Reduction of Cd Uptake in Rice (Oryza sativa) Grain Using Different Field Management Practices in Alkaline Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Characterization of Physicochemical Properties
2.3. Soil Amendment Treatments and Research Design for the Small-Scale Experiment Setup
Rice Cultivation and Management
2.4. Implementation of Different Soil Amendment Treatments for Large-Scale Farm Experiment Setup
Combination Treatments and Research Design for the Large-Scale Farm Experiment Setup
2.5. Preparation of Samples for Analysis
2.6. Determination of Seed Germination Index (SGI)
2.7. Determination of Cd Concentrations in Soil and Grain Samples
2.8. Data Analysis
3. Results
3.1. Cd Concentration in the Surrounding Environment
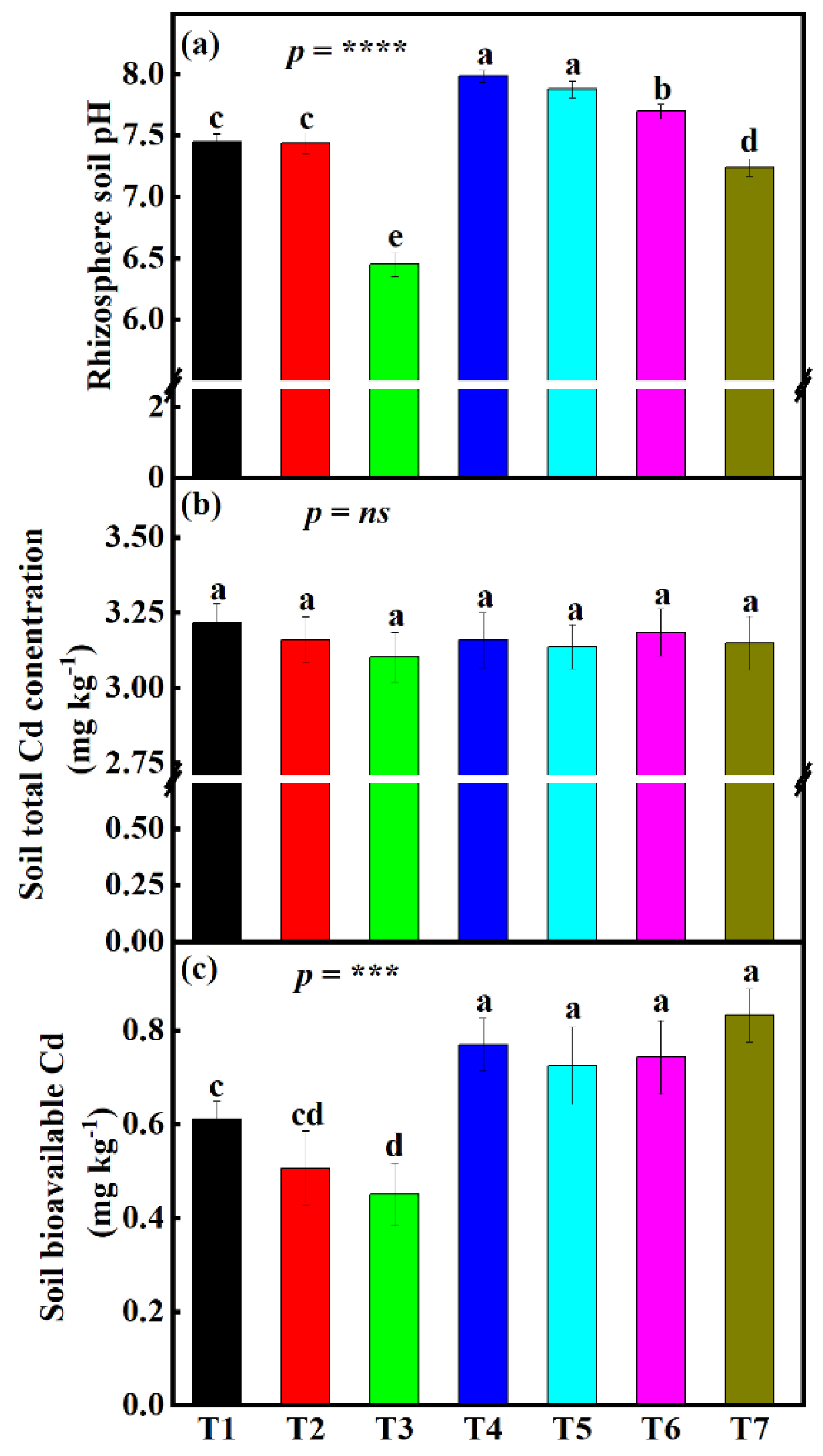
3.2. Influence Different Fertilizer Treatments on Rhizosphere pH Change under the Small-Scale Plot Experiment
3.3. Influence of Different Fertilizer Treatments on Rhizosphere Soil-Available Cd and Soil Total Cd Concentrations under Small-Scale Plot Experiment
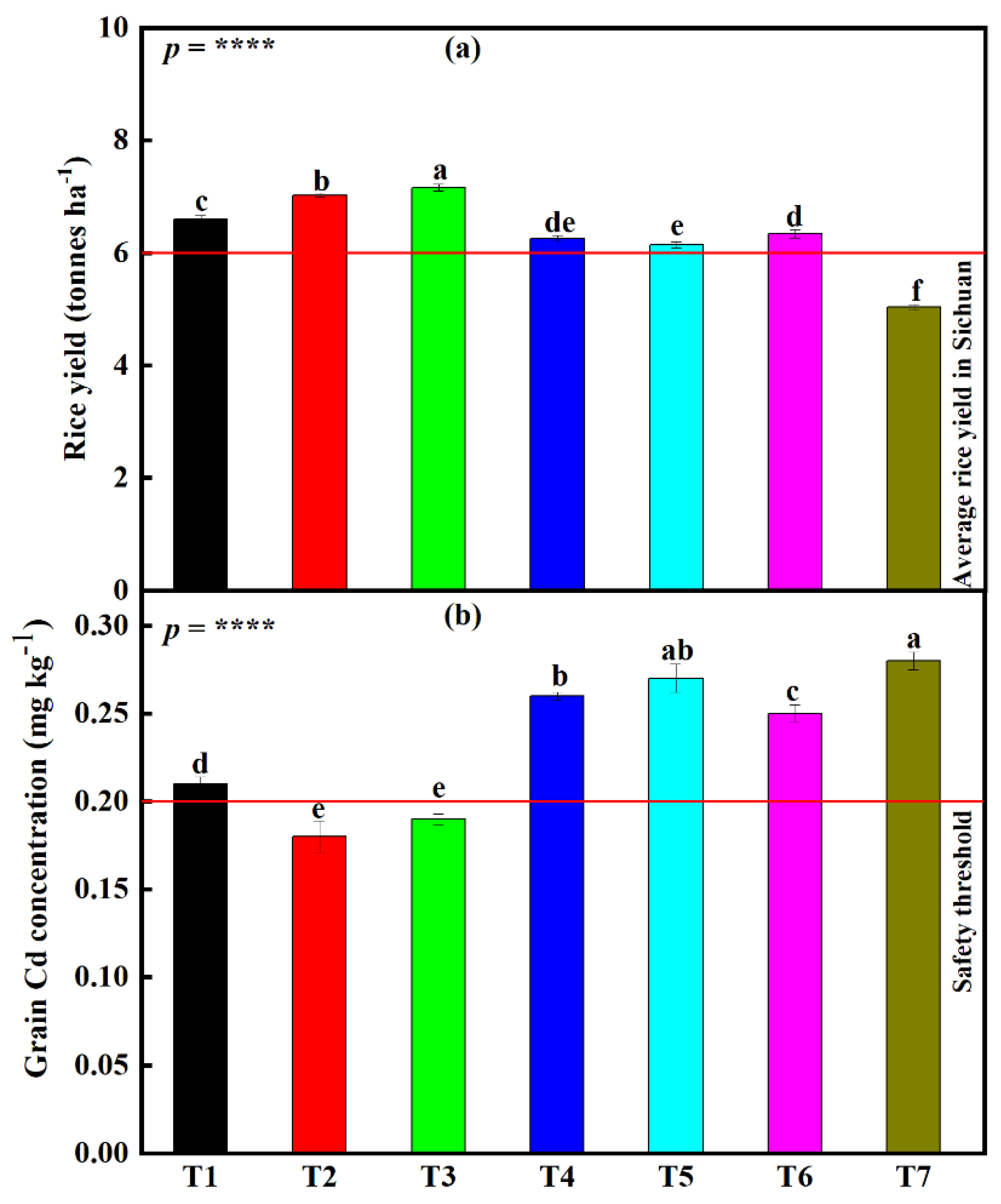
3.4. Influence of the Different Fertilizer Treatments on Rice Seed Germination Index (SGI), Grain Yield, and Grain Cd Concentration under the Small-Scale Plot Experiment
3.5. Effects of Different Soil Amendment Combinations on Rhizosphere pH under the Large-Scale Field Experiment
3.6. Effects of Different Soil Amendment Combinations on Rhizosphere Soil Total Cd Concentrations and Bioavailable Cd under the Large-Scale Field Experiment
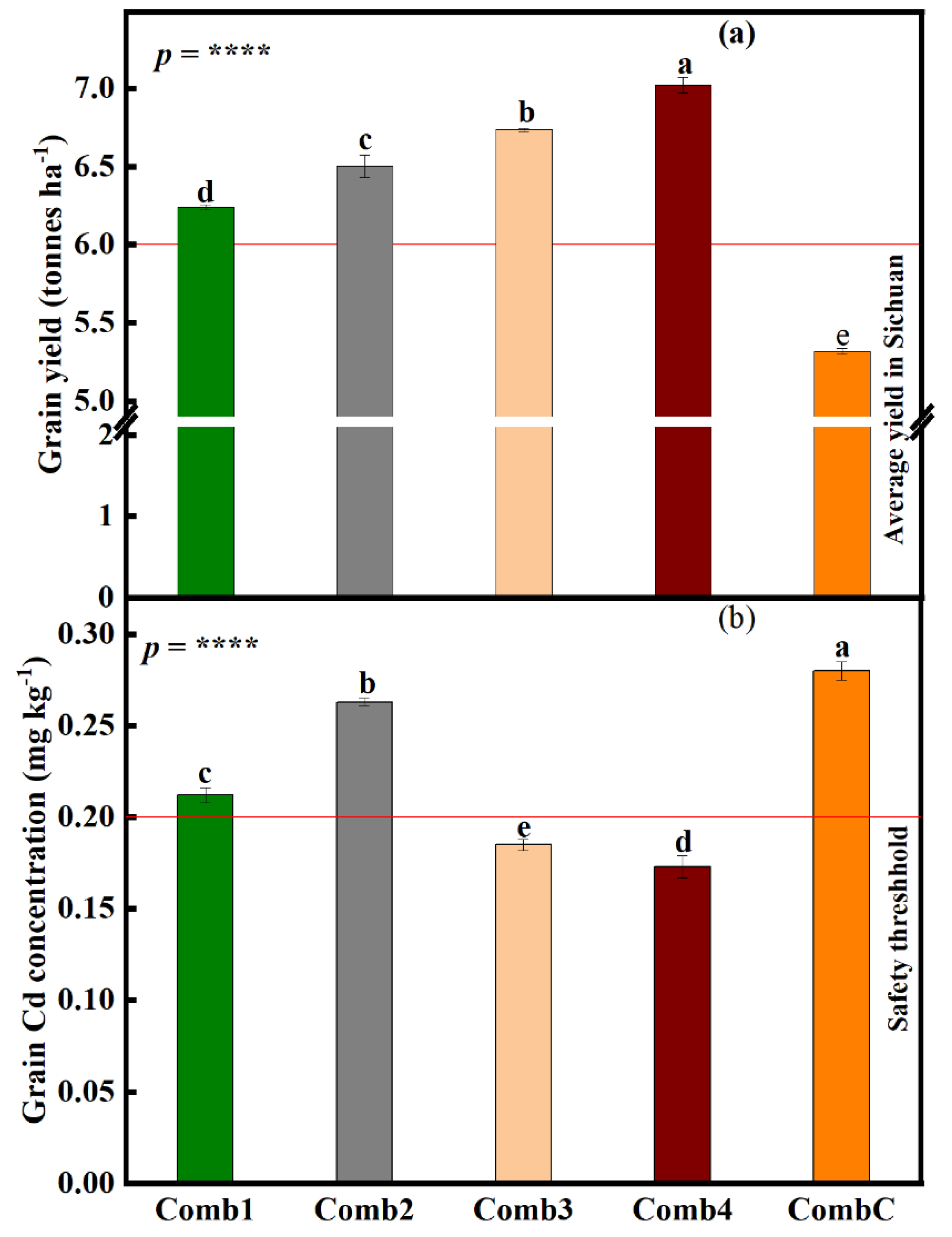
3.7. Effects of Different Combination Treatments on Rice Growth and Yield Parameters under the Large-Scale Field Experiment
3.7.1. Rice Seed Germination Index (SGI), Panicle Length (PL), Number of Tillers per Hill (NTH), and Number of Kernels per Panicle (NKP) at Large Field Scale
3.7.2. Differences in Rice Grain Yield and Grain Cd Concentration under the Large-Scale Field Experiment
3.8. Correlations between Cd Soil and Plant Attributes under the Large Field Scale
4. Discussion
4.1. Humic Acid Effectively Lowers Rhizosphere Soil pH
4.2. Different Amendment Materials Effectively Lowers Rhizosphere Bioavailable Cd
4.3. Soil Amendment Treatments Improve Seedling Germination and Plant Growth
4.4. Different Combination Treatments Variedly Improve Grain Yield and Grain Cd Concentration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, Y.; Li, W.; Yang, Z.; Zhang, Q.; Ji, J. Enrichment and source identification of Cd and other heavy metals in soils with high geochemical background in the karst region, Southwestern China. Chemosphere 2020, 245, 125620. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.; Plusquin, M.; Hogervorst, J.; Roels, H.A.; Celis, H.; Thijs, L.; Vangronsveld, J.; Van Hecke, E.; Staessen, J.A. Environmental exposure to cadmium and risk of cancer: A prospective population-based study. Lancet Oncol. 2006, 7, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.M. Methods for the determination of heavy metals and metalloids in soils. In Heavy Metals in Soils; Springer: Berlin/Heidelberg, Germany, 2013; pp. 97–140. [Google Scholar]
- Devi, U.; Bhattacharyya, K.G. Mobility and bioavailability of Cd, Co, Cr, Cu, Mn and Zn in surface runoff sediments in the urban catchment area of Guwahati, India. Appl. Water Sci. 2018, 8, 18. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kong, D.; Zhang, B.; Kalkhajeh, Y.K.; Zhao, Y.; Huang, J.; Hu, H. Chemical forms of cadmium in soil and its distribution in French marigold sub-cells in response to chelator GLDA. Sci. Rep. 2022, 12, 17577. [Google Scholar] [CrossRef]
- Singh, O.; Labana, S.; Pandey, G.; Budhiraja, R.; Jain, R. Phytoremediation: An overview of metallic ion decontamination from soil. Appl. Microbiol. Biotechnol. 2003, 61, 405–412. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Huang, Y.; Zhou, S. The tolerance and accumulation of Miscanthus sacchariflorus (maxim.) Benth., an energy plant species, to cadmium. Int. J. Phytoremediation 2015, 17, 538–545. [Google Scholar] [CrossRef]
- Han, Y.; Zveushe, O.K.; Dong, F.; Ling, Q.; Chen, Y.; Sajid, S.; Zhou, L.; de Dios, V.R. Unraveling the effects of arbuscular mycorrhizal fungi on cadmium uptake and detoxification mechanisms in perennial ryegrass (Lolium perenne). Sci. Total Environ. 2021, 798, 149222. [Google Scholar] [CrossRef]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef]
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.; Sebastian, A.; Prasad, M.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2022, 52, 675–726. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, N.; Li, X.; Long, J.; Sui, X.; Wu, Y.; Li, J.; Wang, J.; Zhong, H.; Sun, G.Y. Arbuscular mycorrhizal fungi (Glomus mosseae) improves growth, photosynthesis and protects photosystem II in leaves of Lolium perenne L. in cadmium contaminated soil. Front. Plant Sci. 2018, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, M.; Hendrix, S.; Kyndt, T.; Demeestere, K.; Vandamme, D.; Cuypers, A. Short-term effects of cadmium on leaf growth and nutrient transport in rice plants. Plant Sci. 2021, 313, 111054. [Google Scholar] [CrossRef] [PubMed]
- Lien, K.W.; Pan, M.H.; Ling, M.P. Levels of heavy metal cadmium in rice (Oryza sativa L.) produced in Taiwan and probabilistic risk assessment for the Taiwanese population. Environ. Sci. Pollut. Res. 2021, 28, 28381–28390. [Google Scholar] [CrossRef] [PubMed]
- El-Hameed, M.M.A.; Abuarab, M.E.; Al-Ansari, N.; Mottaleb, S.A.; Bakeer, G.A.; Gyasi-Agyei, Y.; Mokhtar, A. Phycoremediation of contaminated water by cadmium (Cd) using two cyanobacterial strains (Trichormus variabilis and Nostoc muscorum). Environ. Sci. Eur. 2021, 33, 135. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Jayalakshmi, N.; Geetha, N.; Sahi, S.V.; Sharma, N.C.; Rene, E.R.; Sarkar, S.K.; Favas, P.J. Accumulation efficiency, genotoxicity and antioxidant defense mechanisms in medicinal plant Acalypha indica L. under lead stress. Chemosphere 2017, 171, 544–553. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Hristozkova, M.; Geneva, M.; Stancheva, I.; Boychinova, M.; Djonova, E. Contribution of arbuscular mycorrhizal fungi in attenuation of heavy metal impact on Calendula officinalis development. Appl. Soil Ecol. 2016, 101, 57–63. [Google Scholar] [CrossRef]
- Khanna, P. Assessment of heavy metal contamination in different vegetables grown in and around urban areas. Res. J. Environ. Toxicol. 2011, 5, 162. [Google Scholar]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.-W. Role of organic amendments on enhanced bioremediation of heavy metal (loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef]
- Meng, J.; Zhong, L.; Wang, L.; Liu, X.; Tang, C.; Chen, H.; Xu, J. Contrasting effects of alkaline amendments on the bioavailability and uptake of Cd in rice plants in a Cd-contaminated acid paddy soil. Environ. Sci. Pollut. Res. 2018, 25, 8827–8835. [Google Scholar] [CrossRef]
- Meng, L.; Huang, T.; Shi, J.; Chen, J.; Zhong, F.; Wu, L.; Xu, J. Decreasing cadmium uptake of rice (Oryza sativa L.) in the cadmium-contaminated paddy field through different cultivars coupling with appropriate soil amendments. J. Soils Sediments 2019, 19, 1788–1798. [Google Scholar] [CrossRef]
- Ito, V.C.; Lacerda, L.G. Black rice (Oryza sativa L.): A review of its historical aspects, chemical composition, nutritional and functional properties, and applications and processing technologies. Food Chem. 2019, 301, 125304. [Google Scholar] [CrossRef] [PubMed]
- Lomthong, T.; Saithong, P. Feasibility of Leum Pua glutinous rice substrate for sugar syrup and vinegar production by raw starch degrading enzyme hydrolysis. Int. Food Res. J. 2019, 26, 1515–1523. [Google Scholar]
- Hu, B.; Shao, S.; Fu, Z.; Li, Y.; Ni, H.; Chen, S.; Zhou, Y.; Jin, B.; Shi, Z. Identifying heavy metal pollution hot spots in soil-rice systems: A case study in South of Yangtze River Delta, China. Sci. Total Environ. 2019, 658, 614–625. [Google Scholar] [CrossRef]
- Xin, F.; Xiao, X.; Dong, J.; Zhang, G.; Zhang, Y.; Wu, X.; Li, X.; Zou, Z.; Ma, J.; Du, G. Large increases of paddy rice area, gross primary production, and grain production in Northeast China during 2000–2017. Sci. Total Environ. 2020, 711, 135183. [Google Scholar] [CrossRef]
- Wen, M.; Ma, Z.; Gingerich, D.B.; Zhao, X.; Zhao, D. Heavy metals in agricultural soil in China: A systematic review and meta-analysis. Eco Environ. Health 2022, 1, 219–228. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, T.; Liu, L.; Ouyang, X. Impact of soil heavy metal pollution on food safety in China. PLoS ONE 2015, 10, e0135182. [Google Scholar] [CrossRef]
- Hoareau, C.E.; Hadibarata, T.; Yılmaz, M. Occurrence of cadmium in groundwater in China: A review. Arab. J. Geosci. 2022, 15, 1455. [Google Scholar] [CrossRef]
- Bian, R.; Chen, D.; Liu, X.; Cui, L.; Li, L.; Pan, G.; Xie, D.; Zheng, J.; Zhang, X.; Zheng, J. Biochar soil amendment as a solution to prevent Cd-tainted rice from China: Results from a cross-site field experiment. Ecol. Eng. 2013, 58, 378–383. [Google Scholar] [CrossRef]
- Huang, C.L.; Bao, L.J.; Luo, P.; Wang, Z.Y.; Li, S.M.; Zeng, E.Y. Potential health risk for residents around a typical e-waste recycling zone via inhalation of size-fractionated particle-bound heavy metals. J. Hazard. Mater. 2016, 317, 449–456. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Kopittke, P.M.; Zhao, F.J. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lu, W.; Bai, C.; Xu, C.; Ye, M.; Zhu, Y.; Yao, L. Cadmium, Arsenic and Mineral Nutrients in Rice, and Potential Risks for Human Health in South China. 2022. Available online: https://www.researchsquare.com/article/rs-1709663/v1 (accessed on 30 October 2022). [CrossRef]
- Lv, L.; Jiao, Z.; Ge, S.; Zhan, W.; Ruan, X.; Wang, Y. Assessment of Cd Pollution in Paddy Soil–Rice System in Silver Mining-Affected Areas: Pollution Status, Transformation and Health Risk Assessment. Int. J. Environ. Res. Public Health 2022, 19, 12362. [Google Scholar] [CrossRef] [PubMed]
- Li, L.F.; Ai, S.Y.; Wang, Y.H.; Tang, M.D.; Li, Y.C. In situ field-scale remediation of low Cd-contaminated paddy soil using soil amendments. Water Air Soil Pollut. 2016, 227, 342. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601, 1591–1605. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, M.Z.; Usman, M.; He, Z.L.; Yang, X. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci. Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef]
- Yu, H.; Wang, J.; Fang, W.; Yuan, J.; Yang, Z. Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci. Total Environ. 2006, 370, 302–309. [Google Scholar] [CrossRef]
- Chi, Y.; Li, F.; Tam, N.F.-Y.; Liu, C.; Ouyang, Y.; Qi, X.; Li, W.C.; Ye, Z. Variations in grain cadmium and arsenic concentrations and screening for stable low-accumulating rice cultivars from multi-environment trials. Sci. Total Environ. 2018, 643, 1314–1324. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Luo, C.; Liu, X.; Wang, S.; Liu, T.; Li, X. Foliar application of two silica sols reduced cadmium accumulation in rice grains. J. Hazard. Mater. 2009, 161, 1466–1472. [Google Scholar] [CrossRef]
- Gao, M.; Zhou, J.; Liu, H.; Zhang, W.; Hu, Y.; Liang, J.; Zhou, J. Foliar spraying with silicon and selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. Sci. Total Environ. 2018, 631, 1100–1108. [Google Scholar] [CrossRef]
- Duan, M.M.; Wang, S.; Huang, D.Y.; Zhu, Q.H.; Liu, S.L.; Zhang, Q.; Zhu, H.H.; Xu, C. Effectiveness of simultaneous applications of lime and zinc/iron foliar sprays to minimize cadmium accumulation in rice. Ecotoxicol. Environ. Saf. 2018, 165, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Rizwan, M.; Ali, S.; Zia ur Rehman, M.; Ishaque, W.; Atif Riaz, M.; Maqbool, A. Effect of foliar-applied iron complexed with lysine on growth and cadmium (Cd) uptake in rice under Cd stress. Environ. Sci. Pollut. Res. 2018, 25, 20691–20699. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Y. Immobilization of Cd in paddy soil using moisture management and amendment. Environ. Sci. Pollut. Res. 2015, 22, 5580–5586. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y. Immobilization remediation of Cd-polluted soil with different water condition. J. Environ. Manag. 2017, 193, 607–612. [Google Scholar] [CrossRef]
- Luo, W.; Yang, S.; Khan, M.A.; Ma, J.; Xu, W.; Li, Y.; Xiang, Z.; Jin, G.; Jia, J.; Zhong, B. Mitigation of Cd accumulation in rice with water management and calcium-magnesium phosphate fertilizer in field environment. Environ. Geochem. Health 2020, 42, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.G.; Kuek, C.; Chaudhry, T.; Khoo, C.S.; Hayes, W.J. Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 2000, 41, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Galiulin, R.V.; Bashkin, V.N.; Galiulina, R.A.; Kucharski, R. Airborne soil contamination by heavy metals in Russia and Poland, and its remediation. Land Contam. Reclam. 2002, 10, 179–187. [Google Scholar] [CrossRef]
- Liang, X.; Han, J.; Xu, Y.; Sun, Y.; Wang, L.; Tan, X. In situ field-scale remediation of Cd polluted paddy soil using sepiolite and palygorskite. Geoderma 2014, 235, 9–18. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Li, Z.; Yang, W.; Zou, J.; Zhou, H.; Zeng, Q.; Liao, B. Effects of calcium magnesium phosphate fertilizer on Cd bioavailability in soil and Cd contents in rice. Acta Sci. Circumst 2017, 37, 2322–2330. [Google Scholar]
- Pei, P.; Sun, Y.; Wang, L.; Liang, X.; Xu, Y. In-situ stabilization of Cd by sepiolite co–applied with organic amendments in contaminated soils. Ecotoxicol. Environ. Saf. 2021, 208, 111600. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Luo, F.; Tu, F.; Rukh, G.; Ye, Z.; Ruan, Z.; Liu, D. Effects of Stabilizing Materials on soil Cd bioavailability and Rice Growth, Cd Uptake and Cd Transport. Front. Environ. Sci. 2022, 2245. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Lin, Q.; Rashid, M.S.; He, Z.; Yang, X. Organic soil additives for the remediation of cadmium contaminated soils and their impact on the soil-plant system: A review. Sci. Total Environ. 2020, 707, 136121. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Liang, T.; Yan, X.; Zhong, L.; Shao, J.; El-Naggar, A.; Guan, C.Y.; Liu, J.; Zhou, Y. A combined management scheme to simultaneously mitigate As and Cd concentrations in rice cultivated in contaminated paddy soil. J. Hazards Mater. 2021, 416, 125837. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, R.; Peng, S.; Liu, Q.; Zhu, X. Effect of humic acid on transformation of soil heavy metals. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; p. 012089. [Google Scholar]
- Gray, C.; Dunham, S.; Dennis, P.; Zhao, F.; McGrath, S. Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud. Environ. Pollut. 2006, 142, 530–539. [Google Scholar] [CrossRef]
- Chen, D.; Chen, D.; Xue, R.; Long, J.; Lin, X.; Lin, Y.; Jia, L.; Zeng, R.; Song, Y. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J. Hazard. Mater. 2019, 367, 447–455. [Google Scholar] [CrossRef]
- Pati, S.; Pal, B.; Badole, S.; Hazra, G.C.; Mandal, B. Effect of silicon fertilization on growth, yield, and nutrient uptake of rice. Commun. Soil Sci. Plant Anal. 2016, 47, 284–290. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Rizwan, M.; Dai, Z.; Yuan, Y.; Hossain, M.M.; Cao, M.; Xiong, S.; Tu, S. Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants. J. Hazard. Mater. 2021, 401, 123393. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, S.; Cai, K.; Huang, F.; Pan, B.; Wang, W. Cd accumulation, biomass and yield of rice are varied with silicon application at different growth phases under high concentration cadmium-contaminated soil. Chemosphere 2020, 242, 125128. [Google Scholar] [CrossRef]
- Zhang, D.; Du, G.; Chen, D.; Shi, G.; Rao, W.; Li, X.; Jiang, Y.; Liu, S.; Wang, D. Effect of elemental sulfur and gypsum application on the bioavailability and redistribution of cadmium during rice growth. Sci. Total Environ. 2019, 657, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Z.Y.; Su, S.W.; Lai, H.Y.; Guo, H.Y.; Chen, T.C.; Chen, Z.S. Remediation techniques and heavy metal uptake by different rice varieties in metal-contaminated soils of Taiwan: New aspects for food safety regulation and sustainable agriculture. Soil Sci. Plant Nutr. 2010, 56, 31–52. [Google Scholar] [CrossRef]
- Anderson, J.; Ingram, J. Tropical Soil Biology and Fertility a Handbook of Methods [Internet]; CAB International: WaHingford, UK, 1993. [Google Scholar]
- Van Reeuwijk, L. Procedures for Soil Analysis; International Soil Reference and Information Center: Wageningen, The Netherland, 2002. [Google Scholar]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic forms. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Amer Society of Agronomy: Madison, WI, USA, 1983; Volume 9, pp. 643–698. [Google Scholar]
- Mehlich, A. Determination of P, Ca, mg, K, Na, and NH4; North Carolina Soil Test Division: Raleigh, NC, USA, 1953; pp. 23–89. [Google Scholar]
- Rowen, D.L. Soil acidity and alkalinity. In Soil Science: Methods and Applications; Pearson Education Limited: New York, NY, USA, 1994; Volume 1. [Google Scholar]
- Pansu, M.; Gautheyrou, J. Exchangeable cations. In Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; pp. 667–676. [Google Scholar]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Liu, L.; Ulhassan, Z.; He, Z.; Yang, X. Sepiolite clay: A review of its applications to immobilize toxic metals in contaminated soils and its implications in soil–plant system. Environ. Technol. Innov. 2021, 23, 101598. [Google Scholar] [CrossRef]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Cao, D.-D.; Hu, J.; Huang, X.-X.; Wang, X.-J.; Guan, Y.-J.; Wang, Z.-F. Relationships between changes of kernel nutritive components and seed vigor during development stages of F1 seeds of sh 2 sweet corn. J. Zhejiang Univ. Sci. B 2008, 9, 964–968. [Google Scholar] [CrossRef]
- Mondal, N.K.; Chittaranjan, D.; Satinath, R.; Datta, J.K.; Arnab, B. Effect of varying cadmium stress on chickpea (Cicer arietinum L.) seedlings: An ultrastructural study. Ann. Environ. Sci. 2013, 7, 59–70. [Google Scholar]
- Nigam, N.; Yadav, V.; Khare, P.; Singh, R.P.; Das, P.; Shanker, K.; Sharma, R.S. Exploring the benefits of biochar over other organic amendments for reducing of metal toxicity in Withania somnifera. Biochar 2019, 1, 293–307. [Google Scholar] [CrossRef]
- Hu, X.; Huang, X.; Zhao, H.; Liu, F.; Wang, L.; Zhao, X.; Gao, P.; Li, X.; Ji, P. Possibility of using modified fly ash and organic fertilizers for remediation of heavy-metal-contaminated soils. J. Clean. Prod. 2021, 284, 124713. [Google Scholar] [CrossRef]
- Xu, C.; Qi, J.; Yang, W.; Chen, Y.; Yang, C.; He, Y.; Wang, J.; Lin, A. Immobilization of heavy metals in vegetable-growing soils using nano zero-valent iron modified attapulgite clay. Sci. Total Environ. 2019, 686, 476–483. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Seshadri, B.; Bolan, N.; Choppala, G.; Kunhikrishnan, A.; Sanderson, P.; Wang, H.; Currie, L.; Tsang, D.C.; Ok, Y.; Kim, G. Potential value of phosphate compounds in enhancing immobilization and reducing bioavailability of mixed heavy metal contaminants in shooting range soil. Chemosphere 2017, 184, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Mindari, W. Effect of humic acid on soil chemical and physical characteristics of embankment. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2016; p. 01028. [Google Scholar]
- Tan, X.; Wang, X.; Chen, C.; Sun, A. Effect of soil humic and fulvic acids, pH and ionic strength on Th (IV) sorption to TiO2 nanoparticles. Appl. Radiat. Isot. 2007, 65, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, Y.; Huang, Q.; Li, B.; Ma, T.; Qin, X.; Zhao, L.; Sun, Y.; Xu, Y. Effects of sepiolite and biochar on the photosynthetic and antioxidant systems of pakchoi under Cd and atrazine stress. J. Environ. Sci. Health B. 2022, 57, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ye, X.; Zhang, Q.; Xiao, W.; Ni, Z.; Yang, L.; Zhao, S.; Hu, J.; Gao, N.; Huang, M. The effect of sepiolite application on rice Cd uptake–A two-year field study in Southern China. J. Environ. Manag. 2020, 254, 109788. [Google Scholar] [CrossRef]
- Xiao, Z.; Peng, M.; Mei, Y.; Tan, L.; Liang, Y. Effect of organosilicone and mineral silicon fertilizers on chemical forms of cadmium and lead in soil and their accumulation in rice. Environ. Pollut. 2021, 283, 117107. [Google Scholar] [CrossRef]
- Gondar, D.; López, R.; Fiol, S.; Antelo, J.; Arce, F. Cadmium, lead, and copper binding to humic acid and fulvic acid extracted from an ombrotrophic peat bog. Geoderma 2006, 135, 196–203. [Google Scholar] [CrossRef]
- Rong, Q.; Zhong, K.; Huang, H.; Li, C.; Zhang, C.; Nong, X. Humic acid reduces the available cadmium, copper, lead, and zinc in soil and their uptake by tobacco. Appl. Sci. 2020, 10, 1077. [Google Scholar] [CrossRef]
- Irving, H.; Andrew, G.; Risdon, E. Reversion: A new procedure in absorptiometry. Nature 1948, 161, 805–806. [Google Scholar] [CrossRef]
- Lu, L.; Liu, G.; Wang, J.; Wu, Y. Bioavailability and mobility of heavy metals in soil in vicinity of a coal mine from Huaibei, China. Hum. Ecol. Risk Assess. 2017, 23, 1164–1177. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.J.; Zahir, Z.A.; Jamil, A. Effect of cadmium on seed germination and seedling growth of four wheat (Triticum aestivum L.) cultivars. Pak. J. Bot 2012, 44, 1569–1574. [Google Scholar]
- Imtiaz, M.; Tu, S.; Xie, Z.; Han, D.; Ashraf, M.; Rizwan, M.S. Growth, V uptake, and antioxidant enzymes responses of chickpea (Cicer arietinum L.) genotypes under vanadium stress. Plant Soil 2015, 390, 17–27. [Google Scholar] [CrossRef]
- Zaman, Q.U.; Rashid, M.; Nawaz, R.; Hussain, A.; Ashraf, K.; Latif, M.; Heile, A.O.; Mehmood, F.; Salahuddin, S.; Chen, Y. Silicon Fertilization: A Step towards Cadmium-Free Fragrant Rice. Plants 2021, 10, 2440. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.; Bandara, D.; Weerasinghe, P.; Iqbal, M.; Wijayawardhana, H. Effect of cadmium on growth parameters and plant accumulation in different rice (Oryza sativa L.) varieties in Sri Lanka. Trop. Agric. Res. 2015, 25, 532–542. [Google Scholar] [CrossRef]
- Murugaiyan, V.; Zeibig, F.; Anumalla, M.; Siddiq, S.A.; Frei, M.; Murugaiyan, J.; Ali, J. Arsenic stress responses and accumulation in rice. In Rice Improvement; Springer: Cham, Switzerland, 2021; pp. 281–313. [Google Scholar]
- Khan, M.; Rolly, N.K.; Al Azzawi, T.N.I.; Imran, M.; Mun, B.G.; Lee, I.J.; Yun, B.W. Lead (Pb)-induced oxidative stress alters the morphological and physio-biochemical properties of rice (Oryza sativa L.). Agronomy 2021, 11, 409. [Google Scholar] [CrossRef]
- Martínez-Cortijo, J.; Ruiz-Canales, A. Effect of heavy metals on rice irrigated fields with waste water in high pH Mediterranean soils: The particular case of the Valencia area in Spain. Agric. Water Manag. 2018, 210, 108–123. [Google Scholar] [CrossRef]
- Duan, Y.; Li, Q.; Zhang, L.; Huang, Z.; Zhao, Z.; Zhao, H.; Du, J.; Zhou, J. Toxic Metals in a Paddy Field System: A Review. Toxics 2022, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Gokulraj, N.; Ravichandran, V.; Boominathan, P.; Soundararajan, R. Influence of Silicon on Physiology and Yield of Rice under Drought Stress. Madras Agric. J. 2018, 105. [Google Scholar] [CrossRef]
- Ramírez-Olvera, S.M.; Trejo-Téllez, L.I.; Gómez-Merino, F.C.; Ruíz-Posadas, L.d.M.; Alcántar-González, E.G.; Saucedo-Veloz, C. Silicon Stimulates Plant Growth and Metabolism in Rice Plants under Conventional and Osmotic Stress Conditions. Plants 2021, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Q.; Tang, T.; Chen, X.; Luo, X. Silicon fertilizer application promotes phytolith accumulation in rice plants. Front. Plant Sci. 2019, 10, 425. [Google Scholar] [CrossRef]
- Bana, R.S.; Jat, G.S.; Grover, M.; Bamboriya, S.D.; Singh, D.; Bansal, R.; Choudhary, A.K.; Kumar, V.; Laing, A.M.; Godara, S. Foliar nutrient supplementation with micronutrient-embedded fertilizer increases biofortification, soil biological activity and productivity of eggplant. Sci. Rep. 2022, 12, 5146. [Google Scholar] [CrossRef]
- Kentelky, E.; Szekely-Varga, Z. Impact of Foliar Fertilization on Growth, Flowering, and Corms Production of Five Gladiolus Varieties. Plants 2021, 10, 1963. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Zaidi, A.; Ameen, F.; Ahmed, B.; AlKahtani, M.D.; Khan, M.S. Heavy metal induced stress on wheat: Phytotoxicity and microbiological management. RSC Adv. 2020, 10, 38379–38403. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Gao, Y.; Kuang, Q.; Wu, Y.; Zhang, Y.; Xu, B.; Chen, Y. Effect of silicon on morpho-physiological attributes, yield and cadmium accumulation in two maize genotypes with contrasting root system size and health risk assessment. Plant Soil. 2022, 477, 117–134. [Google Scholar] [CrossRef]
- Flores, R.A.; Pessoa-de-Souza, M.A.; de Andrade, A.F.; Bueno, A.M.; de Oliveira Abdala, K.; de Souza Júnior, J.P.; de Mello Prado, R.; Santos, G.G.; Mesquita, M. Does foliar application of silicon under natural water stress conditions increase rice yield in subtropical dry regions? Silicon 2022, 14, 3591–3600. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, Z.; Li, X.; Liu, H.; Li, N.; Wei, S. Mitigation of rice cadmium (Cd) accumulation by joint application of organic amendments and selenium (Se) in high-Cd-contaminated soils. Chemosphere 2020, 241, 125106. [Google Scholar] [CrossRef]
- Liang, Y.; Wong, J.; Wei, L. Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 2005, 58, 475–483. [Google Scholar] [CrossRef]
- Ji, X.; Liu, S.; Juan, H.; Bocharnikova, E.A.; Matichenkov, V.V. Effect of silicon fertilizers on cadmium in rice (Oryza sativa) tissue at tillering stage. Environ. Sci. Pollut. Res. 2017, 24, 10740–10748. [Google Scholar] [CrossRef]



| Treatment Codes | Amendment Material Names | Chemical Formulas | Application Rates (kg m−2) |
|---|---|---|---|
| T1 | Sepiolite | Mg4Si6O15(OH)2•6(H2O) | 1.27 |
| T2 | Sodium metasilicate | Na2SiO3 | 0.03 |
| T3 | Humic acid | C187H186O89N9S1 | 0.07 |
| T4 | calcium magnesium phosphate fertilizer | CaMgO4P+ | 0.09 |
| T5 | ferrous sulfate + manganese sulfate | FeSO4 + MgSO4 | 0.07 + 0.007 |
| T6 | Straw biochar | - | 0.75 |
| T7/Control | No chemical added | - | - |
| Treatment Codes | Soil Amendment Options | ||||
|---|---|---|---|---|---|
| Humic Acid | Soil Silicon Fertilizer | Foliar Silicon Fertilizer | Deep Plowing (15–30 cm) | Shallow Plowing (5–10 cm) | |
| Comb1 | + | + | − | + | − |
| Comb2 | + | + | − | − | + |
| Comb3 | + | + | + | + | − |
| Comb4 | + | + | + | − | + |
| CombC | − | − | − | − | + |
| Chemical Properties | Value (Mean ± Standard Error) |
|---|---|
| pH | 7.63 ± 0.7 |
| CEC (mg kg−1) | 12.83 ± 0.9 |
| Exchangeable Ca2+ (mg kg−1) | 17.1 ± 4.7 |
| Exchangeable K+ (mg kg−1) | 76.0 ± 21.2 |
| Exchangeable Fe2+ (mg kg−1) | 476.9 ± 24.8 |
| Exchangeable NH4+ (mg kg−1) | 28.02 ± 0.01 |
| Exchangeable Na+ (%) | 0.001 ± 0.0001 |
| Exchangeable Mn2+ (mg kg−1) | 6.1 ± 0.2 |
| Exchangeable Mg2+ (mg kg−1) | 20.4 ± 0.8 |
| Available P (mg kg−1) | 1.24 ± 0.08 |
| Available NO3− (mg kg−1) | 15.88 ± 0.93 |
| Total soil Cd (mg kg−1) | 3.21 ± 0.03 |
| Irrigation water (mg L−1) | 0.0091 ± 0.0001 |
| Total Cd concentration from old clay building walls (mg kg−1) | 3.8471 ± 0.02 |
| Treatments | Number of Tillers per Hill | Panicle Length (cm) | Number of Kernels per Panicle |
|---|---|---|---|
| Comb1 | 6.20 ± 0.5d | 10.3 ± 0.1c | 33.1 ± 0.9d |
| Comb2 | 7.14 ± 0.4c | 11.4 ± 0.3b | 35.5 ± 7c |
| Comb3 | 8.33 ± 0.5b | 13.8 ± 0.6a | 40.2 ± 1b |
| Comb4 | 8.61 ± 0.8a | 14.2 ± 0.3a | 44.6 ± 1.4a |
| CombC | 5.14 ± 0.4e | 8.3 ± 0.4d | 28.4 ± 2.1e |
| p-value | **** | **** | **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zveushe, O.K.; Ling, Q.; Li, X.; Sajid, S.; Dios, V.R.d.; Nabi, F.; Han, Y.; Dong, F.; Zeng, F.; Zhou, L.; et al. Reduction of Cd Uptake in Rice (Oryza sativa) Grain Using Different Field Management Practices in Alkaline Soils. Foods 2023, 12, 314. https://doi.org/10.3390/foods12020314
Zveushe OK, Ling Q, Li X, Sajid S, Dios VRd, Nabi F, Han Y, Dong F, Zeng F, Zhou L, et al. Reduction of Cd Uptake in Rice (Oryza sativa) Grain Using Different Field Management Practices in Alkaline Soils. Foods. 2023; 12(2):314. https://doi.org/10.3390/foods12020314
Chicago/Turabian StyleZveushe, Obey Kudakwashe, Qin Ling, Xing Li, Sumbal Sajid, Víctor Resco de Dios, Farhan Nabi, Ying Han, Faqin Dong, Fang Zeng, Lei Zhou, and et al. 2023. "Reduction of Cd Uptake in Rice (Oryza sativa) Grain Using Different Field Management Practices in Alkaline Soils" Foods 12, no. 2: 314. https://doi.org/10.3390/foods12020314
APA StyleZveushe, O. K., Ling, Q., Li, X., Sajid, S., Dios, V. R. d., Nabi, F., Han, Y., Dong, F., Zeng, F., Zhou, L., Shen, S., Zhang, W., & Li, Z. (2023). Reduction of Cd Uptake in Rice (Oryza sativa) Grain Using Different Field Management Practices in Alkaline Soils. Foods, 12(2), 314. https://doi.org/10.3390/foods12020314










