Antioxidant and Anti-Inflammatory Mechanisms of Lipophilic Fractions from Polyscias fruticosa Leaves Based on Network Pharmacology, In Silico, and In Vitro Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Chemical Composition Analysis of Lipophilic Fractions from P. fruticosa Leaves
2.3. Chemical-Based Antioxidant Activity Assays
2.4. Cell Culture
2.5. Measurement of NO Production
2.6. Measurement of Reactive Oxygen Species (ROS) Formation
2.7. Western Blot Analysis
2.8. Network Pharmacology Analysis
2.8.1. Prediction of Potential Targets of Compounds in Lipophilic Extracts from P. fruticosa Leaves
2.8.2. Identification of Inflammation- and Antioxidant-Related Target Genes
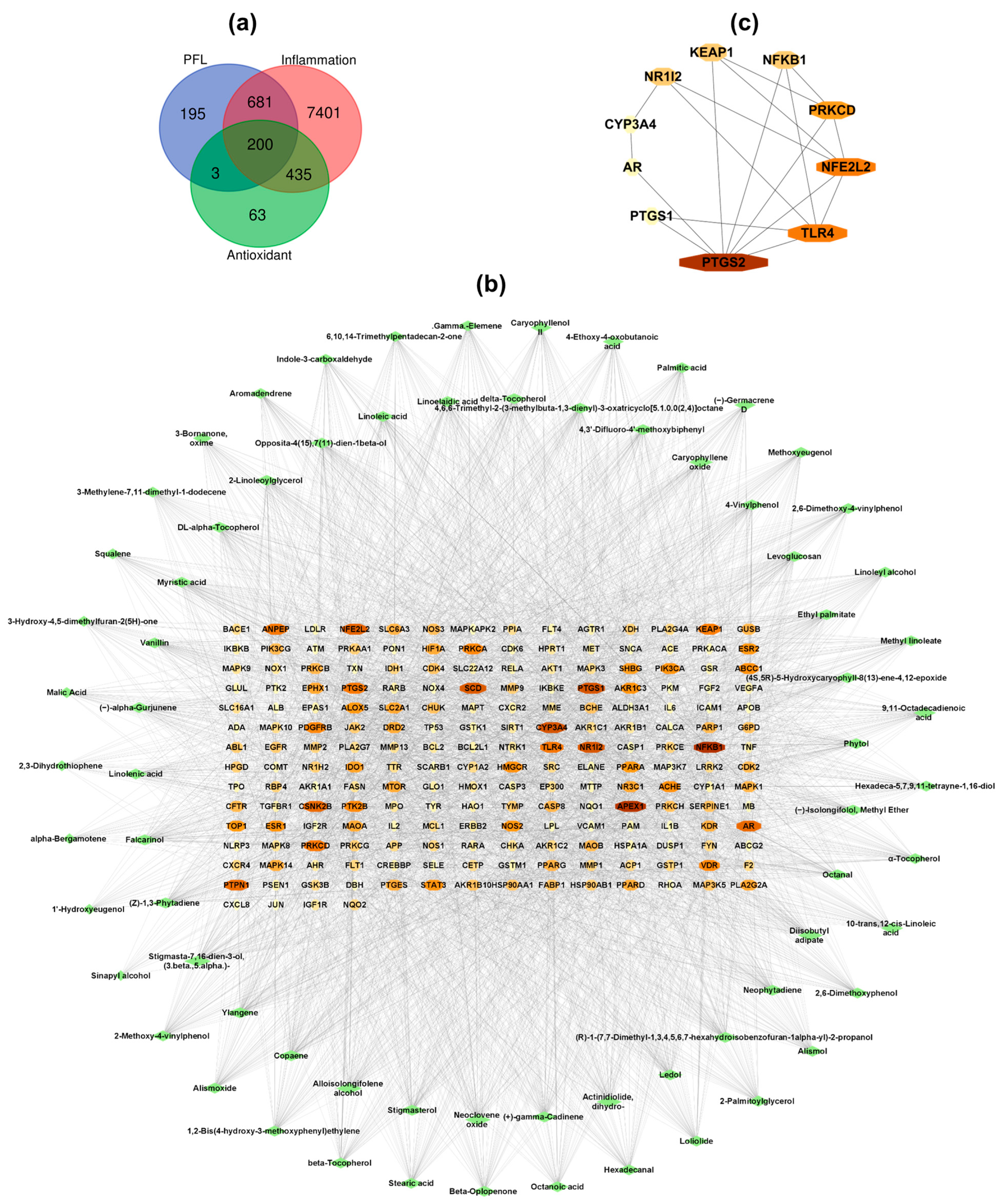
2.8.3. Determination of Common Targets and Construction of Compound–Target and Protein–Protein Interaction Networks
2.8.4. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analyses
2.9. Computational Validation Based on Molecular Docking Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Phytochemcal Profile of Lipophilic Fractions from P. fruticosa Leaves
3.2. Antioxidant and Anti-Inflammatory Mechanisms of Lipophilic Extracts from P. fruticosa Leaves Based on Network Pharmacology Analysis
3.2.1. Network Construction and Analysis
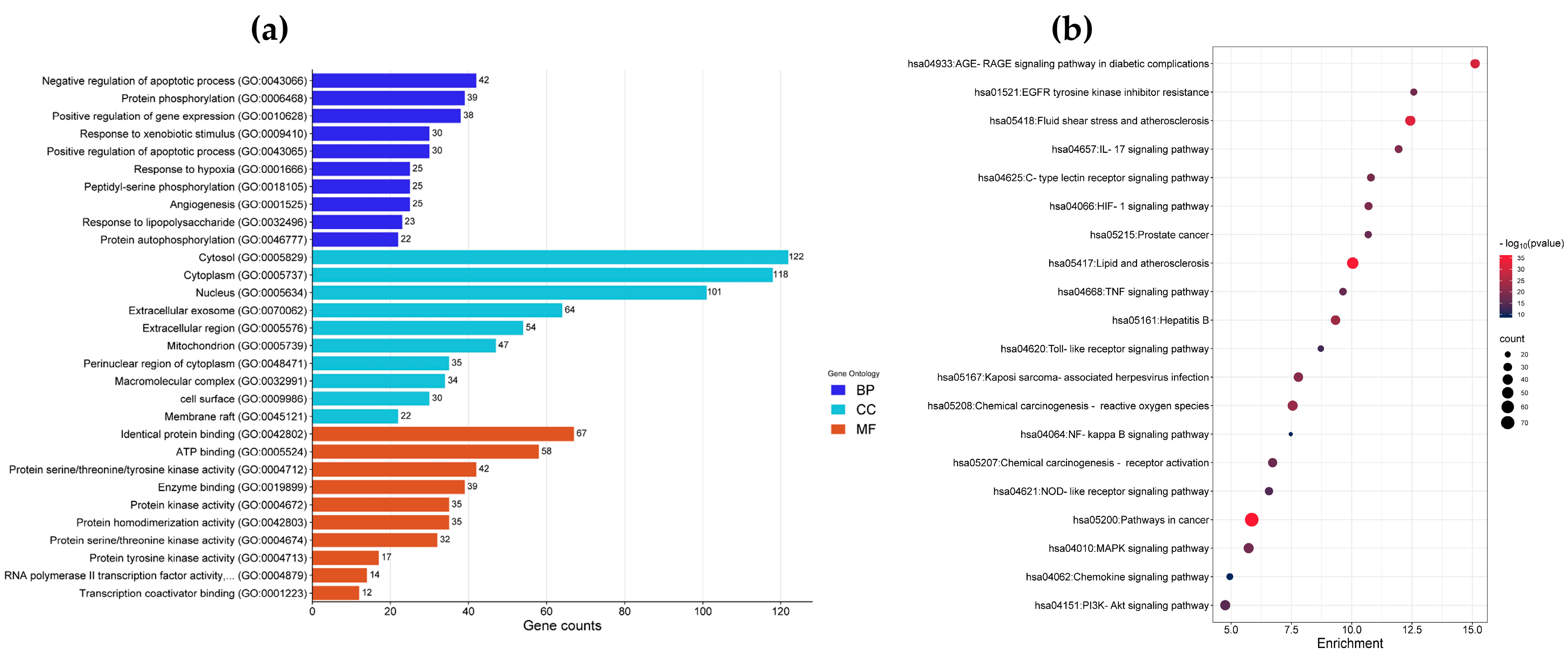
3.2.2. Enrichment Analysis of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways
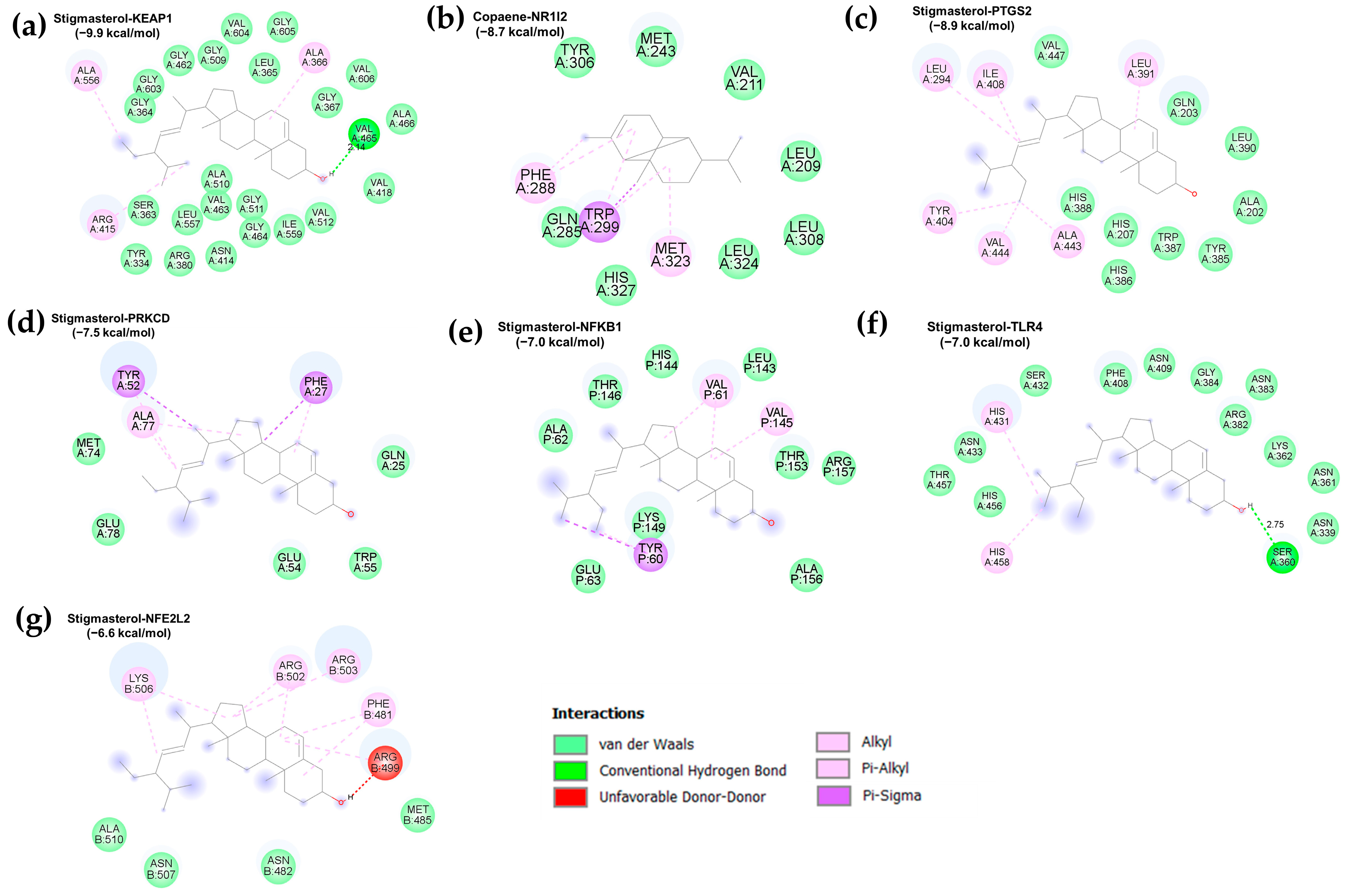
3.3. Molecular Docking Validation
3.4. Chemical-Based Antioxidant Activity of Lipophilic Extracts from P. fruticosa Leaves
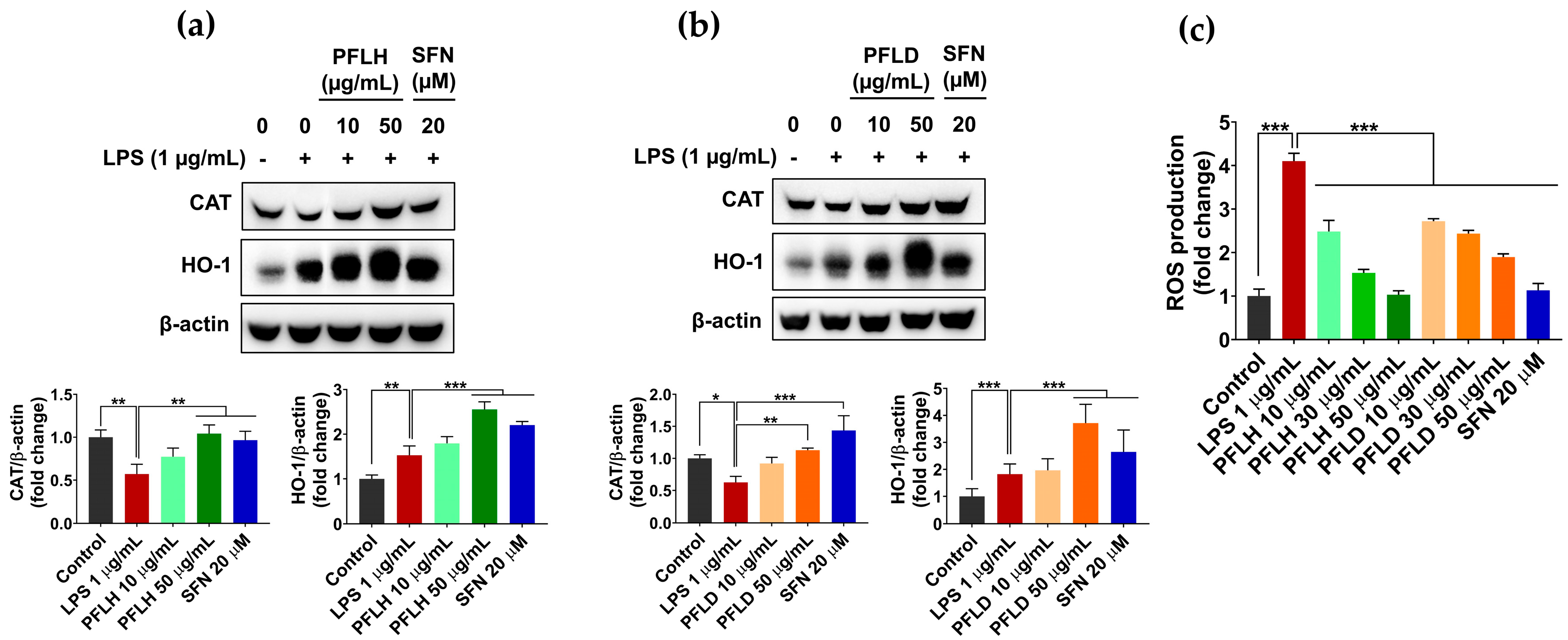
3.5. Antioxidant Activity of Lipophilic Extracts from P. fruticosa Leaves in LPS-Stimulated RAW 264.7 Cells
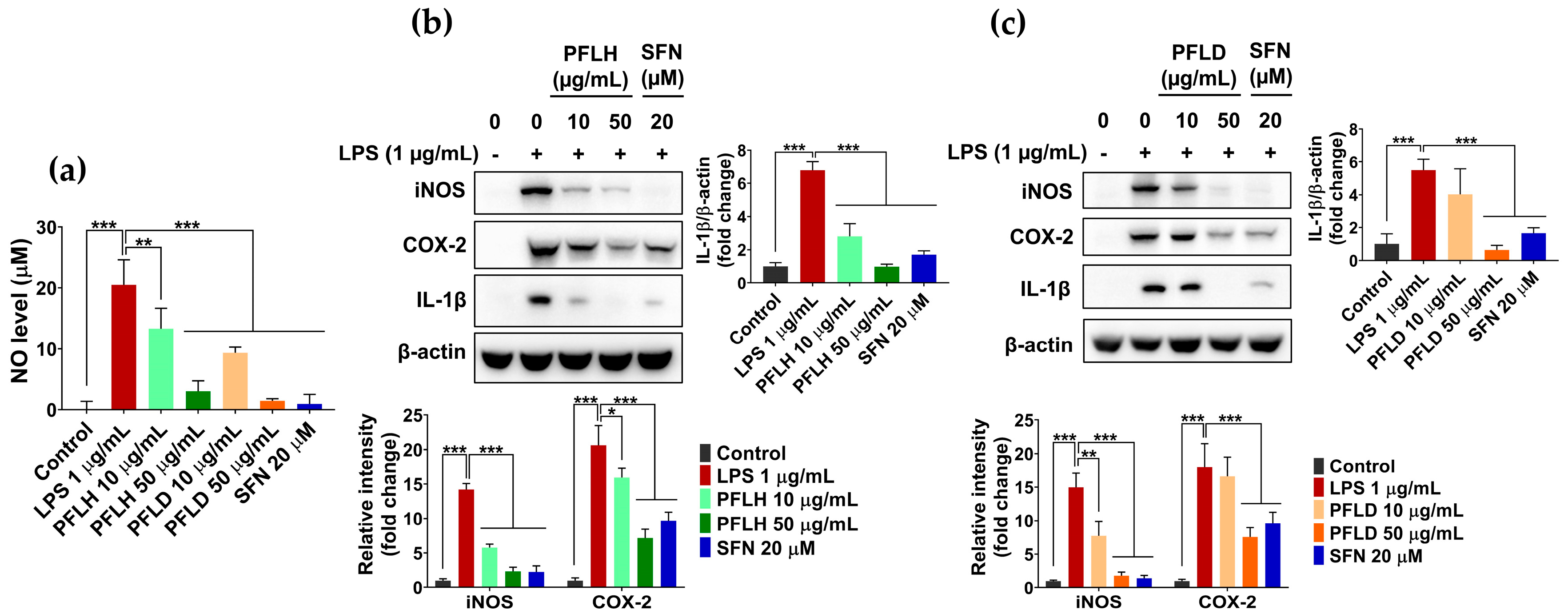
3.6. Anti-Inflammatory Activity of Lipophilic Extracts from P. fruticosa Leaves in LPS-Treated RAW 264.7 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Kıran, T.R.; Otlu, O.; Karabulut, A.B. Oxidative stress and antioxidants in health and disease. J. Lab. Med. 2023, 47, 1–11. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Jeon, H.; Yang, D.; Lee, N.H.; Ahn, M.; Kim, G. Inhibitory Effect of Black Radish (Raphanus sativus L. var. niger) Extracts on Lipopolysaccharide-Induced Inflammatory Response in the Mouse Monocyte/Macrophage-Like Cell Line RAW 264.7. Prev. Nutr. Food Sci. 2020, 25, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Wadley, A.J.; Veldhuijzen van Zanten, J.J.; Aldred, S. The interactions of oxidative stress and inflammation with vascular dysfunction in ageing: The vascular health triad. Age 2013, 35, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Kitamura, M. Bidirectional regulation of NF-κB by reactive oxygen species: A role of unfolded protein response. Free Radic. Biol. Med. 2013, 65, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.T.; Dang, N.H.; Kim, O.; Van Cuong, P.; Dat, N.T.; Hwangbo, C.; Van Minh, C.; Lee, J.-H. Ethanol extract of Polyscias fruticosa leaves suppresses RANKL-mediated osteoclastogenesis in vitro and LPS-induced bone loss in vivo. Phytomedicine 2019, 59, 152908. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, B.; Le, T.T.; Kim, D.W.; Jung, B.H.; Yoo, K.-Y.; Ahn, H.R.; Thuong, P.T.; Tran, T.T.; Pae, A.N.; Jung, S.H.; et al. Neuroprotective Effects of Ethanol Extract of Polyscias fruticosa (EEPF) against Glutamate-Mediated Neuronal Toxicity in HT22 Cells. Int. J. Mol. Sci. 2023, 24, 3969. [Google Scholar] [CrossRef]
- Ly, H.T.; Nguyen, T.T.H.; Le, V.M.; Lam, B.T.; Mai, T.T.T.; Dang, T.P.T. Therapeutic Potential of Polyscias fruticosa (L.) Harms Leaf Extract for Parkinson’s Disease Treatment by Drosophila melanogaster Model. Oxid. Med. Cell. Longev. 2022, 2022, 5262677. [Google Scholar] [CrossRef]
- Asumeng Koffuor, G.; Boye, A.; Kyei, S.; Ofori-Amoah, J.; Akomanin Asiamah, E.; Barku, A.; Acheampong, J.; Amegashie, E.; Kumi Awuku, A. Anti-asthmatic property and possible mode of activity of an ethanol leaf extract of Polyscias fruticosa. Pharm. Biol. 2016, 54, 1354–1363. [Google Scholar] [CrossRef]
- Kubatka, P.; Mazurakova, A.; Samec, M.; Koklesova, L.; Zhai, K.; Al-Ishaq, R.; Kajo, K.; Biringer, K.; Vybohova, D.; Brockmueller, A.; et al. Flavonoids against non-physiologic inflammation attributed to cancer initiation, development, and progression—3PM pathways. EPMA J. 2021, 12, 559–587. [Google Scholar] [CrossRef] [PubMed]
- Brophy, J.J.; Lassak, E.V.; Suksamrarn, A. Constituents of the volatile leaf oils of Polyscias fruticosa (L.) Harms. Flavour Fragr. J. 1990, 5, 179–182. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Jin, X.; Ma, Y.; Yang, Y.; Li, J.; Liang, L.; Liu, R.; Li, Z. A comprehensive application: Molecular docking and network pharmacology for the prediction of bioactive constituents and elucidation of mechanisms of action in component-based Chinese medicine. Comput. Biol. Chem. 2021, 90, 107402. [Google Scholar] [CrossRef]
- Guo, X.; Yu, X.; Zheng, B.; Zhang, L.; Zhang, F.; Zhang, Y.; Li, J.; Pu, G.; Zhang, L.; Wu, H. Network Pharmacology-Based Identification of Potential Targets of Lonicerae japonicae Flos Acting on Anti-Inflammatory Effects. Biomed. Res. Int. 2021, 2021, 5507003. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, R.; Chen, J.; Lv, M.; Xu, X. Network pharmacology analysis of the pharmacological mechanism of Artemisia lavandulaefolia DC. in rheumatoid arthritis. Phytomedicine 2023, 118, 154905. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yang, C.; Wang, Y.; Sun, M.; Bi, C.; Sun, S.; Sun, G.; Hao, J.; Li, L.; Shan, C.; et al. Functional metabolome profiling may improve individual outcomes in colorectal cancer management implementing concepts of predictive, preventive, and personalized medical approach. EPMA J. 2022, 13, 39–55. [Google Scholar] [CrossRef]
- Lim, S.; Choi, A.-H.; Kwon, M.; Joung, E.-J.; Shin, T.; Lee, S.-G.; Kim, N.-G.; Kim, H.-R. Evaluation of antioxidant activities of various solvent extract from Sargassum serratifolium and its major antioxidant components. Food Chem. 2019, 278, 178–184. [Google Scholar] [CrossRef]
- Xue, T.-T.; Yang, Y.-G.; Tang, Z.-S.; Duan, J.-A.; Song, Z.-X.; Hu, X.-H.; Yang, H.-D.; Xu, H.-B. Evaluation of antioxidant, enzyme inhibition, nitric oxide production inhibitory activities and chemical profiles of the active extracts from the medicinal and edible plant: Althaea officinalis. Food Res. Int. 2022, 156, 111166. [Google Scholar] [CrossRef]
- Tabassum, R.; Ashfaq, M.; Tahir, T.; Oku, H. DPPH and Nitric Oxide Free Radical Scavenging Potential of Phenyl Quinoline Derivatives and Their Transition Metal Complexes. J. Mol. Struct. 2022, 134058. [Google Scholar] [CrossRef]
- Yildiz, G.; Demiryürek, A.T.; Sahin-Erdemli, I.; Kanzik, I. Comparison of antioxidant activities of aminoguanidine, methylguanidine and guanidine by luminol-enhanced chemiluminescence. Br. J. Pharmacol. 1998, 124, 905–910. [Google Scholar] [CrossRef]
- Moussa, H.; Dahmoune, F.; Hentabli, M.; Remini, H.; Mouni, L. Optimization of ultrasound-assisted extraction of phenolic-saponin content from Carthamus caeruleus L. rhizome and predictive model based on support vector regression optimized by dragonfly algorithm. Chemom. Intell. Lab. Syst. 2022, 222, 104493. [Google Scholar] [CrossRef]
- Ashmawy, N.S.; Gad, H.A.; Ashour, M.L.; El-Ahmady, S.H.; Singab, A.N.B. The genus Polyscias (Araliaceae): A phytochemical and biological review. J. Herb. Med. 2020, 23, 100377. [Google Scholar] [CrossRef]
- Liu, L.; Xu, F.-R.; Wang, Y.-Z. Traditional uses, chemical diversity and biological activities of Panax L. (Araliaceae): A review. J. Ethnopharmacol. 2020, 263, 112792. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Coniglio, S.; Shumskaya, M.; Vassiliou, E. Unsaturated Fatty Acids and Their Immunomodulatory Properties. Biology 2023, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Dawid, C.; Dunemann, F.; Schwab, W.; Nothnagel, T.; Hofmann, T. Bioactive C17-Polyacetylenes in Carrots (Daucus carota L.): Current Knowledge and Future Perspectives. J. Agric. Food Chem. 2015, 63, 9211–9222. [Google Scholar] [CrossRef]
- Faki, Y.; Er, A. Different Chemical Structures and Physiological/Pathological Roles of Cyclooxygenases. Rambam Maimonides Med. J. 2021, 12, e0003. [Google Scholar] [CrossRef]
- Ferrer, D.M.; Busquets-Cortés, C.; Capó, X.; Tejada, S.; Tur, A.J.; Pons, A.; Sureda, A. Cyclooxygenase-2 Inhibitors as a Therapeutic Target in Inflammatory Diseases. Curr. Med. Chem. 2019, 26, 3225–3241. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Signaling to NF-kB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Pergola, P.E.; Zager, R.A.; Vaziri, N.D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013, 83, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, P.O.; Chen, T. PXR: More Than Just a Master Xenobiotic Receptor. Mol. Pharmacol. 2018, 93, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kluth, D.; Banning, A.; Paur, I.; Blomhoff, R.; Brigelius-Flohé, R. Modulation of pregnane X receptor-and electrophile responsive element-mediated gene expression by dietary polyphenolic compounds. Free Radic. Biol. Med. 2007, 42, 315–325. [Google Scholar] [CrossRef]
- Qiu, Z.; Cervantes, J.L.; Cicek, B.B.; Mukherjee, S.; Venkatesh, M.; Maher, L.A.; Salazar, J.C.; Mani, S.; Khanna, K.M. Pregnane X Receptor Regulates Pathogen-Induced Inflammation and Host Defense against an Intracellular Bacterial Infection through Toll-like Receptor 4. Sci. Rep. 2016, 6, 31936. [Google Scholar] [CrossRef]
- Yazawa, T.; Kawabe, S.; Kanno, M.; Mizutani, T.; Imamichi, Y.; Ju, Y.; Matsumura, T.; Yamazaki, Y.; Usami, Y.; Kuribayashi, M.; et al. Androgen/androgen receptor pathway regulates expression of the genes for cyclooxygenase-2 and amphiregulin in periovulatory granulosa cells. Mol. Cell. Endocrinol. 2013, 369, 42–51. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.S.; Gazi, M.H.; Young, C.Y. The cyclooxygenase 2-specific nonsteroidal anti-inflammatory drugs celecoxib and nimesulide inhibit androgen receptor activity via induction of c-Jun in prostate cancer cells. Cancer Epidemiol. Biomark. Prev. 2003, 12, 769–774. [Google Scholar]
- Chen, C.-C.; Hsieh, T.-F.; Chang, C.-H.; Ma, W.L.; Hung, X.-F.; Tsai, Y.R.; Lin, M.-H.A.; Zhang, C.; Chang, C.; Shyr, C.-R. Androgen receptor promotes the migration and invasion of upper urinary tract urothelial carcinoma cells through the upregulation of MMP-9 and COX-2. Oncol. Rep. 2013, 30, 979–985. [Google Scholar] [CrossRef]
- Li, X.; Bechara, R.; Zhao, J.; McGeachy, M.J.; Gaffen, S.L. IL-17 receptor–based signaling and implications for disease. Nat. Immunol. 2019, 20, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Kim, J.-M.; Park, H.-S.; Yang, A.; Islam, C.; Lakatta, E.G.; Lin, L. AGE-RAGE signal generates a specific NF-κB RelA “barcode” that directs collagen I expression. Sci. Rep. 2016, 6, 18822. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.-L.; Wang, Y.-J.; Lu, Z.-H.; Tian, L.; Xia, Z.-Q.; Wang, K.-L.; Chen, T.; Wang, R.; Feng, Z.-Y.; Shi, G.-P.; et al. Wumei Wan attenuates angiogenesis and inflammation by modulating RAGE signaling pathway in IBD: Network pharmacology analysis and experimental evidence. Phytomedicine 2023, 111, 154658. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Luo, J.; Guo, J.; Yao, X.; Jing, X.; Guo, F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr. Cartil. 2020, 28, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wen, H.; Han, X.; Li, H. Phillygenin protects against osteoarthritis by repressing inflammation via PI3K/Akt/NF-κB signaling: In vitro and vivo studies. J. Funct. Foods 2021, 80, 104456. [Google Scholar] [CrossRef]
- Surh, Y.-J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Malec, V.; Gottschald, O.R.; Li, S.; Rose, F.; Seeger, W.; Hänze, J. HIF-1α signaling is augmented during intermittent hypoxia by induction of the Nrf2 pathway in NOX1-expressing adenocarcinoma A549 cells. Free Radic. Biol. Med. 2010, 48, 1626–1635. [Google Scholar] [CrossRef]
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R.; et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phytother. Res. 2022, 36, 299–322. [Google Scholar] [CrossRef]
- Ashraf, R.; Bhatti, H.N. Chapter 10—Stigmasterol. In A Centum of Valuable Plant Bioactives; Mushtaq, M., Anwar, F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 213–232. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From Preclinical Evidence to Potential Clinical Applications. Front. Pharmacol. 2021, 11, 599959. [Google Scholar] [CrossRef]
- Marahatha, R.; Gyawali, K.; Sharma, K.; Gyawali, N.; Tandan, P.; Adhikari, A.; Timilsina, G.; Bhattarai, S.; Lamichhane, G.; Acharya, A.; et al. Pharmacologic activities of phytosteroids in inflammatory diseases: Mechanism of action and therapeutic potentials. Phytother. Res. 2021, 35, 5103–5124. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Yang, J.; He, J.; Chen, X.; Zhang, H.; Jia, M.; Liu, K.; Jia, C.; Pan, Y.; Wei, J. Stigmasterol alleviates cerebral ischemia/reperfusion injury by attenuating inflammation and improving antioxidant defenses in rats. Biosci. Rep. 2020, 40, BSR20192133. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.S.; Mahomoodally, M.F.; Rengasamy, K.R.R. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef] [PubMed]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activities of the sesquiterpene fraction from Annona reticulata L. bark. Nat. Prod. Res. 2012, 26, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Nair, V.; Bang, W.Y.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of terpenes and polyphenols from three species of Oregano (Lippia graveolens, Lippia palmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2016, 187, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, Y.-Y.; Lee, J.; Jang, Y.-J.; Jang, H.-W. Chemical Composition, Antioxidant, and Anti-Inflammatory Activity of Essential Oil from Omija (Schisandra chinensis (Turcz.) Baill.) Produced by Supercritical Fluid Extraction Using CO2. Foods 2021, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Kobaek-Larsen, M.; Baatrup, G.; Notabi, M.K.; El-Houri, R.B.; Pipó-Ollé, E.; Christensen Arnspang, E.; Christensen, L.P. Dietary Polyacetylenic Oxylipins Falcarinol and Falcarindiol Prevent Inflammation and Colorectal Neoplastic Transformation: A Mechanistic and Dose-Response Study in A Rat Model. Nutrients 2019, 11, 2223. [Google Scholar] [CrossRef] [PubMed]
- Alfurayhi, R.; Huang, L.; Brandt, K. Pathways Affected by Falcarinol-Type Polyacetylenes and Implications for Their Anti-Inflammatory Function and Potential in Cancer Chemoprevention. Foods 2023, 12, 1192. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Truong, V.-L.; Jun, M.; Jeong, W.-S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors 2018, 44, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of Cell Protection by Heme Oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]

| Group | Retention Time | Compound | Molecular Formula | Peak Area (%) | |
|---|---|---|---|---|---|
| PFLH | PFLD | ||||
| Fatty acids and esters (n = 14) | 22.088 | Octanoic acid | C8H16O2 | 0.51 ± 0.05 | 0.97 ± 0.41 |
| 35.66 | Diisobutyl adipate | C14H26O4 | 0.54 ± 0.15 | 0.52 ± 0.24 | |
| 37.298 | Myristic acid | C14H28O2 | 0.40 ± 0.01 | - | |
| 41.518 | Palmitic acid | C16H32O2 | 10.72 ± 0.05 | 5.04 ± 1.95 | |
| 42.154 | Ethyl palmitate | C18H36O2 | 0.45 ± 0.17 | - | |
| 44.102 | Methyl linoleate | C19H34O2 | 0.15 ± 0.01 | - | |
| 44.751 | 10-trans,12-cis-Linoleic acid | C18H32O2 | 8.15 ± 0.12 | 2.85 ± 1.03 | |
| 44.901 | Linolenic acid | C18H30O2 | 7.73 ± 0.04 | 2.56 ± 0.95 | |
| 44.988 | Linoleic acid | C18H32O2 | - | 0.52 ± 0.19 | |
| 45.109 | Linoelaidic acid | C18H32O2 | 0.98 ± 0.01 | - | |
| 45.385 | Stearic acid | C18H36O2 | 2.28 ± 1.08 | 1.1 ± 0.43 | |
| 45.706 | 9,11-Octadecadienoic acid | C18H32O2 | 0.24 ± 0.09 | - | |
| 51.148 | 2-Palmitoylglycerol | C19H38O4 | 2.82 ± 0.17 | 2.51 ± 0.25 | |
| 53.871 | 2-Linoleoylglycerol | C21H38O4 | 2.90 ± 0.24 | - | |
| Furan derivatives (n = 1) | 19.981 | 3-Hydroxy-4,5-dimethylfuran-2(5H)-one | C6H8O3 | - | 0.95 ± 0.03 |
| Hydrocarbons and oxygenated hydrocarbons (n = 4) | 16.99 | Octanal | C8H16O | 0.18 ± 0.01 | 1.49 ± 0.12 |
| 36.497 | Hexadecanal | C16H32O | 0.67 ± 0.03 | - | |
| 39.961 | 3-Methylene-7,11-dimethyl-1-dodecene | C15H28 | - | 1.74 ± 0.02 | |
| 53.857 | Linoleyl alcohol | C18H34O | - | 1.40 ± 0.01 | |
| Polyphenols (n = 9) | 23.663 | 4-Vinylphenol | C8H8O | - | 0.47 ± 0.01 |
| 26.585 | 2-Methoxy-4-vinylphenol | C9H10O2 | - | 2.01 ± 0.87 | |
| 27.498 | 2,6-Dimethoxyphenol | C8H10O3 | - | 0.15 ± 0.05 | |
| 28.919 | Vanillin | C8H8O3 | - | 0.39 ± 0.19 | |
| 32.967 | 2,6-Dimethoxy-4-vinylphenol | C10H12O3 | - | 0.31 ± 0.11 | |
| 36.157 | 4-Allyl-2,6-dimethoxyphenol | C11H14O3 | - | 0.21 ± 0.02 | |
| 37.07 | 1′-Hydroxyeugenol | C10H12O3 | - | 1.79 ± 0.67 | |
| 42.173 | Sinapyl alcohol | C11H14O4 | - | 0.96 ± 0.34 | |
| 53.517 | 1,2-Bis(4-hydroxy-3-methoxyphenyl)ethylene | C16H16O4 | - | 0.34 ± 0.04 | |
| Polyacetylenes (n = 2) | 43.102 | Falcarinol | C17H24O | 7.36 ± 0.24 | - |
| 48.283 | Hexadeca-5,7,9,11-tetrayne-1,16-diol | C16H18O2 | - | 0.94 ± 0.01 | |
| Sterol derivatives (n = 2) | 64.481 | Stigmasterol | C29H48O | 6.53 ± 0.07 | 4.00 ± 1.47 |
| 66.033 | (3beta,5alpha)-Stigmasta-7,16-dien-3-ol | C29H48O | 2.36 ± 0.09 | 1.32 ± 0.46 | |
| Terpenes (n = 28) | 28.459 | Ylangene | C15H24 | 0.06 ± 0.02 | - |
| 28.628 | Copaene | C15H24 | 0.04 ± 0.01 | - | |
| 29.935 | gamma-Elemene | C15H24 | 0.12 ± 0.01 | - | |
| 31.2 | alpha-Bergamotene | C15H24 | 1.65 ± 0.04 | - | |
| 31.431 | (−)-Germacrene D | C15H24 | 0.47 ± 0.08 | - | |
| 32.153 | (+)-gamma-Cadinene | C15H24 | 0.07 ± 0.01 | - | |
| 32.409 | (−)-alpha-Gurjunene | C15H24 | 0.07 ± 0.01 | - | |
| 32.633 | Actinidiolide, dihydro- | C11H16O2 | 0.32 ± 0.01 | 0.23 ± 0.01 | |
| 33.998 | Caryophyllene oxide | C15H24O | 0.18 ± 0.02 | - | |
| 34.885 | Alismol | C15H24O | 0.82 ± 0.01 | - | |
| 35.304 | Opposita-4(15),7(11)-dien-1beta-ol | C15H24O | 0.48 ± 0.04 | - | |
| 35.513 | Alloisolongifolene alcohol | C15H24O | 0.47 ± 0.01 | - | |
| 36.954 | Aromadendrene | C15H24 | 0.10 ± 0.01 | - | |
| 37.302 | 3-Bornanone, oxime | C10H17NO | - | 1.53 ± 0.14 | |
| 37.683 | (4S,5R)-5-Hydroxycaryophyll-8(13)-ene-4,12-epoxide | C15H24O2 | 0.26 ± 0.01 | ||
| 37.96 | Loliolide | C11H16O3 | - | 2.23 ± 1.09 | |
| 38.109 | Alismoxide | C15H26O2 | - | 1.14 ± 0.57 | |
| 39.075 | Neophytadiene | C20H38 | 3.24 ± 0.01 | 3.87 ± 1.47 | |
| 39.18 | 6,10,14-Trimethylpentadecan-2-one | C18H36O | 1.02 ± 0.07 | - | |
| 39.963 | (Z)-1,3-Phytadiene | C20H38 | 1.03 ± 0.01 | - | |
| 40.2 | beta-Oplopenone | C15H24O | - | 1.93 ± 1.03 | |
| 40.792 | Neoclovene oxide | C15H24O | - | 0.85 ± 0.01 | |
| 41.646 | (−)-Isolongifolol methyl ether | C16H28O | - | 2.70 ± 0.21 | |
| 41.958 | Ledol | C15H26O | - | 0.72 ± 0.01 | |
| 42.218 | (R)-1-(7,7-Dimethyl-1,3,4,5,6,7-hexahydroisobenzofuran-1alpha-yl)-2-propanol | C13H22O2 | - | 1.00 ± 0.01 | |
| 43.066 | Caryophyllenol II | C15H24O | - | 0.79 ± 0.30 | |
| 44.4 | Phytol | C20H40O | 5.49 ± 2.65 | 2.08 ± 1.00 | |
| 55.615 | Squalene | C30H50 | 1.66 ± 0.45 | - | |
| Tocols (n = 4) | 57.714 | delta-Tocopherol | C27H46O2 | 0.62 ± 0.08 | - |
| 59.26 | beta-Tocopherol | C28H48O2 | 0.98 ± 0.09 | - | |
| 61.126 | DL-alpha-Tocopherol | C29H50O2 | 0.54 ± 0.25 | - | |
| 69.196 | alpha-Tocopherol | C29H50O2 | 0.96 ± 0.01 | - | |
| Others (n = 7) | 21.773 | 4-Ethoxy-4-oxobutanoic acid | C6H10O4 | - | 1.05 ± 0.40 |
| 23.134 | 2,3-Dihydrothiophene | C4H6S | - | 0.54 ± 0.04 | |
| 24.921 | Malic acid | C4H6O5 | - | 1.29 ± 0.01 | |
| 31.125 | Levoglucosan | C6H10O5 | - | 0.72 ± 0.01 | |
| 36.4 | 4,3′-Difluoro-4′-methoxybiphenyl | C13H10F2O | 0.67 ± 0.04 | - | |
| 38.655 | Indole-3-carboxaldehyde | C9H7NO | - | 0.99 ± 0.01 | |
| 40.602 | 4,6,6-Trimethyl-2-(3-methylbuta-1,3-dienyl)-3-oxatricyclo [5.1.0.0(2,4)]octane | C15H22O | - | 1.11 ± 0.43 | |
| Antioxidant Activity | PFLH | PFLD |
|---|---|---|
| DPPH (IC50, mg/mL) | 1.22 ± 0.03 | 0.73 ± 0.02 * |
| ABTS+ (IC50, mg/mL) | 0.67 ± 0.06 | 0.14 ± 0.02 ** |
| Superoxide radical (IC50, mg/mL) | 0.11 ± 0.04 | 1.11 ± 0.35 ** |
| Hydrogen peroxide (IC50, mg/mL) | 0.26 ± 0.03 | 0.31 ± 0.04 |
| Hydroxyl radical (IC50, mg/mL) | 4.91 ± 2.25 | 0.27 ± 0.06 ** |
| Nitric oxide (IC50, mg/mL) | 6.01 ± 1.57 | 0.39 ± 0.05 * |
| FRAP (mg TE/g dried extract) | 17.89 ± 0.25 | 29.55 ± 0.44 *** |
| PFRAP (mg TE/g dried extract) | 22.18 ± 1.03 | 32.14 ± 2.68 ** |
| TAC (mg TE/g dried extract) | 9.54 ± 0.17 | 130.59 ± 1.75 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rarison, R.H.G.; Truong, V.-L.; Yoon, B.-H.; Park, J.-W.; Jeong, W.-S. Antioxidant and Anti-Inflammatory Mechanisms of Lipophilic Fractions from Polyscias fruticosa Leaves Based on Network Pharmacology, In Silico, and In Vitro Approaches. Foods 2023, 12, 3643. https://doi.org/10.3390/foods12193643
Rarison RHG, Truong V-L, Yoon B-H, Park J-W, Jeong W-S. Antioxidant and Anti-Inflammatory Mechanisms of Lipophilic Fractions from Polyscias fruticosa Leaves Based on Network Pharmacology, In Silico, and In Vitro Approaches. Foods. 2023; 12(19):3643. https://doi.org/10.3390/foods12193643
Chicago/Turabian StyleRarison, Razanamanana H. G., Van-Long Truong, Byoung-Hoon Yoon, Ji-Won Park, and Woo-Sik Jeong. 2023. "Antioxidant and Anti-Inflammatory Mechanisms of Lipophilic Fractions from Polyscias fruticosa Leaves Based on Network Pharmacology, In Silico, and In Vitro Approaches" Foods 12, no. 19: 3643. https://doi.org/10.3390/foods12193643
APA StyleRarison, R. H. G., Truong, V.-L., Yoon, B.-H., Park, J.-W., & Jeong, W.-S. (2023). Antioxidant and Anti-Inflammatory Mechanisms of Lipophilic Fractions from Polyscias fruticosa Leaves Based on Network Pharmacology, In Silico, and In Vitro Approaches. Foods, 12(19), 3643. https://doi.org/10.3390/foods12193643





