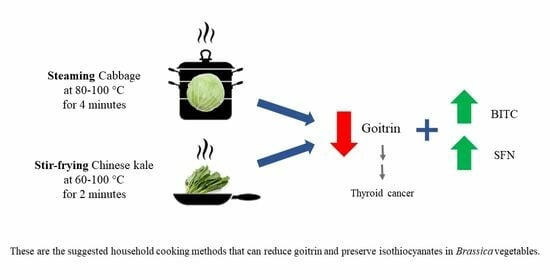
Cooking Methods for Preserving Isothiocyanates and Reducing Goitrin in Brassica Vegetables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Reagents, and Standards
2.2. Cooking Conditions and Sample Collections
2.3. Sample Preparation for Determination of Goitrin, BITC, and Sulforaphane in Cooked and Uncooked Vegetables
2.4. Preparation of Standard Concentrations of Goitrin-NH3, BITC-NH3, and SFN for Standard Calibration Curves
2.5. Determination of the Goitrin, BITC, and SFN in Samples Using LC-MS/MS Assay
2.6. Method Validation
2.7. Data Analysis
3. Results and Discussion
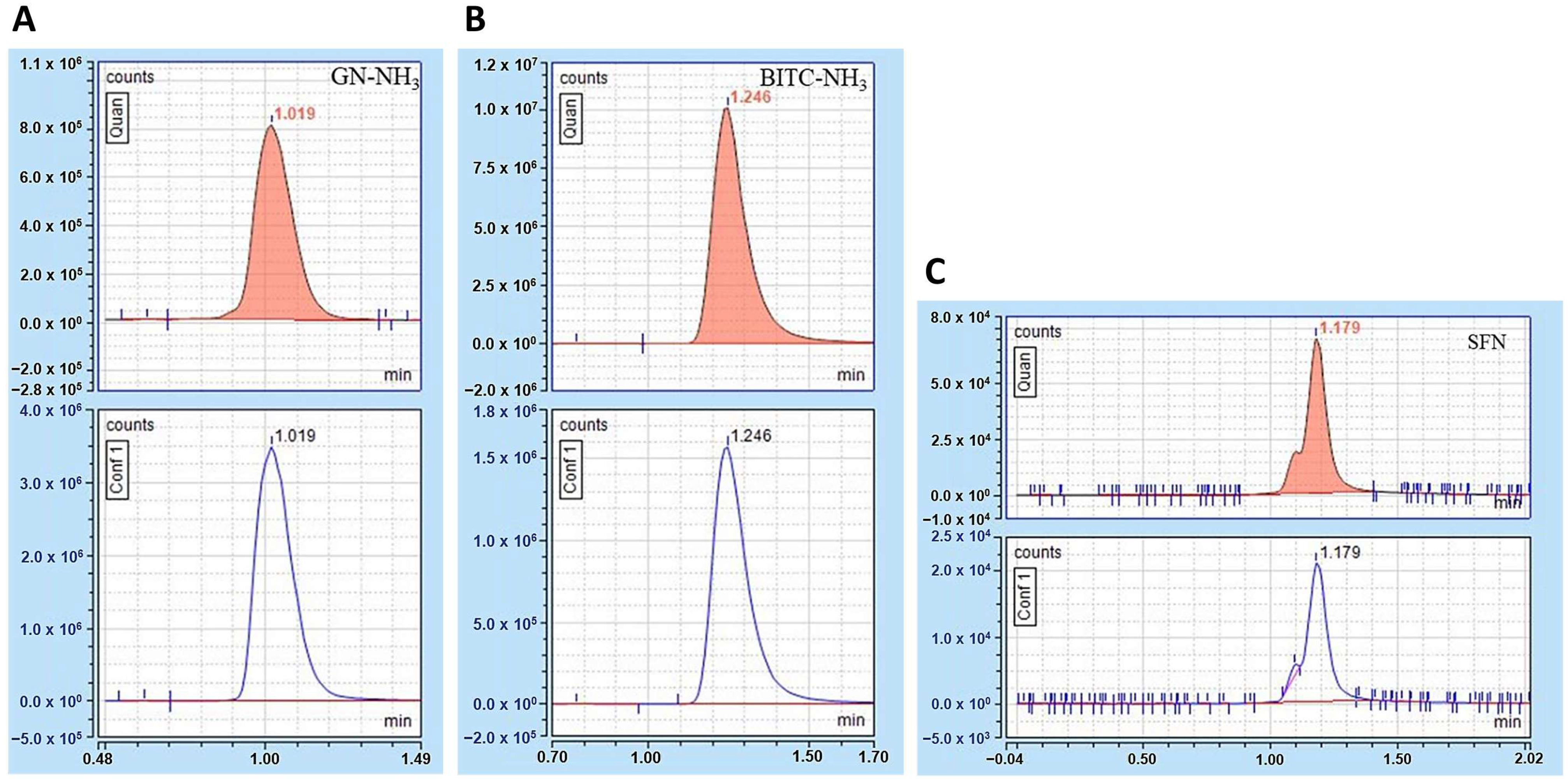
3.1. Chromatograms and Mass Spectra of Goitrin (GN), Benzyl Isothiocyanate (BITC), and Sulforaphane (SFN)
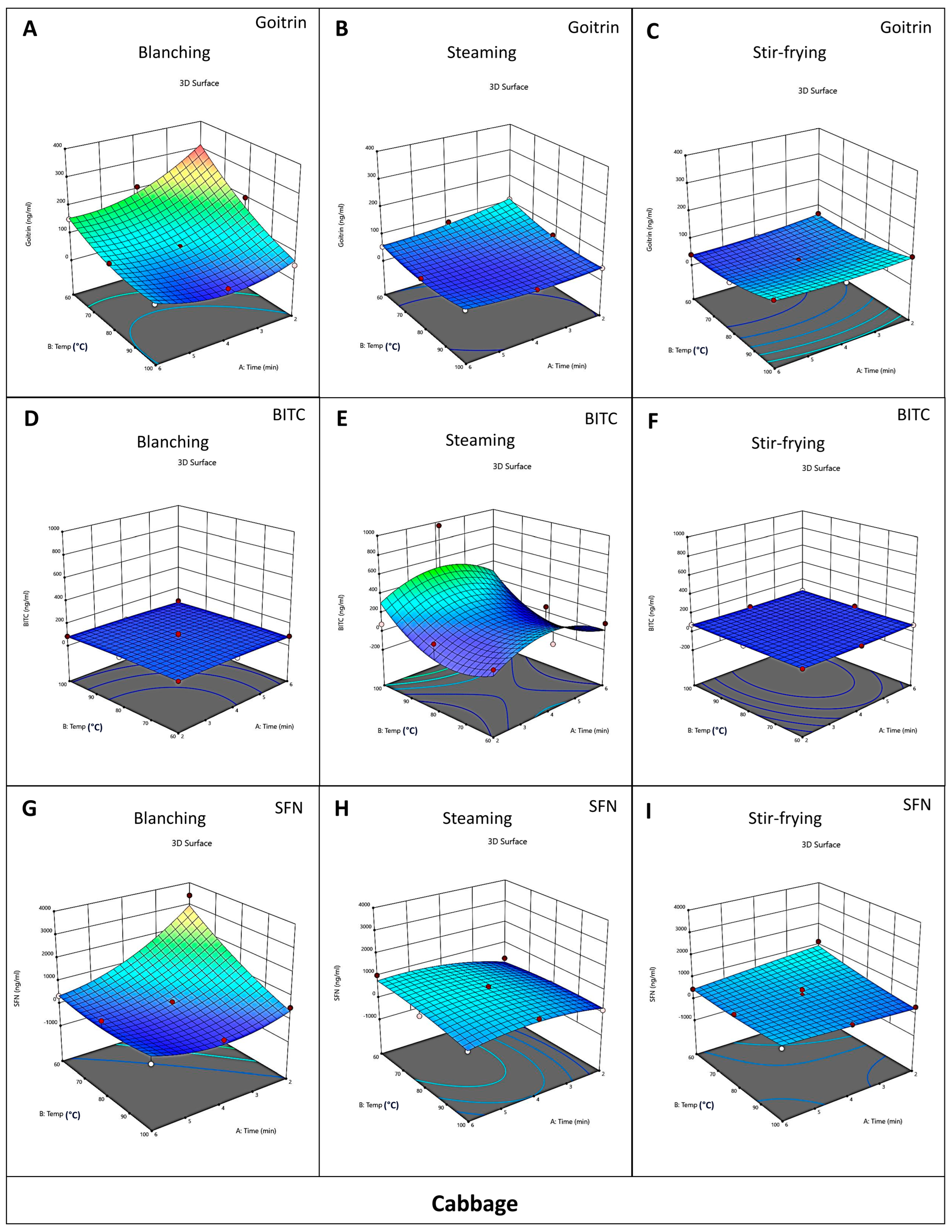
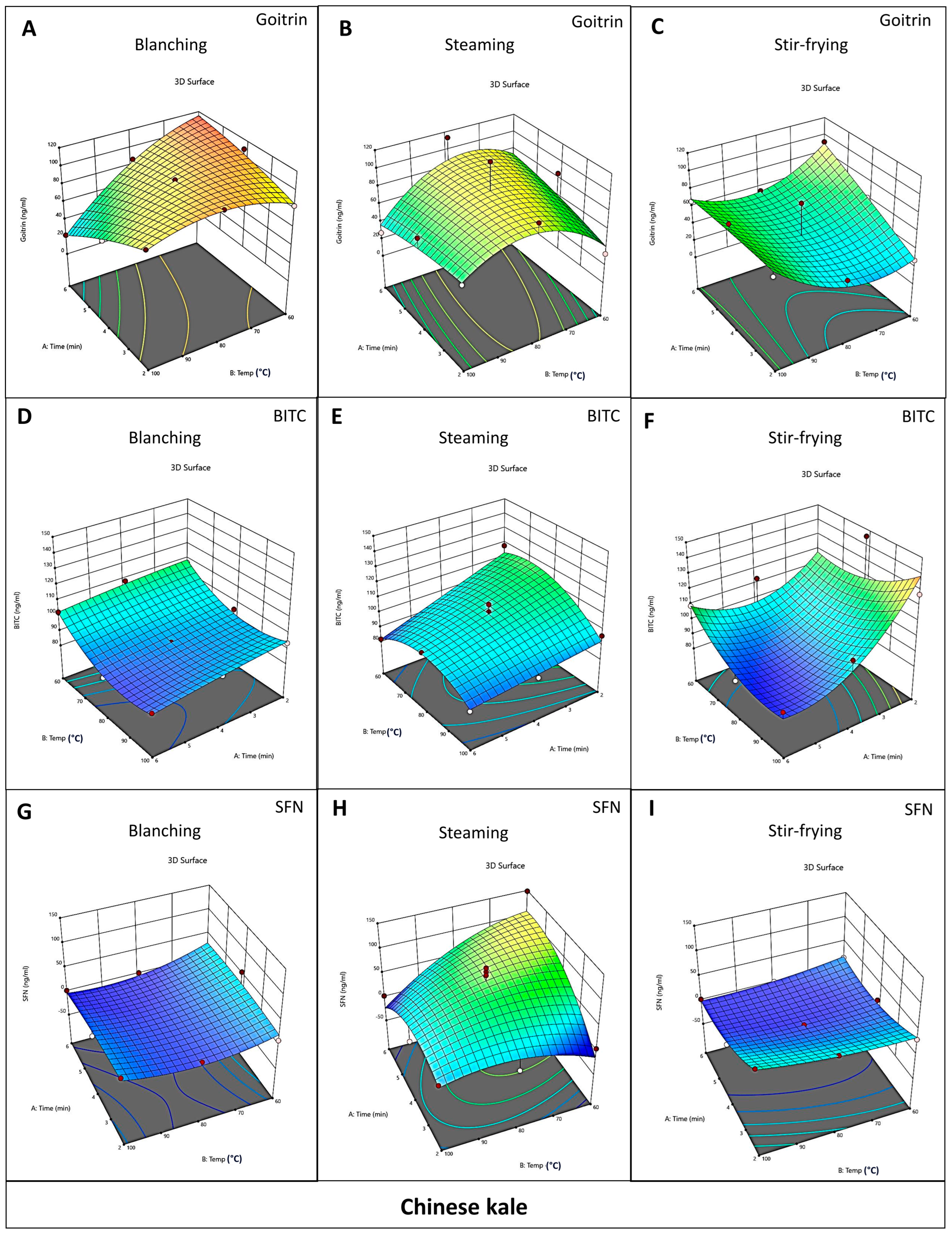
3.2. Effect of Different Cooking Methods on the Content of Goitrin, BITC, and SFN in Cabbage and Chinese Kale
| Cooking Condition | Goitrin (ng/mL) | BITC (ng/mL) | SFN (ng/mL) | ||
|---|---|---|---|---|---|
| Cooking Methods | Cooking Time (min) | Cooking Temp. (°C) | |||
| Uncooked | − | − | 82.3 ± 25.2 abc | 107.2 ± 2.1 cde | 9.7 ± 3.9 g |
| Blanching | 2 | 60 | 84.0 ± 1.0 abcd | 108.4 ± 0.4 bcde | 55.1 ± 8.7 cde |
| 4 | 60 | 109.5 ± 2.8 a | 102.5 ± 0.2 cdefg | 19.6 ± 1.6 fg | |
| 6 | 60 | 112.4 ± 3.7 a | 94.5 ± 0.4 defghi | 10.7 ± 0.6 g | |
| Blanching | 2 | 80 | 104.5 ± 34.6 a | 94.7 ± 0.0 defghi | 61.5 ± 5.1 cd |
| 4 | 80 | 96.6 ± 1.7 ab | 87.7 ± 0.4 fghi | 2.7 ± 0.2 g | |
| 6 | 80 | 86.3 ± 1.9 abcd | 82.1 ± 0.4 hi | 2.4 ± 0.1 g | |
| Blanching | 2 | 100 | 90.4 ± 1.5 abc | 93.7 ± 0.2 defghi | 26.0 ± 2.9 efg |
| 4 | 100 | 57.4 ± 12.1 abcd | 90.0 ± 0.4 efghi | 3.6 ± 0.2 g | |
| 6 | 100 | 22.4 ± 4.3 d | 85.3 ± 0.4 ghi | 2.8 ± 0.2 g | |
| Steaming | 2 | 60 | 33.3 ± 1.1 bcd | 118.0 ± 33.3 bc | 4.9 ± 0.2 g |
| 4 | 60 | 85.3 ± 1.5 abcd | 92.2 ± 0.0 efghi | 26.8 ± 0.7 efg | |
| 6 | 60 | 58.9 ± 26.3 abcd | 81.6 ± 0.2 i | 148.4 ± 6.5 a | |
| Steaming | 2 | 80 | 93.2 ± 19.0 ab | 106.1 ± 0.2 cdef | 5.4 ± 0.7 g |
| 4 | 80 | 81.8 ± 38.0 abcd | 106.3 ± 4.9 cdef | 104.6 ± 7.2 b | |
| 6 | 80 | 113.2 ± 2.2 a | 95.8 ± 0.0 defghi | 3.5 ± 0.5 g | |
| Steaming | 2 | 100 | 56.1 ± 17.3 abcd | 95.9 ± 0.2 defghi | 23.3 ± 30.6 fg |
| 4 | 100 | 62.2 ± 26.5 abcd | 85.4 ± 0.2 ghi | 4.4 ± 0.2 g | |
| 6 | 100 | 27.4 ± 2.9 cd | 82.7 ± 0.2 ghi | 3.5 ± 0.1 g | |
| Stir-frying | 2 | 60 | 27.6 ± 3.5 cd | 102.4 ± 0.4 cdefg | 31.4 ± 11.0 defg |
| 4 | 60 | 60.8 ± 54.9 abcd | 113.1 ± 0.4 bcd | 14.9 ± 1.9 fg | |
| 6 | 60 | 91.8 ± 4.6 abc | 109.1 ± 0.0 bcde | 16.8 ± 9.5 fg | |
| Stir-frying | 2 | 80 | 33.1 ± 4.8 bcd | 145.6 ± 0.4 a | 43.7 ± 2.2 cdef |
| 4 | 80 | 39.9 ± 38.0 bcd | 81.1 ± 0.2 i | 3.4 ± 0.3 g | |
| 6 | 80 | 55.3 ± 9.1 abcd | 81.6 ± 0.4 i | 1.9 ± 0.1 g | |
| Stir-frying | 2 | 100 | 66.1 ± 31.4 abcd | 127.3 ± 0.2 ab | 63.7 ± 39.1 c |
| 4 | 100 | 79.7 ± 3.0 abcd | 101.7 ± 0.2 cdefgh | 6.0 ± 0.1 g | |
| 6 | 100 | 66.3 ± 3.2 abcd | 87.0 ± 0.2 fghi | 3.0 ± 0.2 g | |
| Cooking Condition | Mean of Fold Change/Percent Change (%), Compared to Uncooked Vegetables | ||||
|---|---|---|---|---|---|
| Cooking Methods | Cooking Time (min) | Cooking Temp. (°C) | Goitrin | BITC | SFN |
| Uncooked | − | − | 100% | 100% | 100% |
| Blanching | 2 | 60 | 1.2/+18% | 0.9/−6% | 16.9/+1588% |
| 4 | 60 | 0.8/−17% | 0.8/−18% | 3.4/+245% | |
| 6 | 60 | 0.6/−40% | 0.8/−21% | 1.7/+73% | |
| Blanching | 2 | 80 | 0.8/−21% | 0.9/−13% | 4.2/+321% |
| 4 | 80 | 0.3/−71% | 0.9/−10% | 2.0/+95% | |
| 6 | 80 | 0.4/−61% | 0.5/−46% | 2.5/+152% | |
| Blanching | 2 | 100 | 0.2/−79% | 0.8/−22% | 2.5/+151% |
| 4 | 100 | 0.2/−81% | 0.6/−44% | 0.3/−66% | |
| 6 | 100 | 0.3/−70% | 0.7/−34% | 0.5/−48% | |
| Steaming | 2 | 60 | 0.4/−57% | 0.7/−32% | 1.2/+20% |
| 4 | 60 | 0.3/−68% | 0.8/−17% | 1.4/+36% | |
| 6 | 60 | 0.2/−79% | 0.8/−21% | 5.1/+409% | |
| Steaming | 2 | 80 | 0.2/−75% | 0.8/−16% | 0.7/−26% |
| 4 | 80 | 0.1/−87% | 0.7/−34% | 4.0/+296% | |
| 6 | 80 | 0.2/−82% | 0.6/−41% | 2.2/+117% | |
| Steaming | 2 | 100 | 0.2/−82% | 0.7/−28% | 0.6/−36% |
| 4 | 100 | 0.2/−82% | 0.9/−12% | 3.3/+235% | |
| 6 | 100 | 0.2/−78% | 0.7/−29% | 1.6/+55% | |
| Stir-frying | 2 | 60 | 0.3/−73% | 0.8/−20% | 5.7/+474% |
| 4 | 60 | 0.2/−84% | 0.7/−30% | 2.5/+151% | |
| 6 | 60 | 0.2/−84% | 0.7/−31% | 2.3/+128% | |
| Stir-frying | 2 | 80 | 0.2/−77% | 0.7/−28% | 1.2/+25% |
| 4 | 80 | 0.2/−79% | 0.6/−38% | 2.6/+156% | |
| 6 | 80 | 0.2/−81% | 0.7/−27% | 2.8/+177% | |
| Stir-frying | 2 | 100 | 0.4/−59% | 0.7/−28% | 1.6/+58% |
| 4 | 100 | 0.3/−66% | 0.7/−32% | 2.4/+136% | |
| 6 | 100 | 0.4/−58% | 0.6/−36% | 2.3/+126% | |
| Cooking Condition | Mean of Fold Change/Percent Change (%), Compared to Uncooked Vegetables | ||||
|---|---|---|---|---|---|
| Cooking Methods | Cooking Time (min) | Cooking Temp. (°C) | Goitrin | BITC | SFN |
| Uncooked | − | − | − | − | − |
| Blanching | 2 | 60 | 1.0/+2% | 1.0/+1% | 5.7/+466% |
| 4 | 60 | 1.3/+33% | 1.0/−4% | 2.0/+101% | |
| 6 | 60 | 1.4/+37% | 0.9/−12% | 1.1/+10% | |
| Blanching | 2 | 80 | 1.3/+27% | 0.9/−12% | 6.3/+531% |
| 4 | 80 | 1.2/+17% | 0.8/−18% | 0.3/−72% | |
| 6 | 80 | 1.0/+5% | 0.8/−23% | 0.2/−75% | |
| Blanching | 2 | 100 | 1.1/+10% | 0.9/−13% | 2.7/+167% |
| 4 | 100 | 0.7/−30% | 0.8/−16% | 0.4/−63% | |
| 6 | 100 | 0.3/−73% | 0.8/−20% | 0.3/−72% | |
| Steaming | 2 | 60 | 0.4/−60% | 1.1/+10% | 0.5/−50% |
| 4 | 60 | 1.0/+4% | 0.9/−14% | 2.8/+176% | |
| 6 | 60 | 0.7/−28% | 0.8/−24% | 15.2/+1424% | |
| Steaming | 2 | 80 | 1.1/+13% | 1.0/−1% | 0.6/−45% |
| 4 | 80 | 1.0/−1% | 1.0/−1% | 10.7/+974% | |
| 6 | 80 | 1.4/+38% | 0.9/−11% | 0.4/−64% | |
| Steaming | 2 | 100 | 0.7/−32% | 0.9/−11% | 2.4/+139% |
| 4 | 100 | 0.8/−24% | 0.8/−20% | 0.5/−55% | |
| 6 | 100 | 0.3/−67% | 0.8/−23% | 0.4/−64% | |
| Stir-frying | 2 | 60 | 0.3/−66% | 1.0/−4% | 3.2/+222% |
| 4 | 60 | 0.7/−26% | 1.1/+6% | 1.5/+53% | |
| 6 | 60 | 1.1/+12% | 1.0/+2% | 1.7/+73% | |
| Stir-frying | 2 | 80 | 0.4/−60% | 1.4/+36% | 4.5/+349% |
| 4 | 80 | 0.5/−52% | 0.8/−24% | 0.4/−65% | |
| 6 | 80 | 0.7/−33% | 0.8/−24% | 0.2/−80% | |
| Stir-frying | 2 | 100 | 0.8/−20% | 1.2/+19% | 6.5/+554% |
| 4 | 100 | 1.0/−3% | 0.9/−5% | 0.6/−39% | |
| 6 | 100 | 0.8/−19% | 0.8/−19% | 0.3/−69% | |
3.3. Optimization of Cooking Methods for Cabbage and Chinese Kale
3.4. Strengths and Limitations of This Research
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahey, J.W. Brassica: Characteristics and Properties. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 469–477. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Sorting out the Value of Cruciferous Sprouts as Sources of Bioactive Compounds for Nutrition and Health. Nutrients 2019, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Melim, C.; Lauro, M.R.; Pires, I.M.; Oliveira, P.J.; Cabral, C. The Role of Glucosinolates from Cruciferous Vegetables (Brassicaceae) in Gastrointestinal Cancers: From Prevention to Therapeutics. Pharmaceutics 2022, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Hudlikar, R.; Wang, L.; Wu, R.; Li, S.; Peter, R.; Shannar, A.; Chou, P.J.; Liu, X.; Liu, Z.; Kuo, H.D.; et al. Epigenetics/Epigenomics and Prevention of Early Stages of Cancer by Isothiocyanates. Cancer Prev. Res. 2021, 14, 151–164. [Google Scholar] [CrossRef]
- Rekha, K.; Venkidasamy, B.; Govindasamy, R.; Neralla, M.; Thiruvengadam, M. Isothiocyanates (AITC & BITC) bioactive molecules: Therapeutic potential for oral cancer. Oral Oncol. 2022, 133, 106060. [Google Scholar] [CrossRef] [PubMed]
- Elkashty, O.A.; Tran, S.D. Sulforaphane as a Promising Natural Molecule for Cancer Prevention and Treatment. Curr. Med. Sci. 2021, 41, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Lam-Ubol, A.; Fitzgerald, A.L.; Ritdej, A.; Phonyiam, T.; Zhang, H.; Myers, J.N.; Huang, P.; Trachootham, D. Sensory acceptable equivalent doses of β-phenylethyl isothiocyanate (PEITC) induce cell cycle arrest and retard the growth of p53 mutated oral cancer in vitro and in vivo. Food Funct. 2018, 9, 3640–3656. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [PubMed]
- Kaewsit, N.; Winuprasith, T.; Trachootham, D. Detoxification of heterocyclic aromatic amines from grilled meat using a PEITC-rich vegetable sauce: A randomized crossover controlled trial. Food Funct. 2021, 12, 10411–10422. [Google Scholar] [CrossRef]
- Phikulkhao, N.; Tanaviyutpakdee, P.; Lam-ubol, A.; Trachootham, D. Nutri-phenethyl isothiocyanate Jelly Promotes Detoxification of a Tobacco-specific Oral Carcinogen in Male Active Cigarette Smokers. Cancer Screen. Prev. 2023, 2, 30–41. [Google Scholar] [CrossRef]
- Liu, X.; Lv, K. Cruciferous vegetables intake is inversely associated with risk of breast cancer: A meta-analysis. Breast 2013, 22, 309–313. [Google Scholar] [CrossRef]
- Long, J.; Liu, Z.; Liang, S.; Chen, B. Cruciferous Vegetable Intake and Risk of Prostate Cancer: A Systematic Review and Meta-Analysis. Urol. Int. 2023, 107, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.; Eslick, G.D. Cruciferous vegetables and risk of colorectal neoplasms: A systematic review and meta-analysis. Nutr. Cancer 2014, 66, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.K.; Salwan, P.; Salwan, S. Various Possible Toxicants Involved in Thyroid Dysfunction: A Review. J. Clin. Diagn. Res. 2016, 10, FE01–FE03. [Google Scholar] [CrossRef] [PubMed]
- Boelaert, K. The association between serum TSH concentration and thyroid cancer. Endocr. Relat. Cancer 2009, 16, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.A.; Kim, J. Dietary Factors Affecting Thyroid Cancer Risk: A Meta-Analysis. Nutr. Cancer 2015, 67, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.; Baron-Dubourdieu, D.; Rougier, Y.; Guénel, P. Role of dietary iodine and cruciferous vegetables in thyroid cancer: A countrywide case-control study in New Caledonia. Cancer Causes Control 2010, 21, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Felker, P.; Bunch, R.; Leung, A.M. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutr. Rev. 2016, 74, 248–258. [Google Scholar] [CrossRef]
- Oloyede, O.O.; Wagstaff, C.; Methven, L. The Impact of Domestic Cooking Methods on Myrosinase Stability, Glucosinolates and Their Hydrolysis Products in Different Cabbage (Brassica oleracea) Accessions. Foods 2021, 10, 2908. [Google Scholar] [CrossRef]
- Wang, Z.; Kwan, M.L.; Pratt, R.; Roh, J.M.; Kushi, L.H.; Danforth, K.N.; Zhang, Y.; Ambrosone, C.B.; Tang, L. Effects of cooking methods on total isothiocyanate yield from cruciferous vegetables. Food Sci. Nutr. 2020, 8, 5673–5682. [Google Scholar] [CrossRef]
- Volden, J.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.; Thygesen, I.E.; Wicklund, T. Effect of thermal treatment on glucosinolates and antioxidant-related parameters in red cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chem. 2008, 109, 595–605. [Google Scholar] [CrossRef]
- Baenas, N.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P.; Moreno, D.A. Influence of Cooking Methods on Glucosinolates and Isothiocyanates Content in Novel Cruciferous Foods. Foods 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.N.; Parat, M.O.; Ong, Y.S.; Khaw, K.Y. Anticancer activities of dietary benzyl isothiocyanate: A comprehensive review. Pharmacol. Res. 2021, 169, 105666. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, J.; Ullah, S.; Ahmad, I. Sulforaphane and Its Protective Role in Prostate Cancer: A Mechanistic Approach. Int. J. Mol. Sci. 2023, 24, 6979. [Google Scholar] [CrossRef]
- Ji, Y.; Morris, M.E. Determination of phenethyl isothiocyanate in human plasma and urine by ammonia derivatization and liquid chromatography–tandem mass spectrometry. Anal. Biochem. 2003, 323, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Yahya, H.N.; Oloyede, O.O.; Methven, L.; Wagstaff, C. Changes in rocket salad phytochemicals within the commercial supply chain: Glucosinolates, isothiocyanates, amino acids, and bacterial load increase significantly after processing. Food Chem. 2017, 221, 521–534. [Google Scholar] [CrossRef]
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Q2 (R1), A Validation of Analytical Procedures: Text and Methodology. 2005. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 23 June 2023).
- AOAC. International Guidelines for Standard Method Performance Requirements AOAC Official Methods of Analysis. 2016. Appendix F, 1–18. Available online: https://www.aoac.org/wp-content/uploads/2019/08/app_f.pdf (accessed on 23 June 2023).
- Food and Drug Administration Office of Regulatory Affairs ORA Laboratory Manual Volume II: U.S. Food and Drug Administration. Available online: https://www.fda.gov/media/73920/download (accessed on 23 June 2023).
- Wennberg, M.; Ekvall, J.; Olsson, K.; Nyman, M. Changes in carbohydrate and glucosinolate composition in white cabbage (Brassica oleracea var. capitata) during blanching and treatment with acetic acid. Food Chem. 2006, 95, 226–236. [Google Scholar] [CrossRef]
- Cedrowski, J.; Dąbrowa, K.; Przybylski, P.; Krogul-Sobczak, A.; Litwinienko, G. Antioxidant activity of two edible isothiocyanates: Sulforaphane and erusin is due to their thermal decomposition to sulfenic acids and methylsulfinyl radicals. Food Chem. 2021, 353, 129213. [Google Scholar] [CrossRef]
- Cedrowski, J.; Dąbrowa, K.; Krogul-Sobczak, A.; Litwinienko, G. A Lesson Learnt from Food Chemistry-Elevated Temperature Triggers the Antioxidant Action of Two Edible Isothiocyanates: Erucin and Sulforaphane. Antioxidants 2020, 9, 1090. [Google Scholar] [CrossRef]
- Nugrahedi, P.Y.; Verkerk, R.; Widianarko, B.; Dekker, M. A Mechanistic Perspective on Process-Induced Changes in Glucosinolate Content in Brassica Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 823–838. [Google Scholar] [CrossRef]
- Jones, R.B.; Faragher, J.D.; Winkler, S. A review of the influence of postharvest treatments on quality and glucosinolate content in broccoli (Brassica oleracea var. italica) heads. Postharvest Biol. Technol. 2006, 41, 1–8. [Google Scholar] [CrossRef]
- Gu, Z.-X.; Guo, Q.-H.; Gu, Y.-J. Factors Influencing Glucoraphanin and Sulforaphane Formation in Brassica Plants: A Review. J. Integr. Agric. 2012, 11, 1804–1816. [Google Scholar] [CrossRef]
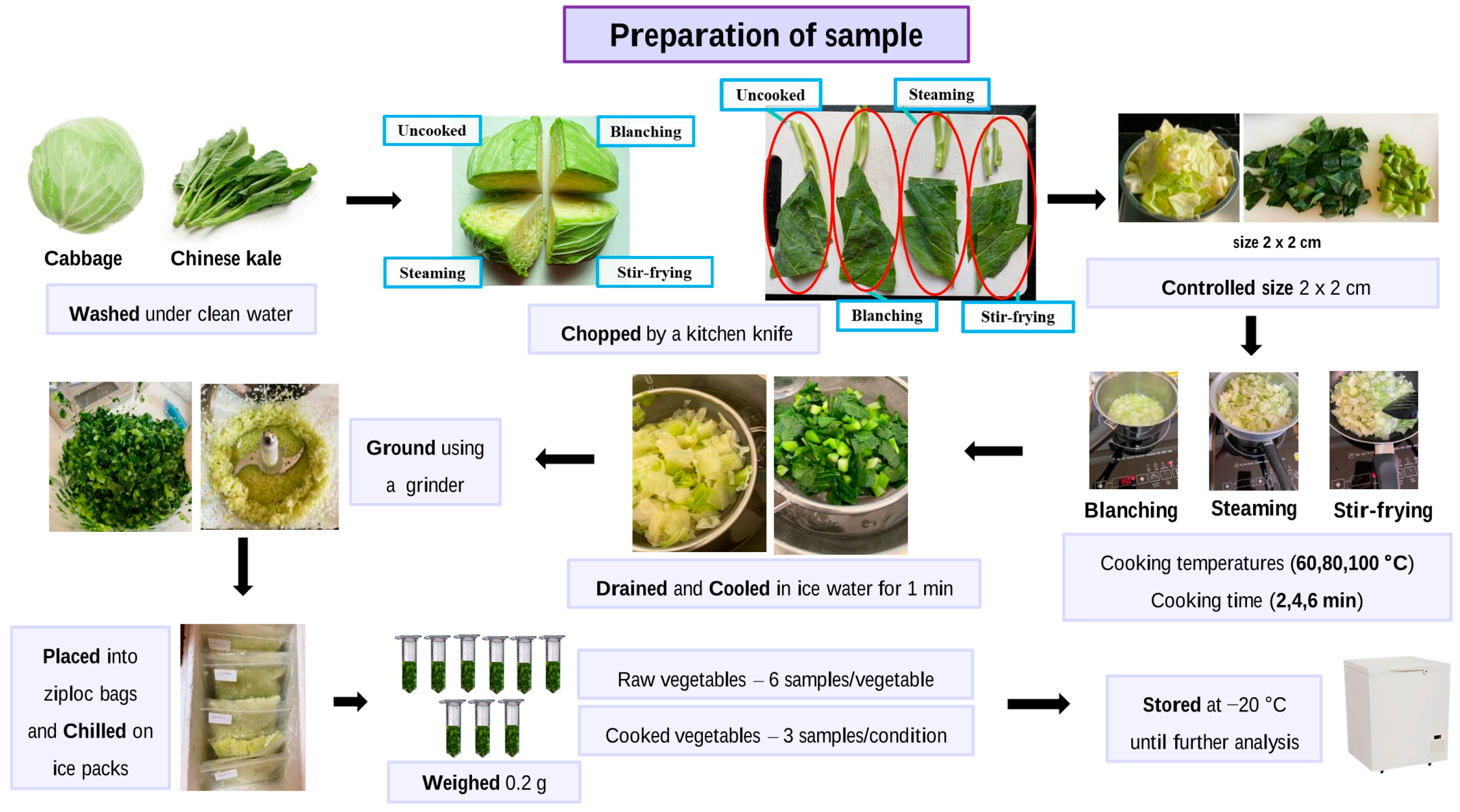
| Cooking Methods | Weight (g) | Water Volume (mL) | Cooking Time (min) | Cooking Temp. (°C) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cabbage | Blanching | 150 | 400 | 2 | 4 | 6 | 60 | 80 | 100 |
| Steaming | 150 | 400 | 2 | 4 | 6 | 60 | 80 | 100 | |
| Stir-frying | 150 | 400 | 2 | 4 | 6 | 60 | 80 | 100 | |
| Chinese kale | Blanching | 150 | 400 | 2 | 4 | 6 | 60 | 80 | 100 |
| Steaming | 150 | 400 | 2 | 4 | 6 | 60 | 80 | 100 | |
| Stir-frying | 150 | 400 | 2 | 4 | 6 | 60 | 80 | 100 | |
| Analyte | R2 | Mean Recovery (%) | Inter-Day Precision (%RSD) | |||||
|---|---|---|---|---|---|---|---|---|
| Cabbage | Chinese Kale | Expected Criteria a | Vegetable Samples | Expected Criteria b | Standard 1 ng/mL | Expected Criteria c | ||
| Goitrin | 0.9997 | 88 | 85 | 60–115 | 2.28 | 21 | 9.56 | 30 |
| BITC | 0.9993 | 93 | 82 | 60–115 | 1.00 | 21 | 2.72 | 30 |
| SFN | 0.9998 | 94 | 90 | 60–115 | 2.44 | 21 | 2.48 | 30 |
| Cooking Condition | Goitrin (ng/mL) | BITC (ng/mL) | SFN (ng/mL) | ||
|---|---|---|---|---|---|
| Cooking Methods | Cooking Time (min) | Cooking Temp. (°C) | |||
| Uncooked | − | − | 251.5 ± 59.6 a | 108.7 ± 1.1 a | 202.8 ± 60.1 f |
| Blanching | 2 | 60 | 296.5 ± 128.4 a | 102.6 ± 1.1 ab | 3423.0 ± 1014.0 a |
| 4 | 60 | 209.8 ± 7.6 ab | 88.8 ± 0.8 bcdefgh | 698.9 ± 162.6 bcdef | |
| 6 | 60 | 152.1 ± 85.1 bcd | 86.0 ± 1.4 bcdefghi | 350.5 ± 101.0 def | |
| Blanching | 2 | 80 | 199.4 ± 40.7 abc | 94.8 ± 9.0 abcde | 853.0 ± 231.7 bcd |
| 4 | 80 | 72.5 ± 13.4 de | 97.8 ± 10.7 abc | 395.5 ± 24.5 cdef | |
| 6 | 80 | 97.6 ± 27.5 bcde | 58.5 ± 0.9 m | 510.1 ± 46.7 bcdef | |
| Blanching | 2 | 100 | 53.2 ± 2.4 de | 84.4 ± 0.4 cdefghij | 509.6 ± 31.9 bcdef |
| 4 | 100 | 48.2 ± 5.6 de | 60.8 ± 20.9 lm | 68.0 ± 15.7 f | |
| 6 | 100 | 76.3 ± 8.5 de | 71.9 ± 0.6 hijklm | 104.6 ± 49.5 f | |
| Steaming | 2 | 60 | 108.5 ± 26.9 bcde | 74.3 ± 0.4 ghijklm | 242.9 ± 59.5 def |
| 4 | 60 | 80.9 ± 5.8 de | 89.9 ± 0.2 bcdefg | 276.7 ± 26.8 def | |
| 6 | 60 | 53.4 ± 4.5 de | 86.0 ± 9.7 bcdefghi | 1033.0 ± 208.1 bc | |
| Steaming | 2 | 80 | 62.8 ± 11.7 de | 91.8 ± 6.3 bcdef | 150.8 ± 7.3 ef |
| 4 | 80 | 33.7 ± 0.6 e | 72.2 ± 0.2 hijklm | 802.6 ± 46.4 bcde | |
| 6 | 80 | 45.5 ± 8.1 de | 64.3 ± 0.2 klm | 440.5 ± 87.3 cdef | |
| Steaming | 2 | 100 | 45.2 ± 15.3 de | 77.8 ± 0.0 fghijkl | 130.6 ± 16.5 ef |
| 4 | 100 | 45.0 ± 1.7 de | 95.5 ± 0.2 abcd | 679.3 ± 36.6 bcdef | |
| 6 | 100 | 55.3 ± 4.8 de | 77.4 ± 0.4 fghijkl | 314.6 ± 62.8 def | |
| Stir-frying | 2 | 60 | 67.9 ± 16.3 de | 86.6 ± 0.2 bcdefghi | 1164.0 ± 21.5 b |
| 4 | 60 | 40.8 ± 6.2 de | 76.6 ± 0.6 fghijkl | 508.2 ± 134.1 bcdef | |
| 6 | 60 | 39.6 ± 14.0 de | 75.4 ± 7.9 fghijklm | 462.2 ± 66.3 cdef | |
| Stir-frying | 2 | 80 | 57.6 ± 0.4 de | 78.1 ± 0.0 efghijk | 253.0 ± 37.8 def |
| 4 | 80 | 52.9 ± 1.4 de | 67.5 ± 0.2 jklm | 519.5 ± 247.9 bcdef | |
| 6 | 80 | 48.1 ± 14.2 de | 79.8 ± 0.2 defghijk | 561.5 ± 81.5 bcdef | |
| Stir-frying | 2 | 100 | 102.4 ± 15.6 bcde | 78.4 ± 0.4 efghijk | 319.7 ± 26.4 def |
| 4 | 100 | 86.3 ± 2.1 cde | 73.4 ± 0.0 ghijklm | 478.8 ± 37.4 cdef | |
| 6 | 100 | 105.1 ± 2.8 bcde | 69.9 ± 0.2 ijklm | 458.5 ± 195.6 cdef | |
| Number | Time (min) | Temp. (°C) | Cooking Method | Goitrin (ng/mL) | BITC (ng/mL) | SFN (ng/mL) | Desirability |
|---|---|---|---|---|---|---|---|
| 1 | 4.26 | 90.92 | Steaming | 33.45 | 278.72 | 681.39 | 0.38 |
| 2 | 2.71 | 60.00 | Stir-frying | 56.30 | 80.10 | 875.54 | 0.29 |
| 3 | 2.68 | 60.00 | Stir-frying | 56.71 | 80.39 | 879.95 | 0.29 |
| 4 | 2.63 | 60.00 | Stir-frying | 57.29 | 80.67 | 885.96 | 0.29 |
| 5 | 2.87 | 60.00 | Stir-frying | 54.50 | 79.11 | 855.86 | 0.29 |
| 6 | 2.96 | 60.00 | Stir-frying | 53.54 | 78.60 | 844.90 | 0.29 |
| 7 | 4.09 | 60.00 | Stir-frying | 43.96 | 74.60 | 704.61 | 0.28 |
| 8 | 4.30 | 60.00 | Stir-frying | 42.66 | 74.35 | 677.52 | 0.27 |
| 9 | 4.70 | 60.00 | Stir-frying | 40.68 | 74.32 | 626.40 | 0.27 |
| 10 | 2.42 | 92.81 | Blanching | 78.21 | 88.59 | 472.44 | 0.18 |
| 11 | 5.89 | 98.65 | Blanching | 73.32 | 63.37 | 343.13 | 0.14 |
| Number | Time (min) | Temp. (°C) | Cooking Method | Goitrin (ng/mL) | BITC (ng/mL) | SFN (ng/mL) | Desirability |
|---|---|---|---|---|---|---|---|
| 1 | 2.00 | 84.56 | Stir-frying | 31.90 | 121.22 | 45.26 | 0.63 |
| 2 | 2.00 | 84.78 | Stir-frying | 32.26 | 121.39 | 45.44 | 0.63 |
| 3 | 2.00 | 84.95 | Stir-frying | 32.54 | 121.51 | 45.57 | 0.63 |
| 4 | 2.00 | 85.10 | Stir-frying | 32.80 | 121.63 | 45.70 | 0.63 |
| 5 | 2.00 | 84.01 | Stir-frying | 31.02 | 120.81 | 44.82 | 0.63 |
| 6 | 2.00 | 83.77 | Stir-frying | 30.65 | 120.65 | 44.64 | 0.63 |
| 7 | 2.00 | 85.86 | Stir-frying | 34.12 | 122.23 | 46.33 | 0.63 |
| 8 | 2.00 | 87.39 | Stir-frying | 37.01 | 123.54 | 47.67 | 0.63 |
| 9 | 2.00 | 62.12 | Stir-frying | 26.49 | 117.22 | 35.37 | 0.61 |
| 10 | 2.00 | 61.75 | Stir-frying | 26.92 | 117.37 | 35.34 | 0.61 |
| 11 | 2.00 | 61.11 | Stir-frying | 27.70 | 117.64 | 35.31 | 0.61 |
| 12 | 2.00 | 60.25 | Stir-frying | 28.84 | 118.04 | 35.28 | 0.61 |
| 13 | 2.00 | 97.00 | Stir-frying | 61.74 | 134.44 | 57.77 | 0.59 |
| 14 | 2.72 | 60.00 | Steaming | 49.52 | 107.39 | 30.35 | 0.46 |
| 15 | 2.74 | 60.00 | Steaming | 49.72 | 107.24 | 31.40 | 0.46 |
| 16 | 2.78 | 60.00 | Steaming | 50.13 | 106.93 | 33.55 | 0.46 |
| 17 | 2.66 | 60.00 | Steaming | 48.91 | 107.85 | 27.12 | 0.46 |
| 18 | 2.80 | 60.00 | Steaming | 50.39 | 106.73 | 34.89 | 0.46 |
| 19 | 2.00 | 60.00 | Blanching | 86.26 | 109.12 | 21.77 | 0.31 |
| 20 | 6.00 | 60.00 | Blanching | 86.45 | 109.10 | 21.90 | 0.31 |
| 21 | 2.32 | 60.29 | Blanching | 86.73 | 108.72 | 21.26 | 0.31 |
| 22 | 6.00 | 63.22 | Stir-frying | 80.98 | 104.01 | 15.86 | 0.30 |
| 23 | 2.32 | 60.00 | Blanching | 89.59 | 108.80 | 24.01 | 0.30 |
| 24 | 4.11 | 60.00 | Stir-frying | 62.49 | 97.11 | 8.11 | 0.29 |
| 25 | 3.80 | 100.00 | Blanching | 62.41 | 90.65 | 15.34 | 0.26 |
| 26 | 3.82 | 100.00 | Blanching | 62.22 | 90.62 | 15.28 | 0.26 |
| 27 | 3.84 | 100.00 | Blanching | 61.81 | 90.56 | 15.14 | 0.26 |
| 28 | 3.91 | 100.00 | Blanching | 60.67 | 90.39 | 14.76 | 0.26 |
| 29 | 4.35 | 100.00 | Blanching | 53.14 | 89.23 | 12.00 | 0.25 |
| 30 | 4.30 | 60.00 | Blanching | 106.45 | 105.99 | 32.20 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panduang, T.; Phucharoenrak, P.; Karnpanit, W.; Trachootham, D. Cooking Methods for Preserving Isothiocyanates and Reducing Goitrin in Brassica Vegetables. Foods 2023, 12, 3647. https://doi.org/10.3390/foods12193647
Panduang T, Phucharoenrak P, Karnpanit W, Trachootham D. Cooking Methods for Preserving Isothiocyanates and Reducing Goitrin in Brassica Vegetables. Foods. 2023; 12(19):3647. https://doi.org/10.3390/foods12193647
Chicago/Turabian StylePanduang, Thanaporn, Pakkapong Phucharoenrak, Weeraya Karnpanit, and Dunyaporn Trachootham. 2023. "Cooking Methods for Preserving Isothiocyanates and Reducing Goitrin in Brassica Vegetables" Foods 12, no. 19: 3647. https://doi.org/10.3390/foods12193647
APA StylePanduang, T., Phucharoenrak, P., Karnpanit, W., & Trachootham, D. (2023). Cooking Methods for Preserving Isothiocyanates and Reducing Goitrin in Brassica Vegetables. Foods, 12(19), 3647. https://doi.org/10.3390/foods12193647