Comparison and Characterization of the Structure and Physicochemical Properties of Three Citrus Fibers: Effect of Ball Milling Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Modified Citrus Fiber by BM
2.3. Determination of Citrus Fiber Components
2.4. Determination of SDF, IDF and TDF Content
2.5. Particle Size Analysis
2.6. Determination of WHC
2.7. Determination of OHC
2.8. Determination of WSC
2.9. Determination of CAC
2.10. Determination of Adsorption Capacity of Sodium Cholate
2.11. Fourier Transform Infrared Spectroscopy (FTIR)
2.12. Scanning Electron Microscopy (SEM)
2.13. Statistical Analysis
3. Results and Discussion
3.1. The Content of IDF, SDF and TDF
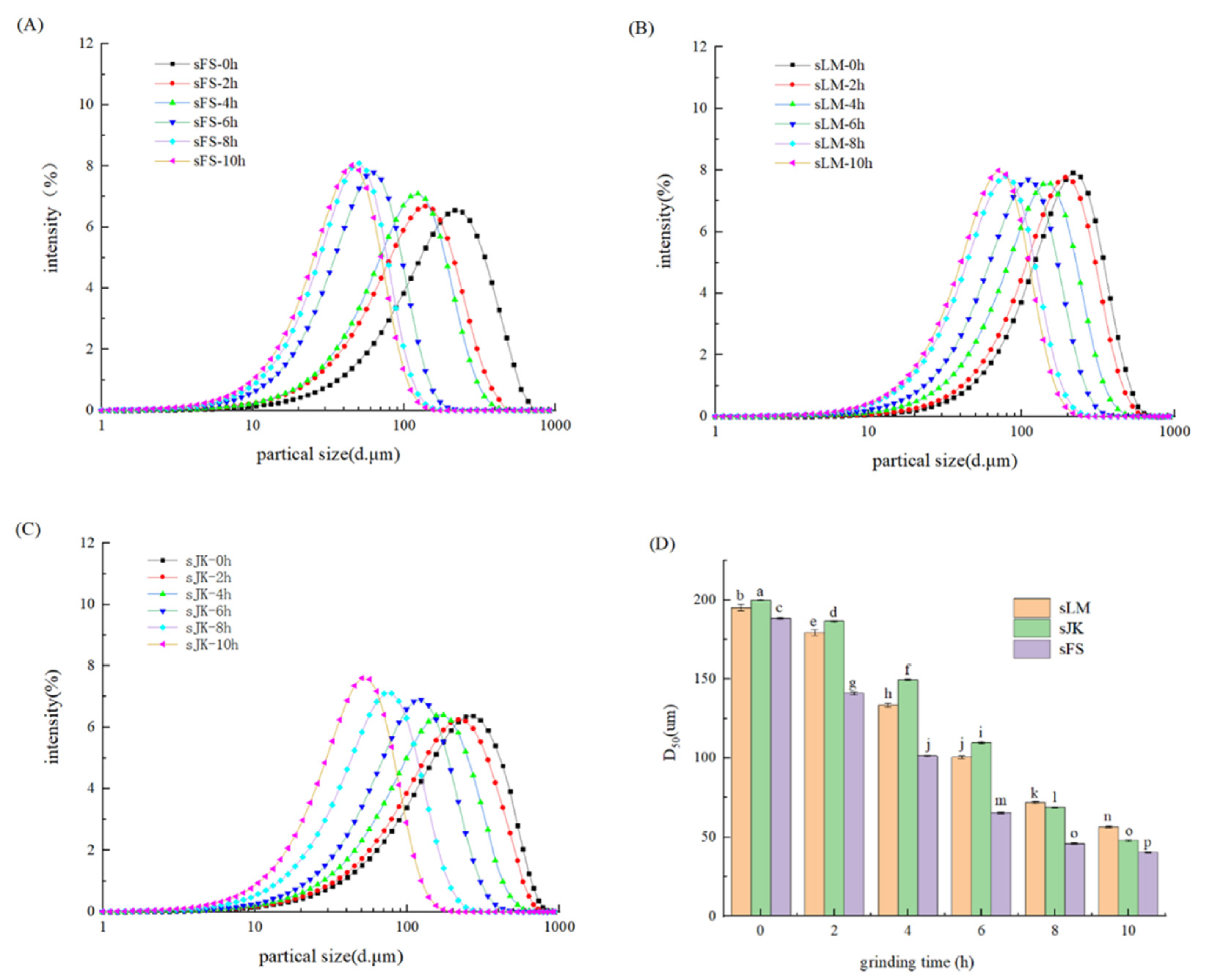
3.2. Particle Size Distribution
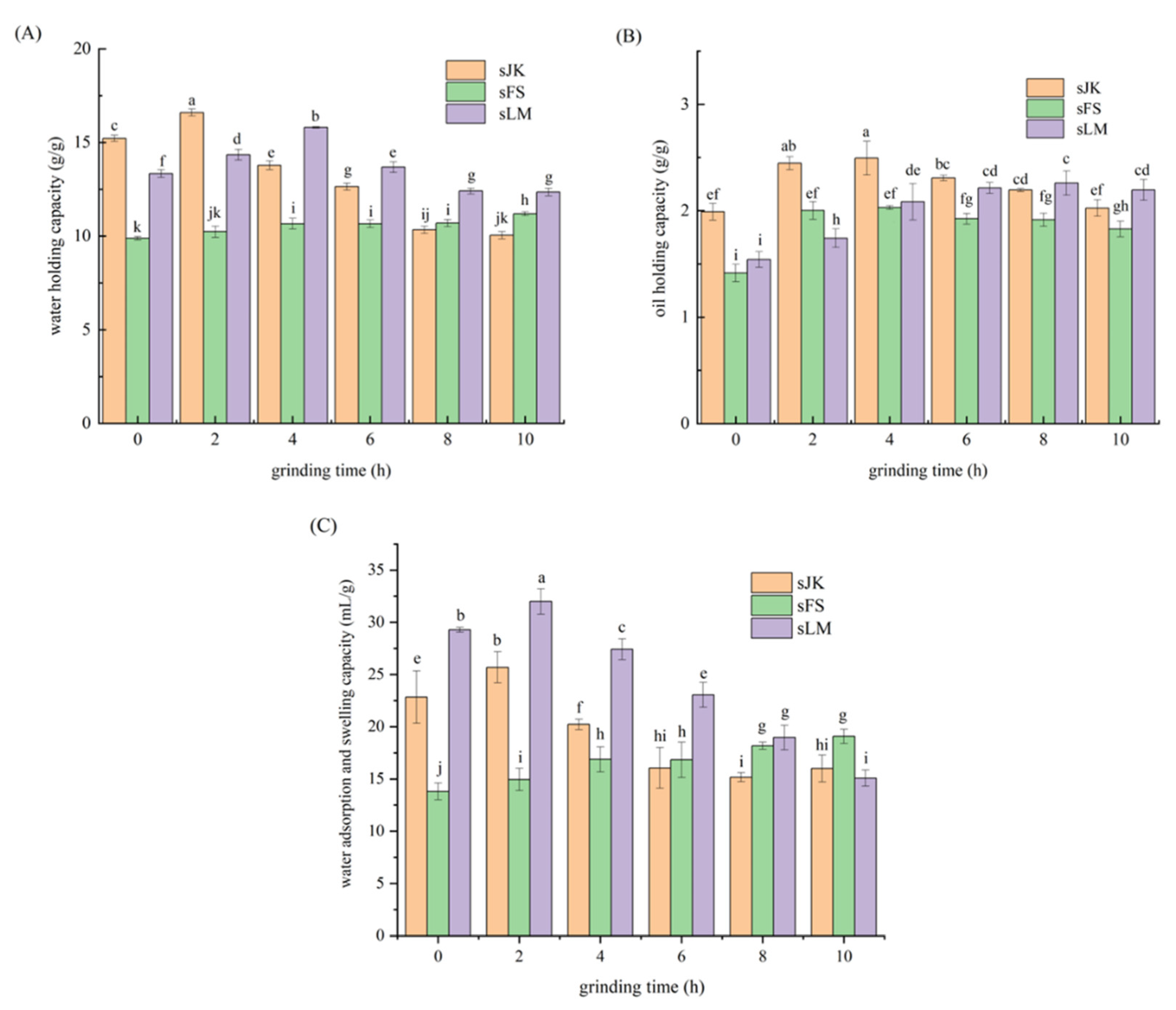
3.3. Analysis of WHC
3.4. Analysis of OHC
3.5. Analysis of WSC
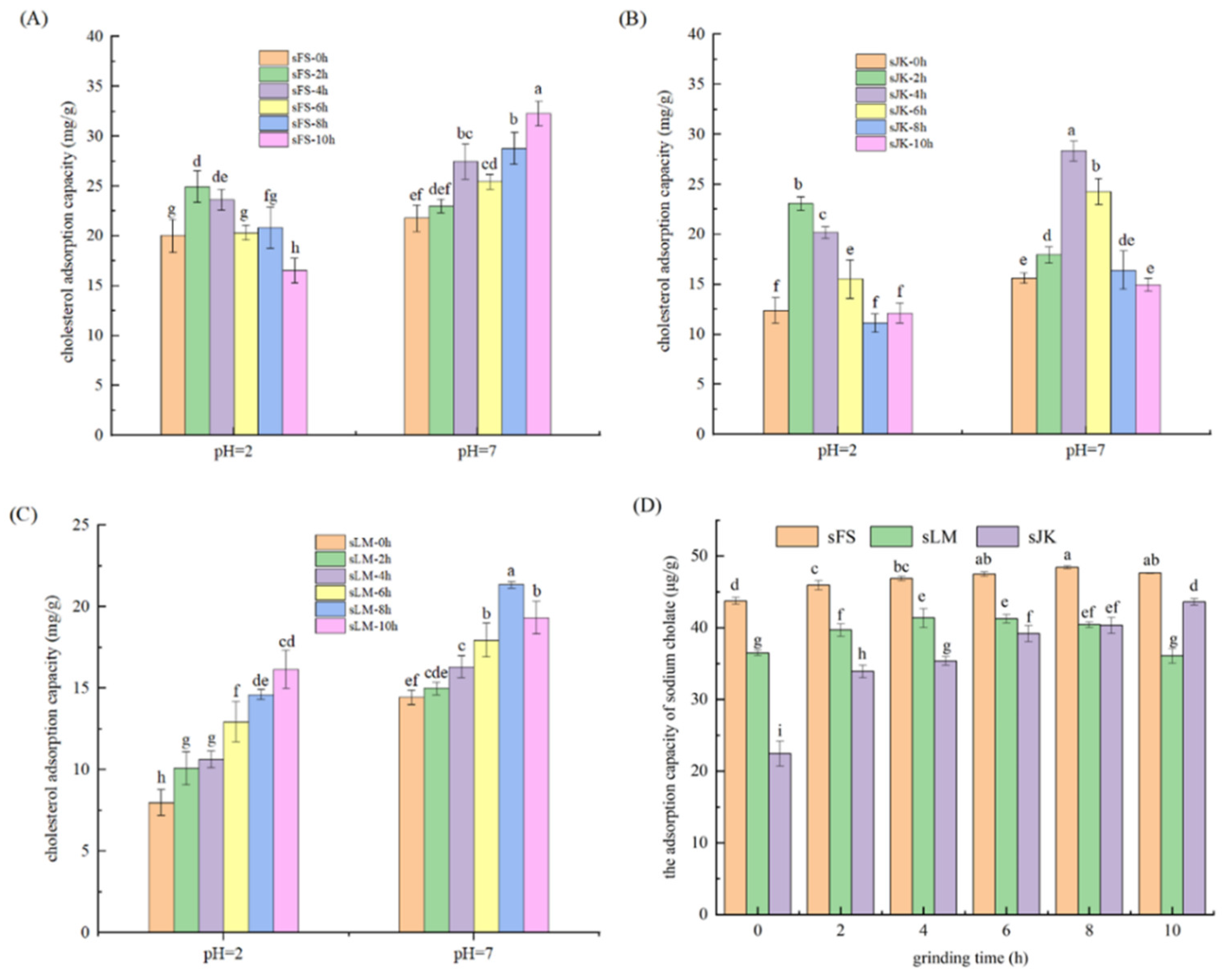
3.6. Analysis of CAC
3.7. Analysis of Adsorption Capacity of Sodium Cholate
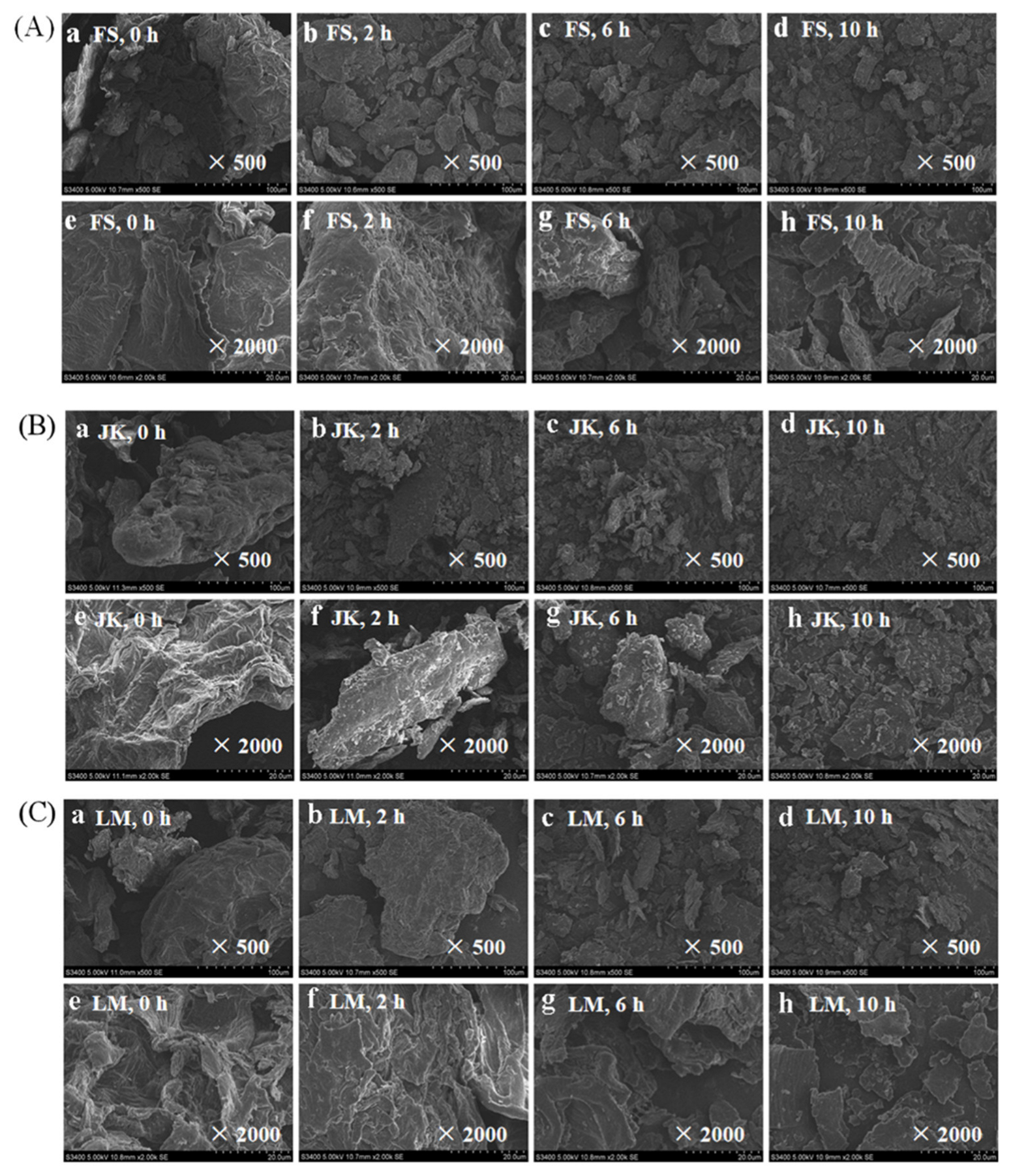
3.8. The Apparent Structure
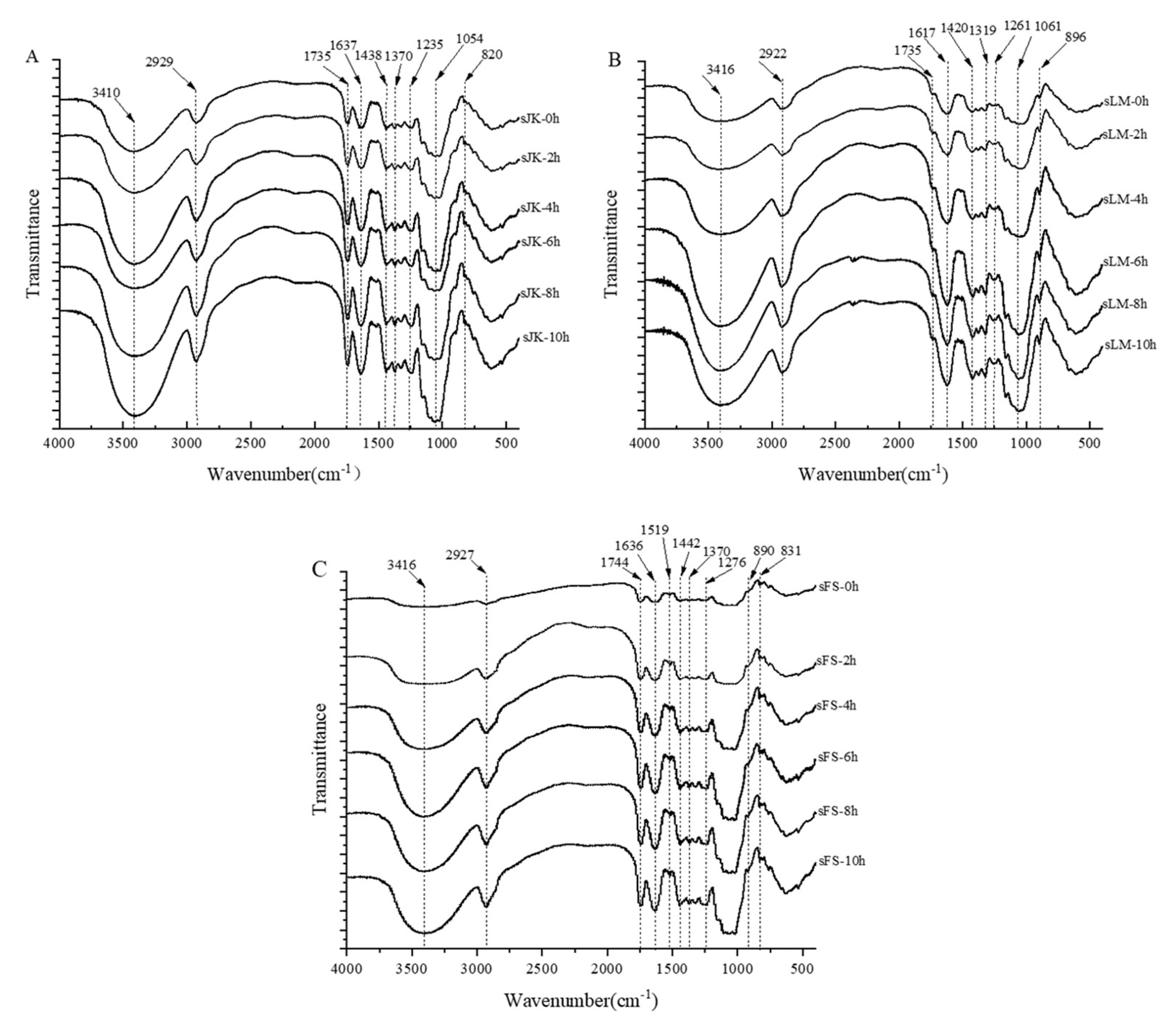
3.9. Analysis of FTIR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, N.; Xia, L.; Ping, Z.; Xueqian, Z.; Ou, Q.; Luqi, H.; Lanping, G.; Wenyuan, G. A Review of Chemical Constituents and Health-Promoting Effects of Citrus Peels. Food Chem. 2021, 65, 130585. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Sharma, K.; Sinha, M.; Baral, E.R.; Koteswararao, R.; Dhyani, A.; Cho, M.H.; Cho, S. Bio-Sorbents, Industrially Important Chemicals and Novel Materials from Citrus Processing Waste as a Sustainable and Renewable Bioresource: A Review. J. Adv. Res. 2020, 23, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Song, L.W.; Qi, J.R.; Liao, J.S.; Yang, X.Q. Enzymatic and Enzyme-Physical Modification of Citrus Fiber by Xylanase and Planetary Ball Milling Treatment. Food Hydrocoll. 2021, 121, 107015. [Google Scholar] [CrossRef]
- Hua, M.; Lu, J.; Qu, D.; Liu, C.; Zhang, L.; Li, S.; Chen, J.; Sun, Y. Structure, Physicochemical Properties and Adsorption Function of Insoluble Dietary Fiber from Ginseng Residue: A Potential Functional Ingredient. Food Chem. 2019, 286, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, X.; Wang, W.; Gu, L.; Hu, C.; Sun, H.; Xu, C.; Hou, J.; Jiang, Z. Lactobacillus Paracasei 24 Attenuates Lipid Accumulation in High-Fat Diet-Induced Obese Mice by Regulating the Gut Microbiota. J. Agric. Food Chem. 2022, 15, 4631–4643. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, J.; Sun, R.; Wang, M.; Wang, K.; Li, Y.; Shang, H.; Hou, J.; Jiang, Z. Lactobacillus Plantarum 23-1 Improves Intestinal Inflammation and Barrier Function through the Tlr4/Nf-Κb Signaling Pathway in Obese Mice. Food Funct. 2022, 13, 5971–5986. [Google Scholar] [CrossRef]
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre Concentrates from Apple Pomace and Citrus Peel as Potential Fibre Sources for Food Enrichment. Food Chem. 2005, 3, 395–401. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Composition, Properties and Health Benefits of Indigestible Carbohydrate Polymers as Dietary Fiber: A Review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, B.; Wen, L.; Wang, F.; Yu, H.; Chen, D.; Su, X.; Zhang, C. Effects of Dietary Fiber on Human Health. Food Sci. Hum. Wellness 2022, 1, 1–10. [Google Scholar] [CrossRef]
- Dervisoglu, M.; Yazici, F. Note. The Effect of Citrus Fibre on the Physical, Chemical and Sensory Properties of Ice Cream. Food Sci. Technol. Int. 2006, 2, 159–164. [Google Scholar] [CrossRef]
- Ozturk, O.K.; Mert, B. The Use of Microfluidization for the Production of Xanthan and Citrus Fiber-Based Gluten-Free Corn Breads. LWT 2018, 96, 34–41. [Google Scholar] [CrossRef]
- Gedikoğlu, A.; Clarke, A.D. Quality Attributes of Citrus Fiber Added Ground Beef and Consumer Acceptance of Citrus Fiber Added Turkish Meat-Balls. Food Health 2019, 5, 205–214. [Google Scholar] [CrossRef]
- Fernández-Ginés, J.M.; Fernández-López, J.; Sayas-Barberá, E.; Sendra, E.; Pérez-Alvarez, J.A. Effect of Storage Conditions on Quality Characteristics of Bologna Sausages Made with Citrus Fiber. J. Food Sci. 2003, 2, 710–714. [Google Scholar] [CrossRef]
- Wang, L.; Shen, C.; Li, C.; Chen, J. Physicochemical, Functional, and Antioxidant Properties of Dietary Fiber from Rosa Roxburghii Tratt Fruit Modified by Physical, Chemical, and Biological Enzyme Treatments. J. Food Process. Preserv. 2020, 44, e14858. [Google Scholar] [CrossRef]
- Liu, T.Y.; Ma, Y.; Yu, S.F.; Shi, J.; Xue, S. The Effect of Ball Milling Treatment on Structure and Porosity of Maize Starch Granule. Innov. Food Sci. Emerg. Technol. 2011, 4, 586–593. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Superfine Grinding Improves Functional Properties and Antioxidant Capacities of Bran Dietary Fibre From Qingke (Hull-Less Barley) Grown in Qinghai-Tibet Plateau, China. J. Cereal Sci. 2015, 65, 43–47. [Google Scholar] [CrossRef]
- Chau, C.F.; Wen, Y.L.; Wang, Y.T. Effects of Micronisation on the Characteristics and Physicochemical Properties of Insoluble Fibres. J. Sci. Food Agric. 2006, 14, 2380–2386. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, R.; Dong, L.; Huang, F.; Tang, X.; Wei, Z.; Zhang, M. Particle Size of Insoluble Dietary Fiber from Rice Bran Affects its Phenolic Profile, Bioaccessibility and Functional Properties. LWT 2018, 87, 450–456. [Google Scholar] [CrossRef]
- Ye, F.; Tao, B.; Liu, J.; Zou, Y.; Zhao, G. Effect of Micronization on the Physicochemical Properties of Insoluble Dietary Fiber from Citrus (Citrus Junos Sieb. Ex Tanaka) Pomace. Food Sci. Technol. Int. 2016, 3, 246–255. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, J.; Zhao, X.; Sun, R.; Sun, C.; Hou, D.; Zhang, X.; Jiang, L.; Hou, J.; Jiang, Z. Oil Bodies Extracted from High-Oil Soybeans (Glycine Max) Exhibited Higher Oxidative and Physical Stability than Oil Bodies from High-Protein Soybeans. Food Funct. 2022, 6, 3271–3282. [Google Scholar] [CrossRef]
- Li, J.; Fu, J.; Ma, Y.; He, Y.; Fu, R.; Qayum, A.; Jiang, Z.; Wang, L. Low Temperature Extrusion Promotes Transglutaminase Cross-Linking of Whey Protein Isolate and Enhances its Emulsifying Properties and Water Holding Capacity. Food Hydrocoll. 2022, 125, 107410. [Google Scholar] [CrossRef]
- Suryanti, V.; Kusumaningsih, T.; Rumingtyas, Y.S. Physicochemical Properties of Dietary Fibers from Artocarpus Camansi Fruit. Pap. Presented IOP Conf. Ser. Mater. Sci. Eng. 2017, 193, 012012. [Google Scholar] [CrossRef]
- Speroni, C.S.; Bender, A.B.B.; Stiebe, J.; Ballus, C.A.; Ávila, P.F.; Goldbeck, R.; Morisso, F.D.P.; da Silva, L.P.; Emanuelli, T. Granulometric Fractionation and Micronization: A Process for Increasing Soluble Dietary Fiber Content and Improving Technological and Functional Properties of Olive Pomace. LWT 2020, 130, 109526. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Yuan, F.; Pan, Q.; Fan, R.; Gao, Y. Physicochemical Characterization of Five Types of Citrus Dietary Fibers. Biocatal. Agric. Biotechnol. 2015, 2, 250–258. [Google Scholar] [CrossRef]
- Dong, J.L.; Wang, L.; Lü, J.; Zhu, Y.Y.; Shen, R.L. Structural, Antioxidant and Adsorption Properties of Dietary Fiber from Foxtail Millet (Setaria Italica) Bran. J. Sci. Food Agric. 2019, 8, 3886–3894. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Li, T.; Gantumur, M.A.; Qayum, A.; Bilawal, A.; Jiang, Z.; Wang, L. Non-Covalent Interaction and Digestive Characteristics Between A-Lactalbumin and Safflower Yellow: Impacts of Microwave Heating Temperature. LWT 2022, 159, 113206. [Google Scholar] [CrossRef]
- Xu, C.; Fu, Y.; Liu, F.; Liu, Z.; Ma, J.; Jiang, R.; Song, C.; Jiang, Z.; Hou, J. Purification and Antimicrobial Mechanism of a Novel Bacteriocin Produced by Lactobacillus Rhamnosus 1.0320. LWT 2021, 137, 110338. [Google Scholar] [CrossRef]
- Deng, M.; Lin, Y.; Dong, L.; Jia, X.; Shen, Y.; Liu, L.; Chi, J.; Huang, F.; Zhang, M.; Zhang, R. Physicochemical and Functional Properties of Dietary Fiber from Pummelo (Citrus Grandis, L. Osbeck) and Grapefruit (Citrus Paradisi Mcfad) Cultivars. Food Biosci. 2021, 40, 100890. [Google Scholar] [CrossRef]
- Marín, F.R.; Soler-Rivas, C.; Benavente-García, O.; Castillo, J.; Pérez-Alvarez, J.A. By-Products from Different Citrus Processes as a Source of Customized Functional Fibres. Food Chem. 2007, 2, 736–741. [Google Scholar] [CrossRef]
- Bender, A.B.B.; Speroni, C.S.; Moro, K.I.B.; Morisso, F.D.P.; dos Santos, D.R.; da Silva, L.P.; Penna, N.G. Effects of Micronization on Dietary Fiber Composition, Physicochemical Properties, Phenolic Compounds, and Antioxidant Capacity of Grape Pomace and its Dietary Fiber Concentrate. LWT 2020, 117, 108652. [Google Scholar] [CrossRef]
- Gao, W.; Chen, F.; Wang, X.; Meng, Q. Recent Advances in Processing Food Powders by Using Superfine Grinding Techniques: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 4, 2222–2255. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, M.; Xu, C.; Liu, Z.; Gu, L.; Ma, J.; Jiang, L.; Jiang, Z.; Hou, J. Effects of Soybean Oil Body as a Milk Fat Substitute on Ice Cream: Physicochemical, Sensory and Digestive Properties. Foods 2022, 11, 1504. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Zhao, J.; Yu, R.; Hussain, M.A.; Qayum, A.; Jiang, Z.; Qu, B. Glycosylated Whey Protein Isolate Enhances Digestion Behaviors and Stabilities of Conjugated Linoleic Acid Oil in Water Emulsions. Food Chem. 2022, 383, 132402. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, K.; Zhao, J.; Sun, R.; Shang, H.; Sun, C.; Liu, L.; Hou, J.; Jiang, Z. Physical and Oxidative Stability of Astaxanthin Microcapsules Prepared with Liposomes. J. Sci. Food Agric. 2022, 11, 4909–4917. [Google Scholar] [CrossRef]
- Protonotariou, S.; Drakos, A.; Evageliou, V.; Ritzoulis, C.; Mandala, I. Sieving Fractionation and Jet Mill Micronization Affect the Functional Properties of Wheat Flour. J. Food Eng. 2014, 134, 24–29. [Google Scholar] [CrossRef]
- Jiang, Z.; Mu, S.; Ma, C.; Liu, Y.; Ma, Y.; Zhang, M.; Li, H.; Liu, X.; Hou, J.; Tian, B. Consequences of Ball Milling Combined with High-Pressure Homogenization on Structure, Physicochemical and Rheological Properties of Citrus Fiber. Food Hydrocoll. 2022, 127, 107515. [Google Scholar] [CrossRef]
- Ramachandraiah, K.; Chin, K.B. Evaluation of Ball-Milling Time on the Physicochemical and Antioxidant Properties of Persimmon by-Products Powder. Innov. Food Sci. Emerg. Technol. 2016, 37, 115–124. [Google Scholar] [CrossRef]
- Jiang, G.; Ramachandraiah, K.; Wu, Z.; Li, S.; Eun, J.B. Impact of Ball-Milling Time on the Physical Properties, Bioactive Compounds, and Structural Characteristics of Onion Peel Powder. Food Biosci. 2020, 36, 100630. [Google Scholar] [CrossRef]
- Ting, Y.; Jiang, Y.; Ho, C.T.; Huang, Q. Common Delivery Systems for Enhancing in Vivo Bioavailability and Biological Efficacy of Nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Zhu, K.; Huang, S.; Peng, W.; Qian, H.; Zhou, H. Effect of Ultrafine Grinding on Hydration and Antioxidant Properties of Wheat Bran Dietary Fiber. Food Res. Int. 2010, 4, 943–948. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Zhang, X.; Devahastin, S. Bioactive Dietary Fiber Powder from Asparagus Leaf by-Product: Effect of Low-Temperature Ball Milling on Physico-Chemical, Functional and Microstructural Characteristics. Powder Technol. 2020, 366, 275–282. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary Fibre and Fibre-Rich by-Products of Food Processing: Characterisation, Technological Functionality and Commercial Applications: A Review. Food Chem. 2011, 2, 411–421. [Google Scholar] [CrossRef]
- Huang, J.Y.; Liao, J.S.; Qi, J.R.; Jiang, W.X.; Yang, X.Q. Structural and Physicochemical Properties of Pectin-Rich Dietary Fiber Prepared from Citrus Peel. Food Hydrocoll. 2021, 110, 106140. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, J.; Qi, J. Functional and Structural Properties of Dietary Fiber from Citrus Peel Affected by the Alkali Combined with High-Speed Homogenization Treatment. LWT 2020, 128, 109397. [Google Scholar] [CrossRef]
- Chau, C.F.; Cheung, P.C. Effects of the Physico-Chemical Properties of Three Legume Fibers on Cholesterol Absorption in Hamsters. Nutr. Res. 1999, 2, 257–265. [Google Scholar] [CrossRef]
- Kethireddipalli, P.; Hung, Y.C.; Phillips, R.D.; Mcwatters, K.H. Evaluating the Role of Cell Wall Material and Soluble Protein in the Functionality of Cowpea (Vigna Unguiculata) Pastes. J. Food Sci. 2002, 1, 53–59. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Yi, C.; Quan, K.; Lin, B. Chemical Composition, Structure, Physicochemical and Functional Properties of Rice Bran Dietary Fiber Modified by Cellulase Treatment. Food Chem. 2021, 342, 128352. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.; Wang, Z.; Hao, Y.; Wang, Y.U.; Yang, Z.; Li, W.; Wang, J. Physicochemical and Functional Properties of Soluble Dietary Fiber from Different Colored Quinoa Varieties (Chenopodium Quinoa Willd). J. Cereal Sci. 2020, 95, 103045. [Google Scholar] [CrossRef]
- Dziedzic, K.; Szwengiel, A.; Górecka, D.; Gujska, E.; Kaczkowska, J.; Drożdżyńska, A.; Walkowiak, J. Effect of Wheat Dietary Fiber Particle Size During Digestion in Vitro on Bile Acid, Faecal Bacteria and Short-Chain Fatty Acid Content. Plant Foods Hum. Nutr. 2016, 2, 151–157. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Yu, H.; Mu, S.; Li, H.; Liu, X.; Zhang, M.; Jiang, Z.; Hou, J. Biological Activities and in Vitro Digestion Characteristics of Glycosylated A-Lactalbumin Prepared by Microwave Heating: Impacts of Ultrasonication. LWT 2022, 158, 113141. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, P.; Li, J.; Wang, X.; Hou, J.; Jiang, Z. Effects of Ultrasound Synergized with Microwave on Structure and Functional Properties of Transglutaminase-Crosslinked Whey Protein Isolate. Ultrason. Sonochem. 2022, 83, 105935. [Google Scholar] [CrossRef]
- Tewari, J.C.; Dixit, V.; Cho, B.K.; Malik, K.A. Determination of Origin and Sugars of Citrus Fruits Using Genetic Algorithm, Correspondence Analysis and Partial Least Square Combined with Fiber Optic Nir Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 3, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
| Sample | IDF | SDF | TDF | IDF: SDF |
|---|---|---|---|---|
| sFS-0h | 46.42 ± 0.28 j | 30.82 ± 0.18 c | 77.24 ± 1.00 e | 1.51 |
| sFS-2h | 45.72 ± 0.49 jk | 30.91 ± 061c | 76.64 ± 0.83 e | 1.48 |
| sFS-4h | 44.88 ± 0.73 k | 31.52 ± 0.37 bc | 76.40 ± 0.72 ef | 1.42 |
| sFS-6h | 43.31 ± 0.92 l | 31.93 ± 0.07 b | 75.25 ± 0.99 fg | 1.36 |
| sFS-8h | 42.35 ± 0.23 lm | 32.19 ± 0.34 ab | 74.55 ± 0.10 g | 1.31 |
| sFS-10h | 41.31 ± 0.45 m | 32.73 ± 0.61a | 74.05 ± 0.16 g | 1.26 |
| sJK-0h | 65.28 ± 0.25 cd | 18.99 ± 0.11 j | 84.27 ± 0.33 c | 3.44 |
| sJK-2h | 62.86 ± 0.74 e | 20.76 ± 0.61 hi | 83.63 ± 0.72 cd | 3.03 |
| sJK-4h | 61.41 ± 1.13 f | 21.70 ± 1.4 gh | 83.10 ± 0.27 cd | 2.82 |
| sJK-6h | 59.32 ± 0.33 g | 23.63 ± 0.53 f | 82.95 ± 0.19 cd | 2.51 |
| sJK-8h | 57.78 ± 0.66 h | 25.09 ± 0.30 e | 82.87 ± 0.65 d | 2.30 |
| sJK-10h | 56.17 ± 1.28 i | 26.50 ± 0.38 d | 82.67 ± 1.28 d | 2.12 |
| sLM-0h | 70.48 ± 0.56 a | 17.97 ± 1.05 j | 88.46 ± 0.73 a | 3.92 |
| sLM-2h | 69.61 ± 0.51 a | 18.23 ± 0.97 j | 87.85 ± 1.30 a | 3.82 |
| sLM-4h | 67.35 ± 0.94 b | 20.08 ± 0.54 i | 87.43 ± 1.46 a | 3.35 |
| sLM-6h | 66.45 ± 0.76 bc | 20.92 ± 0.07 hi | 87.38 ± 0.68 b | 3.18 |
| sLM-8h | 65.94 ± 1.05 c | 21.04 ± 0.91 hi | 86.98 ± 0.13 b | 3.13 |
| sLM-10h | 64.35 ± 0.47 d | 22.41 ± 0.23 g | 86.53 ± 0.35 b | 2.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Zhang, M.; Huang, Y.; Ma, C.; Mu, S.; Li, H.; Liu, X.; Ma, Y.; Liu, Y.; Hou, J. Comparison and Characterization of the Structure and Physicochemical Properties of Three Citrus Fibers: Effect of Ball Milling Treatment. Foods 2022, 11, 2665. https://doi.org/10.3390/foods11172665
Jiang Z, Zhang M, Huang Y, Ma C, Mu S, Li H, Liu X, Ma Y, Liu Y, Hou J. Comparison and Characterization of the Structure and Physicochemical Properties of Three Citrus Fibers: Effect of Ball Milling Treatment. Foods. 2022; 11(17):2665. https://doi.org/10.3390/foods11172665
Chicago/Turabian StyleJiang, Zhanmei, Minghan Zhang, Yuxuan Huang, Chenglong Ma, Sinan Mu, Hongyu Li, Xianqi Liu, Yue Ma, Yue Liu, and Juncai Hou. 2022. "Comparison and Characterization of the Structure and Physicochemical Properties of Three Citrus Fibers: Effect of Ball Milling Treatment" Foods 11, no. 17: 2665. https://doi.org/10.3390/foods11172665
APA StyleJiang, Z., Zhang, M., Huang, Y., Ma, C., Mu, S., Li, H., Liu, X., Ma, Y., Liu, Y., & Hou, J. (2022). Comparison and Characterization of the Structure and Physicochemical Properties of Three Citrus Fibers: Effect of Ball Milling Treatment. Foods, 11(17), 2665. https://doi.org/10.3390/foods11172665






