Carthamus tinctorius L. Seed and Taraxacum coreanum Attenuate Oxidative Stress Induced by Hydrogen Peroxide in SH-SY5Y Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Sample Preparation
2.1.2. Reagents
2.2. DPPH Radical Scavenging Activity
2.3. Hydroxyl Radical (·OH) Scavenging Activity
2.4. Culture and Treatment of Cells
2.5. Cell Viability
2.6. Hoechst 33,342 Staining
2.7. LDH Release
2.8. ROS Production
2.9. NO Generation
2.10. Western Blotting
2.11. Statistic Evaluation
3. Results
3.1. Effects of CTS, TC, and Their Combination on Free Radical Scavenging Activities
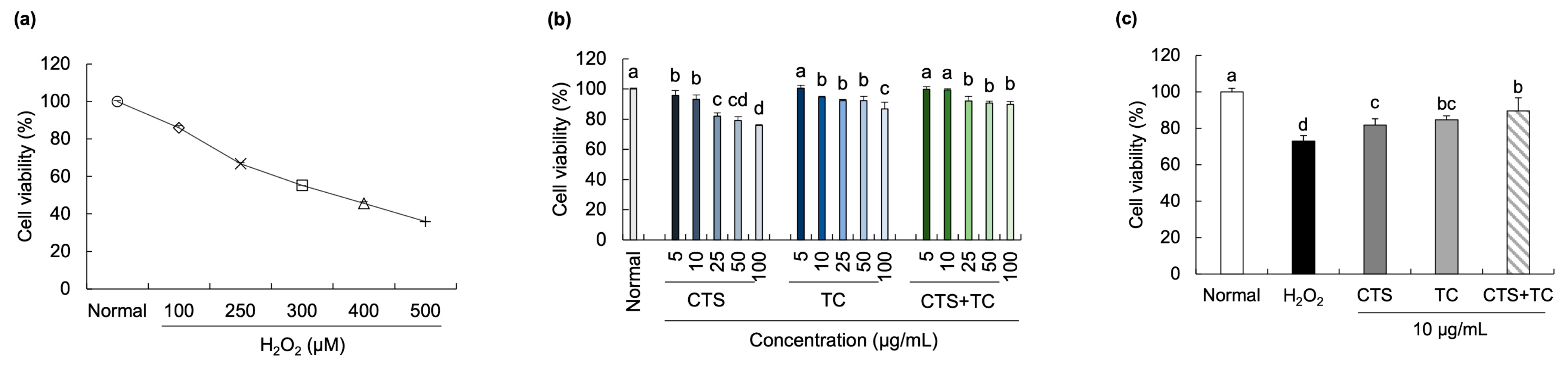
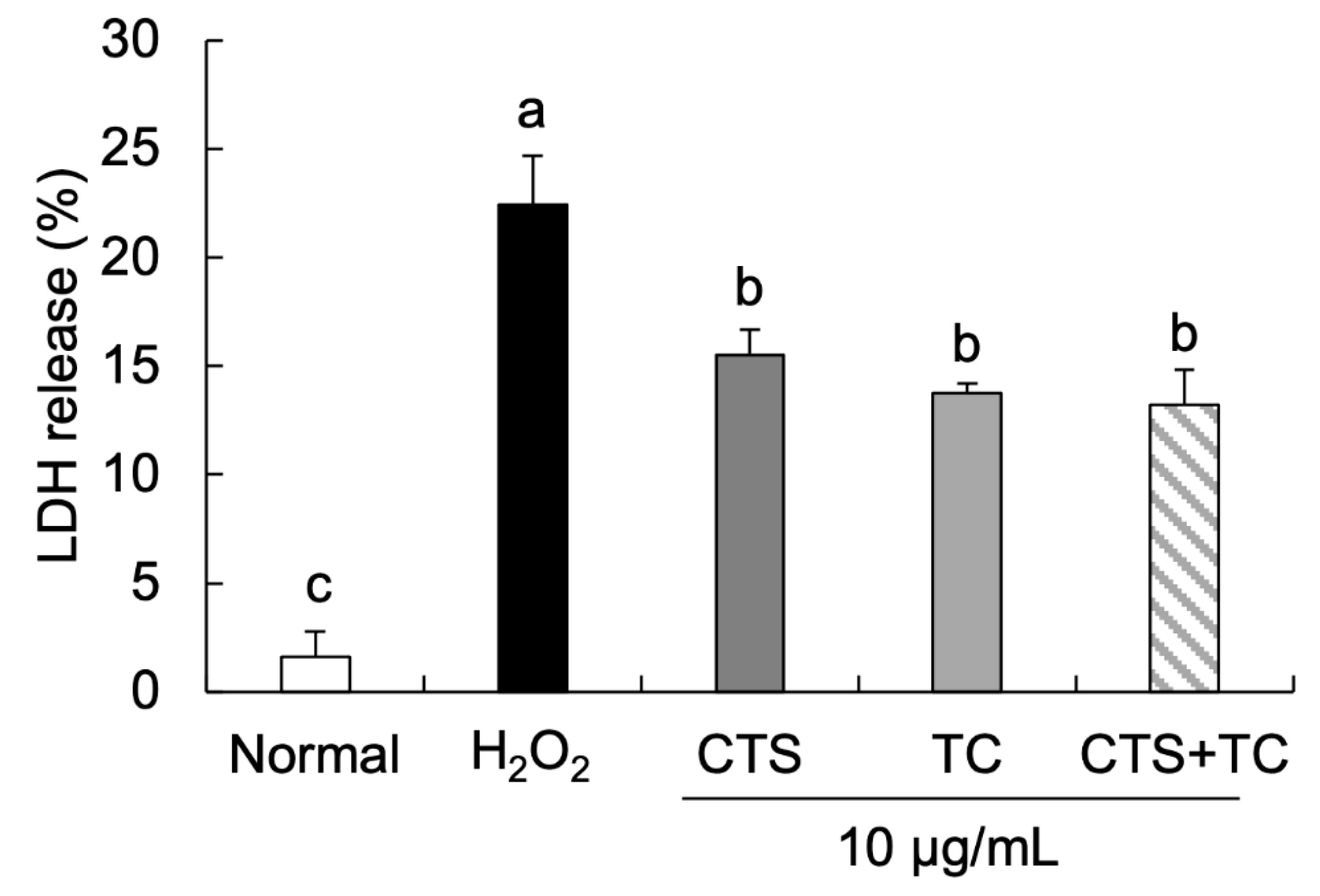
3.2. Effects of CTS, TC, and Their Combination on Neuronal Cell Damage in H2O2-Stimulated SH-SY5Y Cells
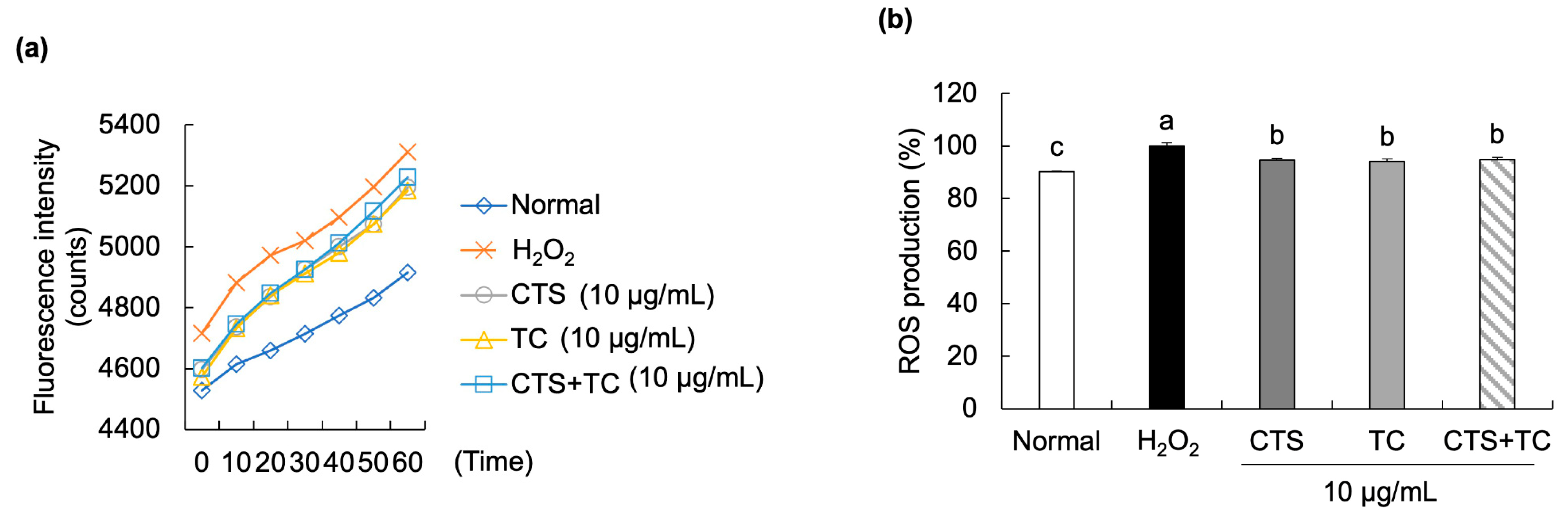
3.3. Effects of CTS, TC, and Their Combination on ROS Production in H2O2-Stimulated SH-SY5Y Cells
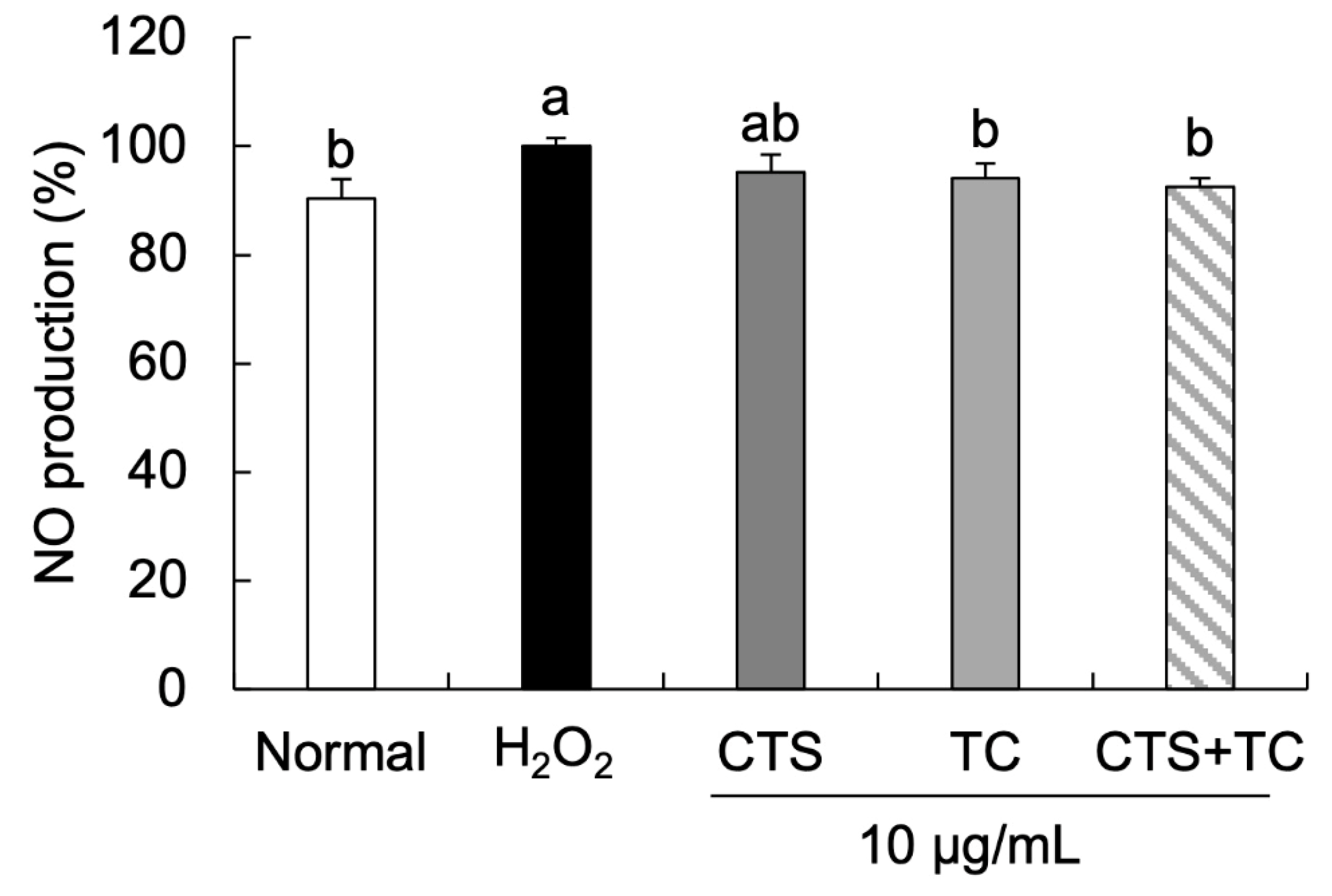
3.4. Effects of CTS, TC, and Their Combination on NO Generation in H2O2-Stimulated SH-SY5Y Cells
3.5. Effects of CTS, TC, and Their Combination on Protein Expression of Antioxidant Enzymes in H2O2-Treated SH-SY5Y Cells
3.6. Effects of CTS, TC, and Their Combination on Bax and Bcl-2 Protein Expressions in H2O2-Stimulated SH-SY5Y Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irshad, M.; Chaudhuri, P.S. Oxidant-antioxidant system: Role and significance in human body. Indian J. Exp. Biol. 2002, 40, 1233–1239. [Google Scholar] [PubMed]
- Wei, Y.-H.; Lu, C.-Y.; Wei, C.-Y.; Ma, Y.-S.; Lee, H.-C. Oxidative stress in human aging and mitochondrial disease-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chin. J. Physiol. 2001, 44, 1–12. [Google Scholar]
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 2019, 18, 100173. [Google Scholar] [CrossRef]
- Dumont, M.; Beal, M.F. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic. Biol. Med. 2011, 51, 1014–1026. [Google Scholar] [CrossRef]
- Landete, J.M. Dietary intake of natural antioxidants: Vitamins and polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef]
- Kang, G.-H.; Chang, E.-J.; Park, S.-W. Antioxidative activity of phenolic compounds in roasted safflower (Carthamus tinctorius L.) seeds. J. Food Sci. Nutr. 1999, 4, 221–225. [Google Scholar]
- Asgarpanah, J.; Kazemivash, N. Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L. Chin. J. Integr. Med. 2013, 19, 153–159. [Google Scholar] [CrossRef]
- Kim, M.-J.; Bae, G.-S.; Choi, S.B.; Jo, I.-J.; Kim, D.-G.; Shin, J.-Y.; Lee, S.-K.; Kim, M.-J.; Park, S.-J.; Song, H.-J. The anti-inflammatory effect of Taraxacum coreanum on lipopolysaccharide induced inflammatory response on RAW 264.7 cells. Korean J. Herbol. 2014, 29, 21–26. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, M.J.; Kwon, D.Y.; Kang, E.S.; Kang, S.; Park, S. Gastroprotective actions of Taraxacum coreanum Nakai water extracts in ethanol-induced rat models of acute and chronic gastritis. J. Ethnopharmacol. 2017, 208, 84–93. [Google Scholar] [CrossRef]
- Mo, E.J.; Ahn, J.H.; Jo, Y.H.; Kim, S.B.; Hwang, B.Y.; Lee, M.K. Inositol derivatives and phenolic compounds from the roots of Taraxacum coreanum. Molecules 2017, 22, 1349. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, A.Y.; Park, C.H.; Shin, Y.S.; Cho, E.J. Protective effect of Carthamus tinctorius L. seed on oxidative stress and cognitive impairment induced by chronic alcohol consumption in mice. Food Sci. Biotechnol. 2018, 27, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Park, C.H.; Cho, E.J.; Kim, J.H.; Seo, W.T. Protective effects of Carthamus tinctorius L. seed on C6 glial cells treated with ethanol. J. Appl. Biol. Chem. 2021, 64, 69–74. [Google Scholar] [CrossRef]
- Yoon, C.-S.; Ko, W.; Lee, D.-S.; Kim, D.-C.; Kim, J.; Choi, M.; Beom, J.S.; An, R.-B.; Oh, H.; Kim, Y.-C. Taraxacum coreanum protects against glutamate-induced neurotoxicity through heme oxygenase-1 expression in mouse hippocampal HT22 cells. Mol. Med. Rep. 2017, 15, 2347–2352. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.; Bernhardt, T.; Moeller, H.-J.; Heuser, I.; Frölich, L. Combination therapy in Alzheimer’s disease: A review of current evidence. CNS Drugs 2004, 18, 827–844. [Google Scholar] [CrossRef]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic effects of Chinese herbal medicine: A comprehensive review of methodology and current research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef]
- He, M.T.; Kim, J.-H.; Kim, J.H.; Park, C.H.; Cho, E.J. Combination of Carthamus tinctorius L. seed and Taraxacum coreanum exerts synergistic effects on learning and memory function by regulating metabolism of amyloid beta in mice. J. Funct. Foods 2020, 72, 104048. [Google Scholar] [CrossRef]
- Kim, J.H.; He, M.T.; Kim, M.J.; Park, C.H.; Lee, J.Y.; Shin, Y.S.; Cho, E.J. Protective effects of combination of Carthamus tinctorius L. seed and Taraxacum coreanum on scopolamine-induced memory impairment in mice. Korean J. Med. Crop Sci. 2020, 28, 85–94. [Google Scholar] [CrossRef]
- Hatano, T.; Edamatsu, R.; Hiramatsu, M.; MORI, A.; Fujita, Y.; Yasuhara, T.; Yoshida, T.; Okuda, T. Effects of the interaction of tannins with co-existing substances. VI.: Effects of tannins and related polyphenols on superoxide anion radical, and on 1, 1-Diphenyl-2-picrylhydrazyl radical. Chem. Pharm. Bull. 1989, 37, 2016–2021. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C. Ferrous-salt-promoted damage to deoxyribose and benzoate. The increased effectiveness of hydroxyl-radical scavengers in the presence of EDTA. Biochem. J. 1987, 243, 709–714. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing cell death by nuclear staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087205. [Google Scholar] [CrossRef]
- Davinelli, S.; Di Marco, R.; Bracale, R.; Quattrone, A.; Zella, D.; Scapagnini, G. Synergistic effect of L-Carnosine and EGCG in the prevention of physiological brain aging. Curr. Pharm. Des. 2013, 19, 2722–2727. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.Y.; Kim, M.R.; Yu, S.H.; Kim, M.J.; Shim, K.-S.; Shin, E.; Lee, J.J.; Lee, Y.C. Combined extract of Vitis vinifera L. And Centella asiatica synergistically attenuates oxidative damage induced by hydrogen peroxide in human umbilical vein endothelial cells. Prev. Nutr. Food Sci. 2020, 25, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Al-Surmi, N.; El-Dengawy, R.; Khalifa, A. Chemical and nutritional aspects of some safflower seed varieties. Int. J. Food Process. Technol. 2016, 7, 585. [Google Scholar]
- Lee, A.Y.; Choi, J.M.; Lee, S.; Kim, H.Y.; Lee, S.; Cho, E.J. The protective effects of the ethyl acetate fraction and flavonoids from Taraxacum coreanum against oxidative stress in neuronal cells induced by hydrogen peroxide and amyloid beta. Korean J. Pharmacogn. 2013, 44, 263–268. [Google Scholar]
- He, M.; Kim, J.; Park, C.; Cho, E. Herbal mixture of Carthamus tinctorius L. seed and Taraxacum coreanum attenuates amyloid beta-induced cognitive dysfunction in vivo. Foods 2022, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Park, C.; Shin, Y.; Kim, J.; Cho, E. N-Feruloyl serotonin attenuates neuronal oxidative stress and apoptosis in Aβ25–35-treated human neuroblastoma SH-SY5Y Cells. Molecules 2023, 28, 1610. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef]
- Foti, M.C. Use and abuse of the DPPH• radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxid. Med. Cell. Longev. 2011, 2011, 809696. [Google Scholar] [CrossRef]
- Lee, M.H.; Kang, H.; Lee, K.; Yang, G.; Ham, I.; Bu, Y.; Kim, H.; Choi, H.-Y. The aerial part of Taraxacum coreanum extract has an anti-inflammatory effect on peritoneal macrophages in vitro and increases survival in a mouse model of septic shock. J. Ethnopharmacol. 2013, 146, 1–8. [Google Scholar] [CrossRef]
- Lee, J.-J.; Oh, H.-K. Nutritional composition and antioxidative activity of different parts of Taraxacum coreanum and Taraxacum officinale. J. Korean Soc. Food Cult. 2015, 30, 362–369. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. In Cancer Cell Culture: Methods and Protocols; Cree, I., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 237–245. [Google Scholar]
- Allen, S.; Sotos, J.; Sylte, M.; Czuprynski, C. Use of Hoechst 33342 staining to detect apoptotic changes in bovine mononuclear phagocytes infected with Mycobacterium avium subsp. paratuberculosis. Clin. Diagn. Lab. Immunol. 2001, 8, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Bucevičius, J.; Lukinavičius, G.; Gerasimaitė, R. The use of hoechst dyes for DNA staining and beyond. Chemosensors 2018, 6, 18. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb. Protoc. 2018, 2018, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Xie, J.; Wang, Y.; Wang, S.; Wu, S.; Wang, Q.; Ding, H. Protective effects of aloe-emodin on scopolamine-induced memory impairment in mice and H2O2-induced cytotoxicity in PC12 cells. Bioorg. Med. Chem. Lett. 2014, 24, 5385–5389. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Liu, H.; Mao, J.; Chu, W.; Li, Q.; Alvarez, P.J.J.; Qu, X.; Zhu, D. Photochemistry of dissolved black carbon released from biochar: Reactive oxygen species generation and phototransformation. Environ. Sci. Technol. 2016, 50, 1218–1226. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. In Advanced Protocols in Oxidative Stress II; Armstrong, D., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 57–72. [Google Scholar]
- Nirmaladevi, D.; Venkataramana, M.; Chandranayaka, S.; Ramesha, A.; Jameel, N.M.; Srinivas, C. Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cell. Mol. Neurobiol. 2014, 34, 973–985. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Lee, A.Y.; Lee, S.; Kim, H.Y.; Lee, S.; Cho, E.J. Anti-inflammatory effects of luteolin and luteoloside from Taraxacum coreanum in RAW264.7 macrophage cells. Appl. Biol. Chem. 2016, 59, 747–754. [Google Scholar] [CrossRef]
- Brown, G.C. Nitric oxide and neuronal death. Nitric Oxide 2010, 23, 153–165. [Google Scholar] [CrossRef]
- Hussain, A.; Saikia, V.; Ramteke, A. Nitric oxide and modulatory effects of the root extracts of phlogacanthus tubiflorus against oxidative stress induced by hydrogen peroxide. Free Radic. Antioxid. 2012, 2, 9–12. [Google Scholar] [CrossRef]
- Bayr, H. Reactive oxygen species. Crit. Care Med. 2005, 33, S498–S501. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef]
- Nimrouzi, M.; Ruyvaran, M.; Zamani, A.; Nasiri, K.; Akbari, A. Oil and extract of safflower seed improve fructose induced metabolic syndrome through modulating the homeostasis of trace elements, TNF-α and fatty acids metabolism. J. Ethnopharmacol. 2020, 254, 112721. [Google Scholar] [CrossRef]
- Lee, H.-H.; Lee, S.-Y. Cytotoxic and antioxidant effects of Taraxacum coreanum Nakai. and T. officinale WEB. extracts. Korean J. Crop Sci. 2008, 16, 79–85. [Google Scholar]
- Yoon, J.-S.; Song, S.-Y.; Cheong, M.-J.; Kim, D.-S.; Lee, H.-H. The effect of the hot water extract from Taraxacum coreanum Nakai on hepatocarcinogenesis induced by N-nitrosodiethylamine in rats. Korean J. Pharmacogn. 2014, 45, 62–68. [Google Scholar]
- Walensky, L. BCL-2 in the crosshairs: Tipping the balance of life and death. Cell Death Differ. 2006, 13, 1339–1350. [Google Scholar] [CrossRef]
- Raisova, M.; Hossini, A.M.; Eberle, J.; Riebeling, C.; Orfanos, C.E.; Geilen, C.C.; Wieder, T.; Sturm, I.; Daniel, P.T. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J. Investig. Dermatol. 2001, 117, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.R.; Zhang, L.; Hu, J.J.; Sun, L.; Du, G.H. Neuroprotective effects of tetramethylpyrazine on hydrogen peroxide-induced apoptosis in PC12 cells. Cell Biol. Int. 2007, 31, 438–443. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, A.Y.; Kim, J.H.; Seong, S.H.; Jang, G.Y.; Cho, E.J.; Choi, J.S.; Kwon, J.; Kim, Y.O.; Lee, S.W.; et al. Protective effect of safflower seed on cisplatin-induced renal damage in mice via oxidative stress and apoptosis-mediated pathways. Am. J. Chin. Med. 2018, 46, 157–174. [Google Scholar] [CrossRef] [PubMed]



| Concentration (µg/mL) | DPPH Radical Scavenging Activity (%) | ||
|---|---|---|---|
| CTS | TC | CTS + TC | |
| 50 | 2.43 ± 4.34 c | 10.31 ± 4.28 c | 11.61 ± 3.44 d |
| 100 | 13.83 ± 3.27 b | 20.73 ± 3.17 b | 38.21 ± 3.42 c |
| 250 | 55.17 ± 10.88 a | 63.31 ± 5.71 a | 78.36 ± 5.50 b |
| 500 | 60.29 ± 7.35 a | 64.97 ± 4.65 a | 86.67 ± 5.92 a |
| IC50 (µg/mL) | 302.63 | 239.80 | 138.34 |
| Concentration (µg/mL) | OH Radical Scavenging Activity (%) | ||
|---|---|---|---|
| CTS | TC | CTS + TC | |
| 50 | 39.41 ± 1.27 c | 41.41 ± 3.67 c | 54.72 ± 0.71 c |
| 100 | 43.38 ± 3.31 bc | 46.86 ± 4.16 b | 55.87 ± 4.63 c |
| 250 | 45.81 ± 1.86 b | 51.67 ± 0.96 b | 64.99 ± 1.81 b |
| 500 | 52.19 ± 4.28 a | 58.46 ± 3.12 a | 78.87 ± 3.95 a |
| IC50 (µg/mL) | 400.66 | 167.34 | 44.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.T.; Park, C.H.; Shin, Y.S.; Kim, J.H.; Cho, E.J. Carthamus tinctorius L. Seed and Taraxacum coreanum Attenuate Oxidative Stress Induced by Hydrogen Peroxide in SH-SY5Y Cells. Foods 2023, 12, 3617. https://doi.org/10.3390/foods12193617
He MT, Park CH, Shin YS, Kim JH, Cho EJ. Carthamus tinctorius L. Seed and Taraxacum coreanum Attenuate Oxidative Stress Induced by Hydrogen Peroxide in SH-SY5Y Cells. Foods. 2023; 12(19):3617. https://doi.org/10.3390/foods12193617
Chicago/Turabian StyleHe, Mei Tong, Chan Hum Park, Yu Su Shin, Ji Hyun Kim, and Eun Ju Cho. 2023. "Carthamus tinctorius L. Seed and Taraxacum coreanum Attenuate Oxidative Stress Induced by Hydrogen Peroxide in SH-SY5Y Cells" Foods 12, no. 19: 3617. https://doi.org/10.3390/foods12193617
APA StyleHe, M. T., Park, C. H., Shin, Y. S., Kim, J. H., & Cho, E. J. (2023). Carthamus tinctorius L. Seed and Taraxacum coreanum Attenuate Oxidative Stress Induced by Hydrogen Peroxide in SH-SY5Y Cells. Foods, 12(19), 3617. https://doi.org/10.3390/foods12193617





