Dynamic Prediction Model for Initial Apple Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Materials and Instruments
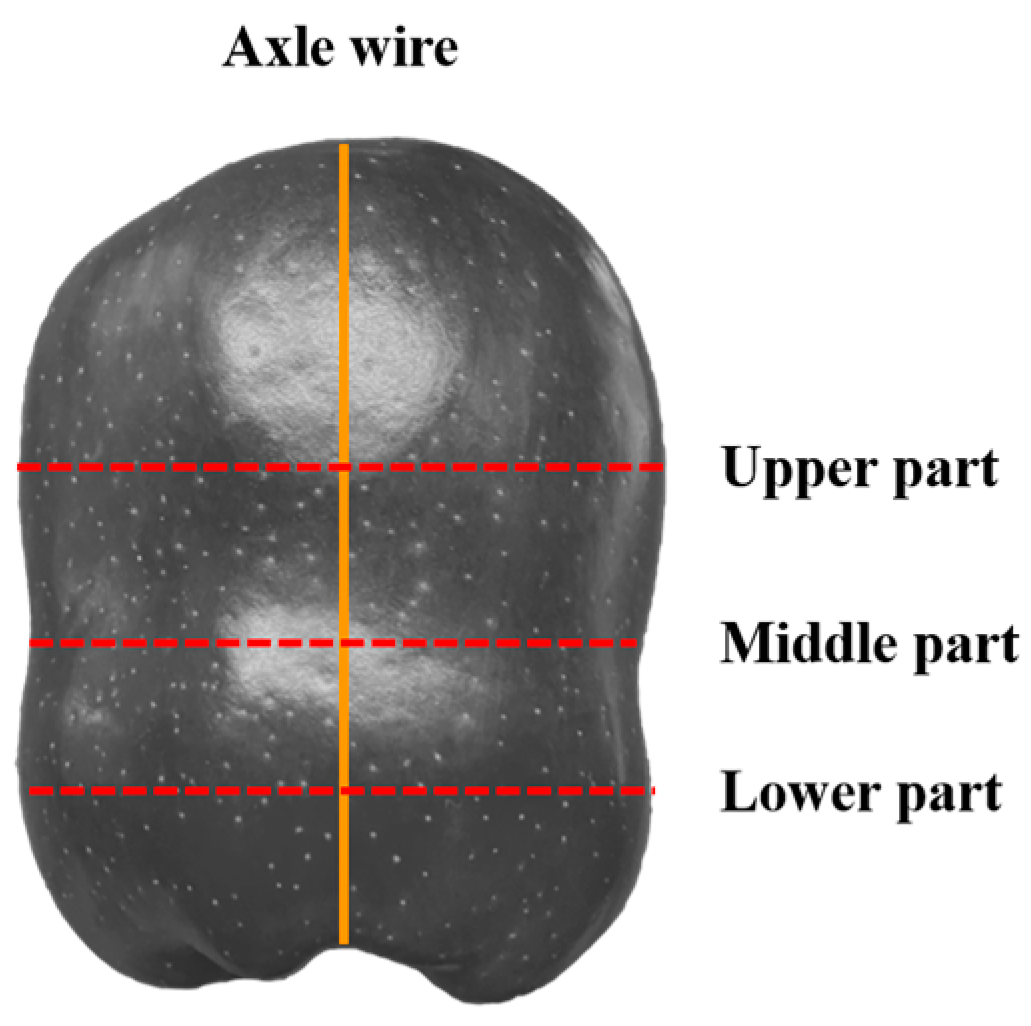
2.2. Observation and Localization Methods of Damage
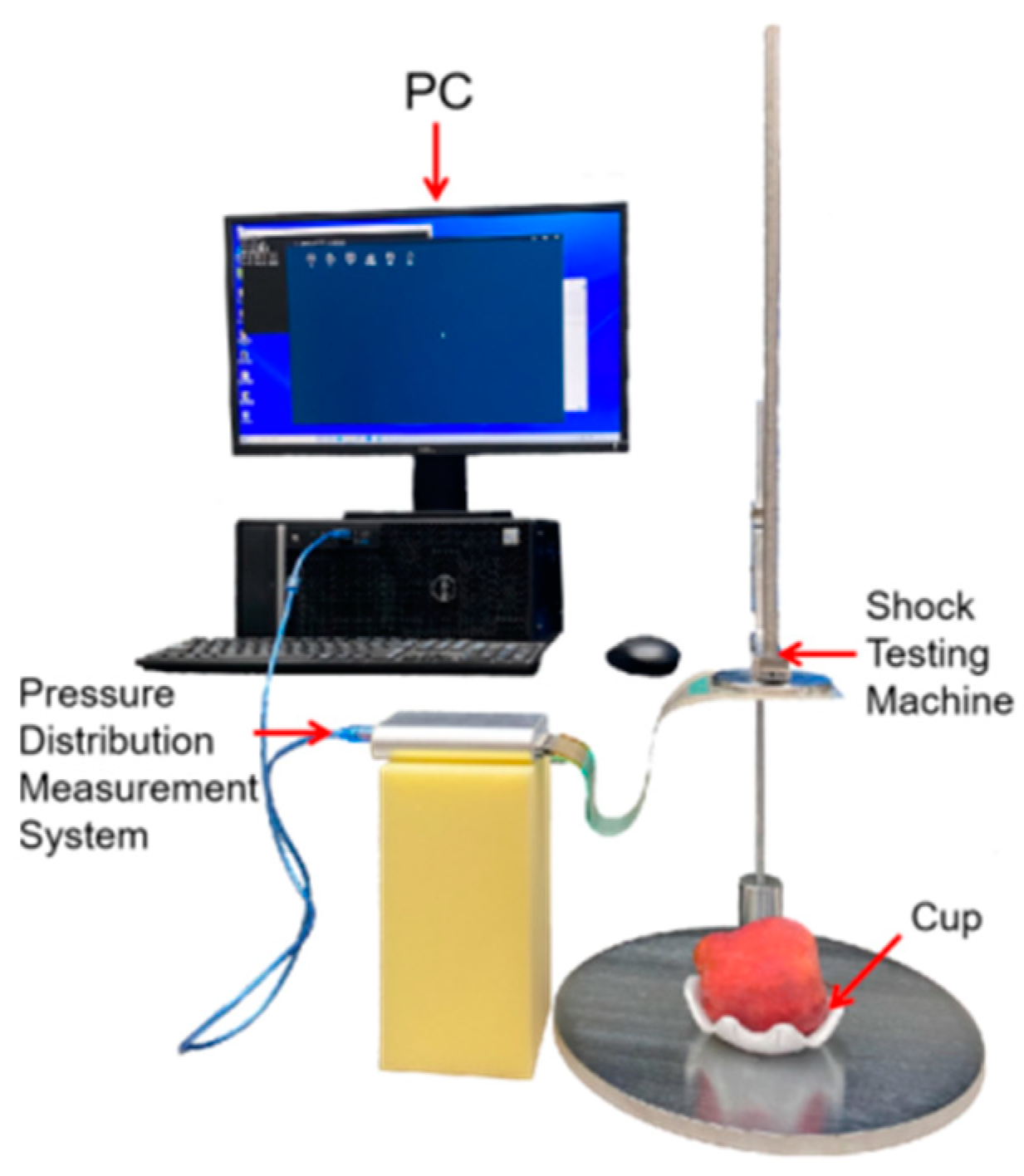
2.3. Experiment of Mechanical Damage
2.4. Collection of the Microscopic Images of the Damaged Parts
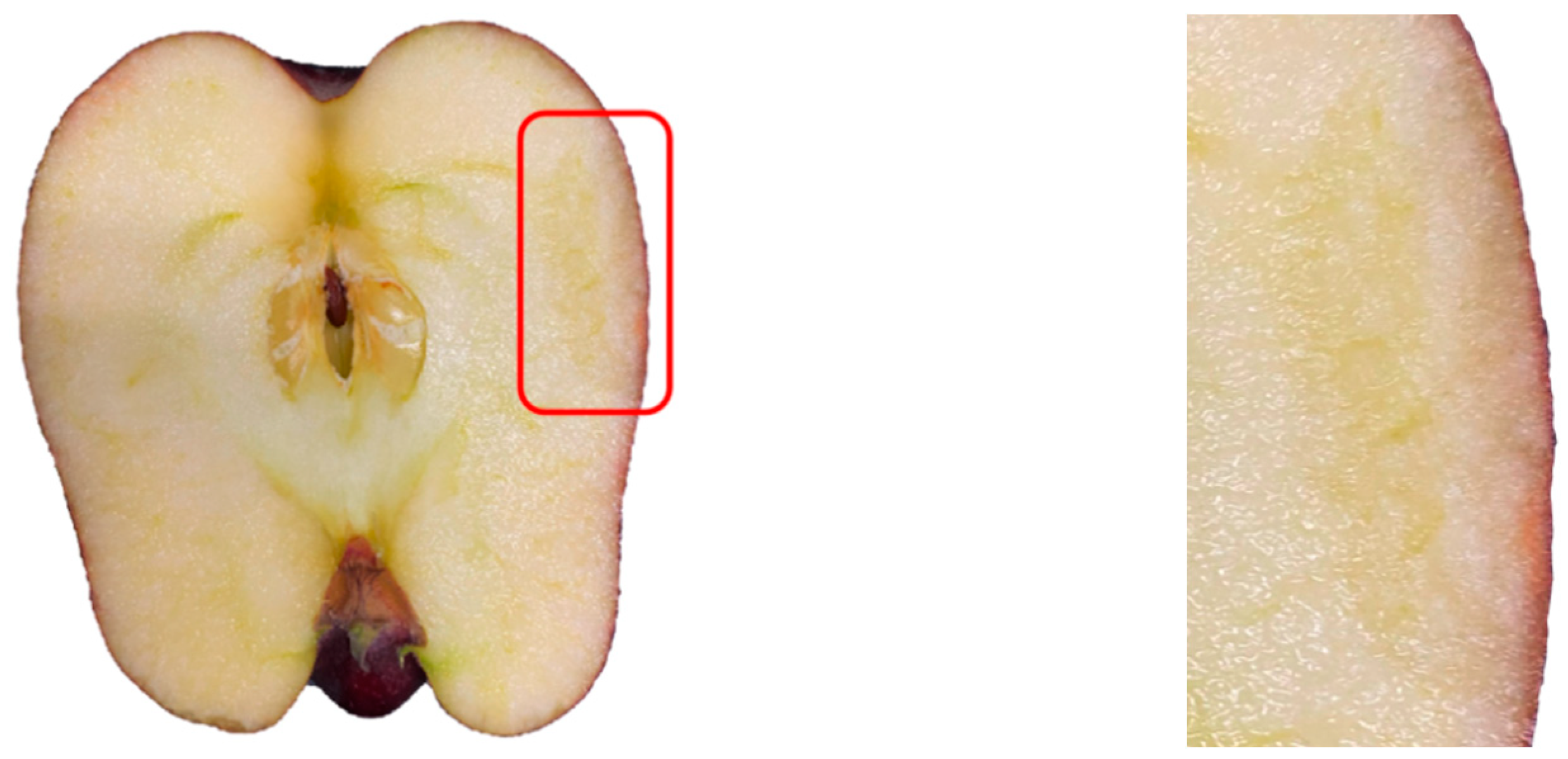
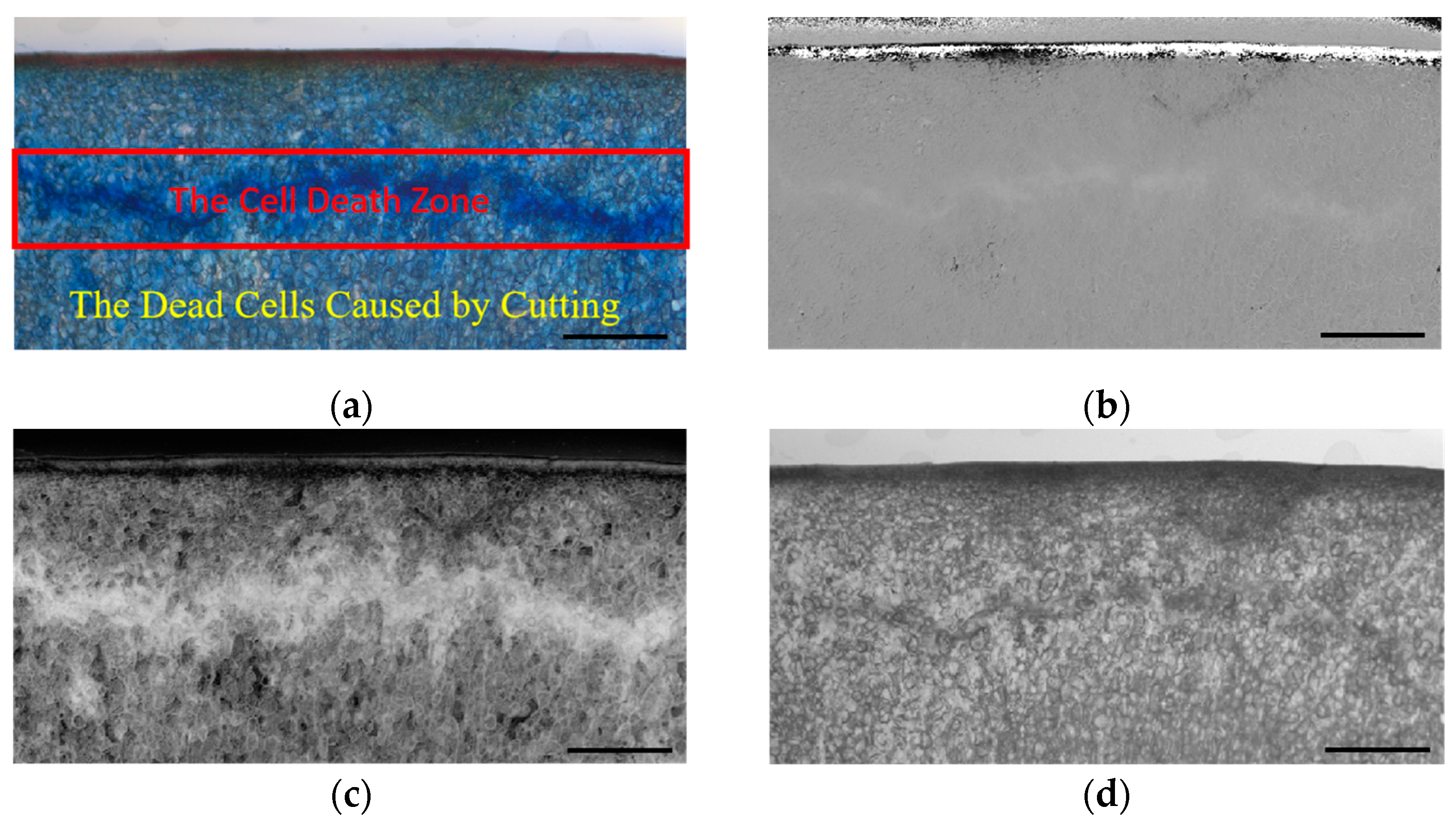
2.5. Determination of Cell Death Zone
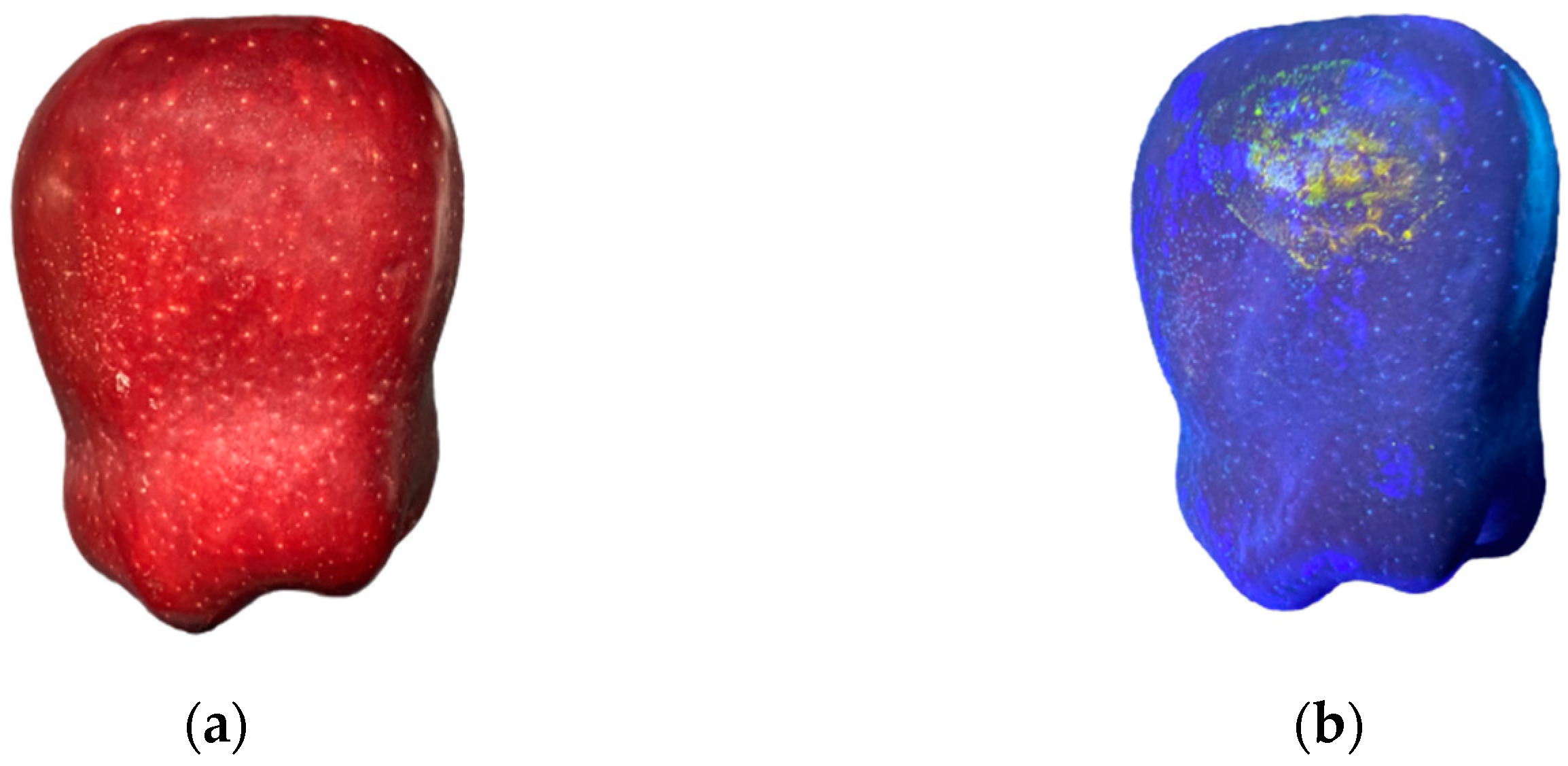
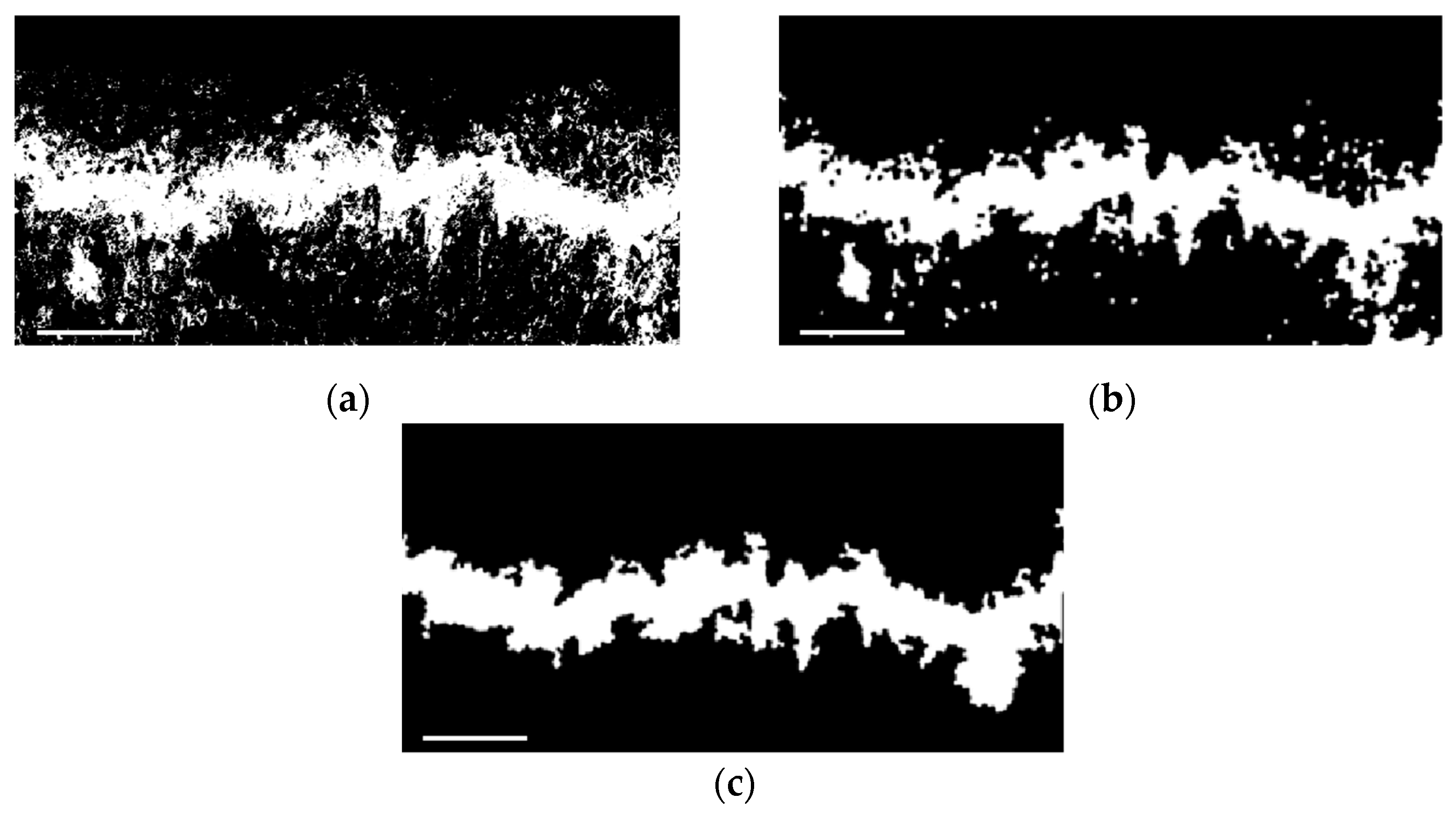
2.5.1. Extraction of Damage Area
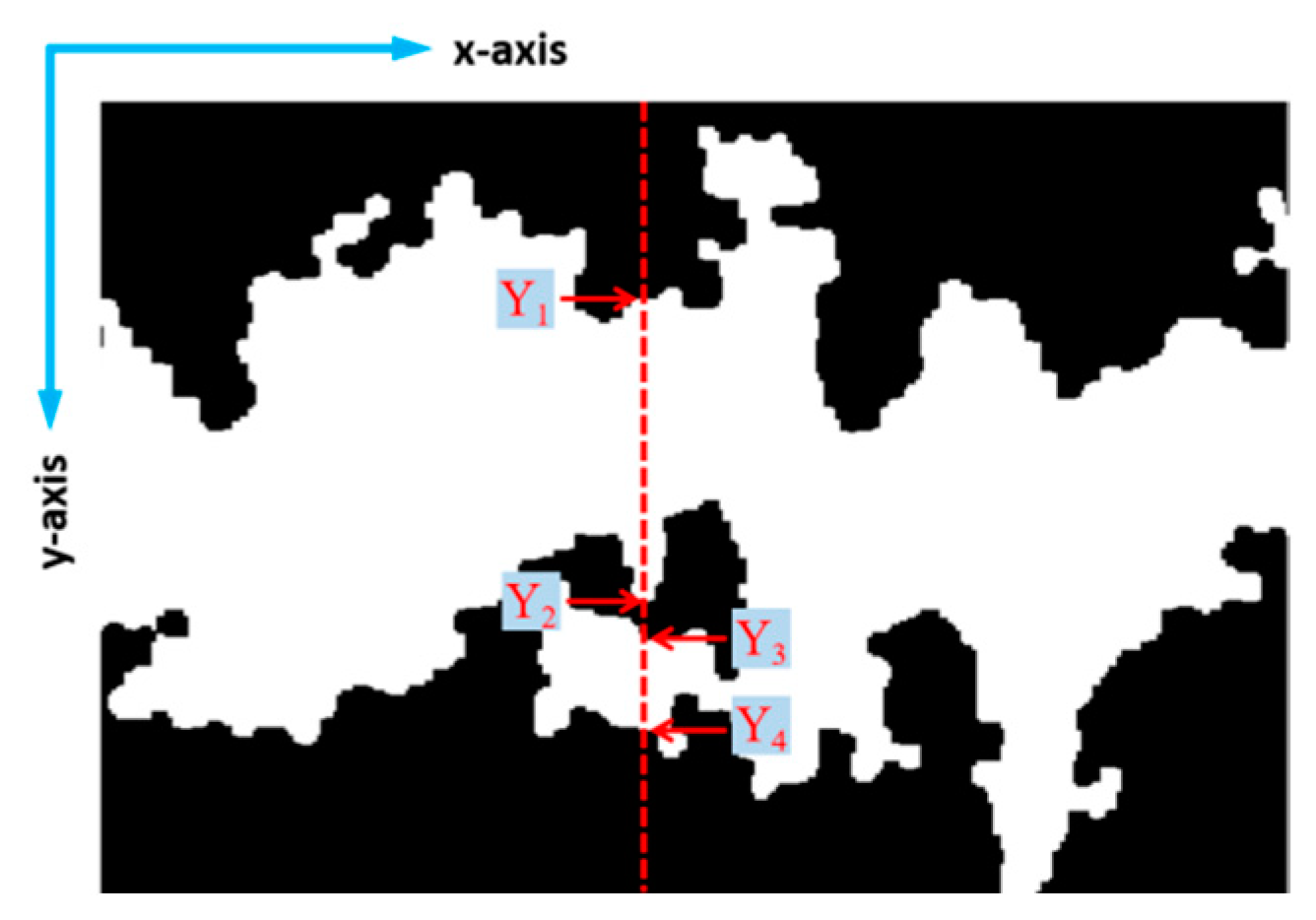
2.5.2. Collection of Damage Data
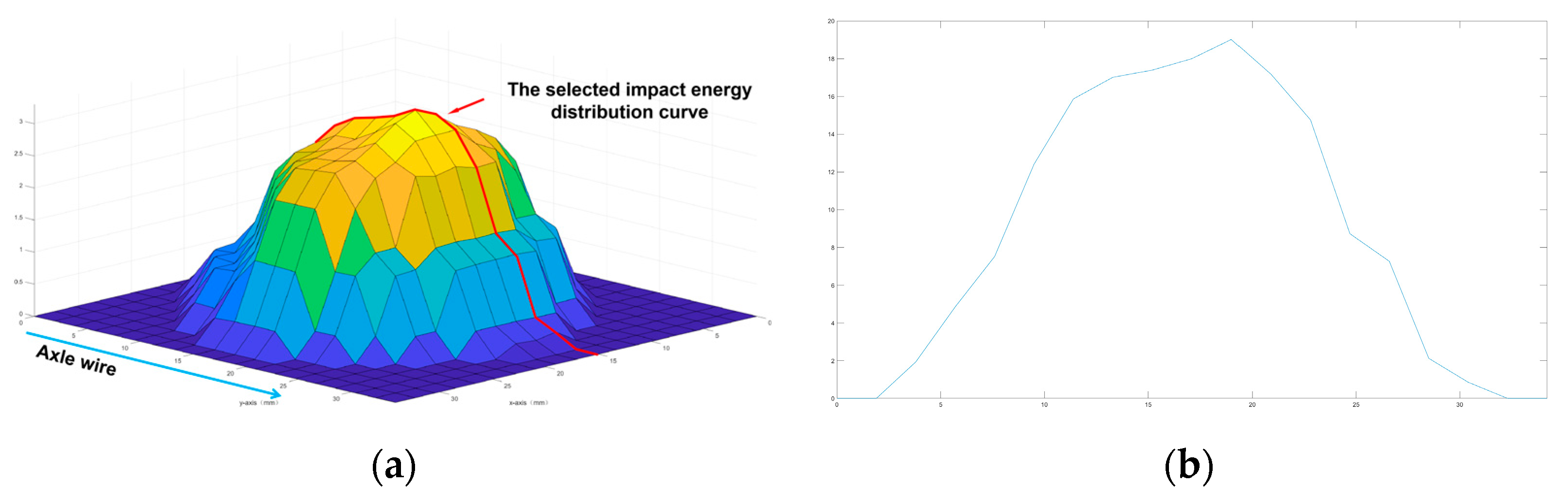
2.6. Collection of the Data for Impact Energy Distribution
2.7. Experiment of Cell Size Distribution
2.8. Experiment for the Determination of Biological Yield Points
3. Results and Discussion
3.1. Theoretical Analysis of the Mechanism of the Phenomenon of the Cell Death Zone
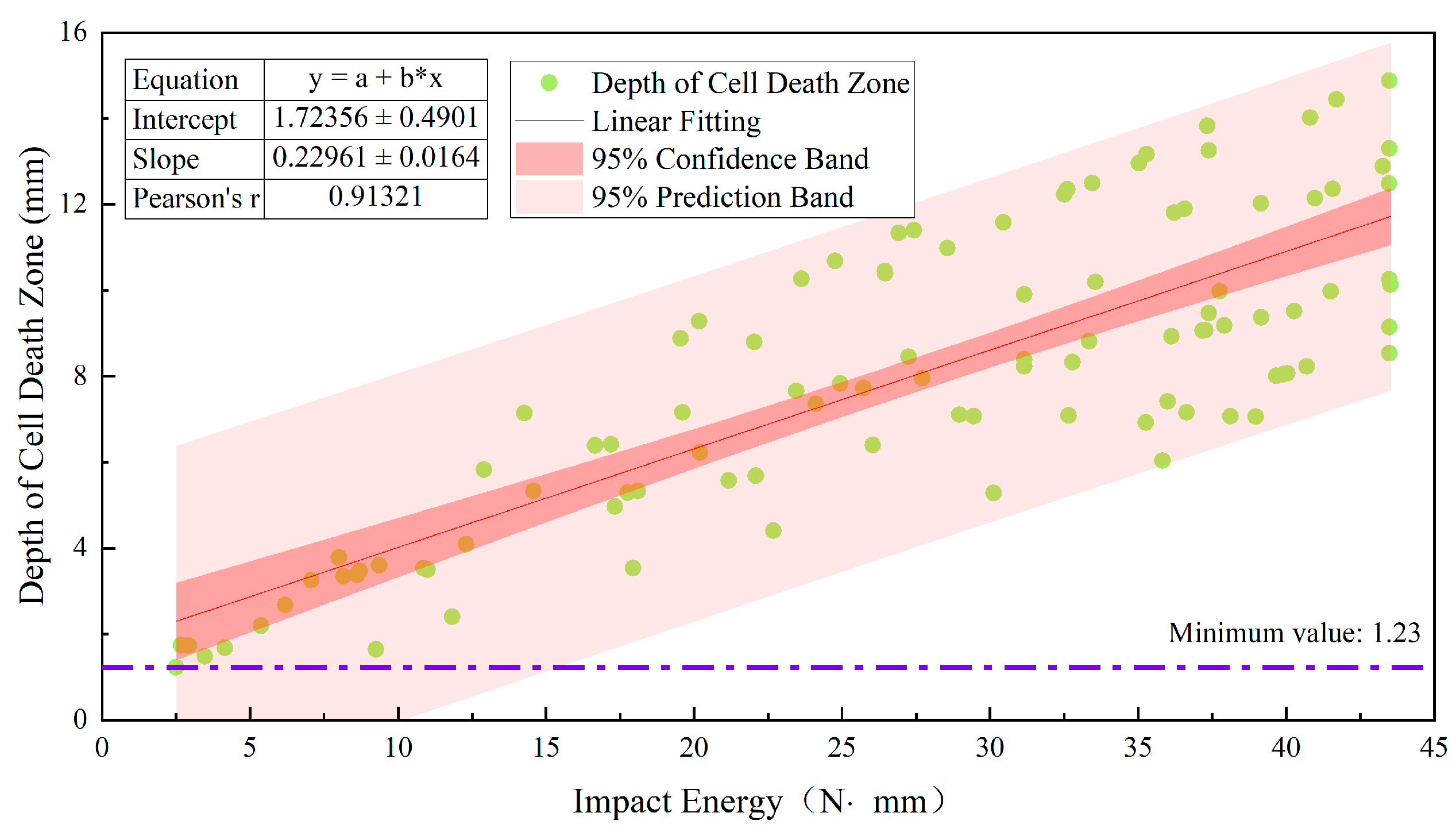
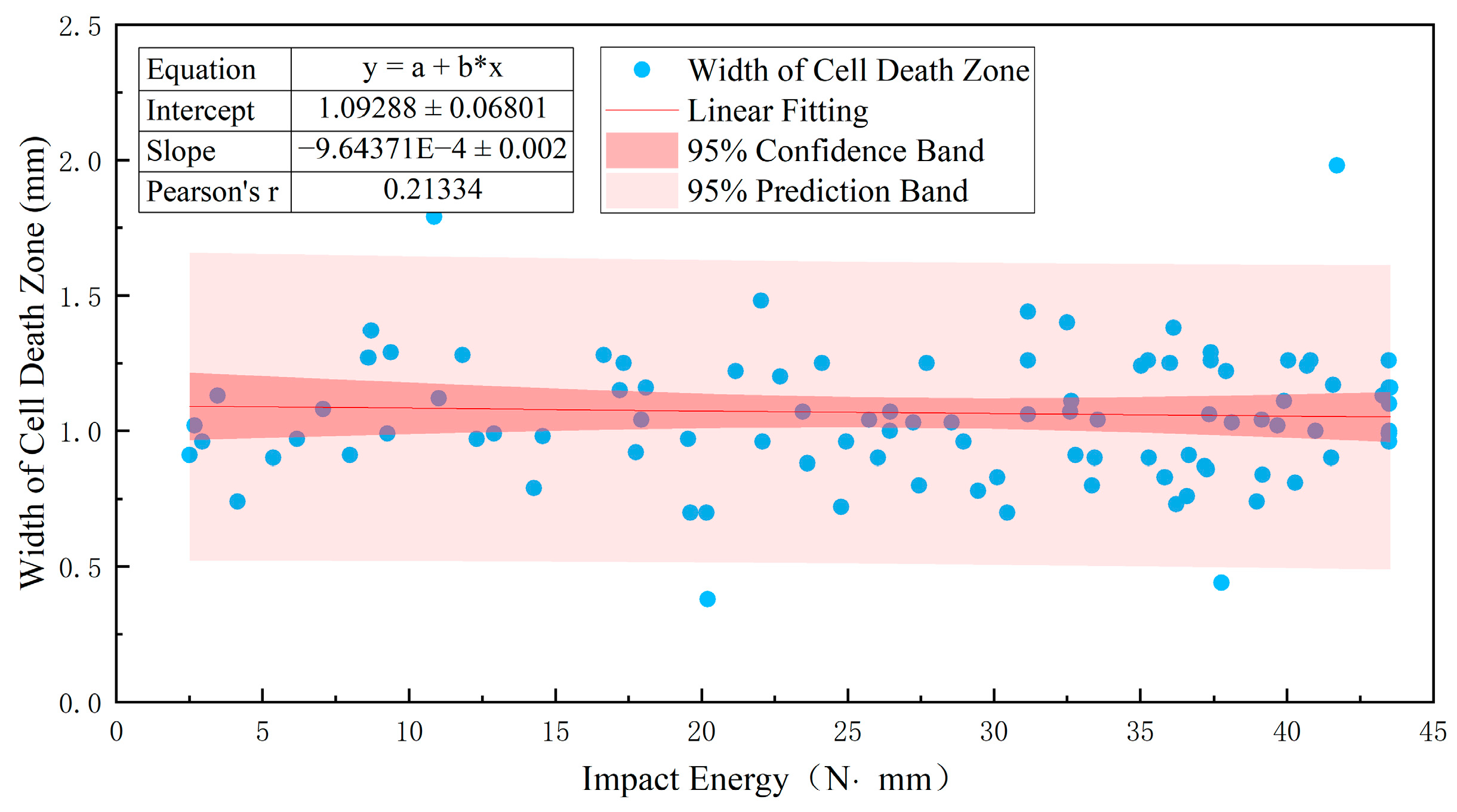
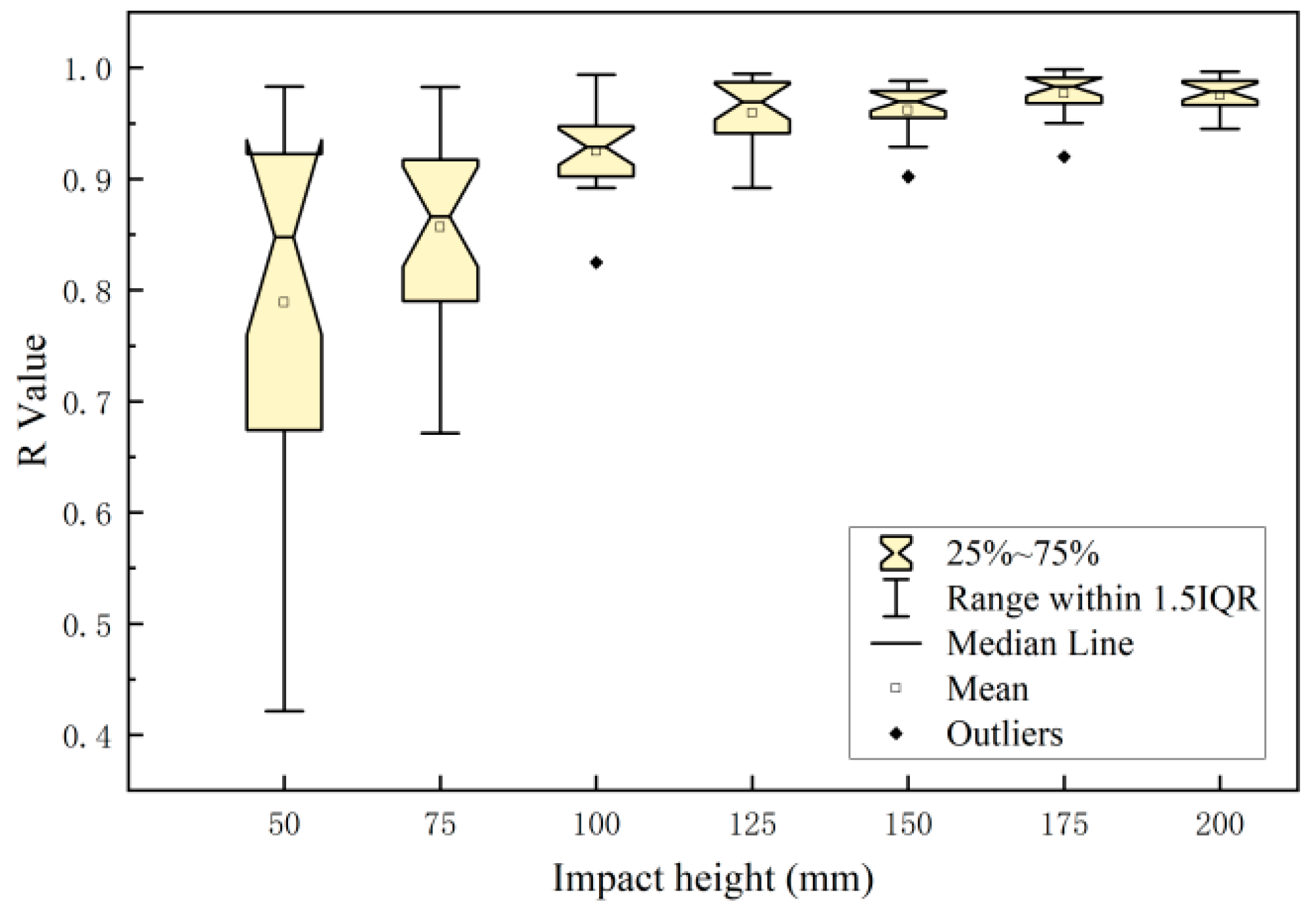
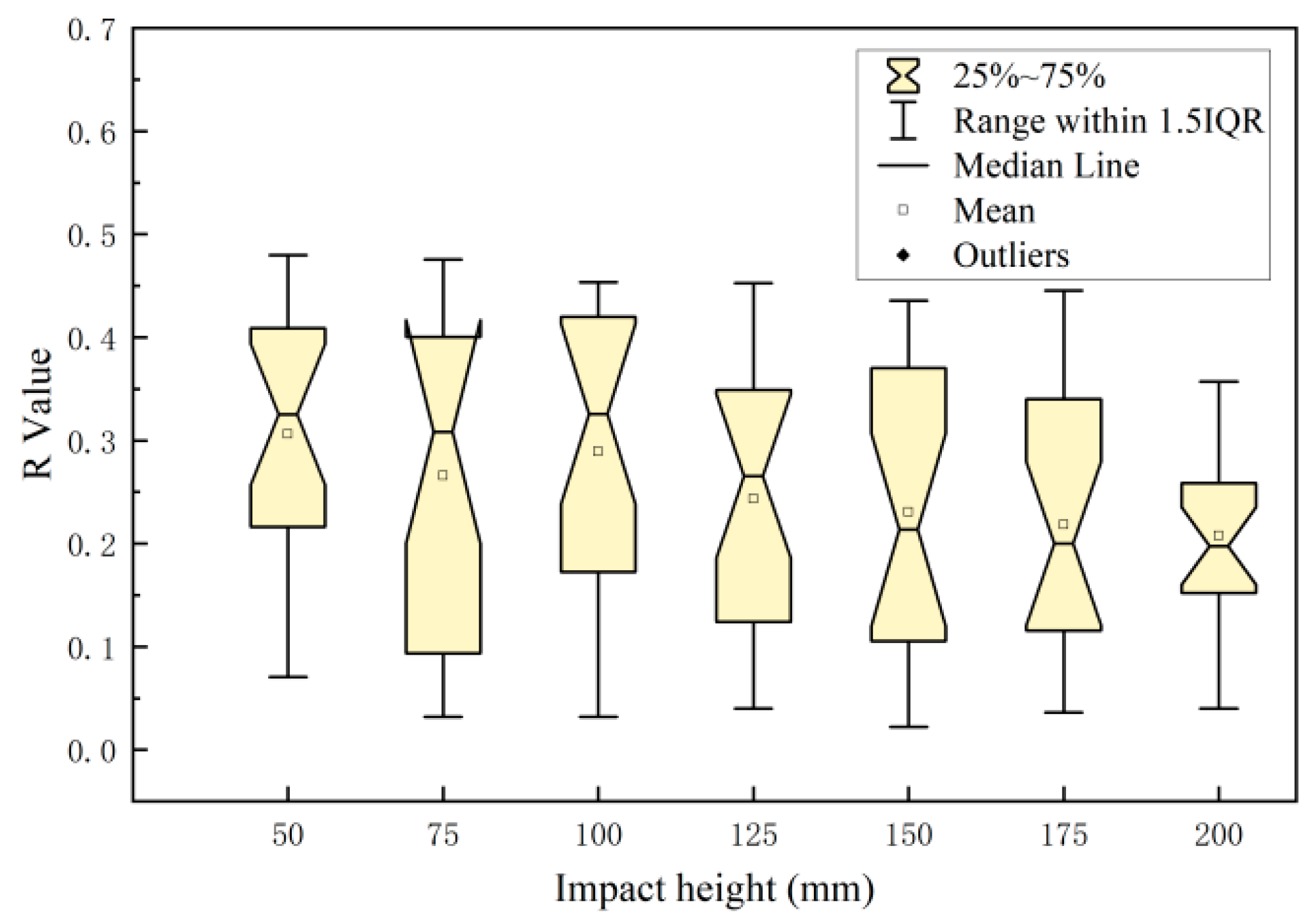
3.2. Relationship between Damage Parameters and Impact Energy Distribution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Harvest and Postharvest Factors Affecting Bruise Damage of Fresh Fruits. Hortic. Plant J. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- GB/T 10651-2008; Fresh Apples. Standardization Administration of the People’s Republic of China: Beijing, China, 2008; Volume 16. Available online: http://c.gb688.cn/bzgk/gb/showGb?type=online&hcno=39F27DD712D12CB6B8AA606228978445 (accessed on 5 July 2023).
- United States Department of Agriculture. United States Standards for Grades of Apples. In The Federal Register/FIND; United States Department of Agriculture: Washington, DC, USA, 2019; Volume 84. Available online: https://www.ams.usda.gov/sites/default/files/media/Apple_Standards.pdf (accessed on 5 July 2023).
- UNECE STANDA. Apples. 2020. Available online: https://unece.org/sites/default/files/2020-12/50_Apples.pdf (accessed on 5 July 2023).
- Kyle, D.; Isabel, S.; Matthew, H. Susceptibility of Impact Damage to Whole Apples Packaged Inside Molded Fiber and Expanded Polystyrene Trays. Foods 2021, 10, 1980. [Google Scholar] [CrossRef]
- Opara, U.L.; Pathare, P.B. Bruise damage measurement and analysis of fresh horticultural produce—A review. Postharvest Biol. Technol. 2014, 91, 9–24. [Google Scholar] [CrossRef]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Bruise damage susceptibility of pomegranates (Punica granatum L.) and impact on fruit physiological response during short term storage. Sci. Hortic. 2019, 246, 664–674. [Google Scholar] [CrossRef]
- Lu, L.-X.; Wang, Z.-W. Dropping Bruise Fragility and Bruise Boundary of Apple Fruit. Trans. ASABE 2007, 50, 1323–1329. [Google Scholar] [CrossRef]
- Ahmad, D. Application of sorting dependent criterias in determination of volume and area bruising of “Golab-Kohanz” apple in Iran. Genetika 2012, 44, 177–187. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.; Castro-García, S.; Blanco-Roldán, G.L.; Ferguson, L.; Rosa, U.A.; Gil-Ribes, J.A. Table olive cultivar susceptibility to impact bruising. Postharvest Biol. Technol. 2013, 86, 100–106. [Google Scholar] [CrossRef]
- Stopa, R.; Szyjewicz, D.; Komarnicki, P.; Kuta, Ł. Limit values of impact energy determined from contours and surface pressure distribution of apples under impact loads. Comput. Electron. Agric. 2018, 154, 1–9. [Google Scholar] [CrossRef]
- Fu, H.; Du, W.; Yang, J.; Wang, W.; Wu, Z.; Yang, Z. Bruise measurement of fresh market apples caused by repeated impacts using a pendulum method. Postharvest Biol. Technol. 2023, 195, 112143. [Google Scholar] [CrossRef]
- Wang, L.-J.; Zhang, Q.; Song, H.; Wang, Z.-W. Mechanical damage of ‘Huangguan’ pear using different packaging under random vibration. Postharvest Biol. Technol. 2022, 187, 111847. [Google Scholar] [CrossRef]
- Ahmad, F.; Zaidi, S.; Arshad, M. Postharvest quality assessment of apple during storage at ambient temperature. Heliyon 2021, 7, e07714. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Guo, W.; Huang, X.; Du, R.; Zhu, X. Effect of pressing and impacting bruises on optical properties of kiwifruit flesh. Postharvest Biol. Technol. 2021, 172, 111385. [Google Scholar] [CrossRef]
- Kleynen, O.; de la Cierva, S.; Destain, M.F. Evolution of pressure distribution during apple compression tests measured with tactile sensors. Acta Hortic. 2003, 604, 591–596. [Google Scholar] [CrossRef]
- Hu, Z.-H. Plant Anatomy; Higher Education Press: Beijing, China, 2010. Chapter 5–8. (In Chinese) [Google Scholar]
- Salguero-Linares, J.; Serrano, I.; Ruiz-Solani, N.; Salas-Gomez, M.; Phukan, U.J.; Gonzalez, V.M.; Bernardo-Faura, M.; Valls, M.; Rengel, D.; Coll, N.S. Robust transcriptional indicators of immune cell death revealed by spatiotemporal transcriptome analyses. Mol. Plant 2022, 15, 1059–1075. [Google Scholar] [CrossRef]
- American Society of Agricultural and Biological. Engineers Compression Test of Food Materials of Convex Shape. ASAE S368.4 DEC2000(R2008). 2008. Available online: https://webstore.ansi.org/preview-pages/ASABE/preview_ASAE+S368.4+DEC2000+(R2017).pdf (accessed on 7 July 2023).
- Mitsuhashi-Gonzalez, K.; Pitts, M.J.; Fellman, J.K.; Curry, E.A.; Clary, C.D. Bruising Profile of Fresh Apples Associated with Tissue Type and Structure. Appl. Eng. Agric. 2010, 26, 509–517. [Google Scholar] [CrossRef]
- Li, H.-G.; Liu, J.-L.; Dang, M.-L.; Yang, H.-J.; Fan, X.-G.; Yang, Y.-Z.; Zhao, Z.-Y. Comparative analysis of the fruit tissue structure of the new variety ‘Ruiyang’ of apples and its parents. J. Fruit Sci. 2018, 35, 1182–1189. (In Chinese) [Google Scholar] [CrossRef]
- Seymour, G.B.; Manning, K.S.; Eriksson, E.M.; Popovich, A.; King, G.J. Genetic identification and genomic organization of factors affecting fruit texture. J. Exp. Bot. 2002, 53, 2065–2071. Available online: https://www.jstor.org/stable/23697404 (accessed on 7 July 2023). [CrossRef]
- Alvarez, M.D.; Saunders, D.E.J.; Vincent, J.F.V. Fracture properties of stored fresh and osmotically manipulatedapple tissue. Eur. Food Res. Technol. 2000, 211, 284–290. [Google Scholar] [CrossRef]
- Gao, G.-F. Introduction to Stress Waves in Solids; Science Press: Beijing, China, 2022. Chapter 7. (In Chinese) [Google Scholar]
- Diels, E.; Odenthal, T.; Keresztes, J.; Vanmaercke, S.; Verboven, P.; Nicolaï, B.; Saeys, W.; Ramon, H.; Smeets, B. Development of a visco-elastoplastic contact force model and its parameter determination for apples. Postharvest Biol. Technol. 2016, 120, 157–166. [Google Scholar] [CrossRef]
- Liwei, W.; Dan, H. Energy dissipation study in impact: From elastic and elastoplastic analysis in peridynamics. Int. J. Solids Struct. 2022, 234–235, 111279. [Google Scholar] [CrossRef]
- Waymel, R.F.; Wang, E.; Awasthi, A.; Geubelle, P.H.; Lambros, J. Propagation and dissipation of elasto-plastic stress waves in two dimensional ordered granular media. J. Mech. Phys. Solids 2018, 120, 117–131. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Li, L.-Y.; Thornton, C. Energy dissipation during normal impact of elastic and elastic–plastic spheres. Int. J. Impact Eng. 2005, 32, 593–604. [Google Scholar] [CrossRef]
- Vaz-Romero, A.; Mercier, S.; Rodríguez-Martínez, J.A.; Molinari, A. A comparative study of the dynamic fragmentation of non-linear elastic and elasto-plastic rings: The roles of stored elastic energy and plastic dissipation. Mech. Mater. 2019, 132, 134–148. [Google Scholar] [CrossRef]
- Liu, T.-L.; Zhang, H.; Wu, F.-Q.; Liu, C.-B. Experimental study on energy dissipation characteristics of frozen soil under uniaxial impact load. China Mine Eng. 2023, 52, 1–6. (In Chinese) [Google Scholar] [CrossRef]
- Stropek, Z.; Gołacki, K. Quantity assessment of plastic deformation energy under impact loading conditions of selected apple cultivars. Postharvest Biol. Technol. 2016, 115, 9–17. [Google Scholar] [CrossRef]
- Reid, M. Fruit quality and its biological basis. Postharvest Biol. Technol. 2003, 29, 107–108. [Google Scholar] [CrossRef]
- Ebrahim, C.; Ebrahim, A.; Alavi, N.A.; Rahman, S. Determination of dynamic deformation behavior of Golden Delicious apple using finite element method and its validation by scanning electron microscopy. Sci. Hortic. 2023, 307, 111531. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Zhu, Y.; Zhang, X.; Wu, Z.; Rao, X. Dynamic Prediction Model for Initial Apple Damage. Foods 2023, 12, 3732. https://doi.org/10.3390/foods12203732
Xu T, Zhu Y, Zhang X, Wu Z, Rao X. Dynamic Prediction Model for Initial Apple Damage. Foods. 2023; 12(20):3732. https://doi.org/10.3390/foods12203732
Chicago/Turabian StyleXu, Tao, Yihang Zhu, Xiaomin Zhang, Zheyuan Wu, and Xiuqin Rao. 2023. "Dynamic Prediction Model for Initial Apple Damage" Foods 12, no. 20: 3732. https://doi.org/10.3390/foods12203732
APA StyleXu, T., Zhu, Y., Zhang, X., Wu, Z., & Rao, X. (2023). Dynamic Prediction Model for Initial Apple Damage. Foods, 12(20), 3732. https://doi.org/10.3390/foods12203732





