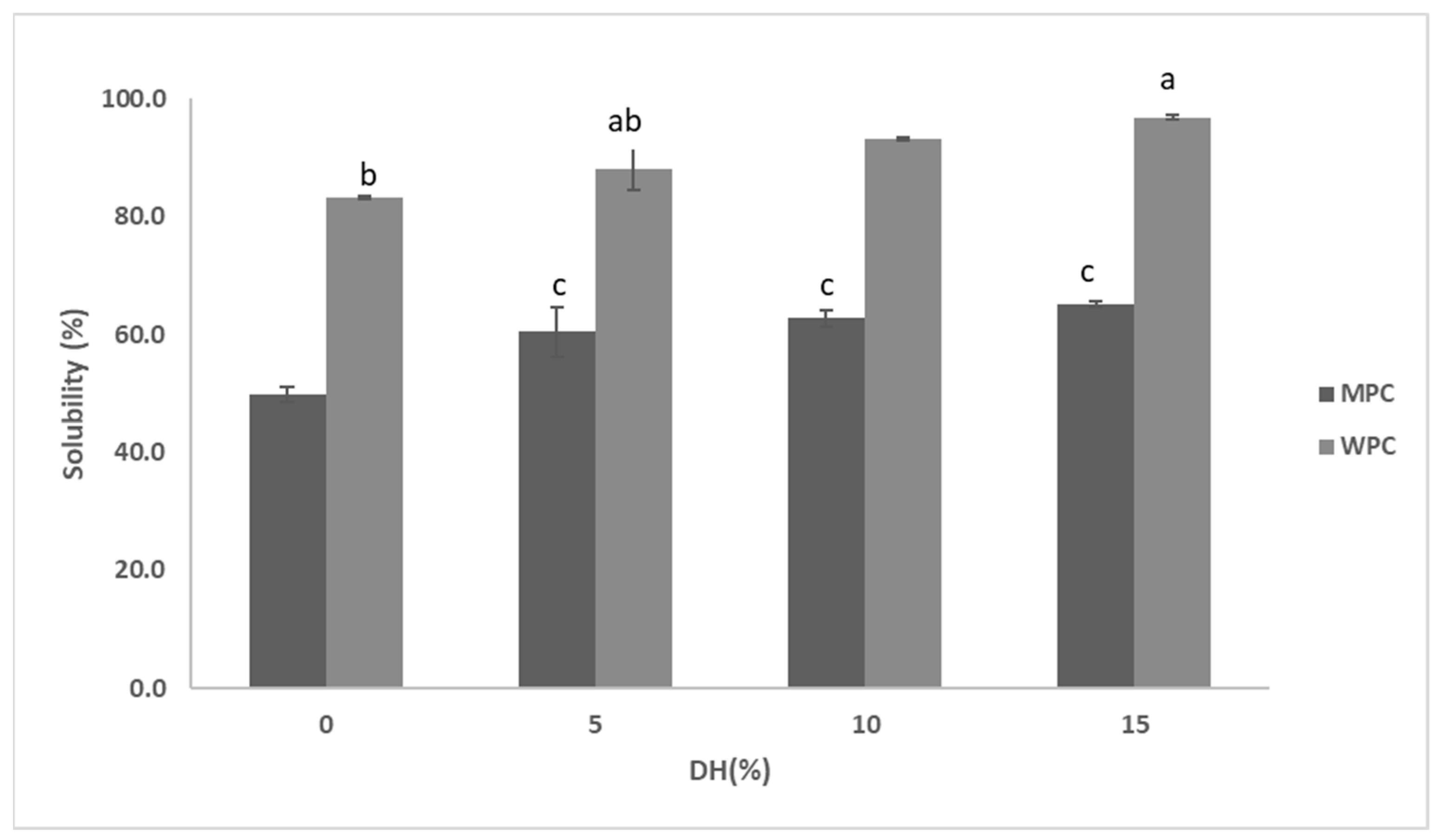
2.6.1. Determination of Protein Solubility
2.6.2. Determination of Heat Stability
2.6.3. Determination of Emulsifying Properties
2.6.4. Determination of Foaming Properties
2.7. Statistical Analysis
3. Results
3.1. Changes in Particle Size and Composition of Milk Protein Hydrolysates Obtained by Actinidin-Induced Hydrolysis of Milk Protein Concentrate and Whey Protein Concentrate
3.2. Modification of Secondary Structure in Milk Protein Hydrolysates Obtained by Actinidin
3.3. Functional Properties of Hydrolysates
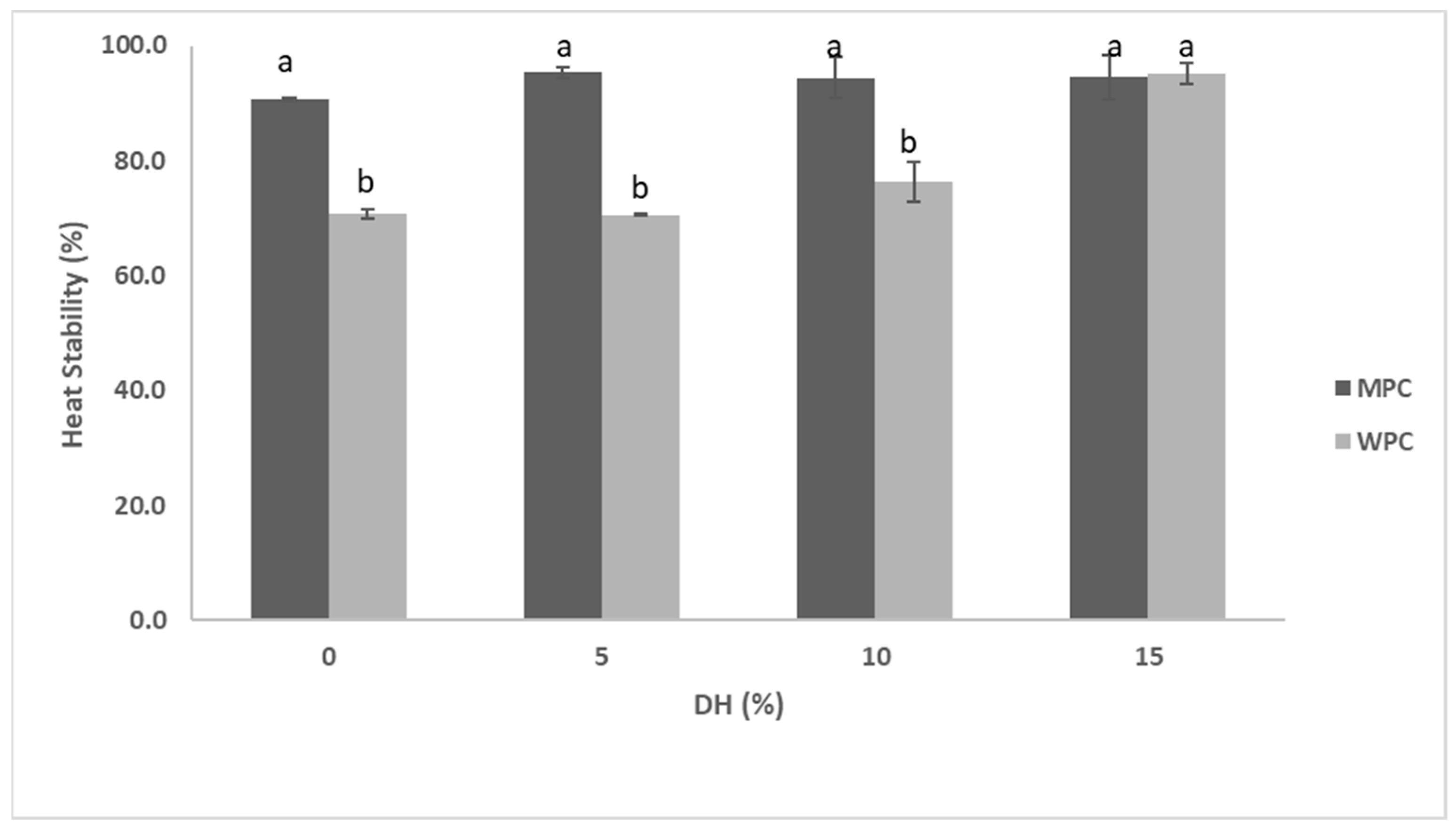
3.3.1. Functional Properties of MPC Hydrolysed by Actinidin
3.3.2. Functional Properties of WPC Hydrolysates Obtained by Actinidin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anema, S.G.; Pinder, D.N.; Hunter, R.J.; Hemar, Y. Effects of storage temperature on the solubility of milk protein concentrate (MPC85). Food Hydrocoll. 2006, 20, 386–393. [Google Scholar] [CrossRef]
- Banach, J.C.; Lin, Z.; Lamsal, B.P. Enzymatic modification of milk protein concentrate and characterization of resulting functional properties. LWT-Food Sci. Technol. 2013, 54, 397–403. [Google Scholar] [CrossRef]
- Havea, P. Protein interactions in milk protein concentrate powders. Int. Dairy J. 2006, 16, 415–422. [Google Scholar] [CrossRef]
- Singh, H. Milk Protein Products|Functional Properties of Milk Proteins. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 887–893. [Google Scholar]
- Srinivasan, M.; Singh, H.; Munro, P.A. Formation and stability of sodium caseinate emulsions: Influence of retorting (121 °C for 15 min) before or after emulsification. Food Hydrocoll. 2002, 16, 153–160. [Google Scholar] [CrossRef]
- Le, T.T.; Deeth, H.C.; Bhandari, B.R.; Alewood, P.F.; Holland, J.W. A proteomic approach to detect lactosylation and other chemical changes in stored milk protein concentrate. Food Chem. 2012, 132, 655–662. [Google Scholar] [CrossRef]
- Iordache, M.; Jelen, P. High Pressure microfluidization treatment of heat denatured whey protein for improved functionality. Innov. Food Sci. Emerg. Technol. 2003, 4, 367–376. [Google Scholar] [CrossRef]
- Ryan, G.; Nongonierma, A.B.; O’Regan, J.; FitzGerald, R.J. Functional properties of bovine milk protein isolate and associated enzymatic hydrolysates. Int. Dairy J. 2018, 81, 113–121. [Google Scholar] [CrossRef]
- Damodaran, S. Protein Stabilization of Emulsions and Foams. J. Food Sci. 2005, 70, R54–R66. [Google Scholar] [CrossRef]
- Kaur, S.; Huppertz, T.; Vasiljevic, T. Milk protein hydrolysis by actinidin: Influence of protein source and hydrolysis conditions. Int. Dairy J. 2021, 118, 105029. [Google Scholar] [CrossRef]
- Kaur, S.; Huppertz, T.; Vasiljevic, T. Actinidin-induced hydrolysis of milk proteins: Effect on antigenicity. LWT-Food Sci. Technol. 2022, 161, 113294. [Google Scholar] [CrossRef]
- Baker, E.N.; Boland, M.J.; Calder, P.C.; Hardman, M.J. The specificity of actinidin and its relationship to the structure of the enzyme. Biochim. Biophys. Acta 1980, 616, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Lara, L.R.; Tello-Solís, S.; Gómez-Ruiz, L.; García-Garibay, M.; Rodríguez-Serrano, G. Degradation of α-lactalbumin and β-lactoglobulin by actinidin. Food Biotechnol. 2003, 17, 117–128. [Google Scholar] [CrossRef]
- Al-Shamsi, K.A.; Mudgil, P.; Hassan, H.M.; Maqsood, S. Camel milk protein hydrolysates with improved technofunctional properties and enhanced antioxidant potential in in vitro and in food model systems. J. Dairy Sci. 2018, 101, 47–60. [Google Scholar] [CrossRef]
- Severin, S.; Xia, W.S. Enzymatic hydrolysis of whey proteins by two different proteases and their effect on the functional properties of resulting protein hydrolysates. J. Food Biochem. 2006, 30, 77–97. [Google Scholar] [CrossRef]
- Jenness, R.; Koops, J. Preparation and properties of a salt solution which simulates milk ultrafiltrate. Neth. Milk Dairy J. 1962, 16, 153–164. [Google Scholar]
- Bogahawaththa, D.; Buckow, R.; Chandrapala, J.; Vasiljevic, T. Comparison between thermal pasteurization and high pressure processing of bovine skim milk in relation to denaturation and immunogenicity of native milk proteins. Innov. Food Sci. Emerg. Technol. 2018, 47, 301–308. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, ML, USA, 2000. [Google Scholar]
- Dermiki, M.; FitzGerald, R.J. Physicochemical and gelling properties of whey protein hydrolysates generated at 5 and 50 °C using Alcalase® and Neutrase®, effect of total solids and incubation time. Int. Dairy J. 2020, 110, 104792. [Google Scholar] [CrossRef]
- Gruppi, A.; Dermiki, M.; Spigno, G.; FitzGerald, R.J. Impact of Enzymatic Hydrolysis and Heat Inactivation on the Physicochemical Properties of Milk Protein Hydrolysates. Foods 2022, 11, 516. [Google Scholar] [CrossRef]
- Liyanaarachchi, W.S.; Ramchandran, L.; Vasiljevic, T. Controlling heat induced aggregation of whey proteins by casein inclusion in concentrated protein dispersions. Int. Dairy J. 2015, 44, 21–30. [Google Scholar] [CrossRef]
- Grewal, M.K.; Huppertz, T.; Vasiljevic, T. FTIR fingerprinting of structural changes of milk proteins induced by heat treatment, deamidation and dephosphorylation. Food Hydrocoll. 2018, 80, 160–167. [Google Scholar] [CrossRef]
- Dissanayake, M.; Liyanaarachchi, S.; Vasiljevic, T. Functional properties of whey proteins microparticulated at low pH. J. Dairy Sci. 2012, 95, 1667–1679. [Google Scholar] [CrossRef]
- Cameron, D.R.; Weber, M.E.; Idziak, E.S.; Neufeld, R.J.; Cooper, D.G. Determination of interfacial areas in emulsions using turbidimetric and droplet size data: Correction of the formula for emulsifying activity index. J. Agric. Food Chem. 1991, 39, 655–659. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Phillips, L.G.; German, J.B.; O’Neill, T.E.; Foegeding, E.A.; Harwalkar, V.R.; Kilara, A.; Lewis, B.A.; Mangino, M.E.; Morr, C.V.; Regenstein, J.M.; et al. Standardized Procedure for Measuring Foaming Properties of Three Proteins: A Collaborative Study. J. Food Sci. 1990, 55, 1441–1444. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Garmany, 2009; pp. 498–544. [Google Scholar] [CrossRef]
- Dissanayake, M.; Vasiljevic, T. Functional properties of whey proteins affected by heat treatment and hydrodynamic high-pressure shearing. J. Dairy Sci. 2009, 92, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Althouse, P.J.; Dinakar, P.; Kilara, A. Screening of proteolytic enzymes to enhance foaming of whey protein isolates. J. Food Sci. 1995, 60, 1110–1112. [Google Scholar] [CrossRef]
- Abd-El-Salam, M.H.; El-Shibiny, S.; Salem, A. Factors affecting the functional properties of whey protein products: A review. Food Rev. Int. 2009, 25, 251–270. [Google Scholar] [CrossRef]
- Fox, P.F. Milk proteins, general and historical aspects. In Advanced Dairy Chemistry-Proteins: Part A, 2nd ed.; Fox, P.F., McSweeney, P.L.H., Eds.; Kluwer Academic; Plenum Publishers: New York, NY, USA, 2003; pp. 1–49. [Google Scholar]
- Zhu, H.; Damodaran, S. Heat-induced conformational changes in whey protein isolate and its relation to foaming properties. J. Agric. Food Chem. 1994, 42, 846–855. [Google Scholar] [CrossRef]
- Sikand, V.; Tong, P.S.; Vink, S.; Walker, J.H. Effect of powder source and processing conditions on the solubility of milk protein concentrates 80. Milchwissenschaft 2012, 67, 300–303. [Google Scholar]
- Khalesi, M.; FitzGerald, R.J. Insolubility in milk protein concentrates: Potential causes and strategies to minimize its occurrence. Crit. Rev. Food Sci. Nutr. 2021, 62, 6973–6989. [Google Scholar] [CrossRef]
- Boland, M.; Singh, J. Chapter 429—Actinidain. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: London, UK, 2013; pp. 1879–1884. [Google Scholar]
- Qi, P.X.; Ren, D.; Xiao, Y.; Tomasula, P.M. Effect of homogenization and pasteurization on the structure and stability of whey protein in milk. J. Dairy Sci. 2015, 98, 2884–2897. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, P.; Unruh, J.; Wickham, E.; Farrell, H. Secondary structure of bovine αS2-casein: Theoretical and experimental approaches. J. Dairy Sci. 2001, 84, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.M., Jr.; Cooke, P.H.; King, G.; Hoagland, P.D.; Groves, M.L.; Kumosinski, T.F.; Chu, B. Particle sizes of casein submicelles and purified κ-casein: Comparisons of dynamic light scattering and electron microscopy with predictive three-dimensional molecular models. ACS Symp. Ser. 1996, 650, 61–81. [Google Scholar] [CrossRef]
- Castro, R.J.S.D.; Sato, H.H. Comparison and synergistic effects of intact proteins and their hydrolysates on the functional properties and antioxidant activities in a simultaneous process of enzymatic hydrolysis. Food Bioprod. Process. 2014, 92, 80–88. [Google Scholar] [CrossRef]
- Wijayanti, H.B.; Bansal, N.; Deeth, H.C. Stability of whey proteins during thermal processing: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1235–1251. [Google Scholar] [CrossRef]
- Oldfield, J.; Singh, H.; Taylor, M.W.; Pearce, K.N. Heat induced interactions of β-lactoglobulin and α-lactalbumin with the casein micelle in pH-adjusted skim milk. Int. Dairy J. 2000, 10, 509–518. [Google Scholar] [CrossRef]
- Rose, D. Factors affecting the pH-sensitivity of the heat stability of milk from individual cows. J. Dairy Sci. 1961, 44, 1405–1413. [Google Scholar] [CrossRef]
- Fang, Y.; Selomulya, C.; Ainsworth, S.; Palmer, M.; Chen, X.D. On quantifying the dissolution behaviour of milk protein concentrate. Food Hydrocoll. 2011, 25, 503–510. [Google Scholar] [CrossRef]
- Gauthier, S.F.; Pouliot, Y. Functional and Biological Properties of Peptides Obtained by Enzymatic Hydrolysis of Whey Proteins1. J. Dairy Sci. 2003, 86, 78–87. [Google Scholar] [CrossRef]
- Slattery, H.; Fitzerald, R.J. Functional properties and bitterness of sodium caseinate hydrolysates prepared with a Bacillus proteinase. J. Food Sci. 1998, 63, 418–422. [Google Scholar] [CrossRef]
- Singh, A.M.; Dalgleish, D.G. The emulsifying properties of hydrolyzates of whey proteins. J. Dairy Sci. 1998, 81, 918–924. [Google Scholar] [CrossRef]
- Monahan, F.J.; McClements, D.J.; Kinsella, J.E. Polymerization of whey proteins in whey protein-stabilized emulsions. J. Agric. Food Chem. 1993, 41, 1826–1829. [Google Scholar] [CrossRef]
- Phillips, L.G.; Schulman, W.; Kinsella, J.E. pH and Heat Treatment Effects on Foaming of Whey Protein Isolate. J. Food Sci. 1990, 55, 1116–1119. [Google Scholar] [CrossRef]
- Grufferty, M.B.; Mulvihill, D.M. Emulsifying and foaming properties of protein isolates prepared from heated milks. Int. J. Soc. Dairy Technol. 1991, 44, 13–19. [Google Scholar] [CrossRef]
- Lee, S.Y.; Morr, C.V.; Ha, E.Y.W. Structural and functional properties of caseinate and whey protein isolate as affected by temperature and pH. J. Food Sci. 2006, 57, 1210–1229. [Google Scholar] [CrossRef]
- Van der Ven, C.; Gruppen, H.; de Bont, D.B.; Voragen, A.G. Correlations between biochemical characteristics and foam-forming and-stabilizing ability of whey and casein hydrolysates. J. Agric. Food Chem. 2002, 50, 2938–2946. [Google Scholar] [CrossRef]
- Kuehler, C.A.; Stine, C.M. Effect of enzymatic hydrolysis on some functional properties of whey proteins. J. Food Sci. 1974, 39, 379382. [Google Scholar] [CrossRef]

| MPH (%DH) | Particle Diameter (nm) | Zeta Potential (mV) | ||||||
|---|---|---|---|---|---|---|---|---|
| MPC | WPC | MPC | WPC | |||||
| MPC Bulk | MPC Supernatant | WPC Bulk | WPC Supernatant | MPC Bulk | MPC Supernatant | WPC Bulk | WPC Supernatant | |
| 0 | 295 ± 4 b | 282 ± 4 a | 426 ± 1 a | 386 ± 3 a | −2 ± 0.3 a | −15 ± 0.0 ab | −9 ± 0.0 a | −9 ± 0.4 a |
| 5 | 281 ± 2 d | 233 ± 12 b | 419 ± 3 b | 381 ± 1 b | −4 ± 0.6 b | −16 ± 1.3 b | −10 ± 0.4 b | −10 ± 0.4 b |
| 10 | 290 ± 3 c | 180 ± 8 c | 409 ± 4 c | 365 ± 1 c | −7 ± 0.0 c | −14 ± 0.2 a | −10 ± 1.9 b | −12 ± 2.2 c |
| 15 | 343 ± 5 a | 171 ± 5 d | 410 ± 3 c | 353 ± 3 d | −3 ± 1.9 ab | −18 ± 0.0 c | −13 ± 0.4 c | −14 ± 0.2 d |
| Protein | Proportion of Proteins Remaining Relative to Control (%) | |||||
|---|---|---|---|---|---|---|
| DH (%) | ||||||
| 5 | 10 | 15 | 5 | 10 | 15 | |
| MPC | Solubility | |||||
| Whole Sample | Supernatant | |||||
| αs-CN | 54.6 ± 3.2 aE | 27.4 ± 2.1 cD | 8.1 ± 0.5 eD | 38.7 ± 3.4 bC | 14.8 ± 0.9 dB | 3.0 ± 0.2 fB |
| β-CN | 57.4 ± 0.0 aD | 30.5 ± 0.0 bC | 7.0 ± 0.0 eE | 27.0 ± 0.0 cE | 19.2 ± 0.0 dA | 4.6 ± 3.6 fA |
| κ-CN | 43.7 ± 0.1 aG | 19.8 ± 0.0 bE | 5.9 ± 0.0 dF | 20.2 ± 0.0 bG | 7.0 ± 0.0 cD | 0.0 ± 0.0 eD |
| β-LG | 61.5 ± 3.6 bC | 31.9 ± 3.3 cC | 9.6 ± 1.0 eC | 63.7 ± 3.5 aA | 13.4 ± 0.0 dC | 2.0 ± 0.0 fC |
| α-LA | 46.0 ± 4.1 aF | 18.3 ± 1.7 cF | 0.0 ± 0.0 dG | 43.0 ± 1.3 bB | 0.0 ± 0.0 dE | 0.0 ± 0.0 dD |
| WPC | Whole sample | Supernatant | ||||
| β-LG | 81.2 ± 3.9 aB | 60.6 ± 1.7 bA | 25.5 ± 0.5 dA | 36.8 ± 0.5 cD | 19.0 ± 0.0 eA | 0.0 ± 0.0 fD |
| α-LA | 83.5 ± 1.6 aA | 45.7 ± 2.0 bB | 21.5 ± 0.5 dB | 23.9 ± 0.6 cF | 0.0 ± 0.0 eE | 0.0 ± 0.0 eD |
| Proportion of Proteins Remaining Relative to Control (%) during Heat Stability | ||||||
|---|---|---|---|---|---|---|
| DH (%) | 5 | 10 | 15 | 5 | 10 | 15 |
| Protein | Supernatant (MPC) | Supernatant (WPC) | ||||
| αs-CN | 28.2 ± 2.1 aD | 13.4 ± 0.4 bC | 10.8 ± 0.3 cC | - | - | - |
| β-CN | 32.3 ± 1.8 aC | 21.0 ± 1.6 bB | 20.0 ± 1.7 cB | - | - | - |
| κ-CN | 28.0 ± 1.3 aD | 11.1 ± 0.9 bD | 9.7 ± 0.9 cD | - | - | - |
| β-LG | 51.7 ± 2.6 aA | 32.3 ± 2.2 cA | 25.7 ± 2.3 dA | 48.5 ± 3.1 bA | 22.3 ± 2.5 eA | 0.0 ± 0.0 fA |
| α-LA | 38.0 ± 2.8 bB | 0.0 ± 0.0 dE | 0.0 ± 0.0 dE | 47.7 ± 2.1 aB | 11.3 ± 4.5 cB | 0.0 ± 0.0 dA |
| Proportion of Proteins Remaining Relative to Control (%) during Foaming | ||||||
|---|---|---|---|---|---|---|
| DH (%) | 5 | 10 | 15 | 5 | 10 | 15 |
| Protein | Foam Drained Liquid (MPC) | Foam Drained Liquid (WPC) | ||||
| αs-CN | 18.0 ± 1.3 aE | 7.8 ± 0.3 bD | 3.4 ± 0.2 cD | - | - | - |
| β-CN | 27.1 ± 0.5 aC | 13.8 ± 0.5 bB | 2.8 ± 0.0 cE | - | - | - |
| κ-CN | 26.3 ± 1.2 aD | 11.3 ± 0.0 bC | 5.0 ± 0.3 cC | - | - | - |
| β-LG | 33.2 ± 2.4 aB | 15.1 ± 0.0 cA | 9.4 ± 0.0 dA | 33.8 ± 0.7 aA | 18.7 ± 1.5 bB | 15.4 ± 0.6 cA |
| α-LA | 36.8 ± 0.3 aA | 11.6 ± 0.0 dC | 7.3 ± 0.0 eB | 26.4 ± 2.4 bB | 25.7 ± 2.3 cA | 4.7 ± 0.4 fB |
| MPH | Band Assignment | Degree of Hydrolysis (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (0) | 5 | 10 | 15 | ||||||
| Band Frequency (cm−2) | Peak Area % | Band Frequency (cm−2) | Peak Area % | Band Frequency (cm−2) | Peak Area % | Band Frequency (cm−2) | Peak Area % | ||
| MPC | β-sheet | 1638–1608, 1691–1681 | 42.4 ± 8.4 dB | 1640–1608, 1693–1681 | 45.2 ± 1.2 cB | 1640–1609, 1693–1681 | 47.7 ± 3.1 bB | 1640–1610, 1693–1682 | 53.2 ± 1.0 aA |
| Random coil | 1647–1643 | 10.6 ± 1.9 bG | 1648–1643 | 11.6 ± 4.3 aG | 1646–1642 | 8.4 ± 1.4 cG | 1646–1642 | 8.2 ± 0.4 cD | |
| α-helix | 1662–1651 | 22.1 ± 3.3 aC | 1662–1650 | 22.5 ± 1.4 aC | 1662–1650 | 19.8 ± 1.6 bC | 1662–1650 | 15.9 ± 0.8 cB | |
| β-turn | 1677–1666 | 17.4 ± 1.2 bE | 1678–1667 | 17.6 ± 5.3 bE | 1678–1667 | 18.8 ± 1.4 aD | 1677–1666 | 16.1 ± 1.4 cB | |
| WPC | β-sheet | 1639–1610, 1691–1681 | 49.6 ± 1.3 bA | 1639–1610, 1691–1680 | 49.6 ± 0.8 bA | 1640–1610, 1690–1681 | 53.3 ± 5.0 aA | 1640–1610, 1691–1681 | 53.4 ± 1.5 aA |
| Random coil | 1648–1643 | 9.0 ± 0.7 bH | 1647–1643 | 11.2 ± 0.4 aG | 1646–1642 | 8.7 ± 1.8 bG | 1646–1642 | 9.0 ± 0.3 bC | |
| α-helix | 1663–1650 | 20.6 ± 1.3 aD | 1663–1650 | 18.3 ± 1.5 bD | 1661–1649 | 14.8 ± 3.2 dE | 1660–1649 | 15.9 ± 1.1 cB | |
| β-turn | 1677–1666 | 15.5 ± 1.4 bF | 1678–1667 | 16.8 ± 1.2 aF | 1677–1666 | 11.7 ± 2.4 cF | 1678–1666 | 15.8 ± 2.0 bB | |
| MPH (%DH) | Foam Stability (s) | Overrun (%) | EAI (m2 g−1) | Adsorbed Protein (mg−1 mL−1) | ESI (h) |
|---|---|---|---|---|---|
| Hydrolysates from MPC | |||||
| 0 | 1260 ± 8 d | 344.8 ± 4 c | 17.45 ± 0.0 c | 2.33 ± 0.2 a | 23.9 ± 0.1 d |
| 5 | 1904 ± 10 c | 349.7 ± 3 c | 13.80 ± 0.4 d | 1.72 ± 0.1 b | 23.8 ± 0.1 d |
| 10 | 2160 ± 6 b | 358.5 ± 2 b | 11.58 ± 0.8 e | 1.56 ± 0.1 bc | 22.9 ± 0.2 e |
| 15 | 2454 ± 2 a | 406 ± 3 a | 9.49 ± 0.1 f | 1.50 ± 0.2 c | 22.5 ± 0.5 f |
| Hydrolysates from WPC | |||||
| 0 | 7 ± 1 h | 0 ± 0.0 f | 20.33 ± 0.3 a | 0.59 ± 0.1 f | 25.1 ± 0.5 a |
| 5 | 120 ± 2 e | 247.6 ± 4 e | 20.24 ± 0.9 a | 0.73 ± 0.1 de | 24.8 ± 0.7 b |
| 10 | 105 ± 6 f | 252.1 ± 4 e | 19.22 ± 0.7 b | 0.77 ± 0.1 d | 24.6 ± 0.4 bc |
| 15 | 40 ± 4 g | 270.4 ± 3 d | 17.45 ± 0.2 c | 0.80 ± 0.0 d | 24.5 ± 0.3 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, S.; Vasiljevic, T.; Huppertz, T. Influence of Actinidin-Induced Hydrolysis on the Functional Properties of Milk Protein and Whey Protein Concentrates. Foods 2023, 12, 3806. https://doi.org/10.3390/foods12203806
Kaur S, Vasiljevic T, Huppertz T. Influence of Actinidin-Induced Hydrolysis on the Functional Properties of Milk Protein and Whey Protein Concentrates. Foods. 2023; 12(20):3806. https://doi.org/10.3390/foods12203806
Chicago/Turabian StyleKaur, Surjit, Todor Vasiljevic, and Thom Huppertz. 2023. "Influence of Actinidin-Induced Hydrolysis on the Functional Properties of Milk Protein and Whey Protein Concentrates" Foods 12, no. 20: 3806. https://doi.org/10.3390/foods12203806
APA StyleKaur, S., Vasiljevic, T., & Huppertz, T. (2023). Influence of Actinidin-Induced Hydrolysis on the Functional Properties of Milk Protein and Whey Protein Concentrates. Foods, 12(20), 3806. https://doi.org/10.3390/foods12203806






