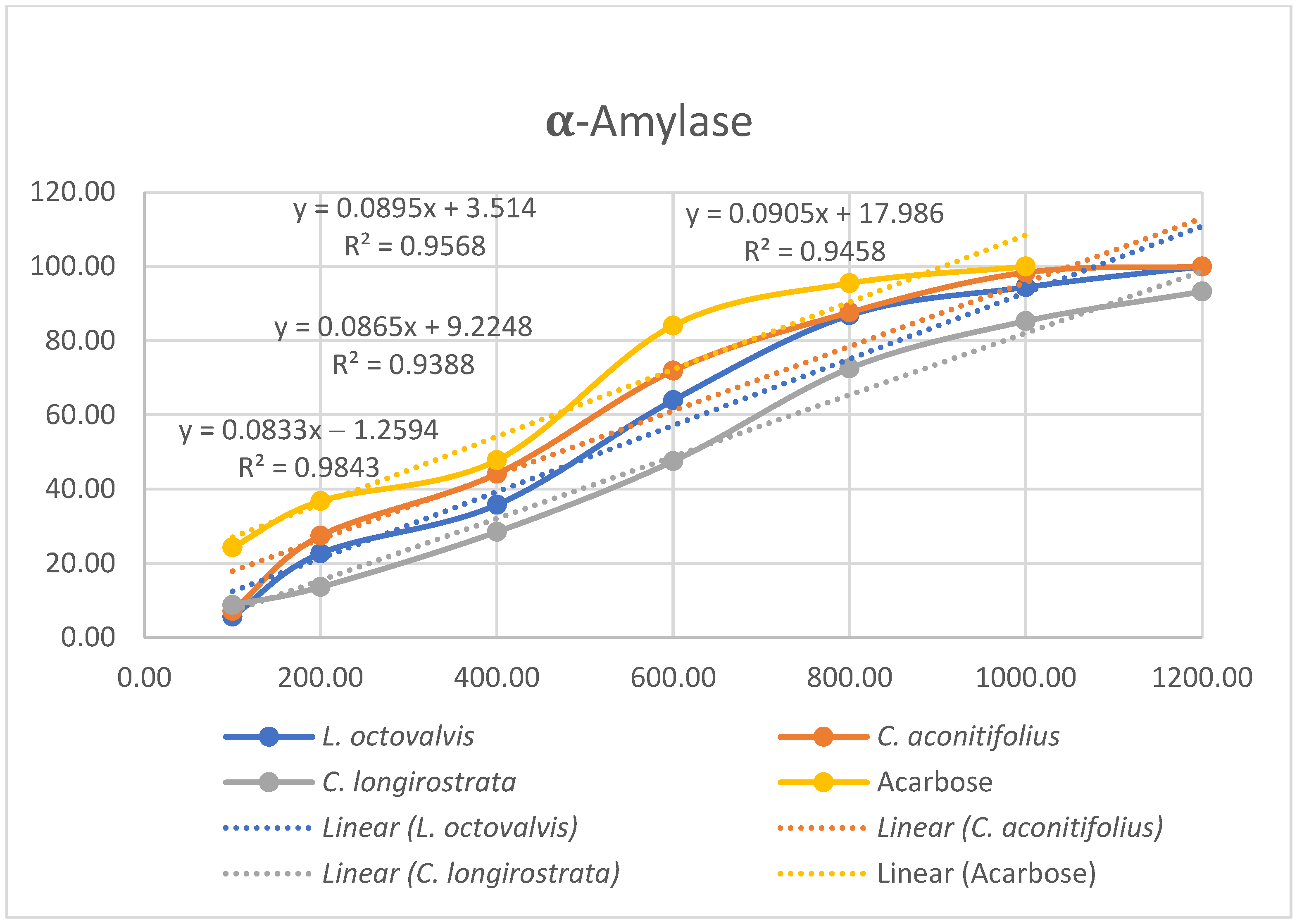
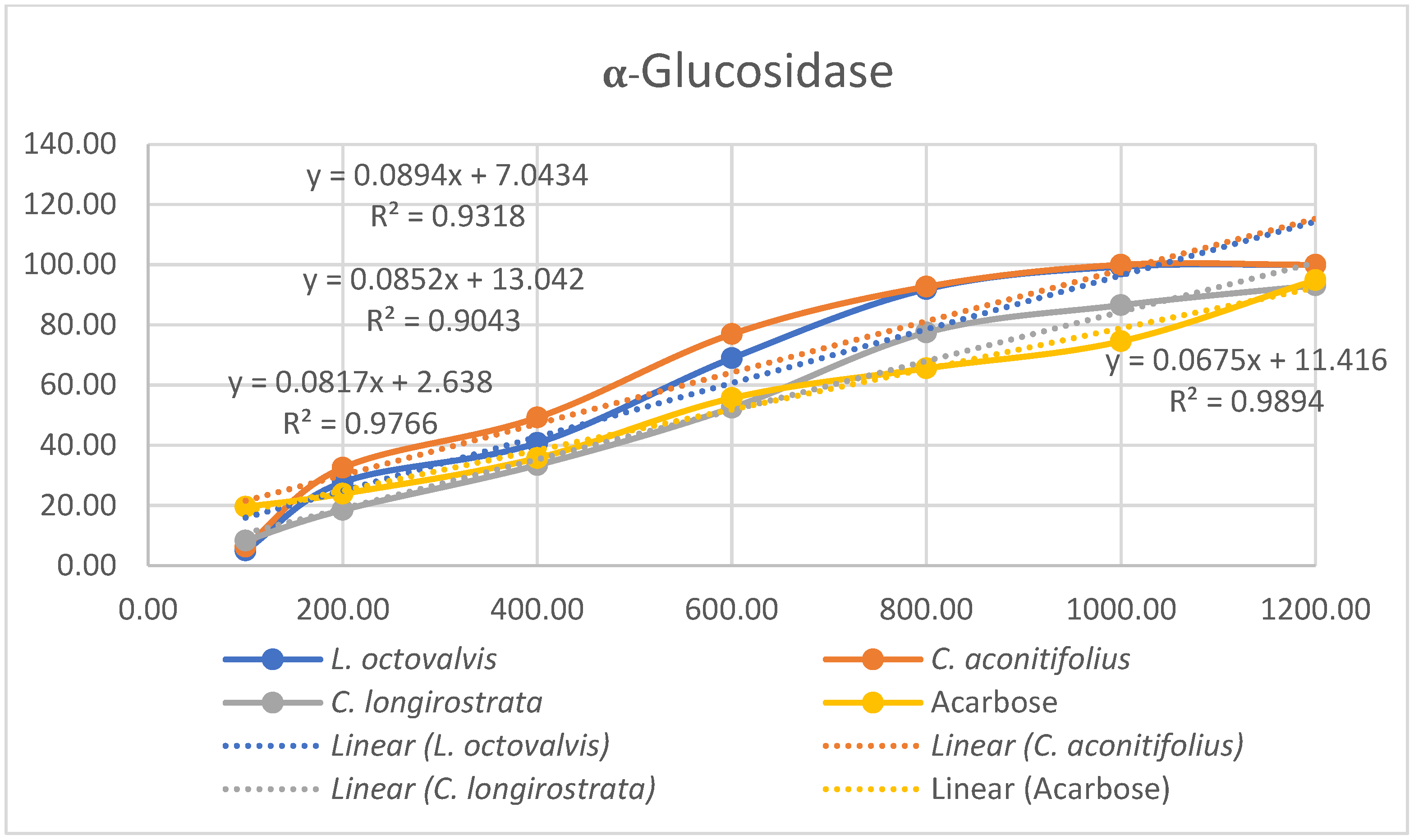
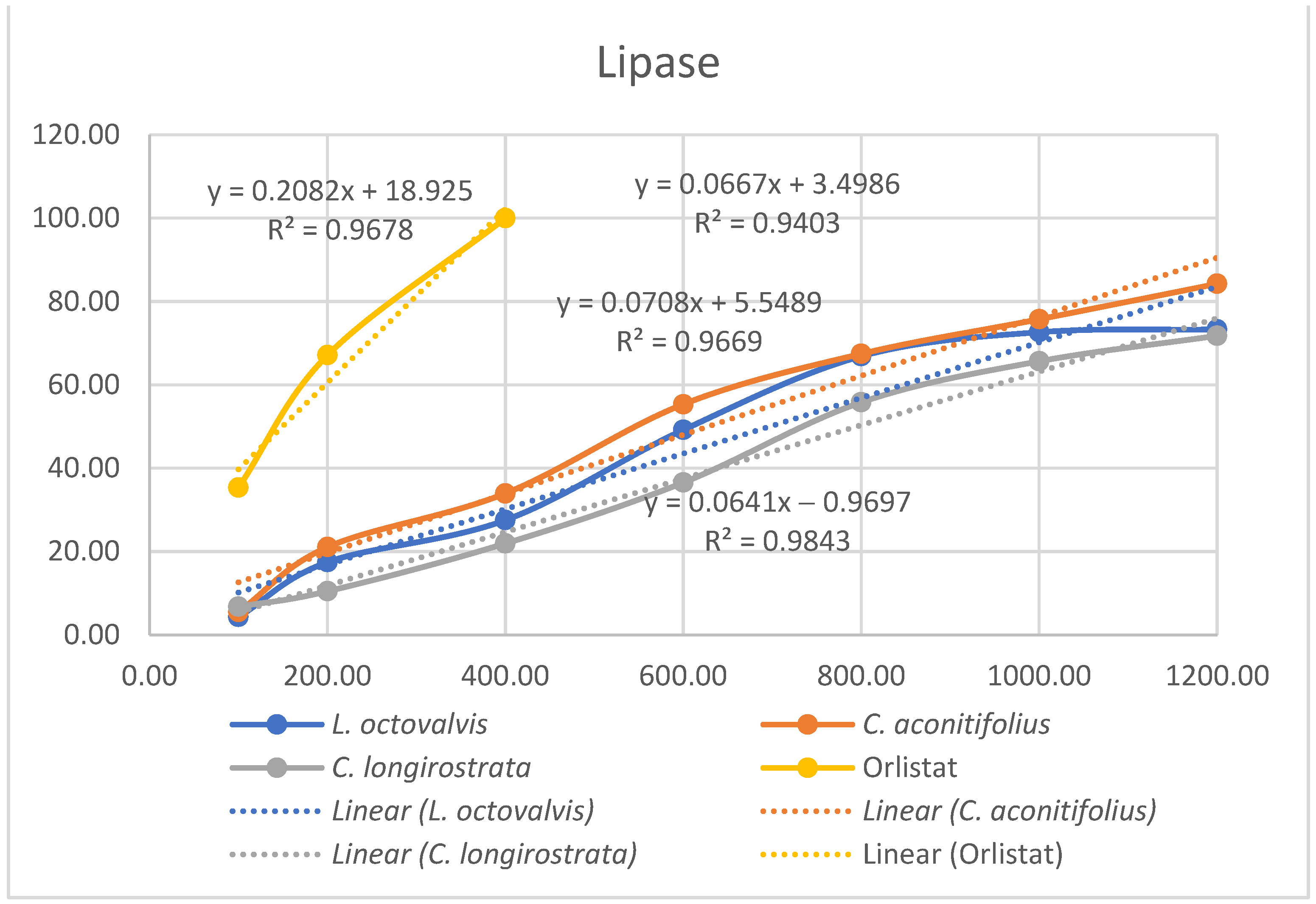
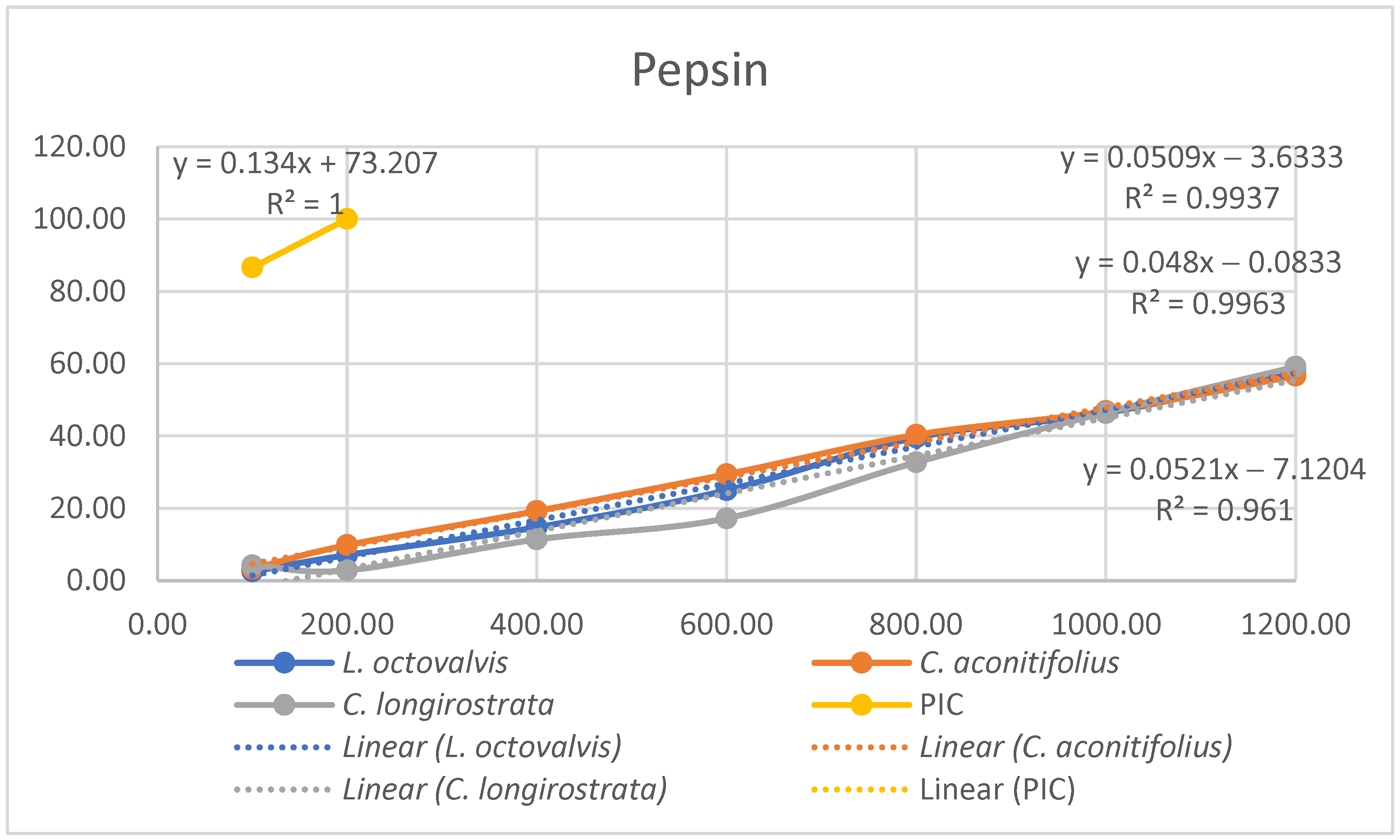
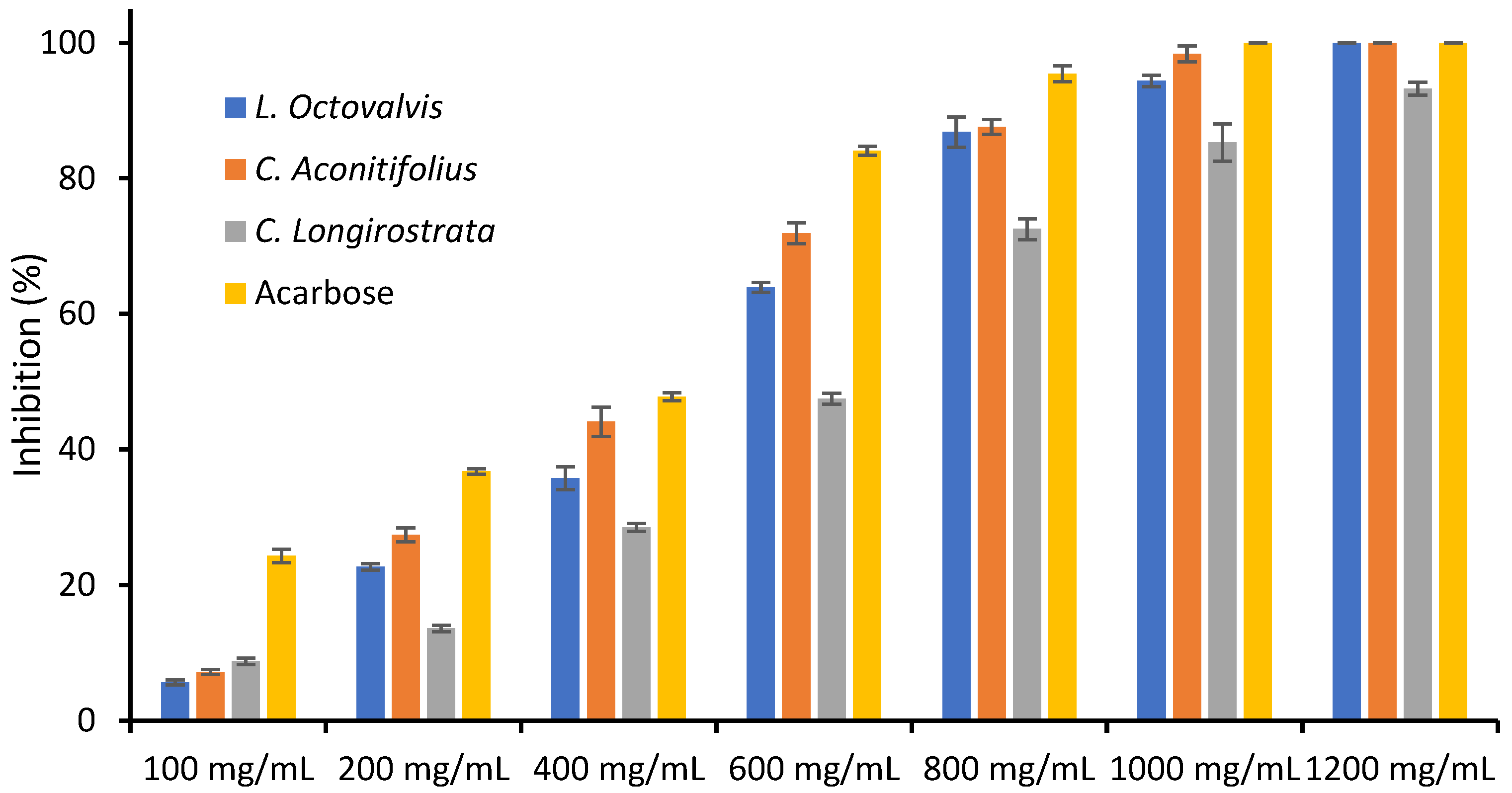
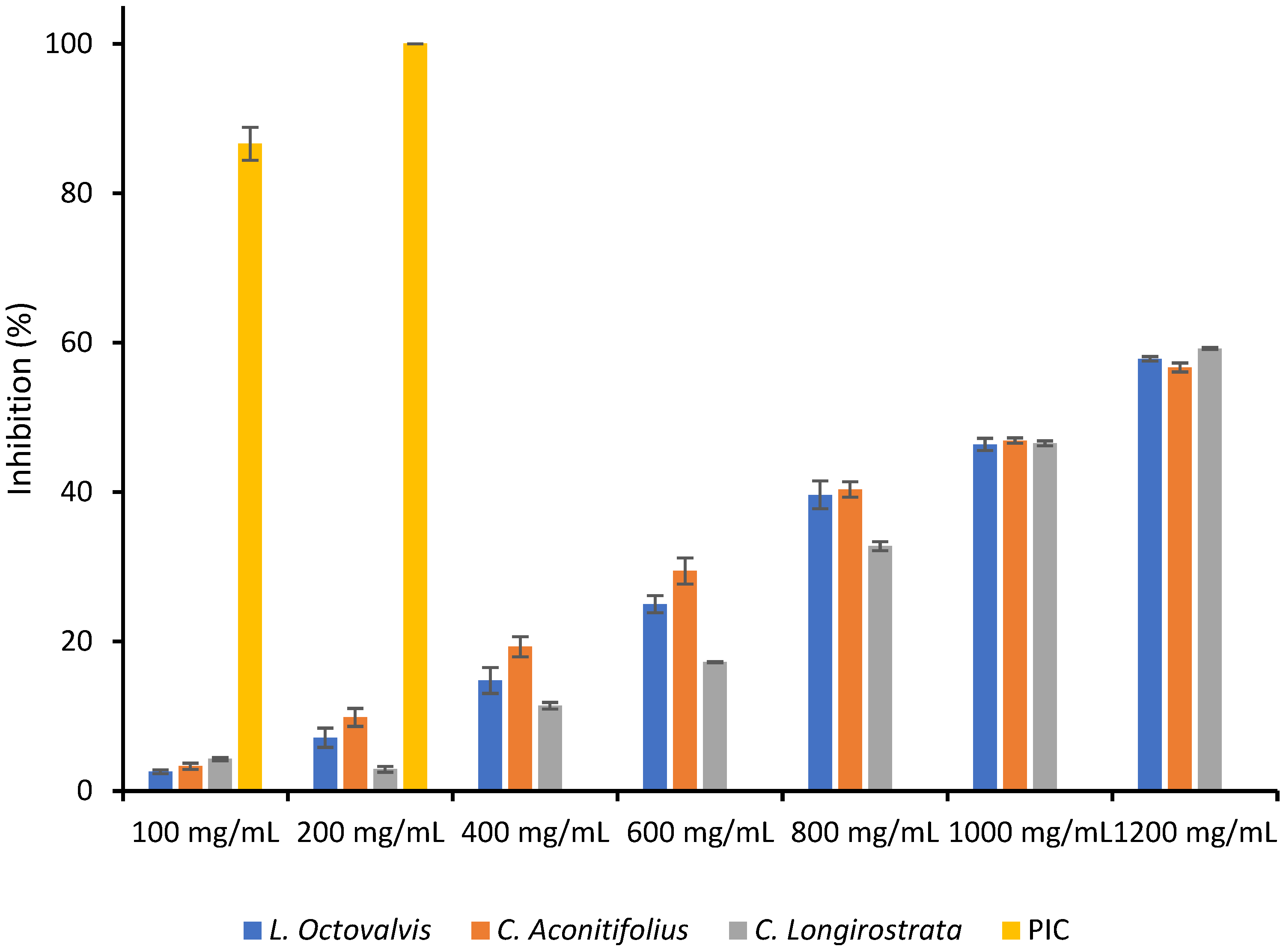
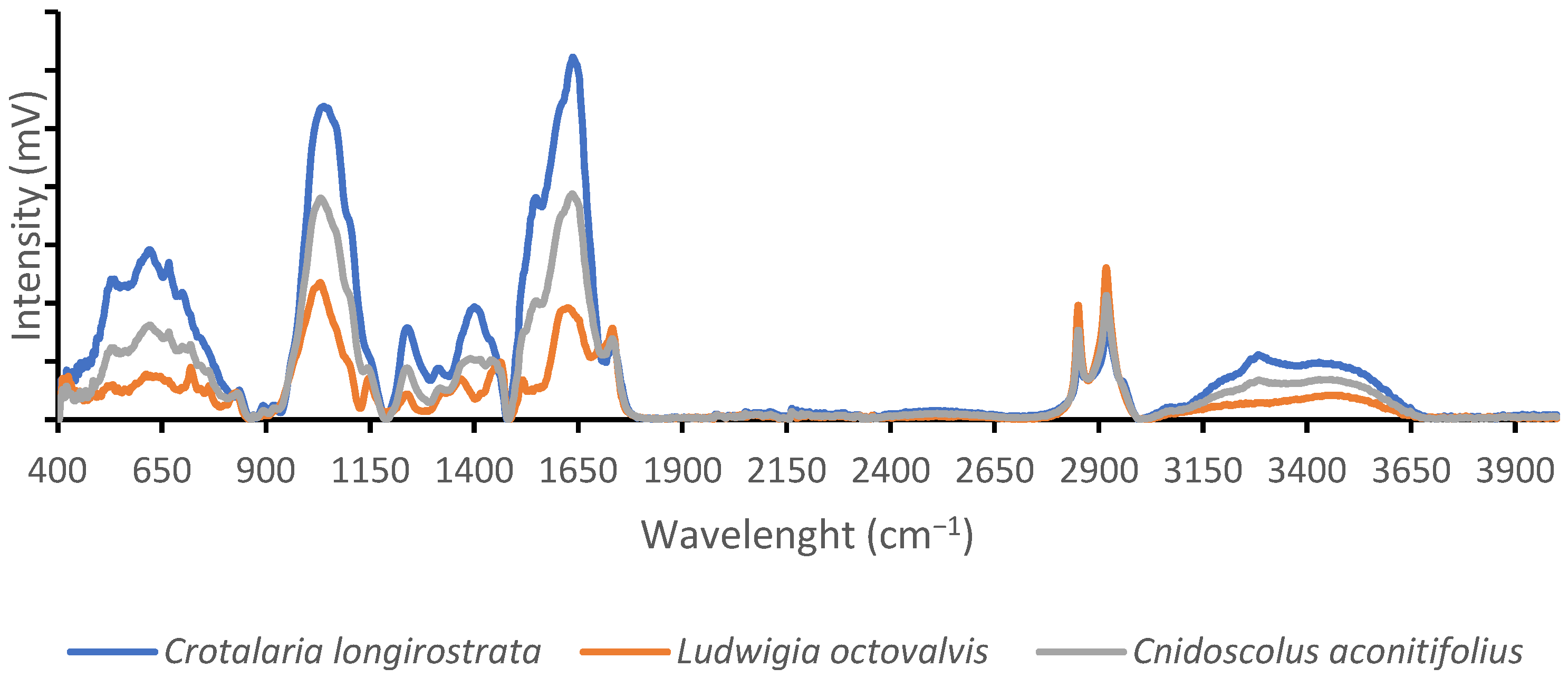
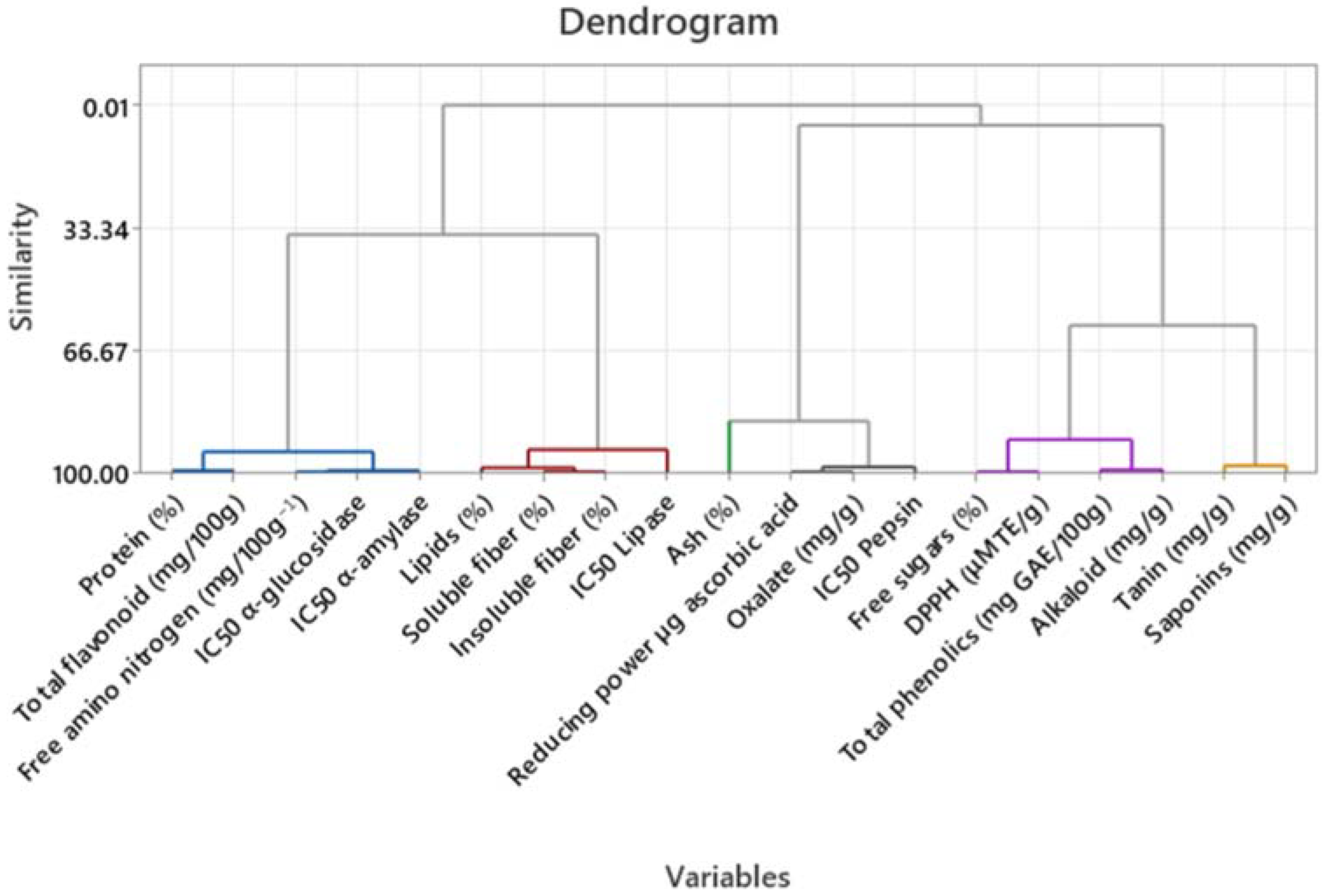
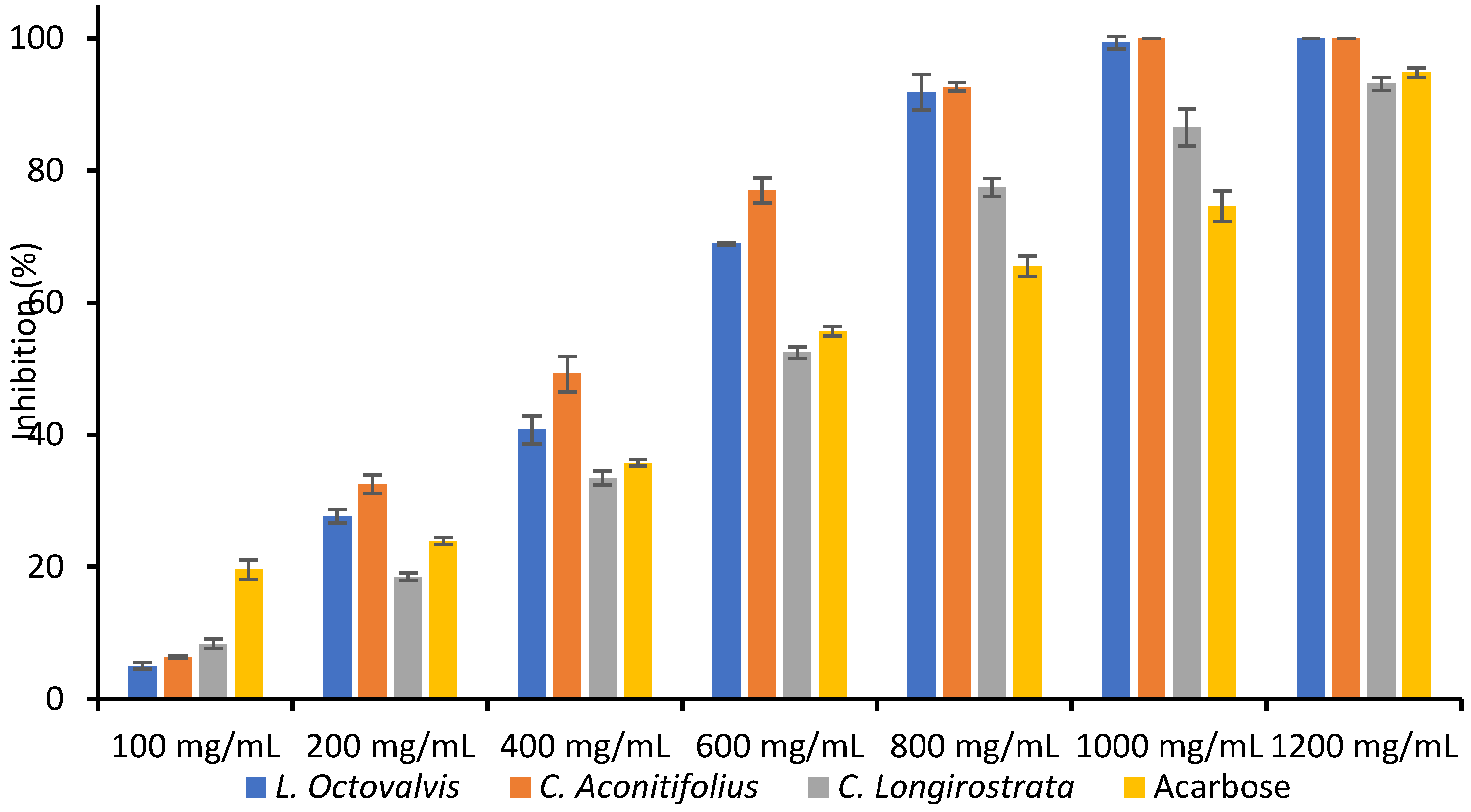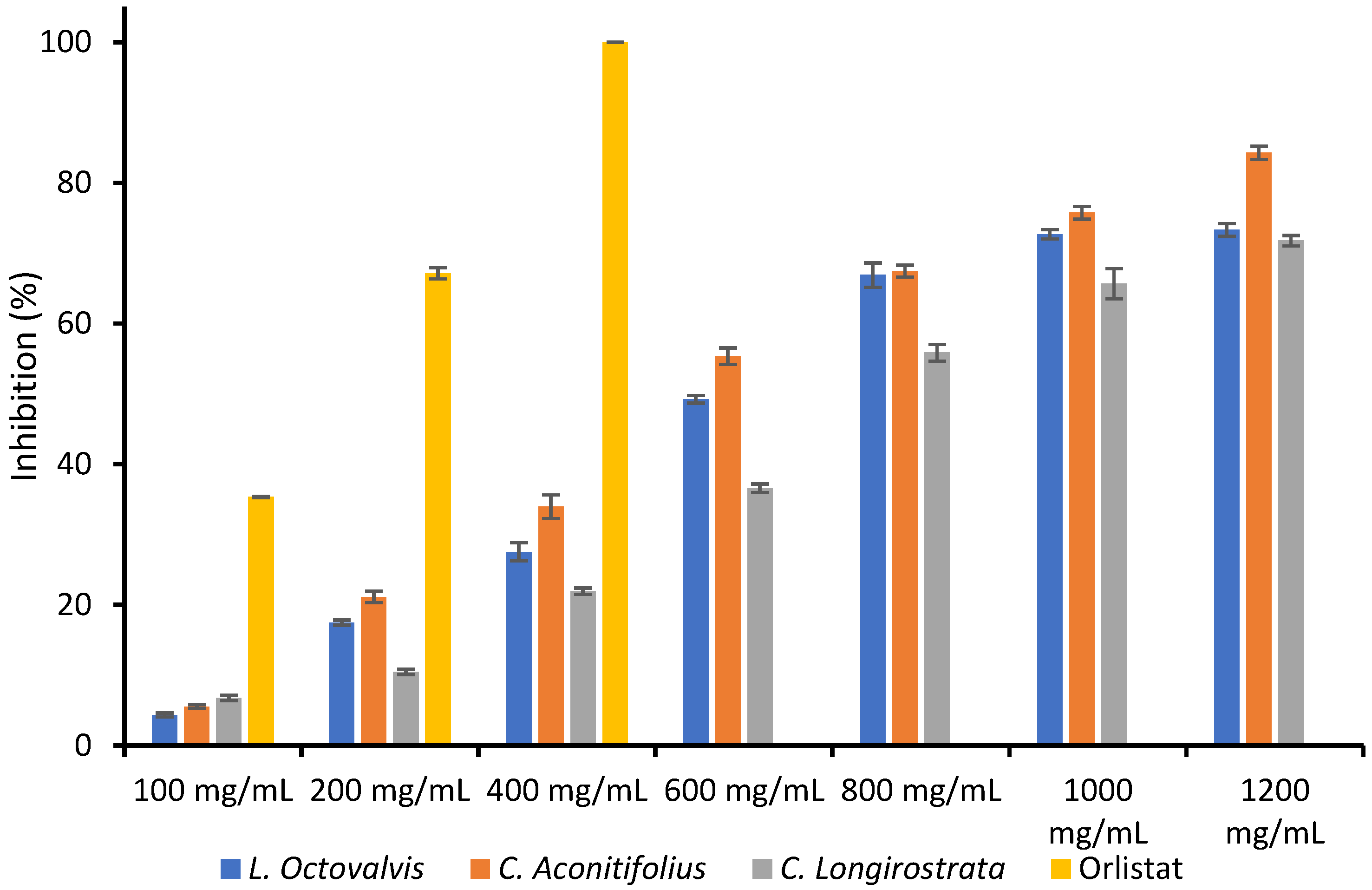4.1. Proximal Composition
One of the objectives of this study was to determine the chemical composition of
L. octovalvis (Jacq.) P.H.Raven,
C. aconitifolius, and
C. longirostrata to gain a better understanding of the nutritional properties of each plant. Oyagbemi et al. (2011) conducted a proximate analysis of
C. aconitifolius, revealing protein content at 14.61%, fat at 2.58%, ash at 9.98%, and fiber at 9.88%. When comparing our results solely with the parameters examined by Oyagbemi et al., we found that the protein content reported in our study (12.52%) was lower than their finding (14.61%). Conversely, our data showed higher fat content (5.67%) compared to theirs (2.58%), and lower ash content (6.66%) in contrast to their value of 9.98%. Notably, our fiber content (54.33%) was substantially higher than their reported value (9.885%). These differences might be influenced by factors such as sample variability, analytical methodologies, environmental conditions, or genetic diversity among the studied
C. aconitifolius specimens. Hence, when comparing nutritional data from different studies, it is crucial to consider these factors for accurate interpretation and understanding of the observed variations [
37].
For
C. longirostrata, a comparative analysis was conducted with a plant of the same genus,
Crotalaria retusa, as reported in the study by Alalade in 2019. The findings revealed notable differences between the two datasets. Specifically, the protein content reported in Alalade et al. (2019) (18.00%) was higher than that observed in our research (14.51%). Likewise, the ash content reported by Alalade et al. (2019) (7.00%) significantly exceeded the value found in our research (0.71%). Furthermore, the insoluble fiber content in our research (31.25%) was substantially higher than the value reported in Alalade et al. (2019) (16.75%) [
38].
These observed variations in protein, ash, and insoluble fiber content may be attributed to several factors, such as differences in plant samples, analytical methods, environmental conditions, or genetic factors that influence the nutritional composition of C. longirostrata. Therefore, it is imperative to consider these factors when comparing nutritional data from various studies to ensure accurate interpretation and comprehension of the discrepancies observed.
A comparison was conducted between
L. octovalvis (Jacq.) P.H.Raven and
Ludwigia grandiflora in a study carried out by Oku et al. in 2020. The choice of Ludwigia grandiflora was due to the unavailability of data for
L. octovalvis in the existing literature. Our experimental findings indicate that
L. octovalvis exhibits a higher protein content (12.70%) compared to the value reported by Oku et al. (10.31%). Additionally, our results show that
L. octovalvis has a lower ash content (6.01%) in contrast to the value presented by Oku et al. (7.89%). Moreover, our data demonstrate a significantly higher level of insoluble fiber in
L. octovalvis (36.31%) compared to Oku et al.’s findings (19.94%) [
39]. These disparities between the datasets for
L. octovalvis should be interpreted with consideration of potential factors such as the comparison with different species that have the same genus, sample source, analytical methods, and experimental procedures, that could contribute to the observed differences.
4.2. Bioactive Compounds
It is important to emphasize that the plant extracts in this study were prepared using hot water instead of an alcoholic solvent. While many research studies commonly employ alcoholic extracts to comprehensively analyze plant composition, the objective of our investigation is to gain insights into the plants from a more realistic perspective, particularly when considering edible plants that are naturally consumed without the extraction of compounds through alcoholic solvents such as methanol or ethanol. The selection of an appropriate solvent and extraction technique is crucial for effectively extracting biologically active compounds from medicinal plants. The polarity of solvents plays a critical role in separating specific compounds with diverse structures and physicochemical properties. Water, ethanol, and glycerol are well-established solvents that are recognized and approved for use in pharmaceutical formulations [
40].
According to Shopska et al. (2019), “free amino nitrogen” (FAN) refers to nitrogen-containing molecules that are readily available, such as amino acids, peptides, and ammonium ions [
41]. The aim of this study was to determine the form in which protein is present, and the results suggest that it contains free amino acids. Amino acids are crucial constituents of plants and serve as the basic building blocks for proteins, the main nitrogen carriers, and signaling molecules. Plant amino acids are produced through three different processes: absorption by roots, reduction in nitrates, and breakdown of ammonium, as explained by Guo et al. (2021) [
42]. Due to the scarce information on the free amino nitrogen content in
L. octovalvis,
C. aconitifolius, and
C. longirostrata, an analysis of FAN content was carried out. When comparing the free amino nitrogen content of
L. octovalvis,
C. aconitifolius, and
C. longirostrata, it can be observed that
C. longirostrata has the highest content, with a mean value of 330.33 ± 12.22 mg/100 g
−1, followed by
L. octovalvis, with a mean value of 259.67 ± 4.16 mg/100 g
−1, and finally,
C. aconitifolius, with a mean value of 225.83 ± 5.55 mg/100 g
−1. Free amino nitrogen includes amino acids, peptides, and ammonium ions and is an important component of plant protein.
Phenolics represent the most extensive category of phytochemicals, playing a pivotal role in the majority of antioxidant activity found within plants and plant-derived products [
43]. The quantification of total phenolics is of significant importance as phenolic compounds serve as vital constituents of plants, exhibiting redox properties that contribute to their antioxidant activity [
44]. Compared to a study made by Yakob et al. in 2012, the total phenolic compounds found in
L. octovalvis using the same methodology was 264.76 ± 0.23 µg GAE/g [
45]. Another study conducted by Lin et al. in 2017 found that the polyphenol content was 146.3 ± 3.1 mg GAE/g [
11], which is lower than the quantity obtained in this research, 652.96 ± 10.58 mg GAE/g. This could be because of a difference in the solvents used (methanol vs. warm water). In the case of
C. aconitifolius, there are few research studies reporting the total phenolic content. In a study conducted by Godínez-Santillán et al. in 2019 [
46], it was found that the total phenolic content was 59.2 ± 2.3 mg CE/g in a methanol:water (50:50) extract. In comparison, in our research, we found a content of 740.57 ± 9.43 mg GAE/100 g, which differs significantly; this was also possibly due to the different solvents used. A study conducted by Jiménez Aguilar and Grusak in 2015 [
47] on
C. longirostrata revealed that the total phenolic compounds ranged from 2.68 ± 0.14 mg GAE/g FW to 3.38 ± 0.24. In comparison, our research found that the total phenolics were 889.66 ± 2.82 mg GAE/100 g. This differs because the result of Aguilar and Grusak’s study is expressed on a fresh weight basis, and ours is expressed on a dry weight basis. Additionally, this difference in results could be attributed to variations in the extraction method employed, as well as the solvent; the previous study utilized 2.5 mL of 90% methanol at 90 °C for 2 h in a water bath, with intermittent vortexing every 30 min. On the other hand, we used hot water at 90 °C for 5 min.
Flavonoids, the largest subgroup among naturally occurring phenolic compounds, are present in various plant components, existing in their unbound state or as glycosides [
43]. Flavonoids, which are widely distributed in plants, have been extensively utilized in traditional herbal remedies. They constitute essential constituents of our diet and are predominantly present in edible plant parts. Flavonoids can be found in various sources such as fruits, vegetables, grains, tree bark, stems, tea, and wine. When addressing complex chronic ailments, conventional treatment methods often rely on polypharmacy, involving the use of multiple medications. It is crucial to recognize that herbal medicines are intricate mixtures comprising diverse elements, both major and minor, and exhibiting multiple targets and processes, thus possessing complex chemical characteristics [
48].
In our investigation, the total flavonoid content in
L. octovalvis was determined to be 337.00 ± 3.61 mg of catechin equivalents per 100 g. However, another study conducted by Pandey et al. in 2023 reported a relatively lower total flavonoid content of 43.9 mg of catechin equivalents per gram. Pandey et al., 2023, employed an extraction method utilizing a mixture of ethanol and water (70:30
v/
v) [
49]. In the case of
C. aconitifolius, the total flavonoid content was determined to be 346.03 ± 4.28 mg of catechin equivalents per 100 g, as indicated in a study conducted by Padilla-Camberos. Another investigation by Padilla-Camberos revealed a flavonoid content of 154.23 ± 3.35 mg of catechin equivalents per milliliter, utilizing an ethyl acetate extract [
50]. The observed significant difference in results could potentially be attributed to the use of different solvents. For
C. longirostrata, we determined that the total flavonoid content was 531.53 ± 10.07 mg of catechin equivalents per 100 g. However, Mendez-Lopez et al. (2022) reported a flavonoid content of 20.8–35.3 mg/g [
51], demonstrating significant discrepancies. These variations can also be attributed to the choice of solvents, specifically hexane in this case.
Trolox, a synthetic analog of α-tocopherol that can dissolve in water, is commonly used as a standard compound to evaluate the Trolox equivalent antioxidant capacity (TEAC) of various food samples. α-tocopherol is widely recognized as the most potent form of vitamin E. The TEAC comparison is based on Trolox’s ability to demonstrate antioxidant activity similar to other natural substances found in food. With its high solubility of approximately 3 mg/mL at neutral pH levels, Trolox is suitable for conducting various antioxidant assays. This allows for the measurement of the antioxidant capacity of food samples by comparing their activity to the known antioxidant capacity of Trolox. Among the most commonly employed methods, a 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay is frequently performed, which involves evaluating the scavenging ability of antioxidants against the DPPH radical [
52].
To validate the findings of this study, a comprehensive review of the literature was conducted to examine previous research on the utilization of 1,1-diphenyl-2-picrylhydrazyl (DPPH) in
L. octovalvis. Although no studies utilizing water extracts were discovered, a study conducted by Yakob et al. in 2012 investigated the application of DPPH using various alcoholic extracts. The outcomes indicated that the methanol extract exhibited the highest DPPH content (1080.84 ± 6.07 μM TE/mg d.w), followed by the ethyl acetate extract (301.48 ± 8.92 μM TE/mg d.w). Conversely, the chloroform extract displayed a lower DPPH content (113.04 ± 1.58 μM TE/mg d.w), while the n-hexane extract exhibited the lowest DPPH content (91.00 ± 0.31 μM TE/mg d.w) [
45]. These findings underscore the significant influence of solvent selection on the DPPH content present in the extracts. In our investigation, a DPPH value of 2.35 ± 0.06 µg ascorbic acid equivalents were determined, thus confirming the substantial impact of solvent choice on the DPPH content.
In the case of
C. aconitifolius, an investigation conducted by Godínez-Santillán et al. in 2019 was discovered, wherein this plant and its DPPH content were examined. It was observed that
C. aconitifolius exhibited a content of 140 ± 6 in the methanol–water (50:50) extract, while the ethanol–water (50:50) extract displayed a content of 102 ± 6 [
46]. These findings diverge significantly from our own research, similar to the DPPH content of
L. octovalvis. Our investigation revealed a DPPH content of 4.34 ± 0.12, representing a substantial difference. This discrepancy may be ascribed to the variances in methodology and the differing solvents employed (methanol, ethanol vs. warm water).
In the case of
C. longirostrata, a comparison was conducted with a previous investigation carried out on
Crotalaria pallida by Alam et al. in 2014. Since no specific studies were found on the same plant species,
Crotalaria pallida was used as it belongs to the same genus [
53]. Comparing the DPPH content between
Crotalaria pallida and
C. longirostrata reveals a significant difference in their antioxidant capacities. The study conducted on
Crotalaria pallida reported a DPPH content of 37.60 μg/mL. In contrast, our investigation on
C. longirostrata found a considerably lower DPPH content of 4.52 ± 0.22 µM TE/g. This discrepancy suggests that
Crotalaria pallida exhibits a higher antioxidant capacity, as indicated by its higher DPPH content, compared to
C. longirostrata. The variation in DPPH content could be attributed to factors such as plant species, extraction methods, or other environmental factors. Further research is needed to elucidate the underlying reasons for this divergence.
Reducing power (FRAP) assay is an efficient and cost-effective technique for directly assessing the overall antioxidant activity of electron-donating antioxidants in a given sample. It involves the reduction of ferric ions (Fe
3+) to ferrous ions (Fe
2+), which triggers a color change, serving as an indicator of the reaction. The method is known for its simplicity, rapidity, and affordability [
47]. Based on our investigation we can conclude that
C. aconitifolius has the highest FRAP value (572.41 ± 4.93 µg ascorbic acid equivalents), indicating stronger total antioxidant activity.
L. octovalvis has a lower FRAP value (443.36 ± 2.14 µg ascorbic acid equivalents), suggesting a moderate level of antioxidant activity. Finally,
C. longirostrata exhibits the lowest FRAP value (411.26 ± 3.92 µg ascorbic acid equivalents), indicating relatively weaker antioxidant activity compared to the other two plants.
To validate the accuracy of our obtained results, a comprehensive literature search was conducted to identify relevant studies that investigated the FRAP (Ferric Reducing Antioxidant Power) content of the same plant species. Although no studies utilizing water extracts were found, a notable study by Yakob et al. in 2012 provided insights into the FRAP content of
L. octovalvis using various alcoholic extracts. Their findings indicated that the methanol extract exhibited the highest FRAP content (1256.88 ± 5.38 μM TE/mg d.w), followed by the ethyl acetate extract (253.45 ± 5.97 μM TE/mg d.w). In contrast, the chloroform extract displayed a relatively lower FRAP content (136.83 ± 3.48 μM TE/mg d.w), while the n-hexane extract demonstrated a similar FRAP content (142.04 ± 3.53 μM TE/mg d.w) [
45]. These results strongly suggest that the choice of solvent for extraction significantly influences the FRAP content of the extracts, with methanol exhibiting the highest antioxidant capacity.
Upon comparing our investigation’s results (443.36 ± 2.14 µg ascorbic acid equivalents) with the findings from the study conducted by Yakob et al. (2012), a notable discrepancy in the FRAP content of the plant extracts became evident. Our investigation reported a significantly higher FRAP content of 443.36 ± 2.14, which contrasts with the values obtained in the Yakob et al. study [
45]. This difference may be attributed to various factors, such as variations in plant samples, extraction methods, or discrepancies in laboratory techniques employed. Further analysis and exploration are required to determine the specific reasons underlying this discrepancy.
In the case of
C. aconitifolius, research conducted by Godínez-Santillán et al. in 2019 was discovered [
46]. In this investigation, the FRAP content of
C. aconitifolius was determined, revealing a content of 133 ± 1 μmol TE/g DW in the methanol–water (50:50) extract, and 86 ± 2 μmol TE/g DW in the ethanol–water (50:50) extract. However, in our own research, we found a content of 572.41 ± 4.93 µg ascorbic acid equivalents. These discrepancies can primarily be attributed to the use of water-only extraction in our study, while their investigation involved a combination with an alcohol extract, leading to potential differences in the measured quantities.
In the case of
C. longirostrata, no FRAP content reports were available. However, in a study conducted by Govindappa et al. in 2011, the FRAP content of the water extract from
Crotalaria pallida was reported as 1323.08 ± 0.03 μmol/L [
54]. In contrast, our research on
C. longirostrata determined a FRAP value of 411.26 ± 3.92 µg ascorbic acid equivalents. These results indicate a significant difference in the FRAP content between the two studies. Various factors could contribute to this disparity, such as variations in the specific plant species, differences in methodology, variations in the growth conditions or harvesting period, and the choice of solvent for extracting and quantifying the FRAP content.