Widely Targeted Metabolomics Was Used to Reveal the Differences between Non-Volatile Compounds in Different Wines and Their Associations with Sensory Properties
Abstract
1. Introduction
2. Materials and Methods
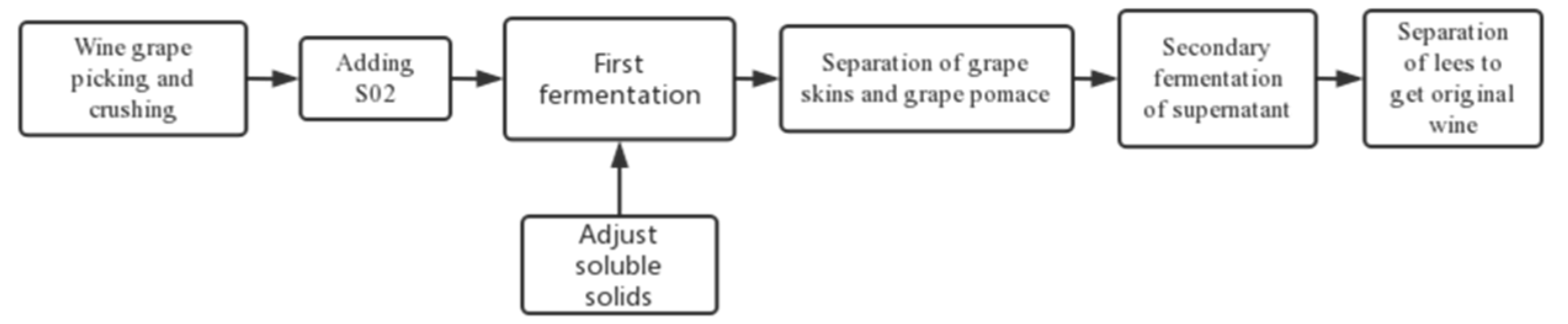
2.1. Harvesting and Fermenting Method
2.2. Detection Methods of Basic Physical and Chemical Indexes
2.3. Color Difference Measurements of Wine Infusions
2.4. Sensory Evaluation of Original Wine of Different Varieties
2.5. Widely-Targeted Metabolomic Analysis of Nonvolatile Metabolites in Raw Grape Wines
2.5.1. Sample Preparation and Extraction
2.5.2. High-Performance Liquid Chromatography Conditions
2.5.3. ESI-QTrap-MS/MS Analysis
2.5.4. Qualitative and Quantitative Metabolite Analyses
2.5.5. Kyoto Encyclopedia of Genes and Genomes (KEGG) Annotations and Metabolic Pathway Analyses of Differential Metabolites
2.6. Association of Non-Volatiles and Sensory Attributes via WGCNA
2.7. Multivariate Data Analysis and Statistical Analysis
3. Results
3.1. Measurement Results of Fundamental Physical and Chemical Indexes of Wine
3.2. CIELAB Parameters of Wine Samples of Different Varieties
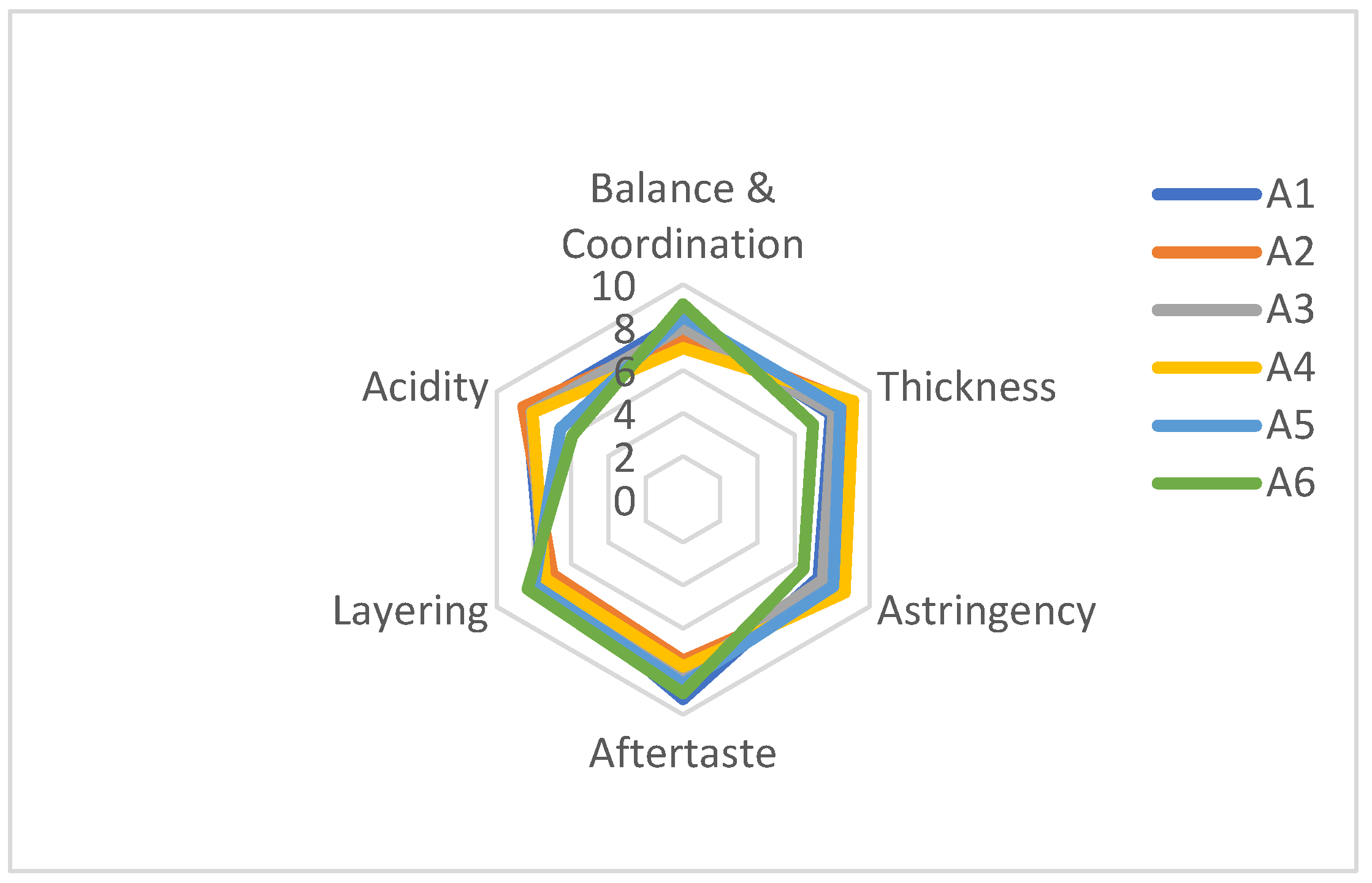
3.3. Sensory Scoring of Wine Samples
3.4. Non-Volatile Metabolite Profiles of Samples of Different Varieties of Wine
3.4.1. Widely Targeted Metabolome Analysis in Wine
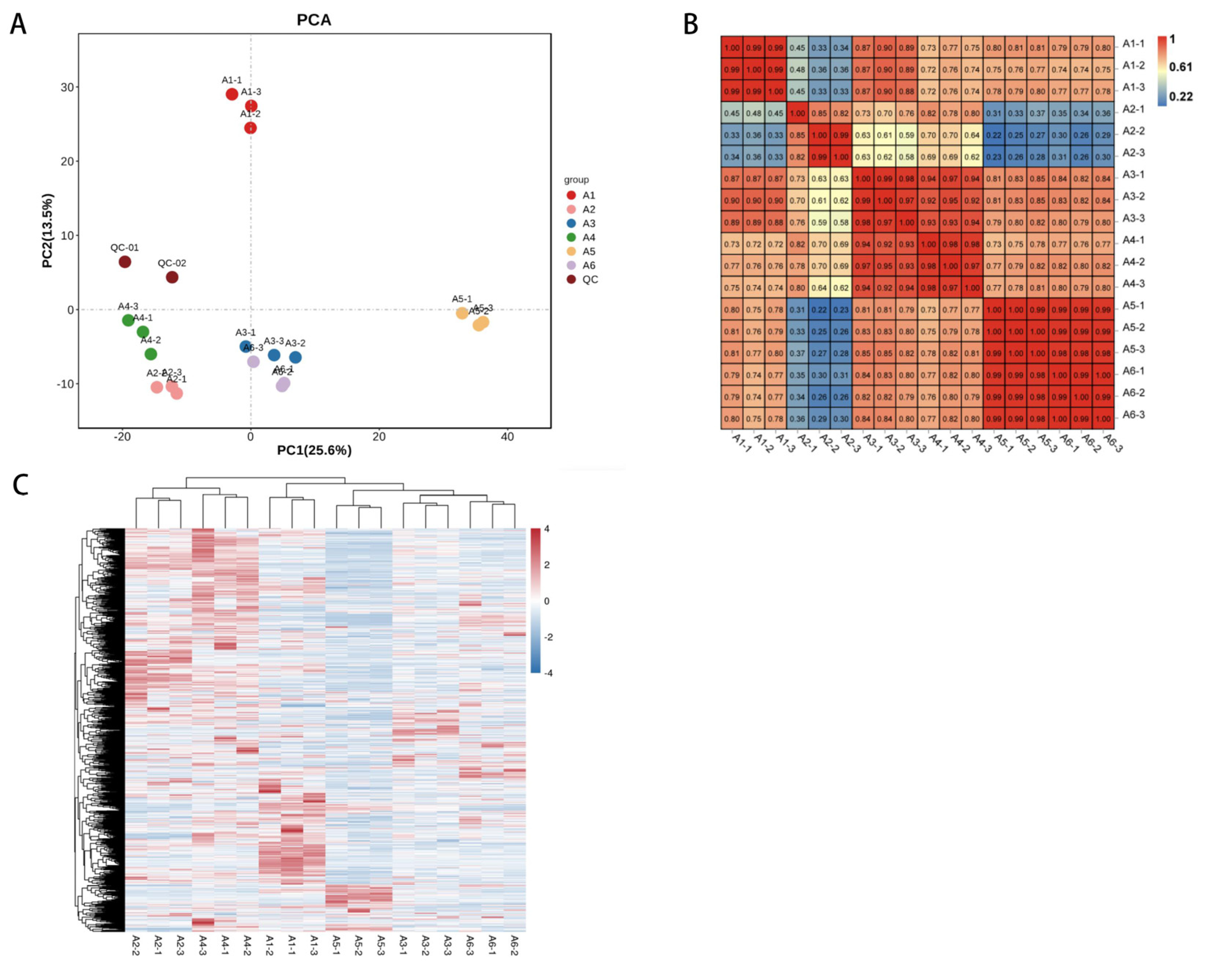
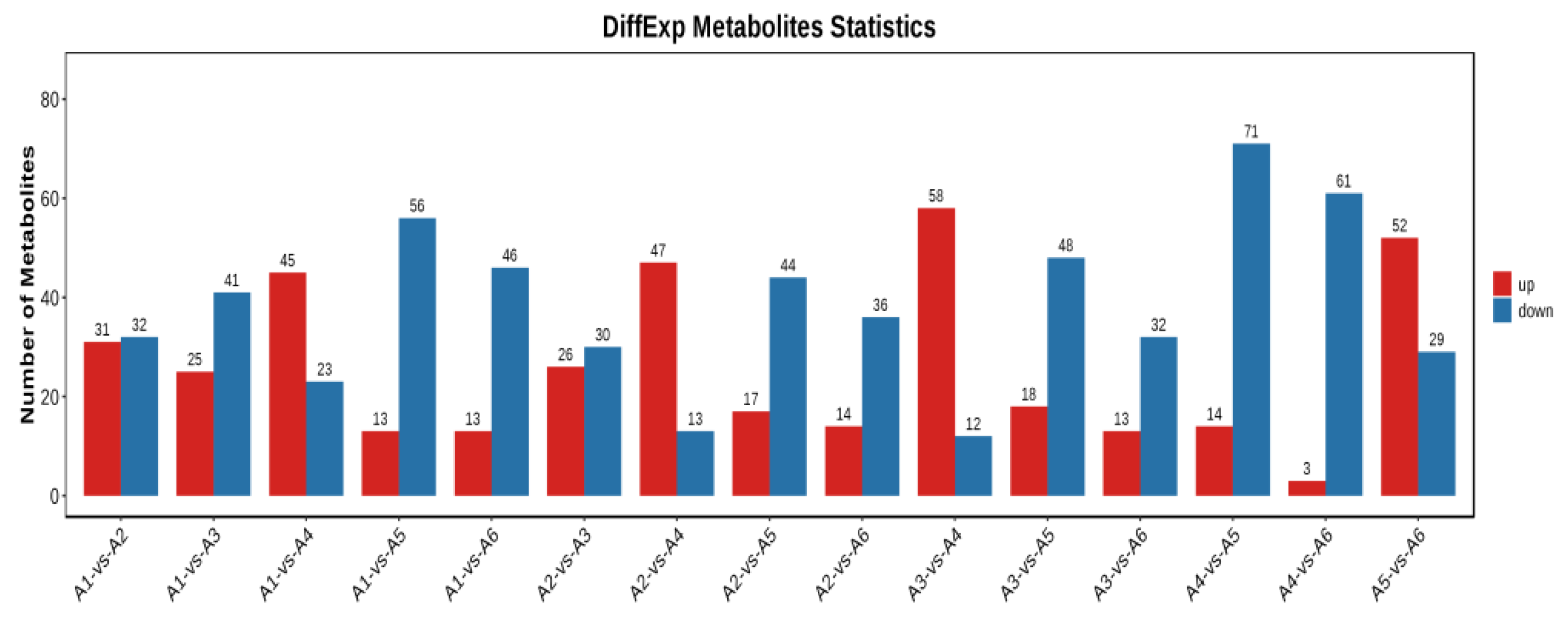
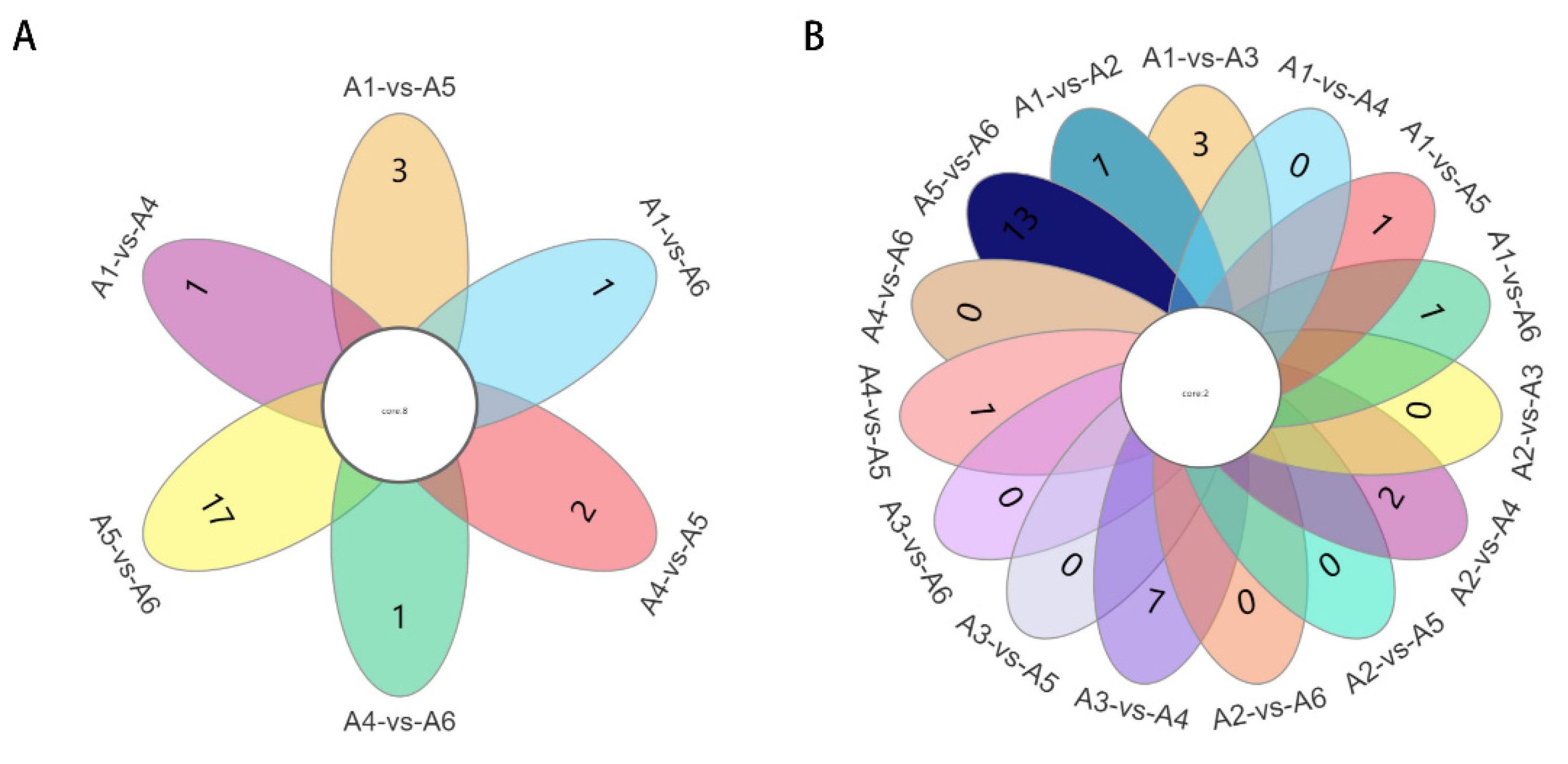
3.4.2. Multivariate Statistical Analysis of Metabolic Profile Differences
3.5. Key Compounds Associated with Wine Flavor Differences
3.6. Identification of Characteristic Metabolites of Each Genotype
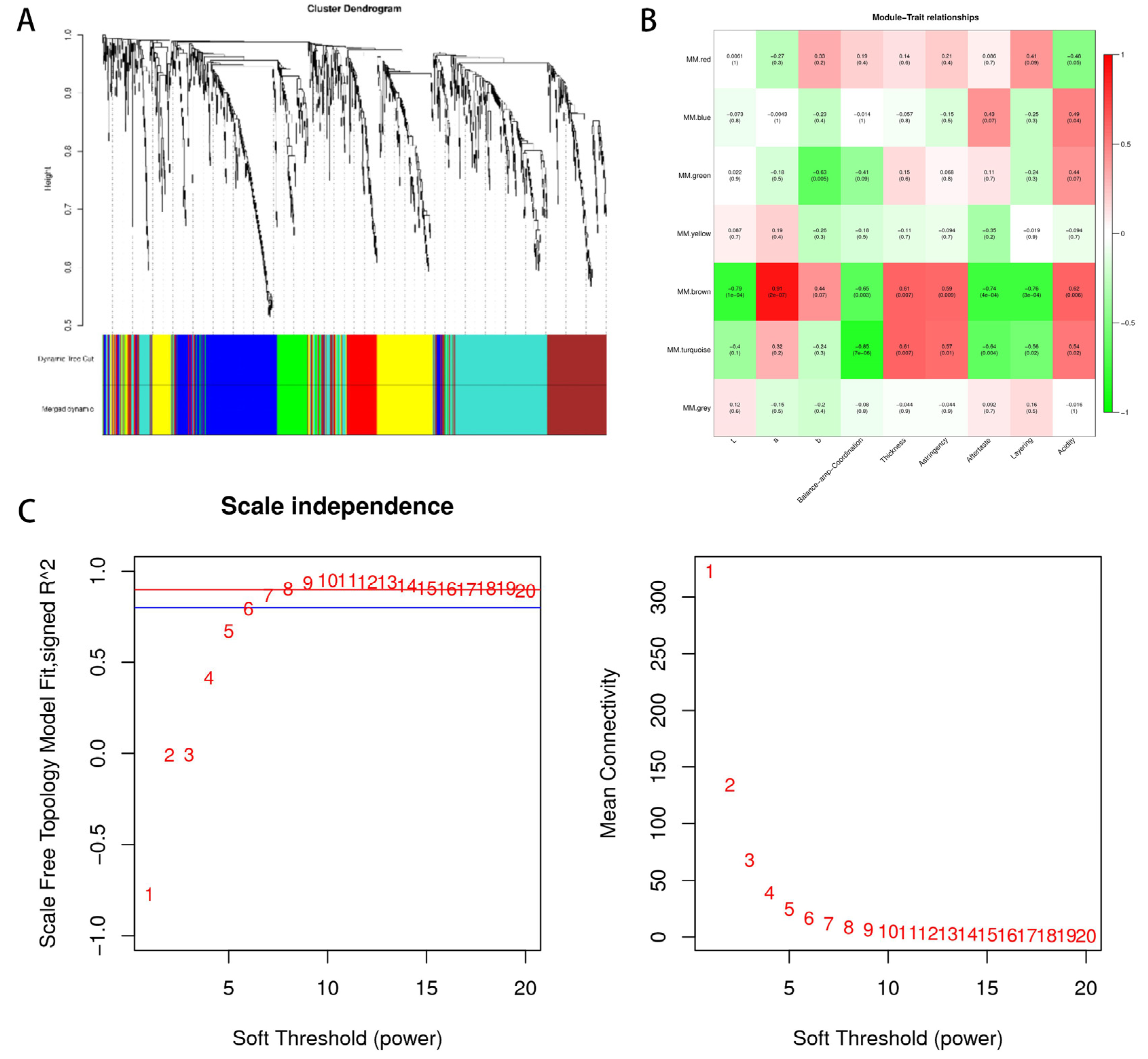
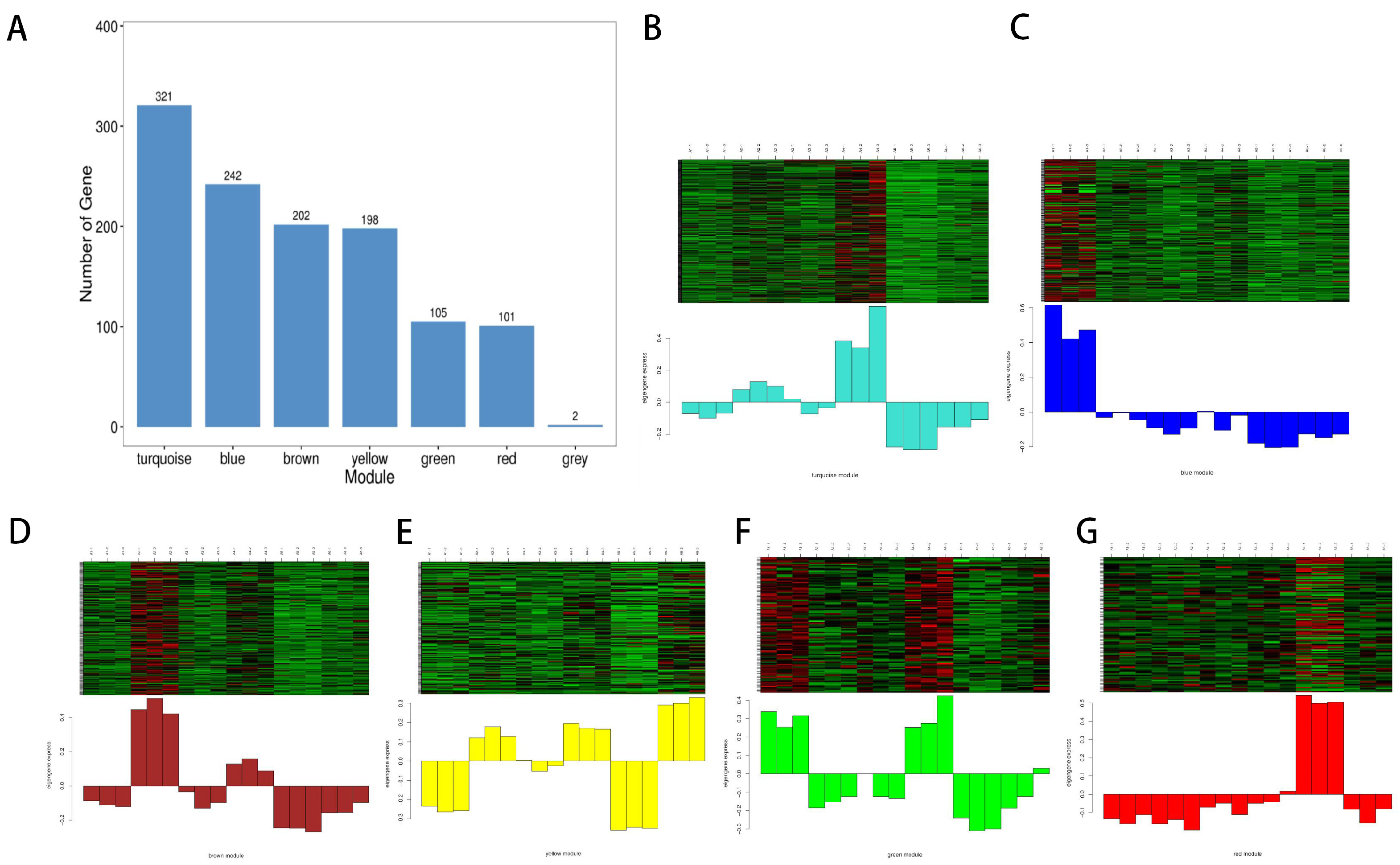
3.7. Non-Volatile Metabolites in Wine Linked to Important Organoleptic Traits through WGCNA
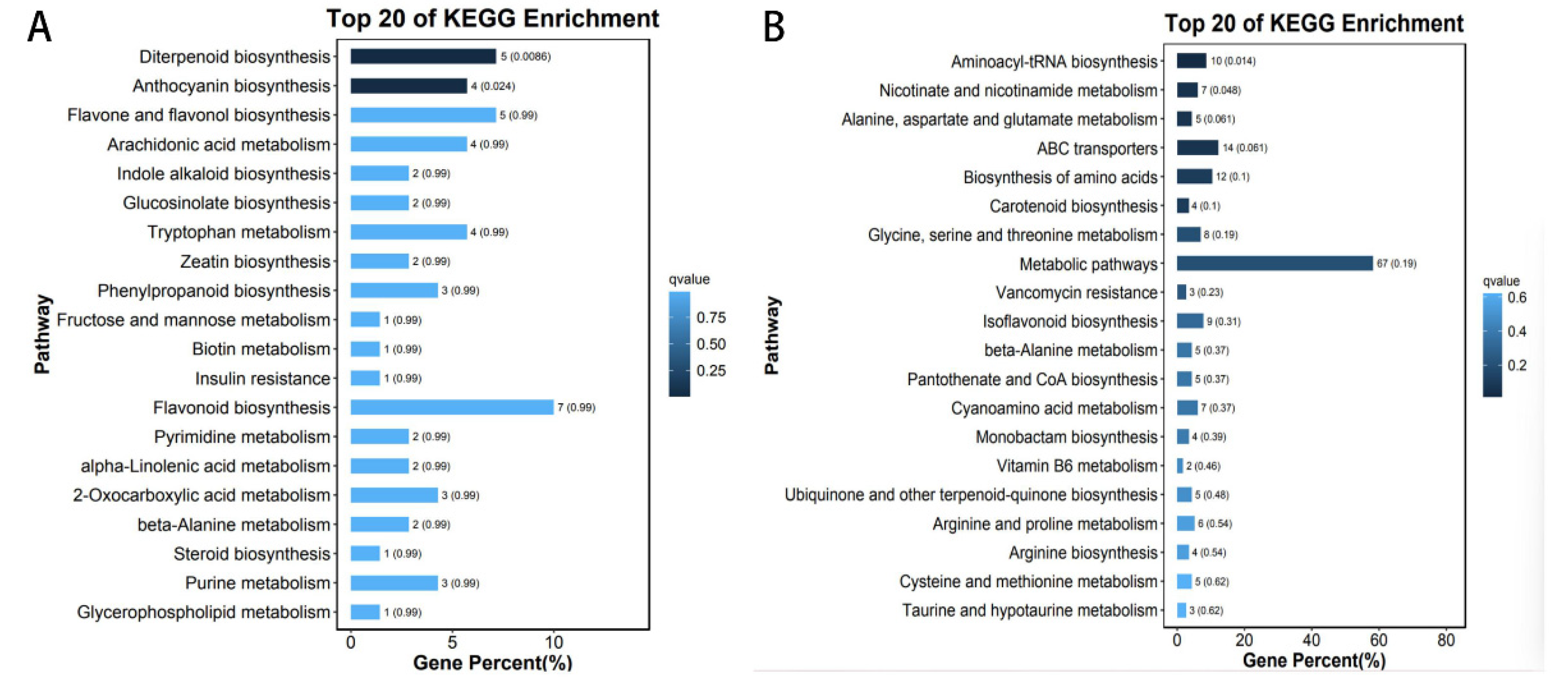
3.8. Classification and Enrichment Analysis of the Metabolite KEGG in the Key Module
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jones-Moore, H.R.; Jelley, R.E.; Marangon, M.; Fedrizzi, B. The interactions of wine polysaccharides with aroma compounds, tannins, and proteins, and their importance to winemaking. Food Hydrocoll. 2021, 123, 107150. [Google Scholar] [CrossRef]
- Josip, V.; Marko, K.; Tina, T.K.; Ivan, M.; Dinko, M.; Marino, V.; Josko, B. Effects of Wine Components in Inflammatory Bowel Diseases. Molecules 2021, 26, 5891. [Google Scholar]
- Nunzia, D.O.; Elisa, M.; Giuseppina, C.; Francesca, C.; Luigi, P.; Luigi, M.; Luisa, B.M.; Angelita, G.; Martino, F. Phenolic Profiles of Red Wine Relate to Vascular Endothelial Benefits Mediated by SIRT1 and SIRT6. Int. J. Mol. Sci. 2021, 22, 5677. [Google Scholar]
- Wang, H.; Tian, X.L.; Yang, C.L.; Han, Y.L.; Shi, X.Q.; Li, H. Wine and health. China Brew. 2022, 41, 1–5. [Google Scholar]
- Salehi, B.; Vlaisavljevic, S.; Adetunji, C.O.; Adetunji, J.B.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Uprety, Y.; Mileski, K.S.; Devkota, H.P.; et al. Plants of the genus Vitis: Phenolic compounds, anticancer properties and clinical relevance. Trends Food Sci. Technol. 2019, 91, 362–379. [Google Scholar] [CrossRef]
- Holst, C.; Becker, U.; Jørgensen, M.E.; Grønbæk, M.; Tolstrup, J.S. Alcohol drinking patterns and risk of diabetes: A cohort study of 70,551 men and women from the general Danish population. Diabetologia 2017, 60, 1941–1950. [Google Scholar] [CrossRef]
- Yao, Q.Y. The cardiovascular benefits of red wine. Food Drug 2006, 72–73. [Google Scholar]
- Cao, W.Y.; Lu, W.P.; Shu, N.; Yang, Y.M.; Fan, S.T. Research progress on wine flavor substances and their influencing factors. China Brew. 2022, 41, 1–7. [Google Scholar]
- Mu, W.S.; Wu, X.Q.; Qi, J.F.; Zhao, J.J.; Feng, J.Y. Analysis of the development situation and market demand characteristics of Chinese wine industry. Sino-Overseas Grapevine Wine 2022, 4, 81–89. [Google Scholar] [CrossRef]
- Shu, N. Study on Fermentation Characteristics and Dry Red Wine Brewing Technology of New Grape New Vitis Amurensis Cultivar ‘Beiguohong’. Master’s Thesis, Chinese Academy of Agricultural Sciences, Xinxiang, China, 2019. [Google Scholar]
- Kim, B.H.; Park, S.K. Volatile aroma and sensory analysis of black raspberry wines fermented by different yeast strains. J. Inst. Brew. 2015, 121, 87–94. [Google Scholar] [CrossRef]
- Jin, Y.N.; Shu, N.; Xie, S.Y.; Cao, W.Y.; Xiao, J.M.; Zhang, B.X.; Lu, W.P. Comparison of ‘Beibinghong’ dry red wines from six producing areas based on volatile compounds analysis, mineral content analysis, and sensory evaluation analysis. Eur. Food Res. Technol. 2021, 247, 1461–1475. [Google Scholar] [CrossRef]
- Gawel, R. Red wine astringency: A review. Aust. J. Grape Wine Res. 1998, 4, 74–95. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Peter, L.; Steve, H. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar]
- Weiyu, C.; Nan, S.; Jinli, W.; Yiming, Y.; Yuning, J.; Wenpeng, L. Characterization of the Key Aroma Volatile Compounds in Nine Different Grape Varieties Wine by Headspace Gas Chromatography–Ion Mobility Spectrometry (HS-GC-IMS), Odor Activity Values (OAV) and Sensory Analysis. Foods 2022, 11, 2767. [Google Scholar]
- Rocío, G.; José, A.M.; Emma, C. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar]
- Lijuan, Z.; Wanfeng, H.; Ayesha, M.; Aamir, I.; Jiaxing, L.; Jiao, Z.; Junjie, L.; Mengjie, K.; Xiaoyun, X.; Siyi, P. Eugenol treatment delays the flesh browning of fresh-cut water chestnut (Eleocharis tuberosa) through regulating the metabolisms of phenolics and reactive oxygen species. Food Chem. X 2022, 14, 100307. [Google Scholar]
- Chen, X.R.; Zhang, B.; Zhang, H.; Xie, Y.S.; Wang, B.; Zhou, X.P.; Li, M.; Han, S.Y. Determination of anthocyanins in red wine by ultra-high performance liquid chromatography tandem triple quadrupole mass spectrometry. Food Ferment. Ind. 2019, 45, 262–268. [Google Scholar] [CrossRef]
- Ba, T.; Ren, Y.L.; Dong, X.Q.; Bourgeois, A. A theoretical and practical exploration of the use of professional oenological tannins in wine. Sino-Overseas Grapevine Wine 2011, 11, 53–57. [Google Scholar] [CrossRef]
- Giuseppa, D.B.; Miriam, P.; Ambrogina, A.; Claudio, M.; Alessia, T.; Rossana, R.; Vincenzo, N.; Vincenzo, L.T.; Giorgia, P.A. Valorization of Traditional Alcoholic Beverages: The Study of the Sicilian Amarena Wine during Bottle Aging. Foods 2022, 11, 2152. [Google Scholar]
- Tian, B.R.; Yuan, M.; Yuan, X.L. FOSS meter—Specific gravity bottle method for the determination of dry leachate in wine. Sino-Overseas Grapevine Wine 2016, 4, 39–41. [Google Scholar] [CrossRef]
- García-Marino, M.; Escudero-Gilete, M.L.; Heredia, F.J.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C. Color-copigmentation study by tristimulus colorimetry (CIELAB) in red wines obtained from Tempranillo and Graciano varieties. Food Res. Int. 2013, 51, 123–131. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-Sanjosé, M.L. Application of absorbance values used in wineries for estimating CIELAB parameters in red wines. Food Chem. 2003, 81, 301–306. [Google Scholar] [CrossRef]
- Li, Y.K.; Han, F.L.; Zhang, Y.L.; Wang, H. Visualization for representation of red wine color based on CIELAB color space. Trans. Chin. Soc. Agric. Mach. 2017, 48, 296–301. [Google Scholar]
- Wang, H.; Chen, X.Y.; Zhang, J.X. Characteristics analysis of young red wines from the eastern food of Helan Mountain based on CIELab color space parameters. Food Sci. 2014, 35, 20–23. [Google Scholar]
- Liu, S.F. Differenced Study of Berry Anthocyanin Accumulation between Vitis Vinifera and Hybrid of Vitis Vinifera × Vitis Amurensis in Red Wine Grapes. Master’s Thesis, Ningxia University, Yinchuan, China, 2020. [Google Scholar]
- Bernal, F.A.; Orduz-Díaz, L.L.; Coy-Barrera, E. Application of Parafac and Opls-Da Analyses on Hplc Fingerprints for the Characterization of Hibiscus sabdariffaCALYXES. Química Nova 2016, 39, 160–166. [Google Scholar]
- Chenchen, M.; Peng, L.; Siyuan, C.; Chang, G.; Xing, T.; Zhaopeng, L.; Ruirong, X. The Identification of APOBEC3G as a Potential Prognostic Biomarker in Acute Myeloid Leukemia and a Possible Drug Target for Crotonoside. Molecules 2022, 27, 5804. [Google Scholar]
- Yu-Zhi, L.; Si, Y.; Pei-Ao, Y.; Dao-Yin, G.; Fang-Li, W.; Zhi, H.; Yu-Yao, Y.; An-Yan, Z.; Xue, T.; Ruo-Qi, Z.; et al. Crotonoside exhibits selective post-inhibition effect in AML cells via inhibition of FLT3 and HDAC3/6. Oncotarget 2017, 8, 103087. [Google Scholar]
- Membrane Proteins—Ion Channels; Study Findings from Cardio-Electrophysiological Research Laboratory Broaden Understanding of Ion Channels (Antiarrhythmic effect of crotonoside by regulating sodium and calcium channels in rabbit ventricular myocytes). Chem. Chem. 2020.
- Peiao, Y.; Lan, Z.; Cheng, P.; Ruoqi, Z. Pharmacokinetics and tissue distribution of crotonoside. Xenobiotica Fate Foreign Compd. Biol. Syst. 2018, 48, 28–36. [Google Scholar]
- Ye, Y.Z.; Luo, H.D. Analysis of evaluation samples for the quality assessment of kits for rapid detection of trimethoprim. China Food Saf. Mag. 2022, 2, 70–72. [Google Scholar] [CrossRef]
- Ji, Y.F.; Zhao, C.Y.; Wan, H.; Duan, M.L.; Xu, X. Synthesis of methotrexate. Chin. J. Synth. Chem. 2008, 16, 716–718. [Google Scholar]
- Ramanathan, M.; Abdul, K.K.; Justin, A. Low dose of L-glutamic acid attenuated the neurological dysfunctions and excitotoxicity in bilateral common carotid artery occluded mice. Behav. Pharmacol. 2016, 27, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wu, T.; Zhang, J.L. Comparative study on quantitative determination of L-glutamic acid in fermentation broth. China Food Addit. 2020, 31, 110–116. [Google Scholar] [CrossRef]
- Wang, J.D.; Li, L.X.; Xu, Q.Y. Study on technology of total nutrient feeding fermentation of L-lysine. China Condiment 2022, 47, 93–97. [Google Scholar]
- Liu, D.D. Incresing the Fermentation Level of L-Lysine via Enhancing Glucose Metabolic Pathways. Master’s Thesis, Jiangnan University, Wuxi, China, 2017. [Google Scholar]
- Lee, J.-E.; Hwang, G.-S.; Berg, F.V.D.; Lee, C.-H.; Hong, Y.-S. Evidence of vintage effects on grape wines using 1 H NMR-based metabolomic study. Anal. Chim. Acta 2009, 648, 71–76. [Google Scholar] [CrossRef]
- Pereira, G.E.; Gaudillere, J.P.; Van Leeuwen, C.; Hilbert, G.; Lavialle, O.; Maucourt, M.; Deborde, C.; Moing, A.; Rolin, D. H-1 NMR and chemometrics to characterize mature grape berries in four wine-growing areas in Bordeaux, France. J. Agric. Food Chem. 2005, 53, 6382–6389. [Google Scholar] [CrossRef]

| Varieties | Flower Type | Species | Parentage | Place of Origin |
|---|---|---|---|---|
| ‘Hassan’ | Bisexual Flower | Vitis amurensis | Wild species | China |
| ‘Zuoshaner’ | Female Flower | Vitis amurensis | Wild species | China |
| ‘Beibinghong’ | Bisexual Flower | Vitis amurensis × Vitis vinifera | ‘Zuoyouhong’ × ‘84–26-53′ | China |
| ‘Shuanghong’ | Bisexual Flower | Vitis amurensis | ‘Tonghuasanhao’ × ‘Shuangqing’ | China |
| ‘Zijingganlu’ | Bisexual Flower | Vitis amurensis | ‘Zuoshaner’ × ‘Hassan’ | China |
| ‘Cabernet Sauvignon’ | Bisexual Flower | Vitis vinifera | ‘Cabernet Franc’ × ‘Sauvignon Blanc’ | France |
| Varieties | Solids (Brix%) | Titratable Acid/g·L−1 | Total Sugar/g·L−1 | Total Anthocyanins/mg·L−1 | Total Phenols (g/L) | Tannin/g·L−1 | pH Value | Dry Extract/g·L−1 | Alcohol (v/v) |
|---|---|---|---|---|---|---|---|---|---|
| ‘Hassan’ | 9.40 ± 0.00 a | 11.00 ± 0.29 c | 4.10 ± 0.12 c | 151.96 ± 6.68 e | 1.61 ± 0.04 d | 2.33 ± 0.31 c | 4.13 ± 0.01 b | 31.50 ± 0.00 d | 13.00 ± 0.00 a |
| ‘Zuoshaner’ | 9.17 ± 0.06 b | 16.25 ± 0.29 a | 5.70 ± 0.35 a | 1477.85 ± 48.31 a | 3.56 ± 0.04 a | 3.59 ± 0.07 b | 3.65 ± 0.01 f | 40.80 ± 0.17 a | 11.00 ± 0.00 c |
| ‘Beibinghong’ | 8.53 ± 0.06 d | 12.69 ± 0.43 b | 2.90 ± 0.24 d | 236.57 ± 14.97 d | 1.39 ± 0.03 e | 1.76 ± 0.05 d | 3.79 ± 0.01 e | 30.90 ± 0.17 e | 12.00 ± 0.00 b |
| ‘Shuanghong’ | 8.67 ± 0.58 c | 12.75 ± 0.00 b | 4.46 ± 0.21 b | 1440.56 ± 75.15 b | 3.00 ± 0.24 b | 3.64 ± 0.09 a | 3.84 ± 0.01 d | 37.13 ± 0.12 c | 13.00 ± 0.00 a |
| ‘Zijingganlu’ | 7.9 ± 0.00 f | 7.38 ± 0.29 e | 4.10 ± 0.12 c | 496.51 ± 10.74 c | 1.90 ± 0.10 c | 1.67 ± 0.10 e | 4.12 ± 0.01 c | 37.40 ± 0.17 b | 12.00 ± 0.00 b |
| ‘Cabernet Sauvignon’ | 8.1 ± 0.00 e | 8.25 ± 0.19 d | 1.65 ± 0.07 e | 108.54 ± 7.28 f | 0.82 ± 0.08 f | 1.36 ± 0.34 f | 4.39 ± 0.01 a | 27.30 ± 0.17 f | 12.00 ± 0.00 b |
| Varieties | L* | a* | b* | Cab* | hab* | ΔEab* |
|---|---|---|---|---|---|---|
| ‘Hassan’ | 66.91 ± 0.06 c | 40.15 ± 0.01 c | 7 ± 0.28 d | 40.75 ± 0.21 d | 9.89 ± 0.48 d | 29.16 d |
| ‘Zuoshaner’ | 41.65 ± 0.10 f | 167.48 ± 0.07 a | 21.76 ± 0.17 a | 168.89 ± 0.10 a | 7.4 ± 0.77 e | 157.30 a |
| ‘Beibinghong’ | 73.76 ± 0.12 b | 35.46 ± 0.07 e | 7.24 ± 0.30 c | 36.2 ± 0.14 e | 11.54 ± 0.28 c | 21.46 e |
| ‘Shuanghong’ | 59.65 ± 0.1 e | 53.3 ± 0.26 b | 4.95 ± 0.07 f | 53.53 ± 0.24 b | 5.31 ± 0.27 f | 44.04 b |
| ‘Zijingganlu’ | 63.22 ± 0.11 d | 39.84 ± 0.07 d | 17.34 ± 0.28 b | 43.46 ± 0.19 c | 23.52 ± 0.4 a | 33.43 c |
| ‘Cabernet Sauvignon’ | 84.65 ± 0.12 a | 17.05 ± 0.14 f | 5.57 ± 0.11 e | 17.93 ± 0.11 f | 18.09 ± 0.25 b |
| Taste | Total | ‘Hassan’ | ‘Zuoshaner’ | ‘Beibinghong’ | ‘Shuanghong’ | ‘Zijinggsnlu’ | ‘Cabernet Sauvignon’ |
|---|---|---|---|---|---|---|---|
| Balance & Coordination | 10 | 8.37 ± 0.12 | 7.63 ± 0.06 | 8.00 ± 0.00 | 7.03 ± 0.06 | 8.50 ± 0.00 | 9.07 ± 0.12 |
| Thickness | 10 | 7.93 ± 0.06 | 9.00 ± 0.00 | 8.07 ± 0.12 | 9.13 ± 0.12 | 8.43 ± 0.12 | 6.97 ± 0.06 |
| Astringency | 10 | 7.27 ± 0.06 | 8.53 ± 0.06 | 7.50 ± 0.00 | 8.67 ± 0.06 | 8.07 ± 0.12 | 6.47 ± 0.06 |
| Aftertaste | 10 | 9.27 ± 0.25 | 7.50 ± 0.00 | 8.17 ± 0.29 | 7.77 ± 0.25 | 8.57 ± 0.12 | 9.00 ± 0.00 |
| Layering | 10 | 7.53 ± 0.15 | 7.00 ± 0.00 | 7.47 ± 0.06 | 7.40 ± 0.10 | 8.00 ± 0.00 | 8.33 ± 0.29 |
| Acidity | 10 | 8.33 ± 0.06 | 8.57 ± 0.12 | 8.13 ± 0.15 | 8.07 ± 0.06 | 6.57 ± 0.12 | 5.93 ± 0.12 |
| Category | Compounds | A1 | A2 | A3 | A4 | A5 | A6 |
|---|---|---|---|---|---|---|---|
| A1, A4, A5, A6 Two-by-Two Comparison of Shared Differential Metabolites | |||||||
| Amino acids | L-Glutamic acid | 8,740,339.55 ± 1,304,003.80 | 22,283,076.63 ± 1,298,588.33 | 19,698,562.46 ± 1,284,131.33 | 32,662,899.86 ± 7,929,513.51 | 844,119.76 ± 205,156.2 | 3,839,362.14 ± 1,319,648.25 |
| Amino acids | L-Lysine; L-Glutamine | 15,534,750.58 ± 1,117,706.63 | 22,766,130.05 ± 1,690,608.93 | 25,430,043.14 ± 2,134,330.58 | 38,305,543.74 ± 12,664,939.83 | 5,927,226.67 ± 815,294.35 | 10,829,465.34 ± 1,061,020.55 |
| Phenols | Trimethoprim | 170,392,569.41 ± 7,239,534.72 | 36,939,619.52 ± 884,872.69 | 91,544,508.75 ± 7,804,644.7 | 58,070,842.15 ± 469,344.95 | 131,185,043.54 ± 10,643,889.16 | 17,080,836.34 ± 527,750.76 |
| Benzimidazoles | Carbendazim | 129,093,442.01 ± 5,991,428.08 | 155,489,246.64 ± 12,991,581.68 | 162,246,727.33 ± 1,609,192.67 | 147,606,817.84 ± 6,560,923.58 | 59,830,134.44 ± 6,447,283.36 | 125,099.58 ± 11,249.83 |
| Nucleotides | Guanine | 283,570.58 ± 49,403.51 | 11,399,025.44 ± 7,981,916.55 | 22,148,403.38 ± 2,181,389.92 | 47,846,898.97 ± 4,624,801.58 | 23,971,289.84 ± 4,091,302.17 | 9,694,707.39 ± 3,002,149.98 |
| Nucleotides | 2’-O-Methyladenosine | 362,280.31 ± 171,861.23 | 121,738,612.1 ± 15,919,248.33 | 89,000,086.69 ± 9,973,817.29 | 98,214,445.33 ± 11,808,605.37 | 58,592,631.05 ± 5,785,864.66 | 25,330,029.91 ± 5,121,593 |
| Alkaloids | Crotonoside | 769,632.02 ± 171,685.55 | 13,377,892.58 ± 2,713,842.41 | 37,705,047.17 ± 3,139,654.42 | 99,257,029.27 ± 8,429,231.15 | 50,940,303.36 ± 5,075,677.01 | 27,310,409.98 ± 3,017,556.38 |
| Carboxylic acids | D-Glutamine | 15,534,750.58 ± 1,117,706.63 | 22,766,130.05 ± 1,690,608.93 | 27,069,147.76 ± 4,471,388.18 | 38,305,543.74 ± 12,664,939.83 | 5,927,226.67 ± 81,5294.35 | 10,829,465.34 ± 1,061,020.55 |
| Category | Compounds | A1, A2, A3, A4, A5, A6 Two-by-two comparison of shared differential metabolites | |||||
| Phenols | Trimethoprim | 170,392,569.41 ± 7,239,534.72 | 36,939,619.52 ± 884,872.69 | 91,544,508.75 ± 7,804,644.7 | 58,070,842.15 ± 469,344.95 | 131,185,043.54 ± 10,643,889.16 | 17,080,836.34 ± 527,750.76 |
| Alkaloids | Crotonoside | 769,632.02 ± 171,685.55 | 13,377,892.58 ± 2,713,842.41 | 37,705,047.17 ± 3,139,654.42 | 99,257,029.27 ± 8,429,231.15 | 50,940,303.36 ± 5,075,677.01 | 27,310,409.98 ± 3,017,556.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Wang, Y.; Lu, W. Widely Targeted Metabolomics Was Used to Reveal the Differences between Non-Volatile Compounds in Different Wines and Their Associations with Sensory Properties. Foods 2023, 12, 290. https://doi.org/10.3390/foods12020290
Cao W, Shu N, Wen J, Yang Y, Wang Y, Lu W. Widely Targeted Metabolomics Was Used to Reveal the Differences between Non-Volatile Compounds in Different Wines and Their Associations with Sensory Properties. Foods. 2023; 12(2):290. https://doi.org/10.3390/foods12020290
Chicago/Turabian StyleCao, Weiyu, Nan Shu, Jinli Wen, Yiming Yang, Yanli Wang, and Wenpeng Lu. 2023. "Widely Targeted Metabolomics Was Used to Reveal the Differences between Non-Volatile Compounds in Different Wines and Their Associations with Sensory Properties" Foods 12, no. 2: 290. https://doi.org/10.3390/foods12020290
APA StyleCao, W., Shu, N., Wen, J., Yang, Y., Wang, Y., & Lu, W. (2023). Widely Targeted Metabolomics Was Used to Reveal the Differences between Non-Volatile Compounds in Different Wines and Their Associations with Sensory Properties. Foods, 12(2), 290. https://doi.org/10.3390/foods12020290





