Thymol Edible Coating Controls Postharvest Anthracnose by Regulating the Synthesis Pathway of Okra Lignin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Isolation and Identification of Pathogens
2.3. Preliminary Verification of Pathogenicity
2.4. Observation of Biological Characteristics of Pathogens
2.5. In Vitro Antifungal Activity of TKL on Mycelium Growth and Spore Germination
2.6. Inhibition of TKL on the Mycelium Weight of Pathogenic Fungus
2.7. The Effects of TKL on the Structure of Pathogenic Fungus Was Observed by SEM
2.8. Effects of TKL on the Intracellular Structure and Enzyme Activity of C. fioriniae
2.9. Effects of Postharvest Coating Wounds of TKL Inoculated with C. fioriniae on Stress Resistance of Okra
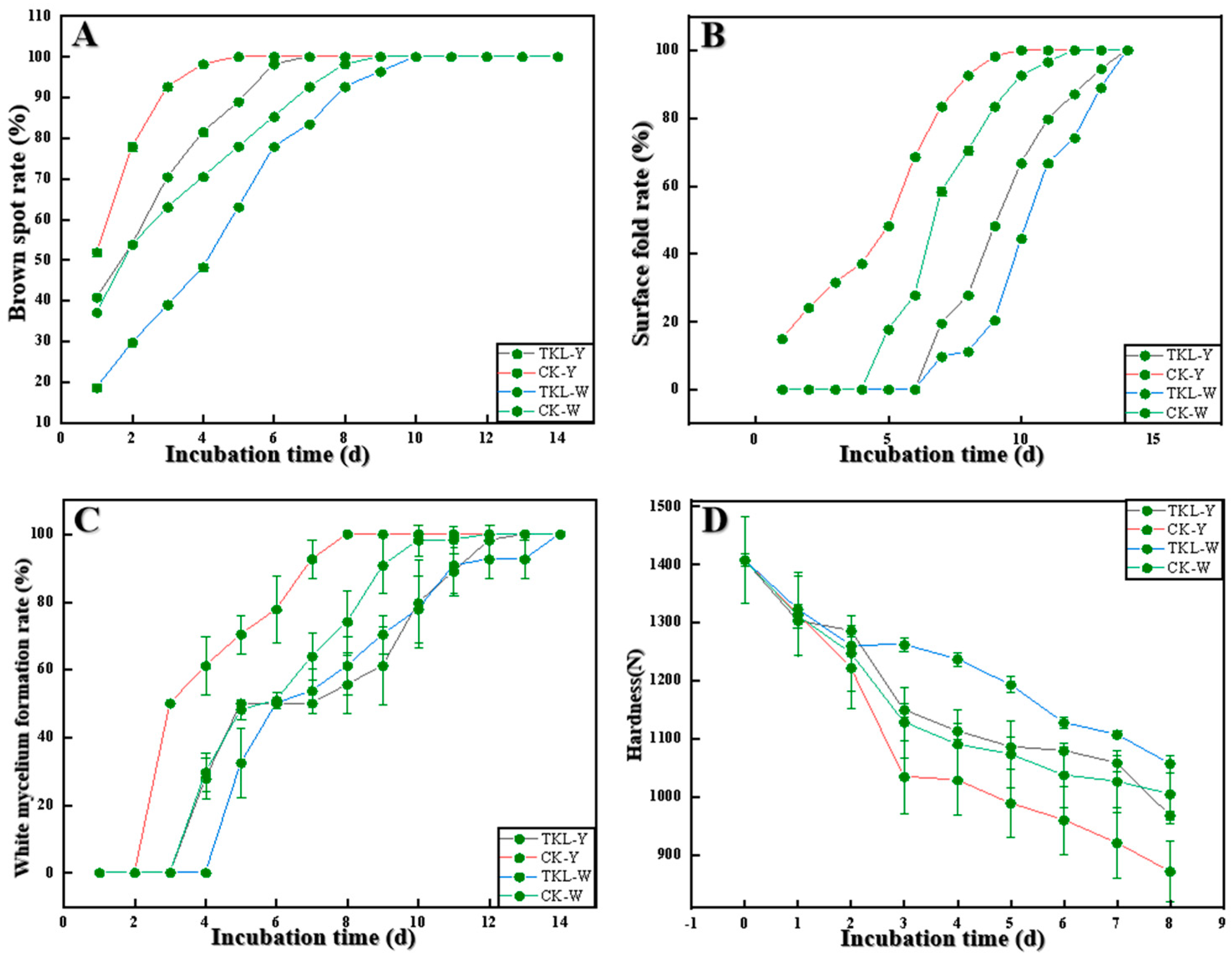
2.9.1. Hardness Determination
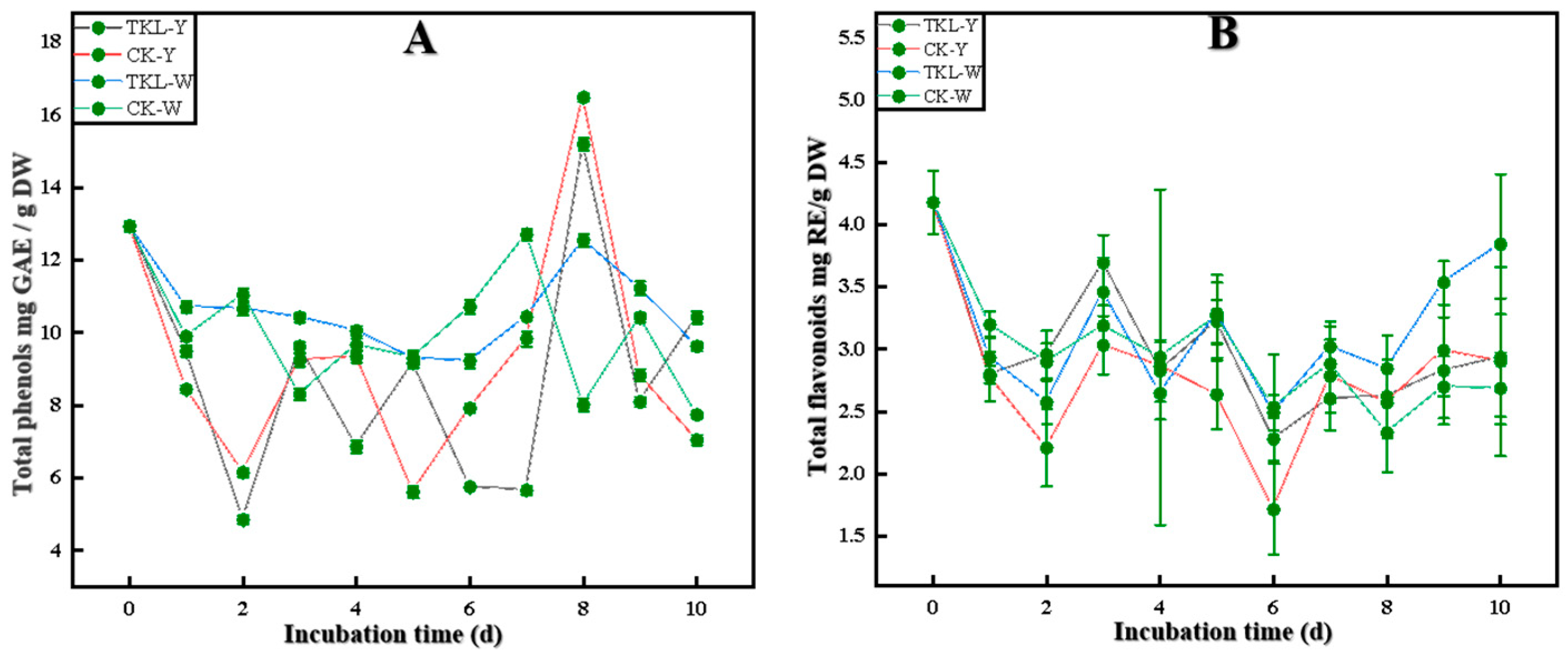
2.9.2. Determination of Total Phenols and Total Flavonoids
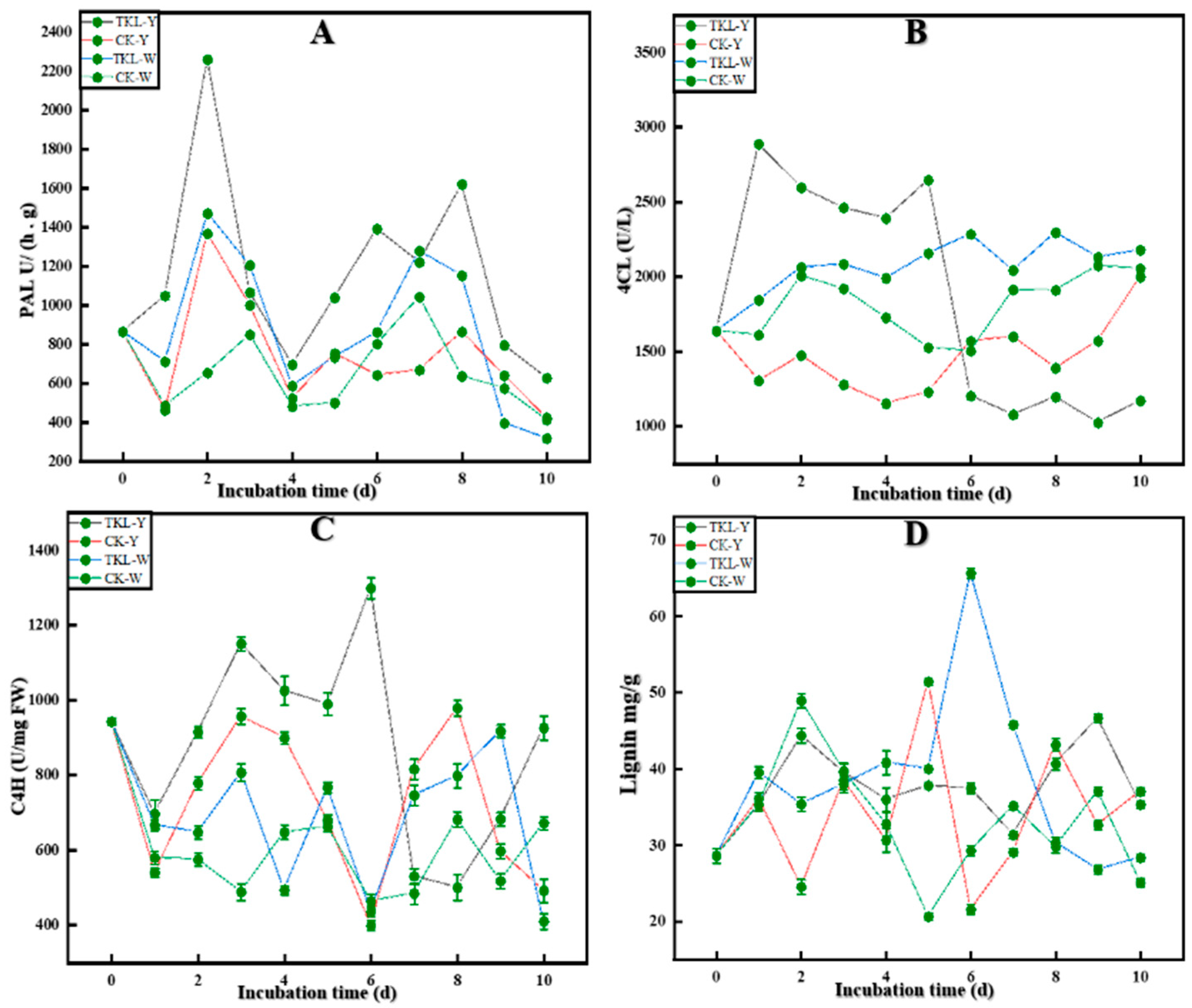
2.9.3. Key Enzyme Index of Okra Lignin Synthesis
2.9.4. Determination of Okra Lignin Content
3. Results
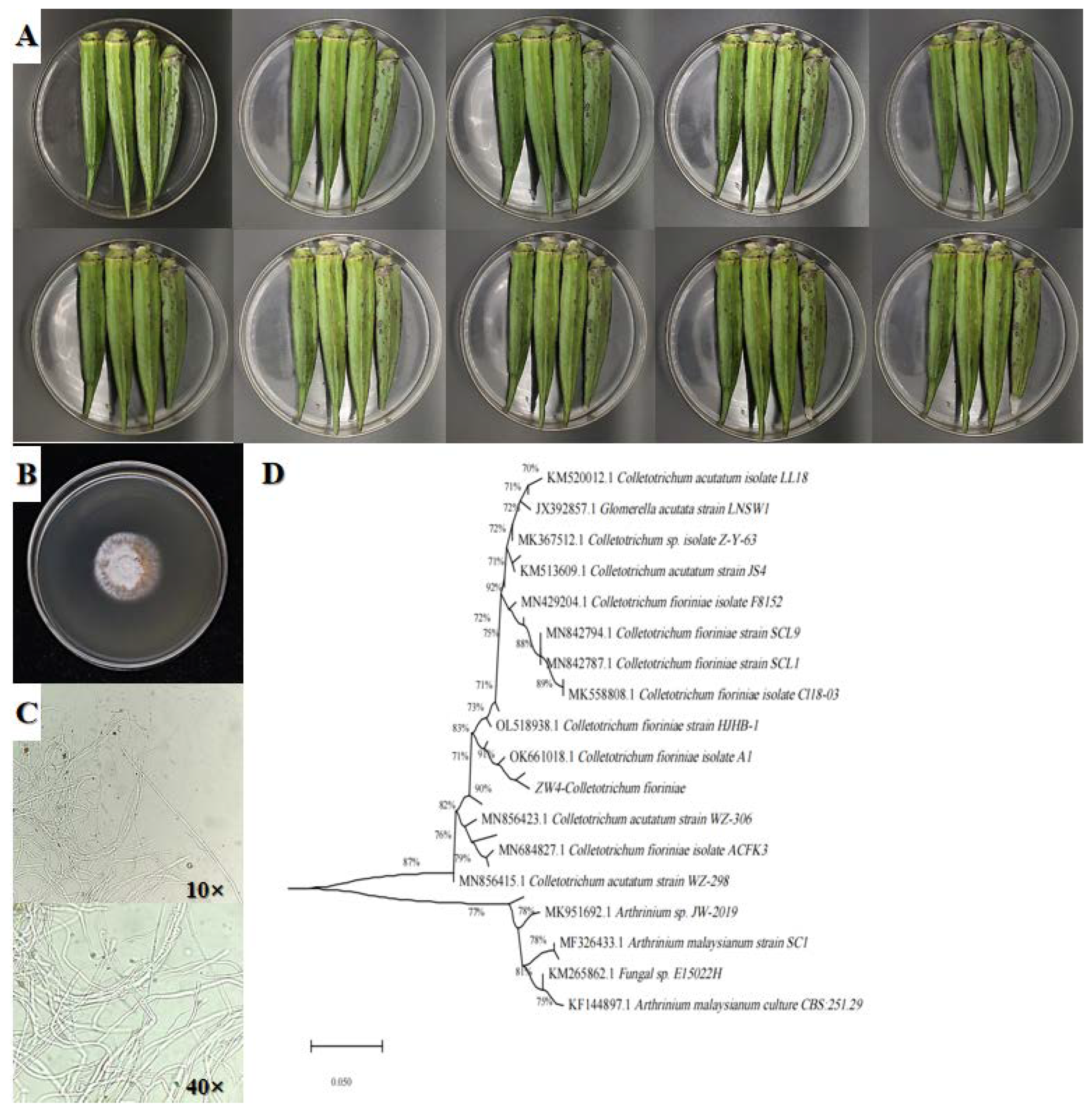
3.1. Isolation and Identification of Pathogens
3.2. Pathogenicity Determination and Biological Characteristics of C. fioriniae
3.3. Inhibitory Effect of TKL on C. fioriniae In Vitro
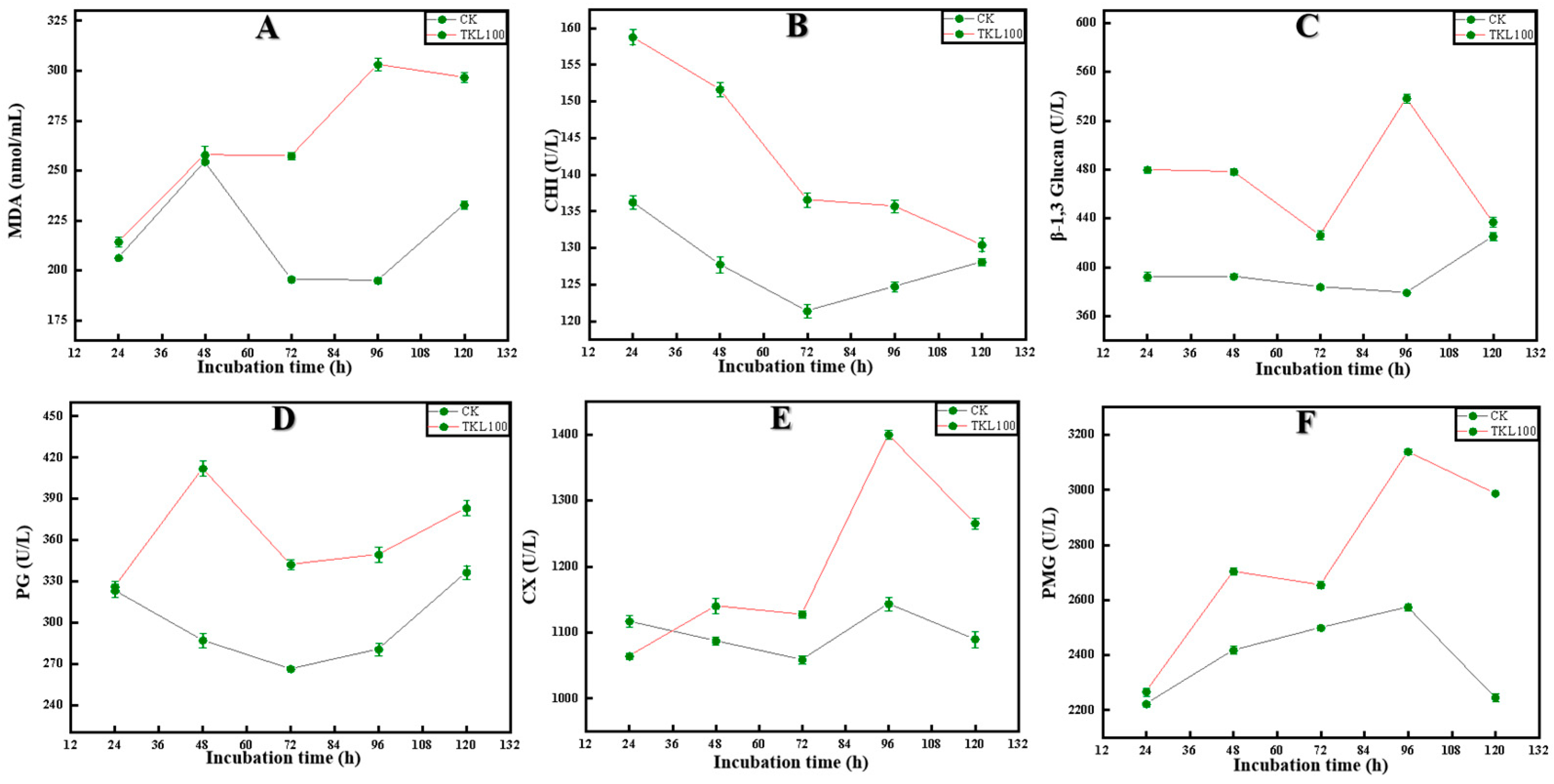
3.4. Effects of TKL on C. fioriniae Enzyme Activities
3.5. Effect of TKL on Surface Disease Changes in Okra Inoculated with the Pathogens
3.6. Effect of TKL on Lignin Synthesis Pathway in Okra Inoculated with the Pathogens
3.6.1. Total Phenols and Total Flavonoids
3.6.2. Key Enzyme Activities in the Lignin Synthesis Pathway
3.6.3. Okra Lignin Content
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dantas, T.L.; Alonso Buriti, F.C.; Florentino, E.R. Okra (Abelmoschus esculentus L.) as a potential functional food source of mucilage and bioactive compounds with technological applications and health benefits. Plants 2021, 10, 1683. [Google Scholar] [CrossRef]
- Elkhalifa, A.; Alshammari, E.; Adnan, M.; Alcantara, J.C.; Awadelkareem, A.M.; Eltoum, N.E.; Mehmood, K.; Panda, B.P.; Ashraf, S.A. Okra (Abelmoschus esculentus) is a potential dietary medicine with nutraceutical importance for sustainable health applications. Molecules 2021, 26, 696. [Google Scholar] [CrossRef] [PubMed]
- Uddin Zim, A.F.M.I.; Khatun, J.; Khan, M.F.; Hossain, M.A.; Haque, M.M. Evaluation of in vitro antioxidant activity of okra mucilage and its antidiabetic and antihyperlipidemic effect in alloxan-induced diabetic mice. Food Sci. Nutr. 2021, 9, 6854–6865. [Google Scholar] [CrossRef] [PubMed]
- Kapadiya, I.B.; Undhad, S.V.; Talaviya, J.R.; Siddhapara, M.R. Evaluation of phytoextracts against Fusarium solani causing root rot of okra. J. Biopestic. 2014, 7, 7–9. Available online: http://www.jbiopest.com/users/LW8/efiles/vol_7_0_7-9.pdf (accessed on 15 June 2022).
- Kwon, J.H.; Jee, H.J. Soft rot of eggplant (Solanum melongena) caused by Choanephora cucurbitarum in Korea. Mycobiology 2005, 33, 163–165. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, W.; Hu, X.; Yan, J.; Liu, Y.; Wang, Z.; Wu, J. Efficacy and mechanism of Thymol/KGM/LG edible coating solution on inhibition of Mucor circinelloides isolated from okra. Front. Microbiol. 2022, 13, 880376. [Google Scholar] [CrossRef]
- Farrag, E. First record of cercospora leaf spot disease on okra plants and its control in Egypt. Plant Pathol. J. 2011, 10, 175–180. [Google Scholar] [CrossRef]
- Muimba-Kankolongo, A. Chapter 11—Vegetable Production. In Food Crop Production by Smallholder Farmers in Southern Africa; Muimba-Kankolongo, A., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 205–274. [Google Scholar]
- Jayawardena, R.; Hyde, K.; Damm, U.; Cai, L.; Liu, M.; Li, X.H.; Yan, J. Notes on currently accepted species of Colletotrichum. Mycosphere 2016, 7, 1192–1260. [Google Scholar] [CrossRef]
- Khodadadi, F.; González, J.B.; Martin, P.L.; Giroux, E.; Bilodeau, G.J.; Peter, K.A.; Doyle, V.P.; Aćimović, S.G. Identification and characterization of Colletotrichum species causing apple bitter rot in New York and description of C. noveboracense sp. nov. Sci. Rep. 2020, 10, 11043. [Google Scholar] [CrossRef]
- Martin, P.L.; Peter, K.A. Quantification of Colletotrichum fioriniae in orchards and deciduous forests indicates it is primarily a leaf endophyte. Phytopathology 2021, 111, 333–344. [Google Scholar] [CrossRef]
- Carneiro, G.A.; Baric, S. Colletotrichum fioriniae and Colletotrichum godetiae causing postharvest bitter rot of apple in South Tyrol (Northern Italy). Plant Dis. 2021, 105, 3118–3126. [Google Scholar] [CrossRef] [PubMed]
- Frederick, R.; Cavin, C.; Thomas, J.L.; Bruckart, W.L., 3rd; Tancos, M.A. Colletotrichum fioriniae infecting invasive Japanese hop (Humulus scandens) in the United States. Plant Dis. 2021, 106, 330. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhai, F.; Zhang, Y.; Wang, D.; Shu, J.; Yao, X. Identification and characterization of Colletotrichum fioriniae and C. fructicola that cause anthracnose in pecan. Front. Plant Sci. 2022, 13, 1043750. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Wang, J.; Bai, Y.; Liu, Y. Transcriptome sequencing of okra (Abelmoschus esculentus L. Moench) uncovers differently expressed genes responding to drought stress. J. Plant Biochem. Biotechnol. 2020, 29, 155–170. [Google Scholar] [CrossRef]
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664. [Google Scholar] [CrossRef]
- Cai, C.; Xu, C.J.; Li, X.; Ferguson, I.; Chen, K. Accumulation of lignin about change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharvest Biol. Technol. 2006, 40, 163–169. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Nie, X.R.; Li, H.Y.; Du, G.; Lin, S.; Hu, R.; Li, H.Y.; Qin, W. Structural characteristics, rheological properties, and biological activities of polysaccharides from different cultivars of okra (Abelmoschus esculentus) collected in China. Int. J. Biol. Macromol. 2019, 139, 459–467. [Google Scholar] [CrossRef]
- Datta, S.; Sarkar, M.; Chowdhury, A.; Rakwal, R.; Agrawal, G.K.; Sarkar, A. A comprehensive insight into the biology of Rhizoctonia solani AG1-IA Kühn, the causal organism of the sheath blight disease of rice. J. Plant Pathol. 2021, 104, 79–98. [Google Scholar] [CrossRef]
- Haruo Shindo; Shozo Kuwatsuka. Behavior of phenolic substances in the decaying process of plants. Soil Sci. Plant Nutr. 1977, 23, 319–332. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the way for lignin valorisation: Recent advances in bioengineering, biorefining and catalysis. Angew. Chem. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [PubMed]
- Phornvillay, S.; Pongprasert, N.; Wongs-Aree, C.; Uthairatanakij, A.; Srilaong, V. Exogenous putrescine treatment delays chilling injury in okra pod (Abelmoschus esculentus) stored at low storage temperature. Sci. Hortic. 2019, 56, 108550. [Google Scholar] [CrossRef]
- Li, D.; Limwachiranon, J.; Li, L.; Zhang, L.; Xu, Y.; Fu, M.; Luo, Z. Hydrogen peroxide accelerated the lignification process of bamboo shoots by activating the phenylpropanoid pathway and programme-d cell death in postharvest storage. Postharvest Biol. Technol. 2019, 153, 79–86. [Google Scholar] [CrossRef]
- Li, C.; Suo, J.; Xuan, L.; Ding, M.; Zhang, H.; Song, L.; Ying, Y. Bamboo shoot-lignification delay by melatonin during low-temperature storage. Postharvest Biol. Technol. 2019, 156, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Chang, J.W.; Saenger, M.; Deering, A. Thymol nanoemulsions formed via spontaneous emulsification: Physical and antimicrobial properties. Food Chem. 2017, 232, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Kumar, A.; Singh, P.P.; Songachan, L.S. Antimicrobial and antioxidant properties of phytochemicals: Current status and future perspective. In Functional and Preservative Properties of Phytochemicals; Academic Press: Cambridge, MA, USA, 2020; pp. 1–45. [Google Scholar] [CrossRef]
- Han, Y.; Ding, J.; Zhang, J.; Li, Q.; Yang, H.; Sun, T.; Li, H. Fabrication and characterization of polylactic acid coaxial antibacterial nanofibers embedded with cinnamaldehyde/tea polyphenol with food packaging potential. Int. J. Biol. Macromol. 2021, 184, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, Q.; Sun, Y.; Chen, B.; Wu, X.; Le, T. Improvement of banana postharvest quality using a novel soybean protein isolate/cinnamaldehyde/zinc oxide bionanocomposite coating strategy. Sci. Hortic. 2019, 258, 108786. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Wan, C.; Kai, W.; Chen, J. Carvacrol delays Phomopsis stem-end rot development in pummelo fruit by maintaining energy status and antioxidant system. Food Chem. 2021, 372, 131239. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, J.; Luo, C.; Yuan, R.; Ge, L. Fabrication of l-menthol contained edible self-healing coating based on guest-host interaction. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124743. [Google Scholar] [CrossRef]
- Florencia Bambace, M.; Mabel Gerard, L.; del Rosario Moreira, M. An approach to improve the safety and quality of ready-to-eat blueberries. J. Food Saf. 2019, 39, e12602. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Chen, T.; Chen, X. A review of the antibacterial activity and mechanisms of plant polysaccharides. Trends Food Sci. Technol. 2022, 123, 264–280. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhu, Z.; Jiao, X.; Shang, Y.; Wen, Y. Encapsulation of thymol in biodegradable nanofiber via coaxial eletrospinning and applications in fruit preservation. J. Agric. Food Chem. 2019, 67, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Sellamuthu, P.S.; Nagarajan, S.K.; Madhavan, T.; Sadiku, E.R. Antifungal activity of wild bergamot (Monarda fistulosa) essential oil against postharvest fungal pathogens of banana fruits. South Afr. J. Bot. 2022, 144, 166–174. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Serrano, M.; Valero, D.; Isabel Rodriguez-Lopez, M.; Gabaldon, J.A.; Castillo, S.; Martinez-Romero, D. Thymol encapsulated into HP-beta-cyclodextrin as an alternative to synthetic fungicides to induce lemon resistance against sour rot decay. Molecules 2020, 25, 4348. [Google Scholar] [CrossRef]
- Saki, M.; ValizadehKaji, B.; Abbasifar, A.; Shahrjerdi, I. Effect of chitosan coating combined with thymol essential oil on physicochemical and qualitative properties of fresh fig (Ficus carica L.) fruit during cold storage. Food Meas. 2019, 13, 1147–1158. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, J.; Hu, C.; Deng, L.; Ritenour, M.A. Use of carvacrol and thymol in shellac coating to control stem-end rot on ‘Ruby Red’ grapefruit and maintain fruit quality during simulated storage and marketing. Sci. Hortic. 2020, 272, 109606. [Google Scholar] [CrossRef]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Mi, H. Characterization of sodium alginate-based films incorporated with thymol for fresh-cut apple packaging. Food Control 2021, 126, 108063. [Google Scholar] [CrossRef]
- Boonruang, K.; Kerddonfag, N.; Chinsirikul, W.; Mitcham, E.J.; Chonhenchob, V. Antifungal effect of poly(lactic acid) films containing thymol and R(-)-carvone against anthracnose pathogens isolated from avocado and citrus. Food Control 2017, 78, 85–93. [Google Scholar] [CrossRef]
- Jogee, P.S.; Ingle, A.P.; Rai, M. Isolation and identification of toxigenic fungi from infected peanuts and efficacy of silver nanoparticles against them. Food Control 2017, 71, 143–151. [Google Scholar] [CrossRef]
- He, J.; Wu, D.; Zhang, Q.; Chen, H.; Li, H.; Han, Q.; Lai, X.; Wang, H.; Wu, Y.; Yuan, J.; et al. Efficacy and mechanism of cinnamon essential oil on inhibition of Colletotrichum acutatum isolated from ‘Hongyang’ Kiwifruit. Front. Microbiol. 2018, 9, 1288. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Shen, Q.; Li, L.Q.; Huang, Y.Q.; Cheung, H.Y. Phytochemical profiles, antioxidant activities of functional herb Abrus cantoniensis and Abrus mollis. Food Chem. 2015, 177, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, J.; Li, M.; Zhang, C.; Zhu, J.; Zhao, Y.; Guo, X.; Ye, J. Study on the flavonoids and pectin contents in different okra (Abelmoschus escullentus L.) accessions. J. Agric. Sci. Bot. 2017, 1, 12–16. [Google Scholar] [CrossRef]
- Lin, S.; Guo, H.; Gong, J.D.B.; Lu, M.; Lu, M.-Y.; Wang, L.; Wu, D.-T. Phenolic profiles, β-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. J. Cereal Sci. 2018, 81, 69–75. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, C.; Guan, X.; Lian, S.; Li, B.; Wang, C. Butylated hydroxytoluene induced resistance against Botryosphaeria dothidea in apple fruit. Front. Microbiol. 2021, 11, 599062. [Google Scholar] [CrossRef]
- Xiang, J.; Yang, C.; Beta, T.; Liu, S.; Yang, R. Phenolic profile and antioxidant activity of the edible tree peony flower and underlying mechanisms of preventive effect on H2O2-induced oxidative damage in Caco-2 Cells. Foods 2019, 8, 471. Available online: https://www.mdpi.com/2304-8158/8/10/471 (accessed on 15 July 2022). [CrossRef]
- Tao, X.; Wu, Q.; Li, J.; Huang, S.; Cai, L.; Mao, L.; Luo, Z.; Li, L.; Ying, T. Exogenous methyl jasmonate regulates phenolic compounds biosynthesis during postharvest tomato ripening. Postharvest Biol. Technol. 2022, 184, 111760. [Google Scholar] [CrossRef]
- Gärtner, A.; Gellerstedt, G.; Tamminen, T. Determination of phenolic hydroxyl groups in residual lignin using a modified UV-method. Nord. Pulp Pap. Res. J. 1999, 14, 163–170. [Google Scholar] [CrossRef]
- de Oliveira, D.R.; Avelino, F.; Mazzetto, S.E.; Lomonaco, D. Microwave-assisted selective acetylation of Kraft lignin: Acetic acid as a sustainable reactant for lignin valorization. Int. J. Biol. Macromol. 2020, 164, 1536–1544. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Li, Y.Z.; Zheng, X.; Tang, H.L.; Zhu, J.W.; Yang, J.M. Increase of β-1, 3-Glucanase and Chitinase Activities in Cotton Callus Cells Treated by Salicylic Acid and Toxin of Verticillium dahliae. J. Integr. Plant Biol. 2003, 45, 802–808. Available online: https://www.jipb.net/EN/Y2003/V45/I7/802 (accessed on 26 November 2022).
- Elkhalifa, A.E.O.; Al-Shammari, E.; Adnan, M.; Alcantara, J.C.; Mehmood, K.; Eltoum, N.E.; Ashraf, S.A. Development and characterization of novel biopolymer derived from Abelmoschus esculentus L. extract and its antidiabetic potential. Molecules 2021, 26, 3609. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, A.; Hayashi, K.; Taniguchi, N.; Naruse, C.; Ozawa, Y.; Shishiyama, J. A new post-harvest disease of okra pods caused by Alternaria alternata. Jpn. J. Phytopathol. 1995, 61, 340–345. [Google Scholar] [CrossRef]
- Grammen, A.; Wenneker, M.; Van Campenhout, J.; Pham, K.T.K.; Van Hemelrijck, W.; Bylemans, D.; Geeraerd, A.; Keulemans, W. Identification and pathogenicity assessment of Colletotrichum isolates causing bitter rot of apple fruit in Belgium. Eur. J. Plant Pathol. 2019, 153, 47–63. [Google Scholar] [CrossRef]
- Matrose, N.A.; Obikeze, K.; Belay, Z.A.; Caleb, O.J. Plant extracts and other natural compounds as alternatives for post-harvest management of fruit fungal pathogens: A review. Food Biosci. 2021, 41, 100840. [Google Scholar] [CrossRef]
- Sun, C.; Li, F.; Wei, M.; Xiang, Z.; Chen, C.; Xu, D. Detection and biological characteristics of Alternaria alternata resistant to difenoconazole from Paris polyphylla var. chinensis, an Indigenous Medicinal Herb. Plant Dis. 2021, 105, 1546–1554. [Google Scholar] [CrossRef]
- Sreelatha, S.; Kumar, N.; Yin, T.S.; Rajani, S. Evaluating the antibacterial activity and mode of action of thymol-loaded chitosan nanoparticles against plant bacterial pathogen xanthomonas campestris pv. campestris. Front. Microbiol. 2022, 12, 792737. [Google Scholar] [CrossRef]
- Ji, S.H.; Kim, T.K.; Keum, Y.S.; Chun, S.-C. The major postharvest disease of onion and its control with thymol fumigation during low-temperature storage. Mycobiology 2018, 46, 242–253. [Google Scholar] [CrossRef]
- Camele, I.; Altieri, L.; De Martino, L.; De Feo, V.; Mancini, E.; Rana, G.L. In vitro control of post-harvest fruit rot fungi by some plant essential oil components. Int. J. Mol. Sci. 2012, 13, 2290–2300. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef]
- Radi, M.; Ahmadi, H.; Amiri, S. Effect of cinnamon essential oil-loaded nanostructured lipid carriers (NLC) Against Penicillium citrinum and Penicillium expansum involved in tangerine decay. Food Bioprocess Technol. 2022, 15, 306–318. Available online: https://link.springer.com/article/10.1007/s11947-021-02737-5 (accessed on 26 November 2022). [CrossRef]
- Wang, X.; Tian, L.; Fu, J.; Liao, S.; Yang, S.; Jia, X.; Gong, G. Evaluation of the membrane damage mechanism of thymol against Bacillus cereus and its application in the preservation of skim milk. Food Control 2022, 131, 108435. [Google Scholar] [CrossRef]
- Tian, L.; Wang, X.; Liu, R.; Zhang, D.; Wang, X.; Sun, R.; Gong, G. Antibacterial mechanism of thymol against Enterobacter sakazakii. Food Control 2021, 123, 107716. [Google Scholar] [CrossRef]
- Ding, J.; Liu, C.; Huang, P.; Zhang, Y.; Hu, X.; Li, H.; Qin, W. Effects of thymol concentration on postharvest diseases and quality of blueberry fruit. Food Chem. 2023, 402, 134227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hao, Y.; Lu, H.; Li, P.; Chen, J.; Shi, Z.; Xie, Y.; Mo, H.; Hu, L. Nano-Thymol emulsion inhibits botrytis cinerea to control postharvest gray mold on tomato fruit. Agronomy 2022, 12, 2973. [Google Scholar] [CrossRef]
- Vellanki, S.; Billmyre, R.B.; Lorenzen, A.; Campbell, M.; Turner, B.; Huh, E.Y.; Lee, S.C. A novel resistance pathway for calcineurin inhibitors in the human-pathogenic mucorales Mucor circinelloides. Microbiology 2020, 11, e02949-19. [Google Scholar] [CrossRef]
- Chakraborty, A.; Fernando, L.D.; Fang, W.; Dickwella Widanage, M.C.; Wei, P.; Jin, C.; Wang, T. A molecular vision of fungal cell wall organization by functional genomics and solid-state NMR. Nat. Commun. 2021, 12, 6346. [Google Scholar] [CrossRef]
- Kang, X.; Kirui, A.; Muszyński, A.; Widanage, M.C.D.; Chen, A.; Azadi, P.; Wang, T. Molecular architecture of fungal cell walls revealed by solid-state NMR. Nat. Commun. 2018, 9, 2747. [Google Scholar] [CrossRef]
- Chatterton, S.; Punja, Z.K. Chitinase and beta-1,3-glucanase enzyme production by the mycoparasite Clonostachys rosea f. catenulata against fungal plant pathogens. Can. J. Microbiol. 2009, 55, 356–367. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Luo, X.; Wu, X.; Ren, J.; Huang, X.; Dong, P. Antifungal activity and mechanism of thymol against Fusarium oxysporum, a pathogen of potato dry rot, and its potential application. Postharvest Biol. Technol. 2022, 192, 112025. [Google Scholar] [CrossRef]
- Lisker, N.; Katan, J.; Henis, Y. Sequential production of polygalacturonase, cellulase, and pectin lyase by Rhizoctonia solani. Can. J. Microbiol. 1975, 21, 1298. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.A.; Barbosa, B.V.R.; Lima, M.T.N.S.; Cardoso, P.G.; Contigli, C.; Pimenta, L.P.S. Antiviral fungal metabolites and some insights into their contribution to the current COVID-19 pandemic. Bioorganic Med. Chem. 2021, 46, 116366. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8363177/ (accessed on 11 September 2022). [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Yang, Y.; Zhong, C.; Notaguchi, M.; Yu, W. A susceptible scion reduces rootstock tolerance to Ralstonia solanacearum in grafted eggplant. Horticulturae 2019, 5, 78. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Sang, Y.; Ma, Y.; Guo, M.; Bai, G.; Chen, G. Influences of ice-temperature storage on cell wall metabolism and reactive oxygen metabolism in Xinjiang (Diaogan) apricot. Postharvest Biol. Technol. 2021, 180, 111614. [Google Scholar] [CrossRef]
- Wei, W.; Xu, L.; Peng, H.; Zhu, W.; Tanaka, K.; Cheng, J.; Sanguinet, K.A.; Vandemark, G.; Chen, W. A fungal extracellular effector inactivates plant polygalacturonase-inhibiting protein. Nat. Commun. 2022, 13, 2213. [Google Scholar] [CrossRef]
- Ranjbar, A.; Ramezanian, A.; Shekarforoush, S.; Niakousari, M.; Eshghi, S. Antifungal activity of thymol against the main fungi causing pomegranate fruit rot by suppressing the activity of cell wall degrading enzymes. LWT 2022, 161, 113303. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, J.; Tschaplinski, T.J.; Tuskan, G.A.; Chen, J.-G.; Muchero, W. Regulation of Lignin Biosynthesis and Its Role in Growth-Defense Tradeoffs. Front. Plant Sci. 2018, 9, 1427. [Google Scholar] [CrossRef]
- Li, X.; Zang, C.; Ge, H.; Zhang, J.; Grierson, D.; Yin, X.-R.; Chen, K. Involvement of PAL, C4H, and 4CL in Chilling Injury-induced Flesh Lignification of Loquat Fruit. HortScience 2017, 52, 127–131. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, F.; Xing, H.; Mao, K.; Chen, G.; Guo, Q.; Chen, J. Correlation Analysis of Lignin Accumulation and Expression of Key Genes Involved in Lignin Biosynthesis of Ramie (Boehmeria nivea). Genes 2019, 10, 389. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6562848/ (accessed on 2 October 2022). [CrossRef]


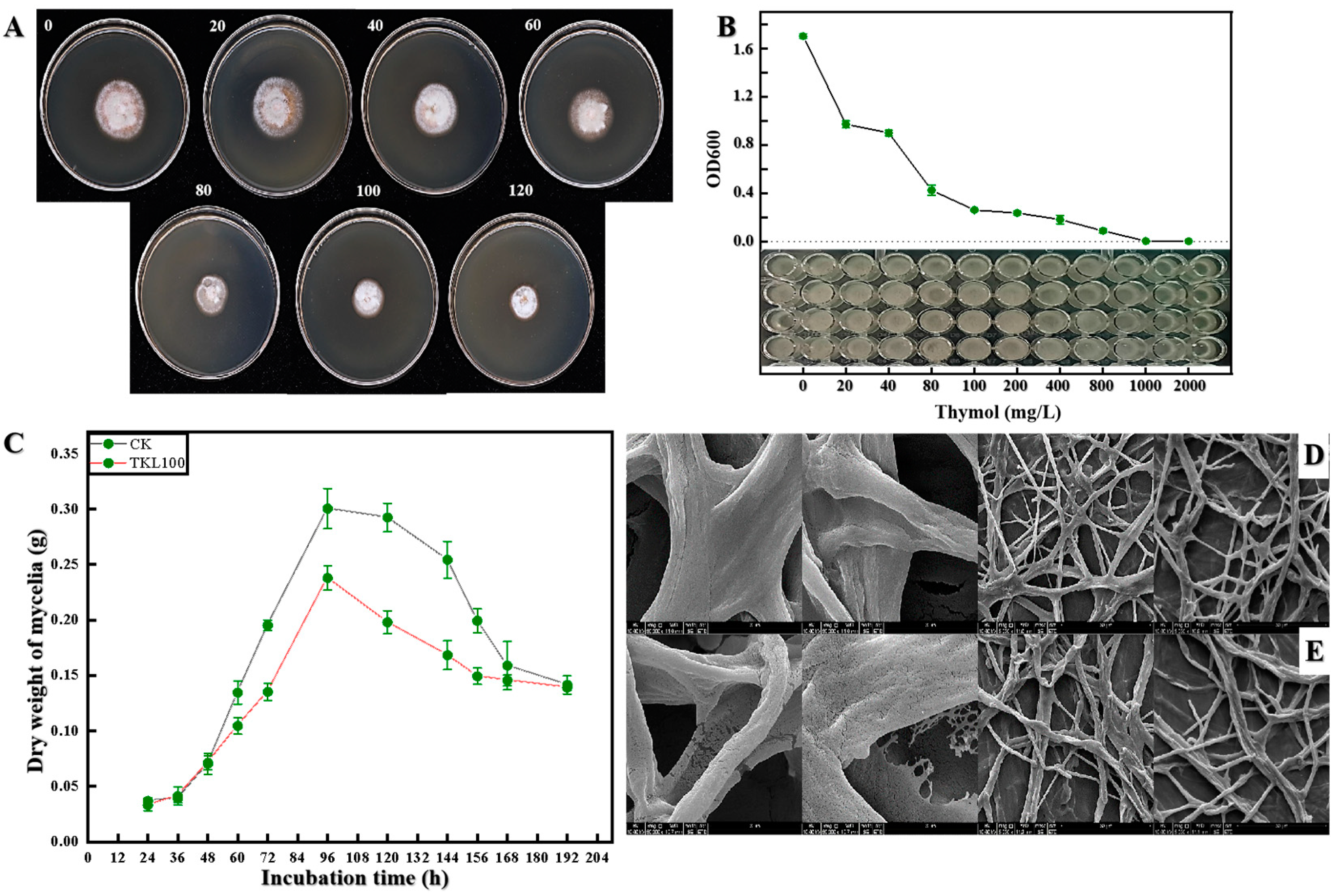
| Bacteriostatic Agent | Thymol Concentration | the Fungus Diameter | Inhibition Rate |
|---|---|---|---|
| TKL 0 | 0 mg/L | 30.12 ± 0.59 a | 0.00% |
| TKL 20 | 20 mg/L | 26.67 ± 0.77 ab | 11.45% |
| TKL 40 | 40 mg/L | 24.23 ± 1.71 bc | 19.57% |
| TKL 60 | 60 mg/L | 18.00 ± 1.00 cd | 40.25% |
| TKL 80 | 80 mg/L | 17.12 ± 1.46 de | 43.16% |
| TKL 100 | 100 mg/L | 14.97 ± 0.73 ef | 50.32% |
| TKL 120 | 120 mg/L | 11.81 ± 0.57 g | 60.81% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Wang, Z.; Li, Y.; Liu, X.; Liu, L.; Yan, J.; Hu, X.; Qin, W. Thymol Edible Coating Controls Postharvest Anthracnose by Regulating the Synthesis Pathway of Okra Lignin. Foods 2023, 12, 395. https://doi.org/10.3390/foods12020395
Zhang Q, Wang Z, Li Y, Liu X, Liu L, Yan J, Hu X, Qin W. Thymol Edible Coating Controls Postharvest Anthracnose by Regulating the Synthesis Pathway of Okra Lignin. Foods. 2023; 12(2):395. https://doi.org/10.3390/foods12020395
Chicago/Turabian StyleZhang, Qinqiu, Zhuwei Wang, Yinglu Li, Xinzhi Liu, Lang Liu, Jing Yan, Xinjie Hu, and Wen Qin. 2023. "Thymol Edible Coating Controls Postharvest Anthracnose by Regulating the Synthesis Pathway of Okra Lignin" Foods 12, no. 2: 395. https://doi.org/10.3390/foods12020395
APA StyleZhang, Q., Wang, Z., Li, Y., Liu, X., Liu, L., Yan, J., Hu, X., & Qin, W. (2023). Thymol Edible Coating Controls Postharvest Anthracnose by Regulating the Synthesis Pathway of Okra Lignin. Foods, 12(2), 395. https://doi.org/10.3390/foods12020395





