Abstract
Obesity is a complex medical condition mainly caused by eating habits, genetics, lifestyle, and medicine. The present study deals with traditional diets like the Mediterranean diet, Nordic diet, African Heritage diet, Asian diet, and DASH, as these are considered to be sustainable diets for curing obesity. However, the bioavailability of phytonutrients consumed in the diet may vary, depending on several factors such as digestion and absorption of phytonutrients, interaction with other substances, cooking processes, and individual differences. Hence, several phytochemicals, like polyphenols, alkaloids, saponins, terpenoids, etc., have been investigated to assess their efficiencies and safety in the prevention and treatment of obesity. These phytochemicals have anti-obesity effects, mediated via modulation of many pathways, such as decreased lipogenesis, lipid absorption, accelerated lipolysis, energy intake, expenditure, and preadipocyte differentiation and proliferation. Owing to these anti-obesity effects, new food formulations incorporating these phytonutrients were introduced that can be beneficial in reducing the prevalence of obesity and promoting public health.
1. Introduction
Obesity is a condition where the body experiences metabolic changes that may cause numerous types of stomach and digestive issues characterized by altered gut flora, inflammation, and intestinal barrier disruption [1]. Obesity can also develop due to numerous interactions involving inheritable, social, and environmental factors. The prevalence of obesity is affected by dietary selection, urban development, and lifestyle [2]. Obesity has a more detrimental effect on longevity in young children and adolescents than adults.
To define an individual of both sexes (male and female) at range of its age to be obese is usually based on body mass index (BMI), where WHO considers an ideal BMI to be 18.5–24.9 by engaging everyday, ideally vigorous, physical exercise (WHO assessed on 26 June 2023). Obesity is associated with an increased chance of developing several well-known cancers, especially liver, pancreatic, kidney, gallbladder, uterine, breast, and colorectal cancer [3]. Obesity is thought to be associated with 4–8% of cancer cases [3]. The prevalence of obesity was shown in the case of meta-analysis investigation, where nearly 37 papers were included and observed that the elevated childhood body mass index (BMI) was linked to an increased risk of adult diabetes (odds ratio OR 1.70; 95%, confidence interval CI 1.30–2.22), coronary heart disease (odds ratio OR 1.20; 9%), and several malignancies, excluding breast cancer or stroke [4]. However, compared to the overall population of physically unfit, obese people, up to 30% of patients classified as obese were found to have lower gut fat content, thinner carotid intima-media, and insulin sensitivity comparable to healthy average-weight humans. The condition known as metabolic syndrome (clustering of abdominal obesity, dyslipidemia, hyperglycemia, and hypertension) has an average prevalence of 31% and increases the risk of cardiovascular disease, cerebrovascular disease, and overall mortality by 1.5 to 2 times, respectively [5]. Relying on plant-based foods, such as fruits, vegetables, and fiber-rich cereals and breads, includes a wide range of phytonutrients, such as vitamins, carotenoids, polyphenols, curcuminoids, polyunsaturated fatty acids, proteins, peptides, dietary fibers, oligosaccharides, and minerals. As a result, phytonutrients in the diet are beneficial and necessary for human health for maintaining a healthy lifestyle [6,7].
Nutrient bioavailability varies depending on a wide range of factors, including the different matrices in which nutrients are present with their chemical nature, binding form, additional foods and their constituents that may enhance or block absorption, post-absorption metabolization, host-associated variables such as health status, inheritance, and age-related habits, as well as individual factors [8]. The bioavailability of the phytonutrient component is also affected by the dietary matrix material and its dispersion through matrix-based alterations during digestion, absorption, metabolism, and distribution throughout the body. It effectively increases the lower oral bioavailability of weakly water-soluble medicines via lipid-based drug delivery systems (LbDDSs) [8]. To understand the pharmacokinetic, in vitro lipolysis analysis and SPECT/CT, in vivo imaging experiments were used to examine the oral absorption of fenofibrate (FF) (natural source from beans, apricots, and apples) from LbDDSs in rodents. Utilizing SPECT/CT imaging, it was demonstrated that even 24 h after delivery, the animal’s stomach contained large levels of the drug and lipid formulation despite absorption improvement, which can be one of the approaches for providing nutrients to the body in order to maximize the health advantages of phytonutrients [9]. The cell membrane’s ability to absorb nutrients also depends on their solubility; as a result, nutrients must be in their soluble form, whereas, the case of releasing insoluble salts by the nutrients, such as chelates with phytic acid, gradually reduces their bioavailability [10,11]. For instance, phytic acid in millet can potentially bind to calcium, zinc, and iron to generate insoluble complexes [12]. Thus, it necessitates that the nutrients be in their soluble form for absorption.
The combination of dietary fat consumption, hormonal changes, and other chronic diseases is one example of the many variables that lead to obesity. Obesity does not depend on the cause led by misinformation and lack of self-control in an individual over their dietary habits. Obesity may be caused due to external or internal factors depending on several factors, including social, physical, and environmental. Environmental factors, such as the availability of food, poor diets, and physical inactivity, management factors (such as the inability to control one’s lifestyle and unhealthy routines), and time factors (such as eating and sleeping patterns) are primarily the reason for the rising rates of obesity [13,14].
Food and nutrition are the primary focuses of obesity therapy since maintaining a healthy weight can be difficult. To prevent obesity, one must continue to modify their intake of nutrients, including protein and legumes, as well as oligosaccharides, polysaccharides, and fiber. Another approach is incorporating vital nutrients into the diet instead of lowering the energy associated with a limited amount of food items [14,15]. Encouraging healthy living practices requires more physical activity that reduces calorie intake and fat content. Since chronic disorders can make obesity worse, they can be controlled by maintaining the homeostasis of our body [16]. When chronic disease prevalence rates rise, the probability of undergoing productivity decreases and growing well-being expenses increase [17].
Current research is based on secondary metabolites with exhibited anti-obesity effects, including phenolics, flavonoids, and terpenoids, along with nobiletin from citrus peel, curcumin from turmeric, and anthocyanins from Hibiscus sabdariffa that act as powerful antioxidants [18], which may be of great importance in controlling lipid profiles and preventing severe consequences of lipid disorders. A diet high in fruits and vegetables, low in fat, and high in fiber can enhance lipid metabolism and help to manage several disorders since plant-based nutrients provide a wide variety of therapeutic effects [19]. A significant relationship between alterations in body composition, disease prevalence, and an increase in phytonutrient intake, indicating phytonutrients, as well as carbohydrates, proteins, and lipids, can help individuals achieve these advantages. These are the prime factors in reducing the prevalence of diseases like obesity [20]. Enhancing the phytonutrient content of fruits, vegetables, and other nutrients along with our understanding of their needs, consumer preferences, and mechanisms of action can be one of the other strategies to control obesity. These findings help them compete better with processed junk food, which is a major contributor to world obesity. As estimated by the World Health Organization (WHO), 600 million individuals worldwide are considered obese individuals [21]. The enhancement of phytonutrients is the focus of several studies that aim to manage the worldwide obesity crisis that drives the growing rate of obesity [22]. The accumulation of excess or abnormal fat, known as obesity, makes it difficult to maintain a healthy weight. A surplus of macronutrients in adipose tissues causes them to produce inflammatory mediators, including tumor necrosis factor and interleukin-6, and decreases the generation of adiponectin, which increases the risk of oxidative stress and a pro-inflammatory state [23]. According to research, phytochemicals can alter pro-inflammatory genes that work by limiting their expression while activating anti-inflammatory genes. This differential gene regulation is regulated by epigenetic changes. By modifying the amounts of pro-inflammatory microRNAs (miRNAs), particularly those that are elevated after nuclear factor-B (NF-B) activation, the researchers in this study demonstrate how phytochemicals might decrease inflammation. These phytochemicals also alter the main inflammatory signaling pathways, such as nuclear factor erythroid 2-related factor 2 (Nrf2), signal transducers and activators of transcription (STAT), and mitogen-activated protein kinases (MAPKs) [24]. The discussion is based on the current scenario of phytonutrients mentioned and that they can potentially act against obesity.
2. Phytonutrients in Regional Diet
2.1. Mediterranean Diet
In the early 1960s, the Mediterranean diet (MD) was described using the ensuing general characteristics. A large variety of plant-based foods, particularly those that are organically produced and abundant in nutrients (such as fruit, vegetables, bread, other cereals, potatoes, beans, nuts, and seeds); fresh fruit as the conventional standard meal; dairy-based products (mainly cheese and yogurt) that are often consumed in small to moderate amounts; whereas, olive oil as the primary source of fat and consumption of fish and poultry in small to moderate amounts are included in this diet [25]. This diet had modest levels of saturated fats as compared to the total fat intake, around 7–8%; nevertheless, given the area analyzed, total fat consumption varied significantly from less than 20% to more than 35% of the total calorie consumption. The Mediterranean diet (MD), which emphasizes a wide variety of plant foods and high polyphenol intake, is known to lower the risk of obesity through dietary patterns [25]. The essential consumption of extra-virgin olive oil (EVOO), vegetables, legumes, fruits, and whole-grain cereals has an impact on body weight by reducing lipid metabolism, oxidative stress, platelet aggregation, and coagulation [26]. The Mediterranean diet resembles some aspects of the traditional dietary practices of the various countries bordering the Mediterranean Sea [27] (Table 1).
The Mediterranean diet can assist in maintaining a healthy diet [28]. The biological components of EVOO that give it its therapeutic properties include fatty acids related to triacylglycerols, free fatty acids, and mono- and diacylglycerols. A variety of lipids like hydrocarbons, sterols, aliphatic alcohols, and tocopherols, as well as volatile compounds like polyphenols have the ability to lower the levels of oxidative stress, overweight and obese total fat in the body, and cholesterol that form in response to insect wounds in olive trees [29]. The main source of olive polyphenols is olive leaves, which are found in various olive tree parts and have the highest oxidative and scavenger capabilities. Fresh olive juice, or EVOO, is only produced by mechanical and physical methods [30]. In contrast, the smallest portion (about 2% of the weight) comprises an extensive set of little substances containing over 230 chemical compounds (aliphatic and triterpene alcohols, sterols, hydrocarbons, volatile compounds, and antioxidants) [31]. It primarily comprises mono- and polyunsaturated fatty acids, accounting for over 98% of the overall weight. Oleuropeoides, flavones, flavonols, flavan-3-ols, and substituted phenols are among the five distinct compounds in olive leaves, including phenolic. Polyphenolic compounds are particularly abundant in EVOO and have a range of advantageous biological effects, including anti-oxidative, anti-inflammatory, antiproliferative, and finally, anti-obesity and hypoglycemic action [32].
2.2. Nordic Diet
The Nordic diet includes additional ingredients like fish, reduced-fat dairy products, potatoes, and vegetable fat, especially apples, pears, berries, root and cruciferous vegetables, cabbage, and whole-grain and rye bread. It is distinguished by its concentration on the intake of healthy regionally specific diets, similar to the MD [33]. The dietary strategy depends on the conventional Okinawan diet [34]. The Okinawan diet is a fiber-rich diet inspired by the nutritional habits of the inhabitants of the Ryukyu Islands, wherein the median lifespan is quite substantial and nearly more. The food is based on traditional Nordic raw food and provides moderately low carbohydrate energy content with higher contents of fiber, fat, and protein [35].
However, it was modified into meals that complemented the Nordic diet and had flavors and nutrients appropriate for Nordic citizens. Traditional Nordic diets heavily influence the food plan, with the inclusion of high-fat content, chicken, fruits, berries, and nuts at its core. Additionally, there are limitations on consuming dairy goods, sugar, white flour, red meat, and chicken that has been processed. The number of foods with a high glycemic index (GI), such as white wheat and sugar, was significantly decreased in favor of nutrients that had minimal impact on blood sugar, effectively supporting lower glycemic activity. With a daily calorie intake of around 1900 kcal, this diet provides a healthy quantity of nutrients such as carbohydrates, proteins, and fats. It stimulates a low glycemic action, the contented of high glycemic index, lower effect, and blood sugar level [36] (Table 1).
The typical daily consumption of grains (wheat, rye, barley, and oat products) and amino acids (fish, egg, milk, and beans) in the Nordic area is 26.7 g for each individual, or around 22% of the total protein consumption. Most cereal protein comes from wheat, which contains approximately 22.1 g per day. Rye, oats, and grain provide about 2.0 g, 0.8 g, and 0.6 g of protein per person per day, respectively. Oats are a grain with unknown potential to produce nutritious, sustainable foods. Oat β-glucan has been shown to promote both gut microbiota and postprandial glucose management, leading to an increase in the production of oat products and the availability of oat meals with health-supporting qualities in several basic food categories [37]. Despite a long heritage of eating oat- and rye-based meals, as well as the rise in specific oat-based cuisines, the predominant source of cereal protein in the Nordic area remains the early stage of consumption of wheat [38]. Furthermore, oat phytochemicals, fish, green tea, berries, and broccoli have been reported to have anti-inflammatory properties, which involvethesuppression of tumor necrosis factor (TNF)-α production andinterleukin (IL)-1β [39].
2.3. African Heritage Diet
The African Heritage diet promotes natural components that can be potentially beneficial for African-American populations since they have much higher risks of long-term health concerns associated with their diets [40]. The study involved participants that comprised African-American individuals between the ages of 18 and 65 who were overweight or obese (body mass index: 25.9–49.9) and included participants who were non-pregnant, without type2 diabetes or suffering from untreated thyroid diseases, and the findings showed an improvement in body weight and cardiovascular diseases (CVDs) risk factor outcome provided with the African Heritage diet [41]. The African Heritage food pyramid strongly emphasizes eating a diet rich in plants, such as vegetables, fruits, grains, beans, and nuts, which reduces body weight and waist edge and reduced blood pressure [42] (Table 1). A broad network of dishes from Africa, African-Americans, the Caribbean, and South America is included in the process, instructing people regarding traditional spices, ingredients, and preparation methods [43].
2.4. Traditional Asian Diets
The lack of potentially adequate evidence pertains to traditional regional dietary practices from other parts of the world that follow similar health-enhancing concepts. The diet of this population might be enhanced without affecting its natural composition simply by restricting the amount of grains consumed and encouraging the consumption of more nutritious fruits and vegetables as dietary sources for carbohydrates and other micronutrients [44], consisting mostly of fish and legumes in comparison to red meat, cultured cuisine, indigenous land and sea veggies, and oils made from sesame, perilla, and sesame seeds [45] (Figure 1).
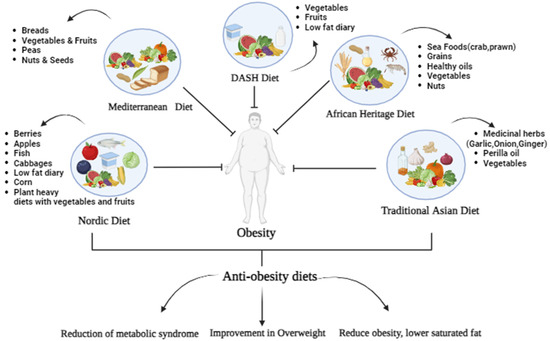
Figure 1.
Dietary patterns of different regions and their outcomes.
The Asian diets that originate from different parts of Asia, like the Korean diet, frequently include a variety of small-portioned foods (including preventing overeating and stability into the total homeostasis of our body and are prepared within the household using seasonal ingredients; as when compared to foods cultivated regardless of the season, fruits that are picked at the peak of their initial pulpiness can offer additional health advantages) [46]. The conventional Korean diet does not contain as many fried items as the Western diet. Dietary factors, including body weight, body mass index that was calculated by weight (kg)/height (m2), body fat, and lean mass, were observed using a bioelectric impedance analyzer and were positively impacted by the Korean diet [45,47].
The Chinese diet comprises rice or noodles, soups, vegetables (mainly broccoli, cabbage, potato, and tomato), steamed bread or dumplings, fruits, vegetables, and soy. In contrast, seafood includes turtles, shrimp, cuttlefish, squid, shark, abalone, and meat [40,48]. The Chinese diet has the ability to reduce the risk of metabolic syndrome, obesity, hypertriglyceridemia, and hypertension [45] (Table 1). According to studies, the diet can lower BMI while maintaining the physique associated by 28% of those who were surveyed for a Western diet roughly for a year after valuation was conducted, represented by 28 in group A (n = 142) that provided the basic traditional Chinese diet (BCTD) and only 6 in group B (n = 142) that providedthe Western standard diet (WSD) [49].
The Japanese diet consists of seaweed, fruits, vegetables, and mushrooms. A Japanese tradition involves using chopsticks and switching between small-portioned items during each course, where the umami flavor found in Japanese cuisine is included to promote satiety and prevent eating excessively [50]. Some Japanese recipes are flavored with umami from konbu, resulting in tastier and healthier meals with lower fat, sugar, and salt levels. Similarly, it has been demonstrated that substituting soy sauce, which is high in umami, for salt can cut down on salt intake by up to 50% without affecting consumer approval [51].
2.5. Dietary Approaches to Stop Hypertension (DASH)
The DASH (Dietary Approaches to Stop Hypertension) dietary plan is an acceptable dietary pattern for people associated with diabetes. In addition to promoting blood pressure control, this dietary pattern has been shown to improve insulin resistance, hyperlipidemia, and overweight/obesity. The DASH diet analysis promotes a diet that emphasizes consuming a range of fruits, veggies, legumes, and whole-grain foods, as well as different sources of animal protein such as seafood, meat, eggs, legumes, nuts, seeds, and soy. Therefore, considering the patient’s general health and nutritional objectives, the optimal caloric intake must be evaluated. The diet followed by DASH may be personalized to meet the needs of those who want to prevent obesity [52]. Individuals who first followed a traditional diet consisting of foods that are typical of American eating habits had considerably lower blood pressure reductions than those who followed a diet rich in fruits, vegetables, low-fat dairy, and foods with lower amounts of saturated and total fat and cholesterol [53] (Table 1).


Table 1.
Dietary patterns of different regions in maintaining obesity.
Table 1.
Dietary patterns of different regions in maintaining obesity.
| Diets | Phytonutrients | Regions | Outcomes | References |
|---|---|---|---|---|
| Mediterranean diet | Fruit, vegetables, breads, other forms of cereals, potatoes, beans, nuts, and seeds | Africa, Asia, and Europe | Monitoring diet, predominantly low in saturated fat; reduced amount 7–8% | [28,29,30] |
| Nordic diet | Apples, pears, berries, root and cruciferous vegetables, cabbages, whole grains, rye bread, intake of fish, low-fat dairy products, potatoes, and vegetable fats | Northern Europe and Europe | Stimulate low glycemic action, the contented of high glycemic index, lower effect, and blood sugar level | [33,35,36] |
| African Heritage diet | A plant-heavy diet with fruits, vegetables, tubers, grains, beans, nuts, healthy oils, and seafood | African, African-American, Caribbean, and South American | Reduced body weight and waist edgesand reduced blood pressure | [41,42,43] |
| Traditional Asian diets | Rice, whole grains, fermented food, indigenous land and sea vegetables, proteins(legumes and fish meat), medicinal herbs (garlic, green onions, andginger), sesame, and perilla oils | Asia | Lower risk of metabolic syndrome, obesity, hypertriglyceridemia, and hypertension | [44,45,48] |
| Dietary Approaches to Stop Hypertension (DASH) | Rich in fruits, vegetables, and low-fat dairy | East Asia | Developments to lower overweight/obesity, low-density lipoprotein cholesterol (LDL-C), and total cholesterol | [52,53] |
3. Bioavailability of Phytonutrients in the Human Metabolism System
Several key factors influence the bioavailability of phytonutrients in the human metabolism system. During digestion, phytonutrients undergo enzymatic breakdown in the gastrointestinal tract before being absorbed through the intestinal wall and entering the bloodstream [54,55]. However, digestion efficiency can vary depending on the specific phytonutrient and an individual’s digestive enzymes.
The presence of other substances in the diet, such as dietary fibers or fats, can interact with phytonutrients and impact their absorption [56,57,58]. For example, dietary fat can enhance the absorption of fat-soluble phytonutrients like carotenoids. Moreover, individual differences, including factors like age [59], genetics [60], gut health [61], and specific health conditions, contribute to variations in phytonutrient bioavailability among individuals. Genetic variations, for instance, can influence the metabolism and absorption of phytonutrients [62].
Furthermore, the synergistic effects of phytonutrients with other compounds in whole foods are crucial for enhancing their bioavailability [63,64,65]. Consuming whole foods rather than phytonutrient supplements is generally recommended to maximize the absorption and bioavailability of phytonutrients, as certain nutrients or compounds in whole foods can enhance their absorption [66].
The processing and preparation methods of plant-based foods also impact the bioavailability of phytonutrients. Factors such as heat, light, and oxidation can lead to the degradation of some phytonutrients. However, certain cooking techniques like steaming can improve the release and absorption of phytonutrients, enhancing their bioavailability [67].
Thus, understanding these key factors is vital for optimizing the bioavailability of phytonutrients and designing dietary strategies that maximize their potential health benefits. By considering digestion and absorption processes, interactions with other substances, the role of the gut microbiota, individual differences, and the effects of cooking and food processing, individuals can make informed choices to enhance the bioavailability of phytonutrients and support overall well-being.
3.1. Digestion and Absorption
Phytonutrients, bioactive compounds derived from plant-based foods, have gained significant attention due to their potential health benefits. However, the bioavailability of phytonutrients, primarily influenced by the efficiency of their digestion and subsequent absorption in the gastrointestinal (GI) tract, plays a crucial role in determining their physiological effects [54]. Upon consumption, phytonutrients enter the GI tract, where various conditions influence their fate. Digestion begins in the oral cavity, where mechanical breakdown and enzymatic action initiate. Once in the stomach, the acidic environment can further facilitate the breakdown of certain phytonutrients [68,69]. However, it is within the small intestine where most phytonutrient digestion occurs. Enzymes, including proteases, lipases, and carbohydrases, act on different classes of phytonutrients, breaking them down into smaller, more absorbable compounds [70].
The efficiency of phytonutrient digestion can vary depending on multiple factors. Firstly, the specific phytonutrient structure plays a role. Some phytonutrients possess complex chemical structures, such as glycosides or conjugated forms, requiring specific enzymes for hydrolysis. The availability and activity of these enzymes vary among individuals, contributing to inter-individual variability in digestion efficiency [71,72,73,74].
Secondly, individual differences in digestive enzyme production and activity can significantly influence phytonutrient digestion. Genetic variations, age, and overall gut health can impact digestive enzymes’ levels and functionality, affecting the breakdown of phytonutrients [55,75]. For instance, lactase deficiency can hinder the digestion of lactose-containing phytonutrients [76].
Once phytonutrients are digested into smaller components, they are absorbed into the bloodstream through the intestinal wall [71]. The absorption process primarily occurs in the small intestine, where various mechanisms are involved [71]. Passive diffusion is the primary mode of absorption for lipophilic phytonutrients, while hydrophilic compounds often require specific transporters for absorption [77,78].
3.2. Interaction with Other Substances
One important aspect is the interaction of phytonutrients with substances such as dietary fibers or fats, which can have both positive and negative effects on their absorption and subsequent physiological effects absorption [56,57,58,79]. Understanding these interactions is crucial for optimizing phytonutrients’ bioavailability and health benefits.
Dietary fibers, present abundantly in fruits, vegetables, and whole grains, can interact with phytonutrients in several ways [63,80]. Firstly, fibers can physically trap phytonutrients, forming complexes that may hinder their absorption [81,82]. However, in some cases, certain types of fibers, particularly soluble fibers, can positively impact phytonutrient bioavailability [83,84]. Soluble fibers can form gel-like structures in the gut, creating a conducive environment for absorbing phytonutrients [85,86].
Dietary fats can also significantly affect the bioavailability of certain phytonutrients, particularly fat-soluble ones [87]. For example, carotenoids, a class of phytonutrients abundant in fruits and vegetables, are better absorbed in dietary fat [88]. Fats can enhance the solubilization of lipophilic phytonutrients, facilitating their incorporation into mixed micelles and subsequent absorption through the intestinal wall [89,90]. Thus, consuming plant-based foods and a source of dietary fat, such as olive oil or avocado, can optimize the absorption and bioavailability of fat-soluble phytonutrients.
Moreover, the specific food matrix in which phytonutrients are found can impact their interaction with other substances and subsequent bioavailability [54]. Whole foods contain a complex mixture of phytonutrients, fibers, fats, proteins, and other components that can act synergistically or competitively. Certain nutrients or compounds in the food matrix can enhance the absorption of phytonutrients through mechanisms such as increased solubility, improved stability, or the facilitation of transport processes [82,91].
3.3. Individual Differences
The bioavailability of phytonutrients, the extent to which they are absorbed and utilized by the body, can exhibit significant inter-individual variation. This variation arises from several factors, including age, genetics, and specific health conditions, which can impact the metabolism and absorption of phytonutrients [92,93].
Genetic variations among individuals can significantly influence the bioavailability of phytonutrients. Genetic polymorphisms in enzymes responsible for phytonutrient metabolism and absorption can affect their efficiency and contribute to individual differences [62,94]. For example, variations in genes encoding phase I and phase II enzymes, such as cytochrome P450s and glucuronosyltransferases, can influence the metabolism and clearance of phytonutrients [95,96,97]. These genetic differences can result in variations in bioavailability and potentially alter the health effects associated with phytonutrient consumption.
Age is another important factor that influences phytonutrient bioavailability. Infants, children, and older people may exhibit differences in the absorption and metabolism of phytonutrients compared to adults [98]. Age-related changes in digestive enzyme production, gastrointestinal physiology, and gut microbiota composition can impact the bioavailability of phytonutrients [72,75,99].
The presence of specific health conditions can also impact the bioavailability of phytonutrients. For example, individuals with malabsorptive disorders, such as celiac disease [100,101,102] or pancreatic insufficiency [103,104], may experience reduced absorption of phytonutrients. Additionally, certain chronic diseases or conditions, such as liver disease [105,106] or kidney dysfunction [105,106], can affect the metabolism and clearance of phytonutrients, altering their bioavailability. Moreover, the use of medications, such as proton pump inhibitors [105,106] or certain antibiotics [105,106], can interfere with the absorption and metabolism of phytonutrients, further contributing to inter-individual differences in bioavailability.
3.4. Cooking and Food Processing
The processing and preparation of plant-based foods can significantly impact the bioavailability of phytonutrients [54]. Various factors, including heat [57], light exposure [107], and oxidation [108], can influence the stability and availability of these compounds. Understanding the effects of different cooking and processing methods is crucial for optimizing the diet’s retention and bioaccessibility of phytonutrients.
One important consideration in cooking plant-based foods is the potential for the heat sensitivity of phytonutrients. Some phytonutrients, such as vitamin C [109] and certain water-soluble compounds [110], are susceptible to degradation when exposed to high temperatures. Prolonged cooking or boiling can lead to the leaching and loss of these heat-sensitive phytonutrients [111]. Therefore, methods like steaming or stirfrying, that involve shorter cooking times and minimal contact with water, are often preferred to preserve the bioavailability of these compounds [112].
Light exposure can also impact the stability and bioavailability of phytonutrients. Some compounds, such as carotenoids [112] and flavonoids [113], are prone to degradation when exposed to light. Therefore, storage and handling practices that minimize light exposure, such as storing fruits and vegetables in opaque containers or wrapping them in protective coverings, can help preserve the integrity of light-sensitive phytonutrients and maintain their bioavailability [114,115].
Oxidation is another important factor to consider in the processing and preparation of plant-based foods. Oxidative processes can degrade phytonutrients and reduce their bioavailability [116]. For example, phenolic compounds widely distributed in plant-based foods are susceptible to oxidation [117,118]. Cutting, peeling, or chopping fruits and vegetables can expose these compounds to oxygen, leading to enzymatic or chemical reactions that result in their degradation [119]. Minimizing exposure to air [120] and using antioxidant-rich ingredients like citrus juices [121] or herbs/spices [122] with high antioxidant content can help mitigate oxidation and preserve phytonutrient bioavailability.
3.5. Bioavailability of Phytonutrients as Anti-Obesity Activities
Scientific studies have provided evidence regarding the bioavailability of phytonutrients with anti-obesity activities, shedding light on their potential role in combating obesity. Several phytonutrients have demonstrated anti-obesity activities, such as polyphenols [123,124,125,126], flavonoids [127,128,129,130], and carotenoids [131,132,133]. These compounds have been shown to regulate various mechanisms involved in energy balance, adipose tissue metabolism, and inflammation, all of which are critical factors in the development of obesity. However, the bioavailability of these phytonutrients is a crucial consideration for their functional efficacy in obesity prevention or treatment [134,135].
The functional bioavailability of phytonutrients for obesity depends not solely on their absorption but also on their interactions with target tissues [136] and their metabolic fate [137]. After absorption, phytonutrients can undergo biotransformation by enzymes, including those in the gut microbiota, leading to the formation of metabolites with distinct biological activities [93]. These metabolites can directly affect adipose tissue metabolism, energy expenditure, and inflammation, contributing to the anti-obesity properties of phytonutrients [138,139].
4. Phytonutrients with Anti-Obesity Effects
There are several anti-obesity drugs in the market; however, their usage has been limited due to their adverse side effects. Anti-obesogenic medications currently on the market fall mostly into two categories. The first is orlistat, which blocks pancreatic lipase activity to reduce intestinal fat absorption, and the second is sibutramine, an anorectic or appetite suppressant. These therapies are pricey and prohibitive, particularly for those in developing nations, and have been associated with adverse health outcomes like increased blood pressure, dry mouth, constipation, headaches, and insomnia [140]. Hence, due to such adverse effects, dietary therapies utilizing naturally occurring bioactive food components have become attractive therapeutic options for treating obesity and metabolic illnesses. Therefore, in recent years, researchers have concentrated on food, especially from plants and animals, in search of components that are efficient and beneficial for lowering obesity and other related problems [141]. These phytonutrients showed potential anti-obesity and anti-diabetes effects by altering the following physiological pathways such as controlling hunger, metabolism, and insulin sensitivity. Consuming phytonutrients is considered a secure, readily accessible, and affordable method of controlling diabetes and obesity [7]. Phytoconstituents possess five potential mechanisms of action to fight against obesity, which include lipase inhibitors, appetite suppressants, thermogenic energy expenditure regulators, adipocyte differentiation regulators, and lipid metabolism regulators [142].
Among phytochemicals, polyphenols, tannins, flavonoids, terpenoids, saponins, alkaloids, steroids, glycosides, and proteins are found in plants, and their respective products are crucial for the treatment of various health problems [142,143]. Polyphenols and flavonoids like resveratrol, quercetin, kaempferol, myricetin, catechins, cyaniding, and anthocyanin [144,145,146] and alkaloids like caffeine, capsaicin, and ephedrine were reported with effective anti-obesity effects via both increased lipolysis as well as thermogenesis along with reduced appetite [147]. Similarly, terpenoids like β-carotenoids, cryptoxanthin, fucoxanthin, zeaxanthin, and lycopene [148] and saponins like ginsenosides [149] showed the potential anti-obesity effect, which is briefly described below. Figure 2 depicts the methodologies adapted for assessing the anti-obesity impact of phytochemicals.
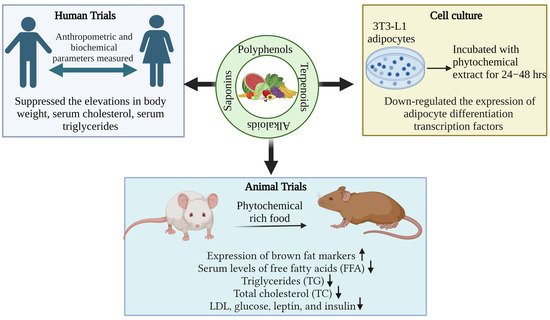
Figure 2.
Methodology adapted for testing anti-obesity effects of phytochemicals.
4.1. Polyphenols
As secondary metabolites, polyphenols constitute a large bioactive substance naturally found in plants, and their presence in diets may help in the beneficial modulation of various factors related to health variables that are related to obesity by consuming dietary polyphenols [124]. Several mechanisms (either an individual or in combination effect) for polyphenols have been reported regarding their anti-obesity functions via inhibiting enzymes and adipocyte differentiation, regulation of lipid metabolism, suppressing appetite or stimulation of energy expenditure, and modulation of microbiota in the gut [150]. The in vitro experiment on catechin from green tea extract (GTE) by Weng et al. [151] showed reduced lipid accumulation by preventing differentiating 3T3-L1 preadipocytes into adipocytes and increased the turning of white adipocytes into brown due to the presence of (-)-Epigallocatechin gallate EGCG. Authors suggested that EGCG could enhance glucose homeostasis in the adipocyte cell line 3T3-L1 by restoring the balance of redox factors and addressing mitochondrial dysfunction. Further, it was also demonstrated that taking GTE at the dose of 583 mg of catechins in a daily diet for 12 weeks caused reductions in the mass of adipose tissue, body weight, and serum levels of LDL-C.Also, GTE supplementation ameliorates lipid accumulation via inhibition of 3T3-L1 adipocytes following conversion of white adipocytes into brown [151]. In another study, Tung et al. [152] studied the browning of 3T3-L1 adipocytes after administration of 20 mM curcumin, thereby raising the expression of brown fat markers (PGC-1, PPAR, UCP1 PRDM16 C/EBP, Tmem26, Cidea, and FGF21Tbx1) and demonstrating that curcumin changes white adipocytes into beige adipocytes. Curcumin reduced TG concentration in brown-like adipocytes by raising mitochondrial CPT-1 and cytochrome C protein levels, which increased fat oxidation. Curcumin also raised pACC, pAMPK/AMPK ratio, and HSL expression to promote lipolysis and inhibit FA formation, respectively [152].
In 2015, Alkhalidy et al. [153] used kaempferol (0.05% in the diet) to enhance skeletal muscle glycolysis, glucose absorption, glycogen synthesis, AMPK activity, and Glut4 expression on the cellular and molecular levels. In addition, adding kaempferol to the diet considerably reduced hyperglycemia in old, obese diabetic mice and retained functional islet mass. These findings suggest that the phytonutrient kaempferol may be taken as a dietary supplement to avoid metabolic diseases linked to aging and obesity [153]. At the cellular level, AMPK restricts gluconeogenesis, regulates mitochondrial biogenesis, and promotes fatty acid oxidation. Similarly, supplementation of MGP (muscadine grape) and MWP (muscadine grape wine) has phytochemical compositions quercetin, myricetin, kaempferol, ellagic acid, cyanidin 3,5-diglucoside, delphinidin 3,5-diglucoside, peonidin 3,5-diglucoside, malvidin 3,5-diglucoside,andpetunidin/pelargonidin 3,5-diglucoside and total anthocyanins feed in the HF diet of mice, showing significantly reduced fat mass by 29 for MGP and 12.5%, for MWP, as compared to the HF group [154]. Compared to mice fed the HF diet alone, the addition of MGP and MWP reduced the liver’s weight by 41.6% and 37.5%, respectively. In contrast, the weight difference between lean and obese mice’s hepatic tissues was 54% [154]. Supplementation of raspberry juice and puree concentrates (RJC and RPC), along with the mixture of ellagic acid (EA) and raspberry ketone (RK), when added to high-fat diets of mice, considerably reduced the amount of body weight increase [155]. Recently, Xu et al. [156] used green tea (Camellia sinensis, Theaceae) extract at diet doses of 400 or 800 mg/kg and reported anti-obesity effects on rats with high-fatdiets for six weeks. The findings showed that green tea extract possesses a suppressive effect on body weight gain and fat buildup due to polyphenols and polysaccharides. Polyphenols, such as caffeine, and polysaccharides enhance the antioxidants and blood lipid levels, reduce serum leptin levels, prevent fatty acid absorption, and lower IL-6 and TNF-gene expression levels. Also, it was demonstrated that polysaccharides and polyphenols collaboratively reduce blood leptin levels with anti-inflammatory effects [156].
In clinical trials performed by Suzuki et al. [157], GTE (green tea extract) enriched (280 and 360 g GTE) in rye bread was used in randomized single-blinded studies for both men and women. They provided 242.1 and 188.3 mg of EGCG, respectively. Compared to the control, GTE-enriched bread consumption caused a significant reduction in waist circumference, i.e., −1.22 cm and was beneficial in maintaining low blood pressure [157]. Figure 3 depicts the chemical structures of polyphenol’s potential as anti-obesity.
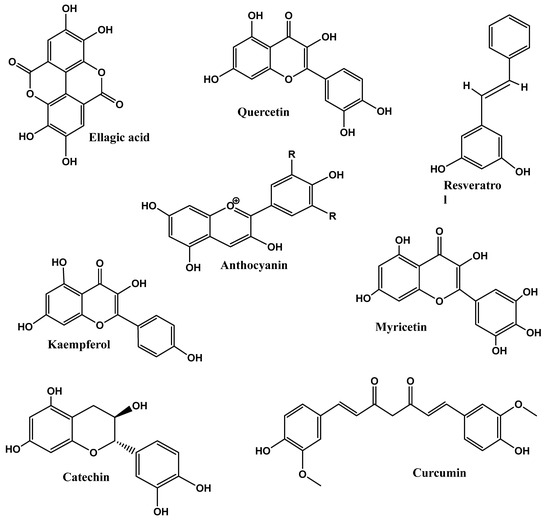

Figure 3.
Chemical structures of phytonutrient potential as anti-obesity.
4.1.1. Phlorizin
Phlorizin (PHZ) is a nutrient found in apples that helps to make one healthier. The amalgamation of PHZ and other strategies aided in curbing weight gain attributed to overconsumption, ultimately resulting in reduced body mass. The outcome of combining PHZ was not due to reduced food intake or energy consumption. The decrease in accumulated fat provided evidence that this combination might result in its better effectiveness [158] (Figure 3). The gut lining’s impairment, which results from excessive intake of fatty food, is mended by the body’s generation of glucagon-like peptide (GLP-2). Overconsumption of fatty foods alters favorable bacteria in the gastrointestinal microbiome. PHZ can help fight obesity by focusing on the relationship between gut bacteria and gut lining [158]. An in vitro study isolated human plasma where the activity was observed bytheinhibition of LDL oxidation at physiological levels, LDL-cholesterol oxidation [159,160]. Phlorizin during in vivo studies was reported to prevent weight gain in mice while their food consumption remained constant. The reduction in body weight corresponded with a decrease in the number of abdominal fats [161]. The PHZ group had less fat and smaller adipose cells than the high-fat diet (HFD) group. The PHZ group exhibited improved lipid levels in both their blood and liver. Individuals with PHZ who exhibited lower levels of certain enzymes involved in fat production, storage, and cholesterol synthesis also showed an evident reduction in liver fat [49,162].
4.1.2. Epigallocatechin Gallate (EGCG)
The consumption of energy enhancement via the increased process of thermogenesis where obesity was caused in male C57BL/6J mice and the phytonutrient epigallocatechin gallate is observed to have the ability to cause changes in blood sugar and triglyceride levels significantly by decreasing/lowering lipid formation in adipose tissues and also by reducing body fat growth [163]. The primary catechin in green tea, (-)-Epigallocatechin-3-gallate (EGCG), offers several possible health benefits, notably a reduction in body weight and possibly adipose tissue weight. The stimulation of AMPK in the liver, skeletal muscle, and white adipose tissue, as well as a decline in caloric intake, are proposed as possible methods by which EGCG may lower body mass index. Epididymal adipose tissue weight and blood lipid properties, such as triglyceride, cholesterol (CHOL), and high- and low-density lipoprotein CHOL (HDL-C, LDL-C) levels, were all significantly impacted by EGCG [164]. The potential of EGCG to regulate body fat content was demonstrated in an in vitro investigation using Escherichia coli and C. elegans strains with the OP50 diet, where the prevention of adipogenesis led to a decrease in the fat content in C. elegans. This was demonstrated by the lowered ATGL-1 gene expression level following EGCG administration [160]. In overweight mice affected by a dietary regimen, EGCG was found to provide benefits in decreasing body fat through reduced calorie intake, enhancing both in vivo and in vitro oxidation of fatty acids and lipolysis [164]. In a clinical trial where 15 women with central obesity were screened to regulate plasma cholesterol and triglycerides, 102 of them with a body mass index (BMI) ≥ 27 kg/m2 and a waist circumference (WC) ≥ 80 cm were observed to be able to decrease body weight and BMI in obese women after a 12-week treatment, alsowitha significant reduction in waist circumference [165] (Figure 3).
4.1.3. Gallic Acid
Gallic acid (GA) is a naturally occurring physiological phenolic acid with a chemical composition of 3-, 4-, and 5-trihydroxy benzoic acid. It can be extracted from vegetables and fruits and is thus prevalent in plant products, such as green tea and fruit juices, which are good for maintaining energy and homeostasis [166]. The ability of gallic acid could initiate an alteration by inhibiting lipogenesis and lowering fat accumulation in obese people. The process of synthesizing fat in our system from components like carbohydrates is called lipogenesis [167]. GA changes the inter-scapular brown adipose tissue’s thermogenic genes and activates the Adenosine 5′-monophosphate-activated protein kinase/Sirtuin 1/Peroxisome proliferator-activated receptor γ coactivator-1 (AMPK/SIRT1/PGC-1) pathway to carry out its favorable metabolic activities [168]. In the in vitro study, the size of adipocytes, because adipocyte hypertrophy is associated with adipose tissue inflammation and metabolic disorder Murine 3T3-L1 preadipocytes and RAW 264 macrophages average fat cell18 size in WAT, was significantly lower in the GA group compared to the control group [169]. In the in vivo reported studies, for the obesity in male C57BL/6 mice, there were formed adipose and fat contents that provided a drastic reduction in lipolysis that was induced, while Fas ligand (FAS), which is a cell surface death receptor, was suppressed to prevent lipogenesis, controlling the degradation of lipids and decreasing fatty tissue and calories [170]. Obese human subjects receiving capsules containing 200 mg of gallic acid and 50 mg of a Chinese herbal decoction three times a day for 24 weeks did not experience weight loss or a decrease in food intake in humans, principally due to the inability to achieve adequate serum levels [167] (Figure 3).
4.1.4. Resveratrol and Quercetin
Quercetin exerts anti-obesity activity via the mitogen-activated protein kinase (MAPK) and 5′-adenine monophosphate-activated protein kinase α1 (AMPKα1) signaling pathways [171]. Resveratrol, a phytoalexin originating from the skin and seeds of grapes and red wine, may also protect against diet-induced obesity in vivo studies and metabolic diseases, including hepatic steatosis and insulin resistance [172]. The anti-obese effect of the combination of resveratrol and quercetin (CQR) is related to a reduction in body weight gain, adipocyte diameter, and adipose tissue weight and an improvement in dyslipidemia in serum. Its anti-obese effect is closely related to its anti-inflammatory properties by which it regulates adipokine release and initiates the AMP-activated protein kinase/Sirtuin 1 (AMPKα1/SIRT1) signaling pathway. These effects indicated that CQR can potentially reduce HFD-induced obesity and inflammation [173]. Inan invitro study in human SGBS adipocytes, it led to the inhibition of adipogenesis by decreasing gene expression levels of the key adipogenic factors peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein α (C/EBPα) and reducing levels of adipokines (ANGPTL4), adipsin, and PAI-1 as well as of glycolysis-associated enzymes ENO2, PFKP, and PFKFB4, all of which are associated with obesity and adipose tissue dysfunction [173]. In the experimental model, male Wistar rats induced obesity where the modulation of gut microbiota was observed to decrease body weight gained significantly, visceral adipose tissue weight, and adipocyte sizes [174]. A clinical trial was conducted in 11 obese, otherwise healthy men by down-regulating genes involved in intercellular junctions, Wnt signaling, angiogenesis, G protein-coupled receptors, and Notch signaling, up-regulating pathways involved in cell cycle regulation, and reducing adipocyte size, thereby, enhancing and improving adipogenesis [175,176] (Figure 3).
4.2. Alkaloids
Another class of bioactive compounds is alkaloids, which are low-molecular-weight and nitrogen-containing compounds. They are heterocyclic rings, which are alkaline due to the presence of a nitrogen atom [177]. These metabolites are divided into various sub-classes based on their precursor (e.g., tryptophan-derived indole alkaloids) and other classes such as piperidine alkaloids, pyrrolidine alkaloids, pyridine alkaloids, tropane alkaloids, quinolizidine alkaloids, etc. [177]. Reserpine from Rauwolfia vomitoria, an alkaloid, reduces blood pressure and treats hypertension by targeting blood vessels. It may also be helpful to boost metabolism and induce the excretion of fluids in obese patients. Alkaloids have anti-obesity effects due to increasing lipolysis and thermogenesis and suppressing hunger [178]. The impact of piperine on adipocyte cell line 3T3-L1 has been demonstrated to block differentiation by down-regulating PPAR, SREPB-1c, and C/EBP, suggesting that it may be useful in the treatment of metabolic disorders [179]. Relatedly, 13-methyl berberine was discovered to have the most powerful anti-adipogenic effects among the 11 protoberberines and two benzophenanthridine alkaloids by Chow et al. [180], who tested them on 3T3-L1 adipocytes. Both PPAR- and CCAAT-enhanced bindings of C/EBP were down-regulated and targeted for gene regulation. The reduction in PPAR, C/EBP, and sterol regulatory element binding protein 1 (SREBP-1) protein levels, as well as the attenuation of this lipid-reducing action by an AMP-activated protein kinase (AMPK) inhibitor followed. It further suggested that this drug’s effects depend on the AMPK signaling pathway. Previously, Ma et al. [181] isolated four main aporphine alkaloids, including 2-hydroxy-1-methoxyaporphine, pronuciferine, nuciferine, and roemerine, were isolated from the leaves of Nelumbo nucifera. These alkaloids exhibited concentration-dependent cytotoxicity in 3T3-L1 cells, and 2-hydroxy-1-methoxyaporphine and pronuciferine significantly increased glucose uptake in differentiated adipocytes. These findings might help explain why lotus leaves are so widely used in China for blood sugar regulation and weight loss [181]. A different component in N. nucifera leaves has a variety of anti-obesity effects. In the investigation by Ahn et al. [182], benzoisoquinoline alkaloids prevent the absorption and storage of fat. While flavonoids prevent fat storage, other alkaloids and metastigmanes are better at preventing fat absorption. As a result, N. nucifera leaves may be used to treat obesity since they control pancreatic lipase and adipocyte differentiation [182].
In the study by Gurung and De [183], authors compared the curcumin and EGCG (epigallocatechin gallate) with the strong alkaloids in cinchona bark, cinchonine demonstrated a higher final body weight reduction rate, and results showed that cinchonine significantly reduced plasma glucose, LDL-+VLDL-cholesterol, HDL-cholesterol, and TG levels in response to the HFD (high-fat diet)-induced hyperlipidemia and hyperglycemia in mice. Cinchonine impacts hyperlipidemia and hyperglycemia caused by an HFD, which is an early sign of metabolic syndrome and other related illnesses. In conclusion, it was suggested that cinchonine significantly reduces adipogenesis by suppressing the WNT and galanin-mediated signaling pathways. This reduces adipose tissue’s inflammation by suppressing the TLR-2- and TLR-4-mediated pro-inflammatory signaling pathways [183]. In another study by Huang et al. [184], lansiumamide B, a new alkaloid discovered in the C. lansium seeds, demonstrated promising anti-obesity effects in rats given an HFD, which could be used as an anti-obesity medication. According to q-RT-PCR analysis results, HFD treatment increased the expression of adipokines, leptin, and the lipogenic markers FAS (fatty acid synthase) and SREBP-1c but not the adipogenic markers AP2 (adipocyte protein 2) and PPAR2, and also lansiumamide B treatment significantly decreased the expressions of leptin, FAS, and SREBP-1c [184]. Mahanimbine, an essential carbazole alkaloid found in Murraya koenigii (curry leaves), prevented HFD-induced hyperlipidemia alongside fat accumulation in adipose tissue and the liver as well as the development of systemic inflammation and oxidative stress. It has been found to enhance the glucose clearance and increase the expression of insulin-responsive genes in the liver and adipose tissue when given daily along with HFD feeding for 12 weeks at both low and high doses, i.e., 2 mg/kg and 4 mg/kg body weight [185]. Ohara et al. [35] studied the effect of the consumption of a G-hesperidin (500 mg)- and caffeine (25, 50, or 75 mg)-based diet for 12 weeks on 75 healthy individuals with moderate BMIs and serum triglycerides. The result of the double-blind randomized design showedareduced abdominal fat area in G-hesperidin with 50 mg caffeine group and G-hesperidin with 75 mg caffeine group. This study suggested that combining both G-hesperidin (500 mg) and caffeine (50 and 75 mg) could be beneficial for treating obesity. Figure 4 represents the chemical structures of the potential of alkaloids as anti-obesity.
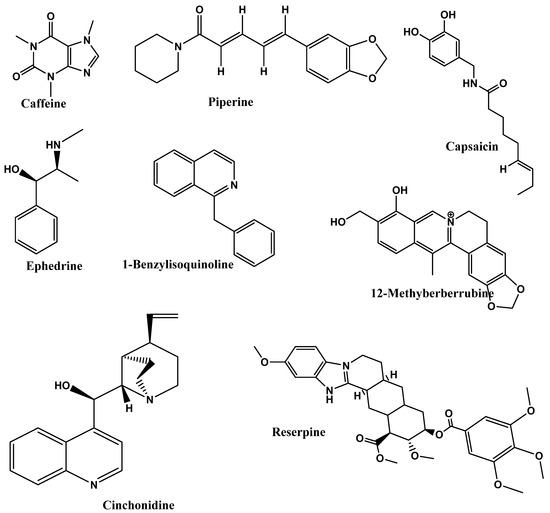
Figure 4.
Chemical structures of potential of alkaloids as anti-obesity.
4.3. Terpenoids
The largest secondary metabolites in nature are terpenes (plants, fungi, marine organisms, and animals). Terpenes are mostly found in essential oils as a major component. It comprises isoprene units with five carbon and eight hydrogen atoms and is regarded as the basic unit of all terpene kinds. There are two isoprene units in monoterpene (C10), three in sesquiterpenes (C15), four in diterpenes (C20), five in sesterpenes (C25), six in triterpenes (C30), and eight in tetraterpenes (C40) [186]. An important site for carotenoids and retinol storage is adipose tissue. According to estimates, 15–20% of the body’s total retinol is stored in the WAT of the rats, primarily in the adipocytes as non-esterified retinol [187]. Adipocytes contain carotenoids mostly connected with triacylglycerol in the lipid droplet and cell membranes. Thus, several reports examined the role of carotenoids in adipogenesis, which may aid in controlling obesity by limiting the buildup of lipids in adipocytes. Most of the observed effects are interfered with nuclear receptors like RAR, RXR, or PPAR to suppress adipocyte development. In addition to producingapo-140-carotenal and suppressing PPAR, PPAR, and RXR activation, -carotene also suppressed adipogenesis by producing all-trans retinoic acid [148]. Adipose tissue, liver, kidney, pancreas, brain, ovaries, gut, and eyes are just a few of the organs and/or tissues in which lycopene demonstrates anti-obesity properties. According to epidemiological research, eating foods high in lycopene may help reduce the chance of developing obesity. Lycopene’s cis-isomers mostly distribute in the liver and adipose tissues and are more accessible and better absorbed than trans-lycopene. In brown adipose tissue (BAT), lycopene can enhance the browning of white adipose tissue (WAT), elevate the expressions of thermogenic genes (UCPs), reduce the expression of fibroblast growth factor-21 (FGF21), and increase the expressions of PPAR, SIRT1, and UCP1 [188]. A xanthophyll with a distinctive structure called fucoxanthin has received much interest for its ability to fight against obesity. An increase in Adrb3 mRNA expression in mice’s WAT, which may be responsible for adaptive thermogenesis via SNS activation and UCP1 expression, may cause the potential anti-obesity benefits of a fucoxanthin diet. Therefore, carotenoid has been proposed as the primary pharmacophore of anti-obesity activity [189]. Zeaxanthin is a form of carotenoid that has been shown to have anti-lipogenesis properties [190]. It has also been shown to impact obesity in C57BL/6J mice fed a high-fat diet by preventing adipocyte 3T3-L1 cell adipogenesis. Zeaxanthin considerably reduced the intracellular lipid content of adipocytes in a dose-dependent manner. Zeaxanthin administered orally at a dose of 20 mg/kg slowed the development of obesity and enhanced dyslipidemia in mice with obesity brought on by a high-fat diet. In vitro and in vivo, it reduces the transcriptional factors and adipocyte-specific genes involved in adipogenesis, demonstrating an anti-adipogenic action. The MDI (0.5 mM 3-isobutyl-1-methylxanthine, 1.0 mM dexamethasone, and 1.0 g mL−1 insulin) and HFD (high-fat diet)induced suppression of AMPK phosphorylation in both adipocytes and epididymal adipose tissues, respectively, via zeaxanthin treatment, which subsequently changed the energy metabolism. These findings confirm that zeaxanthin inhibits lipogenesis, induces AMPK activation, and reduces intracellular lipid content, adipocyte size, and adipose mass [190]. In the previous study, consumption of 90 g pre-meal raw and ripe tomatoes in young women was observed for 4 weeks daily before lunch [191], and biochemical and anthropometric parameters were measured. The study revealed that after 4 weeks, significant reductions in %fat, triglycerides, body weight, fasting blood glucose, uric acid, and cholesterol in young adult women were observed. Tomato in pre-meal is assumed to have high lycopene content, which is responsible for hypolipidemic, hypouricemic, and hypoglycemic effects in young women. Figure 5 represents the chemical structures of the potential of terpenoids as anti-obesity.
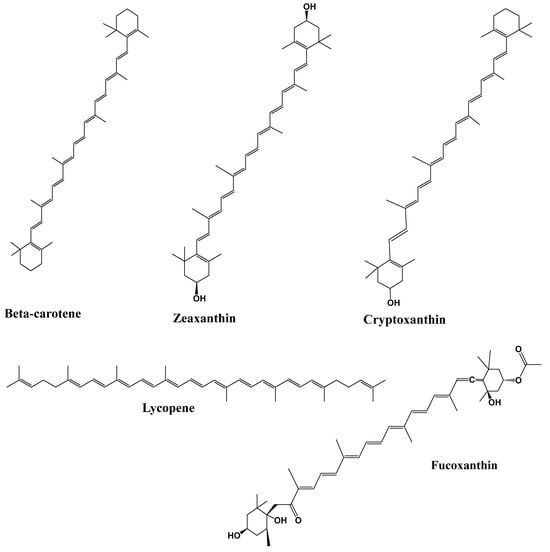
Figure 5.
Chemical structures of potential of terpenoids as anti-obesity.
4.4. Saponins
Many saponins are triterpene glycosides with steroids or triterpenes in the aglycone. Saponin molecules have a variety of lipophilic and lipophobic aglycone moieties that provide emulsifying properties [192]. The most widely recognized saponins are Panax ginseng, Panax japonicas, and Platycodi radix, which have been proven effective in several models to prevent or reduce obesity [193]. Saponin’s glycemic effects appear to be the result of various processes, including the improvement of the elevation of plasma insulin levels, the insulin response, and the stimulation of the pancreas via the production of insulin. For instance, it was demonstrated that saponin platyconic acid, which was isolated from Platycodi radix, promoted insulin-stimulated glucose uptake in 3T3-L1 adipocytes. In contrast, arjunolic acid, discovered in Terminalia arjuna Wight and Am. and other species, had an inhibitory effect on both amylase and glucosidase [194]. The macrophage-conditioned medium (RAW-CM) stimulates adipocytes, red ginseng’s saponin fraction (SF) controls the expression of adipokines, and the expression of adiponectin was elevated (more than two folds). However, its resistance expression was down-regulated (by 40%). SF considerably reduced both MCP-1 by 37% and IL-6 by 25% production in the contact system of adipocytes and macrophages. Also, in another system, the Transwell system, anSF at 100 g/mL showed a dramatically elevated quantity of hemoxygenase-1 (HO-1) by 1.5–3.5 folds and nuclear factor (-derived 2)-like 2 (Nrf2) by 2.8–3.6 folds, by enhancing Nrf2 translocation into the nucleus. Nevertheless, the Nrf2 or HO-1 knockdown condition abrogated the SF-mediated inhibitory effect on producing IL-6 and MCP-1 cytokines. This finding demonstrated the requirement for Nrf2 activation for SF-mediated prevention of obesity-induced inflammation [195].
Findings demonstrate that the stem and leaves of Panax ginseng (SLG) showed significant anti-obesity effects in diet-induced obese mice, as evidenced by reduced serum levels of triglycerides (TGs), free fatty acids (FFAs), low-density lipoprotein (LDL)-cholesterol, total cholesterol (TC), insulin, glucose, and leptin with reduced overall body and liver weight. This was conducted using high-fat diet (HFD)-induced obesity in a mouse model. SLG triggers the up-regulation of CPT-1, PPAR, UCP2, ATGL, and HSL and the down-regulation of PPAR, FAS, CD36, and FATP2 in liver tissue. In addition, comparing the HFD group with the SLG groups, the former group had reduced levels of PPAR, AP2, and leptin mRNA and higher expressions of PPAR, PGC-1, UCP1, and UCP3 genes in adipose tissues. In conclusion, SLG is essential for mice fed an HFD to have anti-obesity effects, possibly due to control of thermogenesis, lipogenesis, and lipolysis [196]. The impacts of ginsenoside (SG) and saponin obtained from sea cucumber (SSC) on enhancing lipid metabolism in C57BL/6 mice fed with an HFD and receiving SSC for eight weeks showed that SSC significantly reduced HF-induced fat mass, lipid levels in both liver and serum, weight gain, and insulin levels in serum and glucose. SSC reduced high-fat diet-induced obesity in C57BL/6 mice primarily by slowing lipid production and speeding up lipid oxidation and glycolysis in the liver [197]. Song et al. [198] conducted a study on 10 obese Korean middle-aged women on the effect of Panax ginseng extract consumption for 8 weeks. Significant changes in body mass index and weight were reported, with slight differences in gut microbiota. The significant reduction in body weight by intake of ginseng extract confirms its beneficial effects on the obese population. However, its effectiveness depends on gut microbiota composition before ginseng intake. Figure 6 represents the chemical structures ofthe potential of saponins as anti-obesity and Table 2 represents the effect of phytonutrients on obesity.
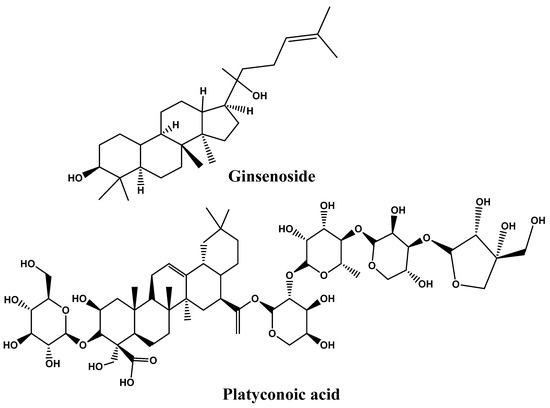
Figure 6.
Chemical structures of potential of saponins as anti-obesity.

Table 2.
Effects of various phytonutrients on obesity (in vitro, animal study, and human study).
4.5. Anthocyanins
Anthocyanins are responsible for the red, blue, and purple colors in vegetables and are reported to possess significant anti-inflammatory properties in obese adipose tissues, whereas anti-obesity mechanisms are associated with anthocyanins [201]. In vitro studies on anthocyanins prevent nuclear factor kappa B (NF-κB) activation, thus decreasing the entire downstream cascade of pro-inflammatory mediators, such as C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor (TNF)-α,and improving gut dysbiosis, restoring a balanced gut microbiota. The IL-6 gene in lipopolysaccharide (LPS)-induced adipose stem cells treats obesity-related inflammation and chronic disease [202] (Figure 7). Male Sprague–Dawley rats induced obesity reduction in food intake through the regulation of neuropeptide Y and γ-aminobutyric acid receptor in the hypothalamus where it has been observed that there is a significant decrease in body weight gain by 15.76% and in daily food intake by 19.10%, as compared to the control [203]. In a clinical trial involving healthy male volunteers, dried purple carrots (118.5 mg/day for 4 weeks) lowered lipids, body composition, and inflammation in obese adults, eventually resulting in an overall reduction in body mass index and low-density lipoprotein cholesterol [204].
Figure 7.
Chemical structures of potential of Anthocyanins as anti-obesity.
Cyanidin
Cyanidin 3-glucoside (C3G) derivatives reduce obesity-induced inflammation by modulating adipocytokines secretion, and this action may be a useful strategy for preventing obesity-associated metabolic pathologies [205]. The bioavailability of cyanidin3-glucoside is only 0.02%, while the microbial degradation product of cyanidin 3-glucoside, 3,4-dihydroxybenzoic acid, has a bioavailability of 44% [206]. In an in vitro study, PC12 cells treated with H2O2 reduce pro-inflammatory markers associated with obesity, such as C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor (TNF)-α, leading to reduced oxidativestress-induced neurotoxicity in PC12 cells treated with H2O2 [202,207]. In vivo studies reported that the ovariectomized female Sprague–Dawley rats induced obesity by stimulating energy expenditure and modulation of lipid metabolism. The resultant activity showed a significant reduction in body weight gained by 32.83%, triglycerides by 24.4%, and LDL by 29.58%, compared to the control of the in vivo study [208]. In clinical trial studies in overweight and obese volunteers that observed changes in other markers of inflammation and lipid metabolism, plasma-oxidized LDL and serum malondialdehyde and hydroxynonenal concentrations decreased (NCT02613715) (Figure 8).
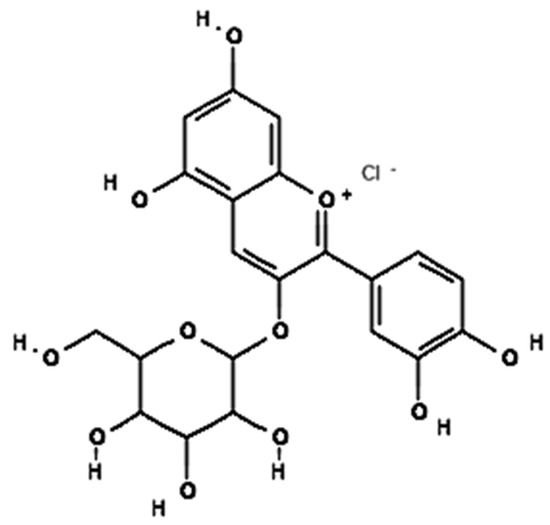
Figure 8.
Chemical structures of potential of Cyanidin 3-glucoside as anti-obesity.
Sources of various phytonutrients, including their active components through various experimental models that are conducted in vivo studies as well as human-related, confirm their effect in the body system involving the fundamental mechanisms of action. The key findings that significantly show a response towards lowering body weight, fat accumulation or deposition, TG, LDL, and an alternative area to target obesity via targeting gut microbiota are shown in Figure 9 and Table 3.
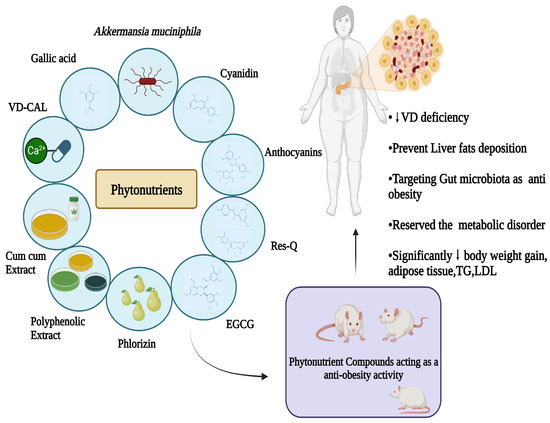
Figure 9.
Phytonutrient mechanisms involved in obesity.

Table 3.
Phytonutrients in various experimental models with their major findings in obesity.
5. Application of Phytonutrient-Based Anti-Obesity Food Supplement Products and Market Trends
In recent years, the increasing incidences of obesity have caught attention all over the world, and inadequate fat intake has been cited as one of the primary causes of obesity and its associated disorders. However, increasing investigation suggests that eating edible vegetable oils may have non-negligible physiological effects, such as reducing inflammation, decreasing blood lipids, and preventing the formation of adipocytes and hunger. Bioactive phytochemicals found in lipids and oils significantly affect obesity, such as phytosterols, phenolic compounds, and tocopherol [212]. In a study, obesity and metabolic syndrome have been linked to dietary intake of polyunsaturated fatty acids (PUFAs). Dietary n-3 PUFAs reduce leptin expression in adipose tissue and may prevent leptin resistance. This study further showed that n-3 PUFAs decrease leptin production, which is controlledby epigenetics, such as reduced methyl-binding protein 2 to the leptin promoter or via histone alteration within the promoter area [213]. Also, the impact of gastrointestinal hormones on fine-tuning hunger has been well-researched and is non-negligible. For example, PYY3-36 (PYY3-36),ghrelin,glucagon-like peptide-1 (GLP-1), pancreatic polypeptide (PP),oxyntomodulin (OXM),amylin, and cholecystokinin have all been demonstrated to impact food intake. Ghrelin enhances appetite and consumption of foods, but all other documented gut hormones have anorectic implications: they promote “satiation” (causing meal cessation) and/or “satiety” [214]. The Phytochemical Index (PI) and carbohydrate consumption had a beneficial correlation. Plant foods have a more beneficial form of carbohydrate than sweetened drinks, white bread, and other meals manufactured from refined flour. Furthermore, phytochemical-rich diets contain components that, like dietary fibers, can influence glucose metabolism. Dietary fibers can lower the glycemic index of meals, reducing glucose absorption and promoting lower insulin production, affecting more favorable physiological responses [215]. To examine the association between DPI (Dietary Phytochemical Index) and pre-diabetes morbidity, three hundred participants were surveyed and divided into 150 pre-diabetics (cases) alongside 150 healthy (controls) groups, and the DPI was determined based on data collected from a 168-item validated diet frequency questionnaire. Controlled cross-over research found that eating vegetables, whole grains, and fruits had a positive effect on FBG (fasting blood glucose) as well as insulin resistance in obese adults with increased FBG [216]. Figure 10 depicts the anti-obesity mechanisms offered by various phytochemicals.
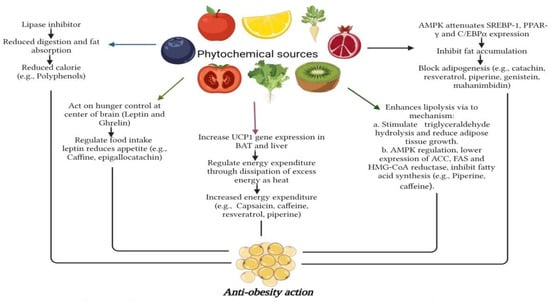
Figure 10.
Depiction of anti-obesity action mechanism via phytochemicals.
Numerous epidemiologic as well as clinical studies have been conducted in recent years because of an increasing interest in preventing obesity with plant-based foods. These studies are crucial for expanding knowledge of the relationship between nutrition and obesity beyond its specific nutrients. According to a 2018 study by Mollica et al. [217], adding Turkish hazelnuts into a high-fat diet was linked to less weight gain, lower food intake, dose-dependent increases in triglycerides, TC, and HDL-C, a reduction in LDL-Cas well as atherogenic index, and a dose-associated reduction in plasma glucose concentration. The mice’s livers were not as affected by a high-fat diet biochemically and morphologically due to hazelnuts. Pomegranate-derived vinegar (PV) was studied by Samad, Azlan, and Ismail [218] and was shown to decrease lipogenesis and increase fatty acid beta-oxidation, making it an effective option for treating obesity. Additionally, consuming PV may increase the expression of the genes for the carnitine palmitoyl transferase 1 alpha (CPT-1a) and peroxisome proliferator-activated receptor alpha (PPARa), as well as phosphorylate AMPK more effectively than acetic acid, suggesting that PV is more effective than acetic acid in reducing obesity. According to Yun et al. [161], the CBVs (citrus-blended vinegars), using different blended ratios according to mandarin vinegar (MV), showed a dose-dependent reduction in intracellular triglyceride content. At 1/100 dilution, CBV2 had the lowest triglyceride content at 136.12 mg/dL, which was 65% lower than MV’s (209.82 mg/dL). Overall, in3T3-L1 cells, CBV was found to have anti-obesity effects by lowering intracellular triglyceride levels, lipid accumulation, and the mRNA levels of genes associated with adipogenesis and lipogenesis. Compared to mice receiving alcohol HFD, the intake of tomato wine enriched with lycopene showed a substantial protective effect, decreasing the buildup of visceral fat depots, such as the perirenal adipose and epididymal depots [219]. Through uncoupled oxidative phosphorylation, brown adipose tissue contributed to thermogenesis by encouraging lipid oxidation and thermogenesis instead of lipid storage. With the addition of tomato wine and lycopene, rats on the HFD gained noticeably browner adipose tissue weight [219]. The recently discovered tetrameric stilbenes (r- and r2-viniferin) were shown to be able to prevent adipocyte differentiation and decrease the total amount of lipids in 3T3-L1 cells by down-regulating the expression of the PPAR, C/EBP, and FABP4 genes. According to these findings, p21-(CDK inhibitor) and Rb-dependent regulation of transcription in 3T3-L1 cells showed thatr2-viniferin repressed the adipogenic process and prevented the cell cycle at the G1-S phase. Pterostilbene, a methylated resveratrol derivative, was reported to suppress the adipocyte development in both 3T3-L1 preadipocytes as well as 3T3-F442A cells when administered for a longterm at low doses (5–10 M) [220].
Along with plant secondary metabolites, dietary fibers also have been paid attention to for their positive correlation with obesity. In this regard, Sung et al. [221] developed a cereal Allium fistulosum extract bar (AFB) to determine the anti-obesity effect of HFD induced in obese mice. AFB therapy in HFD mice decreased body weight increase and glucose and raised blood triacylglycerol, lipid buildup in the liver, insulin levels, adipose tissue, and weight of adipose tissue. Adiponectin and blood HDL-cholesterol levels were also greater in the AFB groups than in the HFD group. In visceral adipose tissue, AFB therapy elevated the mRNA expression of receptor genes PPAR-c, UCP2, and b3-AR. Increased expression of these genes may act as a mediator in the reduction in total fat mass and adipocyte size and also reduce blood lipid levels caused by AFB therapy. Adipose tissue has high levels of PPAR-c expression, which is crucial for adipocyte development and fat storage in the liver. In order to increase fatty acid oxidation in WAT and reduce body weight, uncoupling protein-2, i.e., UCP2, is crucial. The stimulation of these receptors by specific agonists has significant anti-diabetes and anti-obesity benefits by inducing thermogenesis in response to prolonged lipolytic stimulation in rats, which nearly exclusively express b3-Adrenoreceptors (b3-AR) [221]. Recently, the novel biscuits utilized in the study were studied as an effective integrated approach to reducing obesity levels and associated impacts with date fiber (DF) as their primary target [222]. The rats in a diet that consumed biscuits supplemented with 10% DF had the lowest TGslevels, with no difference between them and the rats who ate biscuits with 15% DF, according to the results. The diet2 groups supplemented with 10% DF biscuits demonstrated the lowest levels of VLDL, with no appreciable variations from the orlistat supplement groups (G3). Additionally, other animal models fed biscuits enriched with differing DF levels (diet1 5% DFand diet210% DF) saw a drop in assessed LDL levels [222]. Gorjanović et al. [223] used mice in their report, which were subjected to normal food supplemented along with APF (apple pomace flour) and showed a remarkable reduction in body weight growth (about 39%). Apple polyphenol administration had a curative effect on body weight gain (BWG) and accumulation of fat, as well as enhanced tolerance for glucose in Wistar rats. According to one theory, AP controls the genes responsible for adipogenesis, lipolysis, and fatty acid oxidation. Similarly, in another study, lactic acid-producing bacteria, i.e., LAB, obtained from kefir, which is a popular probiotic drink, and wine grape seed flour (GSF), a prebiotic rich in polyphenols, are linked with metabolic disorders and obesity in (HFD)-induced obese mice. For 9 weeks, these mice were given HFDs containing 6% microcrystalline cellulose (CON), HFD enriched with GSF (5% or 10% GSF), HFD with LAB administered orally, or HFD with a combined dose of GSF and LAB administered orally (GSF+LAB). All GSF and LAB groups exhibited a decline in liver and adipose tissue weights, HF-induced weight gain, plasma lipid concentrations, glucose intolerance, and insulin resistance. Adipose tissue microarray data revealed that the interaction of GSF and LAB impacted genes related to immunological and metabolic disorders, including inflammasome complex assembly [224]. In obese mice induced by an HFD, grape seed flour boosted both energy expenditure (EE) as well as thermogenesis in BAT [225]. UCP1 along with additional metabolism-related genes were likewise impacted by GSF in WAT. The results obtained concluded that GSF reduced diet-induced obesity in mice (C57BL/6J) by improving energy metabolism. The increase in EE by encouraging BAT heat generation and WAT browning is the key factor contributing to the body weight reduction in the GSF-treated mice. Additionally, GSF may reduce several aspects of insulin resistance, glucose tolerance, and plasma biochemical indices. After high doses of GSF therapy, the expressions of various genes are linked to liver diseases, lipid metabolism, and changes in energy metabolism [225].
Recently, the kiwifruit jelly with chenpi (FKJ) designed with the addition of chenpi (30.26%), kiwifruit juice (35%), and pectin (2.88%) with the optimum mix via 3D printing revealed that dietary intake, adipose tissue, and liver weight decreased significantly after the intake of FKJ in a dose-dependent effect. When 3T3-L1 adipocytes are differentiated, it significantly decreases adipogenesis. Research has also shown that chenpi extract might successfully reduce obesity and hepatic steatosis brought on by an HFD [170]. Lim et al. [226] used germinated waxy black rice (GWBR) to assess the anti-obesity effect in 3T3-L1 adipocytes. The results indicated that the hot water extract from the germinated waxy black rice (GWBR) section had the greatest protective impact against lipid accumulation in 3T3-L1 adipocytes, increasing by 41% compared with control cells. GWBR extract lowered adipocyte proliferation and reduced lipid accumulation (more red staining). When 3T3-L1 adipocyte cells were exposed to 1 mg/mL concentration of GWBR extracted in hot water, it showed a substantial increase in expression of CPT-1 mRNA, which is linked to oxidation and UCP2 mRNA expression linked to thermogenesis. In another study, the positive benefits of tea and GTCs on obesity were determined [157]. The catechin EGCG was demonstrated as one of the most potent bioactive molecules among green tea catechins (GTCs). GTCs may slow digestive enzymes and limit absorption, thereby reducing fat. White adipose tissue accumulation and restrained body weight growth promote glucose absorption and increase glucose transporter-4 expression on muscle cell plasma membranes. As a result, green tea’s anti-obesity effects in HFD-induced obesity include overexpression of glucose transporter-4, reduction in absorption, and inhibition of digestive enzymes. In another report, a comparison of commercial anti-obesity medicine, orlistat (11.3%), was performed with SCOBY jackfruit drinks and suggested that jackfruit drinks significantly improved weight management control in HFD-fed obese mice and caused considerable body weight reduction (18.5–20.2%) [227]. Blood composition and signs of inflammation in medicated obese mice showed no negative consequences. Following the SCOBY jackfruit drinks diet treatments, gene expression involved in glucose transport, inflammatory cytokines, lipid biosynthesis, and chemokines in the adipose tissues was dramatically down-regulated. The sequencing of 16S rRNA from the mice’s feces demonstrated that SCOBY jackfruit drinks had changed the makeup of the gut microbiota, with the proliferation of helpful gut microorganisms being boosted in those animals relative to all control groups. Table 4 discusses a few studies where phytochemicals are used in the development of anti-obesity food products.

Table 4.
Application of phytonutrient-based anti-obesity food supplement products.
Consumers could overlook well-intended information without targeted, concentrated campaigns or efforts [235]. Because of this, lifestyle variables, including a healthy diet and exercise, continue to be crucial for preventing and treating obesity [236].
Since there are limited pharmacological options on the market, there is an immediate need for new anti-obesity medications [237]. The majority of the anti-obesity medicines that were authorized and sold, yet, have since been pulled off the market because of dangerous side effects. Figure 11 depicts the recent timeline for the withdrawal of anti-obesity drugs from the market with their reported adverse effects. For example, fenfluramine and dexfenfluramine were pulled off the market in the 1990s due to their negative impact on heart valves. Due to an unfavorable risk-to-benefit ratio, the European Medicines Agency, or EMA, advised the removal of numerous anti-obesity medications from the market in 2000, including phentermine, diethylpropion, and mazindol. Currently, lorcaserin, phentermine, orlistat, naltrexone, and liraglutide are some of the anti-obesity active pharmaceutical components that have been authorized by the US FDA and are available on the market [238]. Only a small number of pharmaceuticals have been assigned for marketing purposes. Among those, some had to be pulled back due to serious side effects or widespread safety concerns with the approved therapies. This is despite significant research investments to find effective medications for treating obesity [239].
Figure 11.
Health implications of anti-obesity drugs withdrawn from market.
Regarding market division by application, one of the key contributors to the total sale of the nutritional product sector is the weight loss category. Over the projected period of 2015–2025, the growth of the weight loss or management category is predicted to rise at a compound yearly growth rate of about 7.4% [240]. Consumer views of the health-promoting properties of fruits, vegetables, and nuts—perceptions based on the phytonutrients found in these foods—have changed current market patterns. Phytonutrients are now added to, orextracted, orformulated in various food items and sold globally [241].
The impact of biotic and abiotic constraints, population income, technological and genetic advancement, the machinery revolution, faster access to information, the development of cities, the social and economic context, and climate changes that affect agricultural products and people’s movement from one region to another, all had a significant impact on agricultural market trends over the past ten years [242]. Indeed, phytonutrients are becoming more and more important in the market. The market for phytonutrients is predicted to develop at a compound annual growth rate (CAGR)of 7.2% between 2015 and 2020, reaching a potential value of USD 4.6 billion. Health problems, including cancer, type 2 diabetes, and cardiovascular illnesses, are the main driving factors for the worldwide phytonutrients market [243]. By dramatically boosting the intake of foods high in sugar, fat, salt, and saturated fats among children and adolescents, both food and beverage marketing in different channels and situations plays a crucial role [124]. The search for anti-obesity medications (AOMs) has become extremely difficult for both technological and societal reasons [244]. Many herbal products around the market make anti-obesity claims and are based on nutraceuticals. Nutraceutical anti-obesity supplements that are marketed include Sri Sri tattva, Jiva, Onelife amla, Lords, Dr. Bhargava, Medilexicon, and Onelife multiman [245]. The majority of anti-obesity medications have been demonstrated to enhance proxy measures of cardiovascular health, such as triglyceride concentration decreases, changes in blood pressure with weight loss, and elevations in HDL-cholesterol concentration [246]. Recent research found that the most often advertised natural weightloss compounds are chitosan, capsaicin, glucomannan, carnitine, and conjugated linoleic acid (CLA). Other widely used herbal compounds in Europe include the unroasted seeds of Coffeaarabica L. (Rubiaceae), Garcinia cambogia (Gaertn.) Desr., and Camellia sinensis (L.) Kuntze (Theaceae) [247].
Several problems were encountered when applying phytochemicals as an anti-obesity supplement/drug. The key reasons that they are frequently citedare poor stability, minor bioavailability, low water solubility, and rapid breakdown by enzymes in the digestive system, liver, kidneys, and other organs. When these phytochemicals are encapsulated, sufficient amounts may be supplied to the desired organs and cells, promoting fat loss and enhancing general metabolic status. These preliminary results, however early, may signal the beginning of a new approach to dealing with the increasing incidence of obesity that may also behighly compliant [136]. Another problem is that hand-made herbal medicines have no way of being controlled or registered, and many nations have poorly regulated markets for them. Many stakeholders have reported multiple instances of adulteration of herbal weight reduction medications. Research of 160 herbal food weight reduction supplements showed that more than 50% had six APIs (active pharmaceutical ingredients) together. In herbal medicines, unreported APIs for sibutramine, sildenafil, phenolphthalein, fluoxetine, and lorcaserin were found [248]. Anti-obesity herbal drugs, including those sold in stores and online, can vary greatly in composition. They have unpredictable high concentrations of active substances and unpredictably negative consequences. In addition to having variable quantities of active chemicals, these products can have unpredictable and potentially hazardous results. They could include adulterants, such as discontinued medications and drug mimics as well as thyroid extracts (e.g., fenfluramine). For all the aforementioned reasons, herbal anti-obesity treatments may directly be harmful or interact negatively with other drugs. Researchers, doctors, and other medical experts need to be conscious of the issue. They need to caution their patients of the possible hazards of using these drugs and their diverse character. Certain drugs and some substances in dietary supplements for weight loss may interact. When combined with other stimulants, coffee and bitter orange, for instance, this may have an additional impact. It has been demonstrated that bitter orange inhibitstheCYP3A4 function, raising blood levels of certain medications like cyclosporine and saquinavir (National Institute of Health, accessed on 7 April 2023). To boost product research and development, market participants are concentrating on consolidation and cooperation methods.
6. Future Perspectives and Conclusions
The rising global prevalence of obesity has led to a pressing need for effective preventive strategies. In this context, phytonutrients have emerged as promising candidates for anti-obesity prevention. Phytonutrients are bioactive compounds in plant-based foods that exhibit various biological activities, including antioxidant, anti-inflammatory, and metabolic modulation properties. These compounds have the potential to influence key mechanisms involved in the development and progression of obesity, making them attractive targets for preventive interventions.
One future perspective is further exploring the molecular mechanisms through which phytonutrients exert their anti-obesity effects. Understanding the specific pathways and targets that phytonutrients modulate can provide valuable insights into their potential as preventive agents. This knowledge can guide the development of targeted interventions that optimize the use of phytonutrients for anti-obesity purposes [249].
Another important aspect of future perspectives is utilizing food waste and by-products to maximize the potential of phytonutrient sources [250,251]. Many phytonutrient-rich parts of plants, such as peels, stems, and leaves, are often discarded as waste. However, these parts can be valuable sources of phytonutrients and bioactive compounds. Innovations in food processing technologies and developing novel food products can help extract and incorporate these phytonutrient-rich components into the regional diet, reducing waste and improving nutritional value.
In conclusion, the future perspective of phytonutrients as anti-obesity prevention is promising. Further research is needed to elucidate the underlying molecular mechanisms, identify specific phytonutrients with potent anti-obesity properties, develop innovative delivery systems, and explore synergistic effects with other bioactive compounds. Harnessing the potential of phytonutrients in preventive interventions can contribute to global efforts to combat obesity and promote public health. The development of innovative food supplement products and functional foods incorporating phytonutrients can offer convenient and effective ways to deliver these bioactive compounds to consumers.
Author Contributions
S.A.S.—Conceptualization, methodology, validation, data curation, writing—original draft, writing—review and editing, visualization, supervision, project administration, investigation, and resources. I.A.H.—Conceptualization and writing—original draft, review and editing. P.S.—Writing—original draft. Y.S.W.—Review. N.G.—Writing—original draft. R.C.-M.—Validation and funding. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from Nobelium Joining Gdańsk Tech Research Community (contract number DEC 33/2022/IDUB/l.1; NOBELIUM nr 036236) is gratefully acknowledged. R. Castro-Muñoz also acknowledges the School of Engineering and Science and the FEMSA-Biotechnology Center at Tecnológico de Monterrey for their support through the Bioprocess (0020209I13) Focus Group.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, 176–185. [Google Scholar]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and cancer: A current overview of epidemiology, pathogenesis, outcomes, and management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Llewellyn, A.; Simmonds, M.; Owen, C.G.; Woolacott, N. Childhood obesity as a predictor of morbidity in adulthood: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B.; Engin, A. The definition and prevalence of obesity and metabolic syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Urasaki, Y.; Le, T.T. Functional Complementation of Anti-Adipogenic Phytonutrients for Obesity Prevention and Management. Nutrients 2022, 14, 4325. [Google Scholar] [CrossRef] [PubMed]
- Shubham, K.; Anukiruthika, T.; Dutta, S.; Kashyap, A.V.; Moses, J.A.; Anandharamakrishnan, C. Iron deficiency anemia: A comprehensive review on iron absorption, bioavailability and emerging food fortification approaches. Trends Food Sci. Technol. 2020, 99, 58–75. [Google Scholar] [CrossRef]
- Tran, T.; Bønløkke, P.; Rodríguez-Rodríguez, C.; Nosrati, Z.; Esquinas, P.L.; Borkar, N.; Plum, J.; Strindberg, S.; Karagiozov, S.; Rades, T.; et al. Using in vitro lipolysis and SPECT/CT in vivo imaging to understand oral absorption of fenofibrate from lipid-based drug delivery systems. J. Control. Release 2020, 317, 375–384. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef]
- Hendriks, S.L.; Viljoen, A.; Marais, D.; Wenhold, F.A.; McIntyre, A.M.; Ngidi, M.S.; Annandale, J.G.; Stewart, D. Considerations for the design of nutrition-sensitive production programmes in rural South Africa. BMC Public Health 2020, 20, 1383. [Google Scholar] [CrossRef] [PubMed]
- Tharifkhan, S.A.; Perumal, A.B.; Elumalai, A.; Moses, J.A.; Anandharamakrishnan, C. Improvement of nutrient bioavailability in millets: Emphasis on the application of enzymes. J. Sci. Food Agric. 2021, 101, 4869–4878. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Mu, M.; Liu, K.; He, Y. Screen time and childhood overweight/obesity: A systematic review and meta-analysis. Child Care Health Dev. 2019, 45, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Neve, K.L.; Isaacs, A. How does the food environment influence people engaged in weight management? A systematic review and thematic synthesis of the qualitative literature. Obes. Rev. 2022, 23, e13398. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes. Rev. 2014, 15, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Plow, M.A.; Moore, S.; Husni, M.E.; Kirwan, J.P. A systematic review of behavioural techniques used in nutrition and weight loss interventions among adults with mobility-impairing neurological and musculoskeletal conditions. Obes. Rev. 2014, 15, 945–956. [Google Scholar] [CrossRef]
- Anderson, E.; Durstine, J.L. Physical activity, exercise, and chronic diseases: A brief review. Sports Med. Health Sci. 2014, 1, 3–10. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Yadav, P.; Vashishth, D.; Sharma, K.; Kumar, A.; Chahal, J.; Dalal, S.; Kataria, S.K. A review on obesity management through natural compounds and a green nanomedicine-based approach. Molecules 2021, 26, 3278. [Google Scholar] [CrossRef]
- Bibi, R.; Jahan, S.; Afsar, T.; Almajwal, A.; Hammadeh, M.E.; Alruwaili, N.W.; Ra/zak, S.; Amor, H. The influence of paternal overweight on sperm chromatin integrity, fertilization rate and pregnancy outcome among males attending fertility clinic for IVF/ICSI treatment. BMC Pregnancy Childbirth 2022, 22, 620. [Google Scholar] [CrossRef]
- Orgeron III, R.; Pope, J.; Erickson, D.; Green, V. Phytonutrients: A Potential Role in Obesity (P08-047-19). Curr. Dev. Nutr. 2019, 3, nzz044.P08-047-19. [Google Scholar] [CrossRef]
- World Obesity. Available online: https://data.worldobesity.org/maps/?area=trends&group=M&year=2020 (accessed on 15 July 2023).
- Martin, C.; Zhang, Y.; Tonelli, C.; Petroni, K. Plants, diet, and health. Annu. Rev. Plant Biol. 2013, 64, 19–46. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Nisar, A.; Jagtap, S.; Vyavahare, S.; Deshpande, M.; Harsulkar, A.; Ranjekar, P.; Prakash, O. Phytochemicals in the treatment of inflammation-associated diseases: The journey from preclinical trials to clinical practice. Front. Pharmacol. 2013, 14, 1177050. [Google Scholar] [CrossRef] [PubMed]
- Issaoui, M.; Delgado, A.M.; Caruso, G.; Micali, M.; Barbera, M.; Atrous, H.; Ouslati, A.; Chammem, N. Phenols, flavors, and the mediterranean diet. J. AOAC Int. 2020, 103, 915–924. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Camposeo, S.; De Gennaro, B.; Pascuzzi, S.; Roselli, L. In the ancient world, virgin olive oil was called “liquid gold” by Homer and “the great healer” by Hippocrates. Why has this mythic image been forgotten? Food Res. Int. 2014, 62, 1062–1068. [Google Scholar] [CrossRef]
- Delgado-Floody, P.; Alvarez, C.; Caamaño-Navarrete, F.; Jerez-Mayorga, D.; Latorre-Román, P. Influence of Mediterranean diet adherence, physical activity patterns, and weight status on cardiovascular response to cardiorespiratory fitness test in Chilean school children. Nutrition 2020, 71, 110621. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Maio, I.D.; Selvaggini, R.; Taticchi, A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2013, 3, 1–23. [Google Scholar] [CrossRef]
- Aloo, S.O.; Ofosu, F.K.; Kim, N.H.; Kilonzi, S.M.; Oh, D.H. Insights on Dietary Polyphenols as Agents against Metabolic Disorders: Obesity as a Target Disease. Antioxidants 2023, 12, 416. [Google Scholar] [CrossRef]
- Jin, T.; Song, Z.; Weng, J.; Fantus, I.G. Curcumin and other dietary polyphenols: Potential mechanisms of metabolic actions and therapy for diabetes and obesity. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E201–E205. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Galbete, C.; Kröger, J.; Jannasch, F.; Iqbal, K.; Schwingshackl, L.; Schwedhelm, C.; Weikert, C.; Boeing, H.; Schulze, M.B. Nordic diet, Mediterranean diet, and the risk of chronic diseases: The EPIC-Potsdam study. BMC Med. 2018, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Willcox, D.C.; Scapagnini, G.; Willcox, B.J. Healthy aging diets other than the Mediterranean: A focus on the Okinawan diet. Mech. Ageing Dev. 2014, 136, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Muroyama, K.; Yamamoto, Y.; Murosaki, S. Erratum to: Oral intake of a combination of glucosyl hesperidin and caffeine elicits an antiobesity effect in healthy, moderately obese subjects: A randomized double-blind placebo-controlled trial. Nutr. J. 2017, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Darwiche, G.; Höglund, P.; Roth, B.; Larsson, E.; Sjöberg, T.; Wohlfart, B.; Steen, S.; Ohlsson, B. An Okinawan-based Nordic diet improves anthropometry, metabolic control, and health-related quality of life in Scandinavian patients with type 2 diabetes: A pilot trial. Food Nutr. Res. 2016, 60, 32594. [Google Scholar] [CrossRef]
- Kårlund, A.; Kolehmainen, M.; Landberg, R.; Poutanen, K. Traditional and new sources of grain protein in the healthy and sustainable Nordic diet. J. Cereal Sci. 2022, 105, 103462. [Google Scholar] [CrossRef]
- FAO/WHO. FAOSTAT. 2018. Available online: https://www.fao.org/faostat/en/#data/FBS (accessed on 20 May 2023).
- Sang, S.; Chu, Y. Whole grain oats, more than just a fiber: Role of unique phytochemicals. Mol. Nutr. Food Res. 2017, 61, 1600715. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.M.; Wilcox, S.; Frongillo, E.A.; Murphy, E.A.; Hutto, B.; Wilson, M.; Davey, M.; Bernhart, J.A.; Okpara, N.; Bailey, S.; et al. Effect of a Plant-Based vs. Omnivorous Soul Food Diet on Weight and Lipid Levels Among African American Adults: A Randomized Clinical Trial. JAMA Netw. 2023, 6, e2250626. [Google Scholar] [CrossRef]
- Bello, A. The Role of Diet in the Control of Hypertension: What Africans Should Know. Available online: https://www.datelinehealthafrica.org/the-role-of-diet-in-the-control-of-hypertension-what-africans-should-know (accessed on 14 September 2023).
- Kuehn, B.M. Heritage diets and culturally appropriate dietary advice may help combat chronic diseases. JAMA 2019, 322, 2271–2273. [Google Scholar] [CrossRef]
- Medagama, A.; Widanapathirana, H. A traditional Asian diet modified to meet nutritional requirements of diabetes, has anything changed? A cross-sectional dietary survey. BMC Nutr. 2015, 1, 4–9. [Google Scholar] [CrossRef]
- Kim, G.N.; Shin, M.R.; Shin, S.H.; Lee, A.R.; Lee, J.Y.; Seo, B.I.; Kim, M.Y.; Kim, T.H.; Noh, J.S.; Rhee, M.H.; et al. Study of antiobesity effect through inhibition of pancreatic lipase activity of Diospyros kaki fruit and Citrus unshiu peel. BioMed Res. Int. 2016, 2016, 1723042. [Google Scholar] [CrossRef] [PubMed]
- Marchiori, D.; Papies, E.K. A brief mindfulness intervention reduces unhealthy eating when hungry, but not the portion size effect. Appetite 2014, 75, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Park, S.H.; Choi, E.K.; Cha, Y.S.; Cho, B.H.; Kim, Y.G.; Kim, M.G.; Song, W.O.; Park, T.S.; Ko, J.K.; et al. Beneficial Effects of Korean Traditional Diets in Hypertensive and Type 2 Diabetic Patients. J. Med. Food 2014, 17, 161–171. [Google Scholar] [CrossRef]
- Ma, G. Food, eating behavior, and culture in Chinese society. J. Ethn. Foods 2015, 2, 195–199. [Google Scholar] [CrossRef]
- Leonetti, F.; Liguori, A.; Petti, F.; Rughini, S.; Silli, L.; Liguori, S.; Bangrazi, S. Effects of basic traditional Chinese diet on body mass index, lean body mass, and eating and hunger behaviours in overweight or obese individuals. J. Tradit. Chin. Med. ChungTsa Chih Ying Wen Pan 2016, 36, 456–463. [Google Scholar] [CrossRef]
- Niu, K.; Momma, H.; Kobayashi, Y.; Guan, L.; Chujo, M.; Otomo, A.; Ouchi, E.; Nagatomi, R. The traditional Japanese dietary pattern and longitudinal changes in cardiovascular disease risk factors in apparently healthy Japanese adults. Eur. J. Nutr. 2016, 55, 267–279. [Google Scholar] [CrossRef]
- Milinovic, J.; Mata, P.; Diniz, M.; Noronha, J.P. Umami taste in edible seaweeds: The current comprehension and perception. Int. J. Gastron. Food Sci. 2021, 23, 100301. [Google Scholar] [CrossRef]
- Campbell, A.P. DASH Eating Plan: An Eating Pattern for Diabetes Management. Diabetes Spectr. 2017, 30, 76–81. [Google Scholar] [CrossRef]
- Salehi-Abargouei, A.; Maghsoudi, Z.; Shirani, F.; Azadbakht, L. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases—Incidence: A systematic review and meta-analysis on observational prospective studies. Nutrition 2013, 29, 611–618. [Google Scholar] [CrossRef]
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap—Deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef] [PubMed]
- Niaz, K.; Shah, M.A.; Khan, F.; Saleem, U.; Vargas, C.; Panichayupakaranant, P. Bioavailability and safety of phytonutrients. In Phytonutrients in Food; Elsevier: Amsterdam, The Netherlands, 2020; pp. 117–136. [Google Scholar] [CrossRef]
- Beane, K.E.; Redding, M.C.; Wang, X.; Pan, J.H.; Le, B.; Cicalo, C.; Young, S.J.; Kim, J.; Lee, J.H.; Shin, E.C.; et al. Effects of dietary fibers, micronutrients, and phytonutrients on gut microbiome: A review. Appl. Biol. Chem. 2021, 64, 36. [Google Scholar] [CrossRef]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent updates on bioaccessibility of phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- Martin, C. The Interface between Plant Metabolic Engineering and Human Health. Curr. Opin. Biotechnol. 2013, 24, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Barraj, L.M.; Herman, D.; Bi, X.; Cheatham, R.; Randolph, R.K. Phytonutrient intake by adults in the United States in relation to fruit and vegetable consumption. J. Acad. Nutr. Diet. 2012, 112, 222–229. [Google Scholar] [CrossRef]
- Elsamanoudy, A.Z.; Neamat-Allah, M.A.M.; Mohammad, F.A.H.; Hassanien, M.; Nada, H.A. The role of nutrition related genes and nutrigenetics in understanding the pathogenesis of cancer. J. Microsc. Ultrastruct. 2016, 4, 115. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mengxue, G.; Guangbo, K.; He, H. The Potential Role of Phytonutrients Flavonoids Influencing Gut Microbiota in the Prophylaxis and Treatment of Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 798038. [Google Scholar] [CrossRef] [PubMed]
- Holst, B.; Gary, W. Nutrients and Phytochemicals: From Bioavailability to Bioefficacy beyond Antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef]
- Liu, R.H. Health Benefits of Fruit and Vegetables Are from Additive and Synergistic Combinations of Phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The Synergistic and Antagonistic Antioxidant Interactions of Dietary Phytochemical Combinations. Crit. Rev. Food Sci. Nutr. 2003, 62, 5658–5677. [Google Scholar] [CrossRef]
- de Kok, T.M.; van Breda, S.G.; Manson, M.M. Mechanisms of combined action of different chemopreventive dietary compounds: A review. Eur. J. Nutr. 2008, 47, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, M.K. Nutrition Benefits and Considerations for Whole Foods Plant-Based Eating Patterns. Am. J. Lifestyle Med. 2022, 16, 284–290. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food Processing Strategies to Enhance Phenolic Compounds Bioaccessibility and Bioavailability in Plant-Based Foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J.D. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Złotek, U.; Zielińska, E. Digestion and bioavailability of bioactive phytochemicals. Int. J. Food Sci. Technol. 2017, 52, 291–305. [Google Scholar] [CrossRef]
- Santhiravel, S.; Bekhit, A.E.D.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The impact of plant phytochemicals on the gut microbiota of humans for a balanced life. Int. J. Mol. Sci. 2022, 23, 8124. [Google Scholar] [CrossRef]
- McGhie, T.K.; Walton, M.C. The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2022, 51, 702–713. [Google Scholar] [CrossRef]
- Walle, T. Absorption and Metabolism of Flavonoids. Free. Radic. Biol. Med. 2004, 36, 829–837. [Google Scholar] [CrossRef]
- Kan, J.; Wu, F.; Wang, F.; Zheng, J.; Cheng, J.; Li, Y.; Yang, Y.; Du, J. Phytonutrients: Sources, bioavailability, interaction with gut microbiota, and their impacts on human health. Front. Nutr. 2022, 9, 960309. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.K.; Zahid, A.; Shah, F.H. Role of food product development in increased food consumption and value addition. In Food Processing for Increased Quality and Consumption; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 455–479. [Google Scholar] [CrossRef]
- Xiao, J.Y.C.; Huang, Q. Edible Nanoencapsulation Vehicles for Oral Delivery of Phytochemicals: A Perspective Paper. J. Agric. Food Chem. 2017, 65, 6727–6735. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, H.; Shahidi, F.; Luo, T.; Deng, Z. Interactions among dietary phytochemicals and nutrients: Role of cell membranes. Trends Food Sci. Technol. 2022, 124, 38–50. [Google Scholar] [CrossRef]
- Harahap, I.A.; Sobral, M.M.C.; Casal, S.; Pinho, S.; Faria, M.A.; Suliburska, J.; Ferreira, I.M. Fat oxidation of fatty fish vs. meat meal diets under in vitro standardized semi-dynamic gastric digestion. Front. Nutr. 2022, 9, 901006. [Google Scholar] [CrossRef] [PubMed]
- Kritchevsky, D. Dietary Fibre in Health and Disease. In Advanced Dietary Fibre Technology; Wiley: Hoboken, NJ, USA, 2000; pp. 147–161. [Google Scholar] [CrossRef]
- Shahidi, F.; Pan, Y. Influence of Food Matrix and Food Processing on the Chemical Interaction and Bioaccessibility of Dietary Phytochemicals: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6421–6445. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Whole grains and phenolic acids: A review on bioactivity, functionality, health benefits and bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef]
- Tosh, S.M.; Bordenave, N. Emerging science on benefits of whole grain oat and barley and their soluble dietary fibers for heart health, glycemic response, and gut microbiota. Nutr. Rev. 2021, 78, 13–20. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in Nanoparticle and Microparticle Delivery Systems for Increasing the Dispersibility, Stability, and Bioactivity of Phytochemicals. Biotechnol. Adv. 2020, 38, 107287. [Google Scholar] [CrossRef]
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 57, 59–81. [Google Scholar] [CrossRef]
- Borel, P.; Dangles, O.; Kopec, R.E. Fat-soluble vitamin and phytochemical metabolites: Production, gastrointestinal absorption, and health effects. Prog. Lipid Res. 2023, 90, 101220. [Google Scholar] [CrossRef] [PubMed]
- Mashurabad, P.C.; Palika, R.; Jyrwa, Y.W.; Bhaskarachary, K.; Pullakhandam, R. Dietary fat composition, food matrix and relative polarity modulate the micellarization and intestinal uptake of carotenoids from vegetables and fruits. J. Food Sci. Technol. 2017, 54, 333–341. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Xiao, H. Excipient foods: Designing food matrices that improve the oral bioavailability of pharmaceuticals and nutraceuticals. Food Funct. 2014, 5, 1320–1333. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J. Food Sci. 2010, 75, R50–R57. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lin, Q.; Zhao, H.; Li, X.; Sang, S.; McClements, D.J.; Long, J.; Jin, Z.; Wang, J.; Qiu, C. Bioaccessibility and bioavailability of phytochemicals: Influencing factors, improvements, and evaluations. Food Hydrocoll. 2022, 135, 108165. [Google Scholar] [CrossRef]
- Porrini, M.; Patrizia, R. Factors Influencing the Bioavailability of Antioxidants in Foods: A Critical Appraisal. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 647–650. [Google Scholar] [CrossRef]
- Boronat, A.; Rodriguez-Morató, J.; Serreli, G.; Fitó, M.; Tyndale, R.F.; Deiana, M.; de la Torre, R. Contribution of biotransformations carried out by the microbiota, drug-metabolizing enzymes, and transport proteins to the biological activities of phytochemicals found in the diet. Adv. Nutr. 2021, 12, 2172–2189. [Google Scholar] [CrossRef]
- Borel, P.; Charles, D. Bioavailability of Fat-Soluble Vitamins and Phytochemicals in Humans: Effects of Genetic Variation. Annu. Rev. Nutr. 2018, 38, 69–96. [Google Scholar] [CrossRef]
- Hoda, M.; Hemaiswarya, S.; Doble, M. Role of phenolic phytochemicals in diabetes management. Role of Phenolic Phytochemicals in Diabetes Management; Springer: Singapore, 2019; pp. 159–173. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, P.; Duan, S.; Wan, X.; Xing, H.; Yang, J.; Zhang, X.; Yao, Z.; Yao, X. Potential determinants for metabolic fates and inhibitory effects of isobavachalcone involving in human cytochrome P450, UDP-glucuronosyltransferase enzymes, and efflux transporters. J. Pharm. Sci. 2021, 110, 2285–2294. [Google Scholar] [CrossRef]
- vel Szic, K.S.; Declerck, K.; Vidaković, M.; Vanden Berghe, W. From inflammaging to healthy aging by dietary lifestyle choices: Is epigenetics the key to personalized nutrition? Clin. Epigenet. 2015, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Giovanni, S. Interactions between Dietary Polyphenols and Aging Gut Microbiota: A Review. BioFactors 2022, 48, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of celiac disease and other gluten related disorders in wheat and strategies for mitigating them. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef]
- Theethira, T.G.; Dennis, M. Celiac disease and the gluten-free diet: Consequences and recommendations for improvement. Dig. Dis. 2015, 33, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Caeiro, C.; Pragosa, C.; Cruz, M.C.; Pereira, C.D.; Pereira, S.G. The role of pseudocereals in celiac disease: Reducing nutritional deficiencies to improve well-being and health. J. Nutr. Metab. 2022, 2022, 8502169. [Google Scholar] [CrossRef]
- Shabbir, M.A.; Mehak, F.; Khan, Z.M.; Ahmad, W.; Khan, M.R.; Zia, S.; Rahaman, A.; Aadil, R.M. Interplay between ceramides and phytonutrients: New insights in metabolic syndrome. Trends Food Sci. Technol. 2021, 111, 483–494. [Google Scholar] [CrossRef]
- Rémond, D.; Shahar, D.R.; Gille, D.; Pinto, P.; Kachal, J.; Peyron, M.A.; Dos Santos, C.N.; Walther, B.; Bordoni, A.; Dupont, D.; et al. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 2015, 6, 13858–13898. [Google Scholar] [CrossRef]
- Shahmohammadi, H.A.; Hosseini, S.A.; Hajiani, E.; Malehi, A.S.; Alipour, M. Effects of green coffee bean extract supplementation on patients with non-alcoholic fatty liver disease: A randomized clinical trial. Hepat. Mon. 2017, 17, e12299. [Google Scholar] [CrossRef]
- Gerges, S.H.; Wahdan, S.A.; Elsherbiny, D.A.; El-Demerdash, E. Non-alcoholic fatty liver disease: An overview of risk factors, pathophysiological mechanisms, diagnostic procedures, and therapeutic interventions. Life Sci. 2021, 271, 119220. [Google Scholar] [CrossRef]
- Ota, S.; Morita, A.; Ohnuki, S.; Hirata, A.; Sekida, S.; Okuda, K.; Ohya, Y.; Kawano, S. Carotenoid dynamics and lipid droplet containing astaxanthin in response to light in the green alga Haematococcus pluvialis. Sci. Rep. 2018, 8, 5617. [Google Scholar] [CrossRef]
- Battino, M.; Giampieri, F.; Cianciosi, D.; Ansary, J.; Chen, X.; Zhang, D.; Gil, E.; Forbes-Hernández, T. The roles of strawberry and honey phytochemicals on human health: A possible clue on the molecular mechanisms involved in the prevention of oxidative stress and inflammation. Phytomedicine 2021, 86, 153170. [Google Scholar] [CrossRef] [PubMed]
- Mercali, G.D.; Jaeschke, D.P.; Tessaro, I.C.; Marczak, L.D.F. Study of vitamin C degradation in acerola pulp during ohmic and conventional heat treatment. LWT-Food Sci. Technol. 2012, 47, 91–95. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Aoyagi, H. Thermal and UV degradation kinetics of water-soluble extracellular pigment produced by Talaromyces purpurogenus. Food Bioprocess Technol. 2022, 15, 606–619. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, J.; Lv, F.; Chen, S.; Chen, J.; Liu, D.; Ye, X. Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes. Food Chem. 2016, 197, 1264–1270. [Google Scholar] [CrossRef]
- Ioannou, I.; Chekir, L.; Ghoul, M. Effect of heat treatment and light exposure on the antioxidant activity of flavonoids. Processes 2020, 8, 1078. [Google Scholar] [CrossRef]
- Shahidi, F.; Chandrasekara, A.; Zhong, Y. Bioactive phytochemicals in vegetables. In Handbook of Vegetables and Vegetable Processing; Wiley: Hoboken, NJ, USA, 2018; Volume 1–2, pp. 181–222. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef]
- Tiwari, U.; Cummins, E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre-and post-harvest food processing operations. Food Res. Int. 2013, 50, 497–506. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic compounds and plant phenolic extracts as natural antioxidants in prevention of lipid oxidation in seafood: A detailed review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Sommano, S.R.; Chanasut, U.; Kumpoun, W. Enzymatic browning and its amelioration in fresh-cut tropical fruits. In Fresh-Cut Fruits and Vegetables; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 51–76. [Google Scholar] [CrossRef]
- Poljsak, B. Strategies for Reducing or Preventing the Generation of Oxidative Stress. Oxidative Med. Cell. Longev. 2011, 2011, 194586. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S. Current Evidence on the Effect of Dietary Polyphenols Intake on Brain Health. Curr. Nutr. Food Sci. 2020, 16, 1170–1182. [Google Scholar] [CrossRef]
- Jabeen, N.S.; Kiruthiga, V.; Vinodhini, A.; Rudrapal, M. Herbs, Spices, and Dietary Constituents as Sources of Phytoantioxidants. In Phytoantioxidants and Nanotherapeutics; Wiley: Hoboken, NJ, USA, 2022; pp. 55–76. [Google Scholar] [CrossRef]
- Duarte, L.; Gasaly, N.; Poblete-Aro, C.; Uribe, D.; Echeverria, F.; Gotteland, M.; Garcia-Diaz, D.F. Polyphenols and their anti-obesity role mediated by the gut microbiota: A comprehensive review. Rev. Endocr. Metab. Disord. 2021, 22, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; D’Angelo, S. Anti-obesity effects of polyphenol intake: Current status and future possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef] [PubMed]
- Jack, B.U.; Malherbe, C.J.; Mamushi, M.; Muller, C.J.; Joubert, E.; Louw, J.; Pheiffer, C. Adipose tissue as a possible therapeutic target for polyphenols: A case for Cyclopia extracts as anti-obesity nutraceuticals. Biomed. Pharmacother. 2019, 120, 109439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X. Interactions of tea polyphenols with intestinal microbiota and their implication for anti-obesity. J. Sci. Food Agric. 2020, 100, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef]
- Song, D.; Cheng, L.; Zhang, X.; Wu, Z.; Zheng, X. The modulatory effect and the mechanism of flavonoids on obesity. J. Food Biochem. 2019, 43, e12954. [Google Scholar] [CrossRef]
- Oliveira, A.K.D.S.; de Oliveira e Silva, A.M.; Pereira, R.O.; Santos, A.S.; Barbosa Junior, E.V.; Bezerra, M.T.; Barreto, R.S.S.; Quintans-Junior, L.J.; Quintans, J.S. Anti-obesity properties and mechanism of action of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7827–7848. [Google Scholar] [CrossRef]
- Varshney, R.; Mishra, R.; Das, N.; Sircar, D.; Roy, P. A comparative analysis of various flavonoids in the regulation of obesity and diabetes: An in vitro and in vivo study. J. Funct. Foods 2019, 59, 194–205. [Google Scholar] [CrossRef]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Gunawan, W.B.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids Composition of Green Algae Caulerpa racemosa and Their Antidiabetic, Anti-Obesity, Antioxidant, and Anti-Inflammatory Properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K. Anti-Obesity Therapy by Food Component: Unique Activity of Marine Carotenoid, Fucoxanthin. Obes. Control. Ther. Open Access 2013, 1, 3267. [Google Scholar] [CrossRef]
- Kim, H.J.; Koo, K.A.; Park, W.S.; Kang, D.M.; Kim, H.S.; Lee, B.Y.; Woo, D.K.; Kwak, S.S.; Ahn, M.J. Anti-obesity activity of anthocyanin and carotenoid extracts from color-fleshed sweet potatoes. J. Food Biochem. 2020, 44, e13438. [Google Scholar] [CrossRef]
- Stefania, D.S.; Clodoveo, M.L.; Cariello, M.; D’Amato, G.; Franchini, C.; Faienza, M.F.; Corbo, F. Polyphenols and obesity prevention: Critical insights on molecular regulation, bioavailability and dose in preclinical and clinical settings. Crit. Rev. Food Sci. Nutr. 2021, 61, 1804–1826. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef]
- Goktas, Z.; Zu, Y.; Abbasi, M.; Galyean, S.; Wu, D.; Fan, Z.; Wang, S. Recent advances in nanoencapsulation of phytochemicals to combat obesity and its comorbidities. J. Agric. Food Chem. 2020, 68, 8119–8131. [Google Scholar] [CrossRef]
- Pekkinen, J.; Rosa, N.N.; Savolainen, O.I.; Keski-Rahkonen, P.; Mykkänen, H.; Poutanen, K.; Micard, V.; Hanhineva, K. Disintegration of wheat aleurone structure has an impact on the bioavailability of phenolic compounds and other phytochemicals as evidenced by altered urinary metabolite profile of diet-induced obese mice. Nutr. Metab. 2014, 11, 1. [Google Scholar] [CrossRef]
- Azhar, Y.; Parmar, A.; Miller, C.N.; Samuels, J.S.; Rayalam, S. Phytochemicals as novel agents for the induction of browning in white adipose tissue. Nutr. Metab. 2016, 13, 89. [Google Scholar] [CrossRef]
- Mele, L.; Bidault, G.; Mena, P.; Crozier, A.; Brighenti, F.; Vidal-Puig, A.; Del Rio, D. Dietary (Poly) phenols, brown adipose tissue activation, and energy expenditure: A narrative review. Adv. Nutr. 2017, 8, 694–704. [Google Scholar] [CrossRef]
- Mopuri, R.; Islam, M.S. Medicinal plants and phytochemicals with anti-obesogenic potentials: A review. Biomed. Pharmacother. 2017, 89, 1442–1452. [Google Scholar] [CrossRef]
- Salvadó, M.J.; Casanova, E.; Fernández-Iglesias, A.; Arola, L.; Bladé, C. Roles of proanthocyanidin rich extracts in obesity. Food Funct. 2015, 6, 1053–1071. [Google Scholar] [CrossRef]
- Balaji, M.; Ganjayi, M.S.; Kumar, G.E.H.; Parim, B.N.; Mopuri, R.; Dasari, S. A review on possible therapeutic targets to contain obesity: The role of phytochemicals. Obes. Res. Clin. Pract. 2016, 10, 363–380. [Google Scholar] [CrossRef]
- Saha, M.; Mukherjee, S.; Das, S.; Chowdhury, M.; Das, M.; Sarkar, S.; Chatterjee, S. Phytonutrient screening and evaluation of in-vitro antibacterial activity of onion and garlic peels: A comparative study with the prospects of waste to wealth. Int. J. Herb. Med. 2022, 10, 39–44. [Google Scholar] [CrossRef]
- Carpene, C.; Gomez-Zorita, S.; Deleruyelle, S.; Carpene, M.A. Novel strategies for preventing diabetes and obesity complications with natural polyphenols. Curr. Med. Chem. 2015, 22, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Jiang, H.; Jiang, B.; Zhang, T.; Cui, S.W.; Jin, Z. Phytonutrients for controlling starch digestion: Evaluation of grape skin extract. Food Chem. 2014, 145, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Shimizu, H.; Okazaki, Y.; Sakaguchi, H.; Taira, T.; Suzuki, T.; Chiji, H. Anthocyanin-rich phytochemicals from aronia fruits inhibit visceral fat accumulation and hyperglycemia in high-fat diet-induced dietary obese rats. J. Oleo Sci. 2015, 64, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Saad, B.; Ghareeb, B.; Kmail, A. Metabolic and epigenetics action mechanisms of antiobesity medicinal plants and phytochemicals. Evid. Based Complement. Altern. Med. 2021, 2021, 9995903. [Google Scholar] [CrossRef] [PubMed]
- Mounien, L.; Tourniaire, F.; Landrier, J.F. Anti-obesity effect of carotenoids: Directimpact on adipose tissue and adipose tissue-driven indirect effects. Nutrients 2019, 11, 1562. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Zhang, C.; Sun, G.; Sun, X. Effect of Panax notoginseng saponins and major anti-obesity components on weight loss. Front. Pharmacol. 2021, 11, 601751. [Google Scholar] [CrossRef]
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Managing obesity through natural polyphenols: A review. Future Foods 2020, 1–2, 100002. [Google Scholar] [CrossRef]
- Weng, G.; Duan, Y.; Zhong, Y.; Song, B.; Zheng, J.; Zhang, S.; Yin, Y.; Deng, J. Plant extracts in obesity: A role of gut microbiota. Front. Nutr. 2021, 8, 727951. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.C.; Hsieh, P.H.; Pan, M.H.; Ho, C.T. Cellular models for the evaluation of the antiobesity effect of selected phytochemicals from food and herbs. J. Food Drug Anal. 2017, 25, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Alkhalidy, H.; Moore, W.; Zhang, Y.; McMillan, R.; Wang, A.; Ali, M.; Suh, K.S.; Zhen, W.; Cheng, Z.; Jia, Z.; et al. Small molecule kaempferol promotes insulin sensitivity and preserved pancreatic β-cell mass in middle-aged obese diabetic mice. J. Diabetes Res. 2015, 2015, 532984. [Google Scholar] [CrossRef]
- Gourineni, V.; Shay, N.F.; Chung, S.; Sandhu, A.K.; Gu, L. Muscadine grape (Vitis rotundifolia) and wine phytochemicals prevented obesity-associated metabolic complications in C57BL/6J mice. J. Agric. Food Chem. 2012, 60, 7674–7681. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Miranda-Garcia, O.; Adamson, A.; Sasaki, G.; Shay, N.F. Development of obesity is reduced in high-fat fed mice fed whole raspberries, raspberry juice concentrate, and a combination of the raspberry phytochemicals ellagic acid and raspberry ketone. J. Berry Res. 2016, 6, 213–223. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Wu, T.; Dai, S.; Xu, J.; Zhou, Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015, 6, 297–304. [Google Scholar] [CrossRef]
- Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial effects of tea and the green tea catechin epigallocatechin-3-gallate on obesity. Molecules 2016, 21, 1305. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, J.; Yi, K.; Peng, L.; Xie, J.; Gou, X.; Peng, T.; Tang, L. Phlorizin ameliorates obesity-associated endotoxemia and insulin resistance in high-fat diet-fed mice by targeting the gut microbiota and intestinal barrier integrity. Gut Microbes 2020, 12, 1842990. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.V.; Needs, P.W. Apple peel bioactive rich extracts effectively inhibit in vitro human LDL cholesterol oxidation. Food Chem. 2013, 138, 463–470. [Google Scholar] [CrossRef]
- Khanam, S.; Mishra, A.; Shahid, A.; Pujari, N.M. Therapeutic indication of Phloridzin: A new Gleam for metabolic disorders. Phytomed. Plus 2022, 2, 100200. [Google Scholar] [CrossRef]
- Yun, Y.R.; Park, B.Y.; Kim, S.H.; Jung, J.H. Antioxidant, anti-obesity, and anti-aging activities of Jeju citrus blended vinegar. Foods 2021, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.K.; Cho, S.J.; Jung, U.J.; Ryu, R.; Choi, M.S. Phlorizin supplementation attenuates obesity, inflammation, and hyperglycemia in diet-induced obese mice fed a high-fat diet. Nutrients 2016, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Mao, L.; Xu, P.; Wang, Y. Effects of (−)-epigallocatechin gallate (EGCG) on energy expenditure and microglia-mediated hypothalamic inflammation in mice fed a high-fat diet. Nutrients 2018, 10, 1681. [Google Scholar] [CrossRef]
- Li, F.; Gao, C.; Yan, P.; Zhang, M.; Wang, Y.; Hu, Y.; Wu, X.; Wang, X.; Sheng, J. EGCG Reduces Obesity and White Adipose Tissue Gain Partly Through AMPK Activation in Mice. Front. Pharmacol. 2018, 9, 1366. [Google Scholar] [CrossRef]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef]
- Doan, K.V.; Ko, C.M.; Kinyua, A.W.; Yang, D.J.; Choi, Y.H.; Oh, I.Y.; Nguyen, N.M.; Ko, A.; Choi, J.W.; Joeng, Y.; et al. Gallic acid regulates body weight and glucose homeostasis through AMPK activation. Endocrinology 2015, 156, 157–168. [Google Scholar] [CrossRef]
- Tanaka, M.; Sugama, A.; Sumi, K.; Shimizu, K.; Kishimoto, Y.; Kondo, K.; Iida, K. Gallic acid regulates adipocyte hypertrophy and suppresses inflammatory gene expression induced by the paracrine interaction between adipocytes and macrophages in vitro and in vivo. Nutr. Res. 2020, 73, 58–66. [Google Scholar] [CrossRef]
- Peng, M.; Gao, Z.; Liao, Y.; Guo, J.; Shan, Y. Development of functional kiwifruit jelly with chenpi (FKJ) by 3D food printing technology and its anti-obesity and antioxidant potentials. Foods 2022, 11, 1894. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Russo, G.L.; Daglia, M.; Nabavi, S.M. Role of quercetin as an alternative for obesity treatment: You are what you eat! Food Chem. 2015, 179, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Fernández-Quintela, A.; Arias, N.; Portillo, M.P. Resveratrol: Anti-obesity mechanisms of action. Molecules 2014, 19, 18632–18655. [Google Scholar] [CrossRef]
- Zhao, L.; Cen, F.; Tian, F.; Li, M.J.; Zhang, Q.; Shen, H.Y.; Shen, X.C.; Du, J. Combination treatment with quercetin and resveratrol attenuates high fat diet-induced obesity and associated inflammation in rats via the AMPKα1/SIRT1 signaling pathway. Exp. Ther. Med. 2017, 14, 5942–5948. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, B.; Shen, J.; Wan, L.; Zhu, Y.; Yi, T.; Xiao, Z. The beneficial effects of quercetin, curcumin, and resveratrol in obesity. Oxidative Med. Cell. Longev. 2017, 2017, 1459497. [Google Scholar] [CrossRef] [PubMed]
- Konings, E.; Timmers, S.; Boekschoten, M.V.; Goossens, G.H.; Jocken, J.W.; Afman, L.A.; Müller, M.; Schrauwen, P.; Mariman, E.C.; Blaak, E. EThe effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int. J. Obes. 2014, 38, 470–473. [Google Scholar] [CrossRef]
- Mongioì, L.M.; Perelli, S.; Condorelli, R.A.; Barbagallo, F.; Crafa, A.; Cannarella, R.; Vignera, S.L.; Calogero, A.E. The role of resveratrol in human male fertility. Molecules 2021, 26, 2495. [Google Scholar] [CrossRef]
- Matsuura, H.N.; Fett-Neto, A.G. Plant alkaloids: Main features, toxicity, and mechanisms of action. Plant Toxins 2015, 2, 1–15. [Google Scholar] [CrossRef]
- Saad, B.; Zaid, H.; Shanak, S.; Kadan, S. Anti-diabetes and anti-obesity medicinal plants and phytochemicals. In Anti-diabetes and Anti-obesity Medicinal Plants and Phytochemicals; Springer: Cham, Switzerland, 2017; pp. 129–144. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Sekhon-Loodu, S.; Mantso, T.; Panayiotidis, M.I. Phytochemicals in regulating fatty acid β-oxidation: Potential underlying mechanisms and their involvement in obesity and weight loss. Pharmacol. Ther. 2016, 165, 153–163. [Google Scholar] [CrossRef]
- Chow, Y.L.; Sogame, M.; Sato, F. 13-Methylberberine, a berberine analogue with stronger anti-adipogenic effects on mouse 3T3-L1 cells. Sci. Rep. 2016, 6, 38129. [Google Scholar] [CrossRef]
- Ma, C.; Wang, J.; Chu, H.; Zhang, X.; Wang, Z.; Wang, H.; Li, G. Purification and characterization of aporphine alkaloids from leaves of Nelumbo nucifera Gaertn and their effects on glucose consumption in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2014, 15, 3481–3494. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, E.S.; Lee, C.; Kim, S.; Cho, S.H.; Hwang, B.Y.; Lee, M.K. Chemical constituents from Nelumbo nucifera leaves and their anti-obesity effects. Bioorganic Med. Chem. Lett. 2013, 23, 3604–3608. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; De, P. Spectrum of biological properties of cinchona alkaloids: A brief review. J. Pharmacogn. Phytochem. 2017, 6, 162–166. [Google Scholar]
- Huang, L.; Li, D.; Xu, Y.S.; Feng, Z.L.; Meng, F.C.; Zhang, Q.W.; Gan, L.S.; Lin, L.G. Clausoxamine, an alkaloid possessing a 1, 3-oxazine-4-one ring from the seeds of Clausena lansium and the anti-obesity effect of lansiumamide B. RSC Adv. 2017, 7, 46900–46905. [Google Scholar] [CrossRef]
- Jagtap, S.; Khare, P.; Mangal, P.; Kondepudi, K.K.; Bishnoi, M.; Bhutani, K.K. Effect of mahanimbine, an alkaloid from curry leaves, on high-fat diet-induced adiposity, insulin resistance, and inflammatory alterations. BioFactors 2017, 43, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S. Introductory Chapter: Terpenes and Terpenoids. In Terpenes and Terpenoids; IntechOpen: Benin City, Nigeria, 2018. [Google Scholar]
- Bonet, M.L.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids in adipose tissue biology and obesity. Carotenoids Nat. Biosynth. Regul. Funct. 2016, 79, 377–414. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qi, J.; Li, L. Phytochemicals as potential candidates to combat obesity via adipose non-shivering thermogenesis. Pharmacol. Res. 2019, 147, 104393. [Google Scholar] [CrossRef]
- Liu, M.; Liu, H.; Xie, J.; Xu, Q.; Pan, C.; Wang, J.; Wu, X.; Sanabil; Zheng, M.; Liu, J. Anti-obesity effects of zeaxanthin on 3T3-L1 preadipocyte and high fat induced obese mice. Food Funct. 2017, 8, 3327–3338. [Google Scholar] [CrossRef]
- Vinha, A.F.; Barreira, S.V.; Costa, A.S.; Alves, R.C.; Oliveira, M.B.P. Pre-meal tomato (Lycopersicon esculentum) intake can have anti-obesity effects in young women? Int. J. Food Sci. Nutr. 2014, 65, 1019–1026. [Google Scholar] [CrossRef]
- Jeepipalli, S.P.; Du, B.; Sabitaliyevich, U.Y.; Xu, B. New insights into potential nutritional effects of dietary saponins in protecting against the development of obesity. Food Chem. 2020, 318, 126474. [Google Scholar] [CrossRef]
- Leal-Díaz, A.M.; Noriega, L.G.; Torre-Villalvazo, I.; Torres, N.; Alemán-Escondrillas, G.; López-Romero, P.; Sánchez-Tapia, M.; Aguilar-López, M.; Furuzawa-Carballeda, J.; Velázquez-Villegas, L.A.; et al. Aguamiel concentrate from Agave salmiana and its extracted saponins attenuated obesity and hepatic steatosis and increased Akkermansia muciniphila in C57BL6 mice. Sci. Rep. 2016, 6, 34242. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of saponins on lipid metabolism: A review of potential health benefits in the treatment of obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Kang, B.; Suh, H.J.; Choi, H.S. Red ginseng-derived saponin fraction suppresses the obesity-induced inflammatory responses via Nrf2-HO-1 pathway in adipocyte-macrophage co-culture system. Biomed. Pharmacother. 2018, 108, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, H.; Zhao, Y.; Zhu, H.; Cai, E.; Gao, Y.; Liu, S.; Yang, H.; Zhang, L. Saponins from stems and leaves of Panax ginseng prevent obesity via regulating thermogenesis, lipogenesis and lipolysis in high-fat diet-induced obese C57BL/6 mice. Food Chem. Toxicol. 2017, 106, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Hu, X.; Zhang, T.; Dong, P.; Li, Z.; Xue, C.; Chang, Y.; Wang, Y. Saponin from sea cucumber exhibited more significant effects than ginsenoside on ameliorating high fat diet-induced obesity in C57BL/6 mice. MedChemComm 2018, 9, 725–734. [Google Scholar] [CrossRef]
- Song, M.Y.; Kim, B.S.; Kim, H. Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. J. Ginseng Res. 2014, 38, 106–115. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, Y.; Cai, Z.; Xu, B. Saponins and flavonoids from adzuki bean (Vigna angularis L.) ameliorate high-fat diet-induced obesity in ICR mice. Front. Pharmacol. 2017, 8, 687. [Google Scholar] [CrossRef]
- Abd Allah, A.L.; Abd-Elrahman, W.M. Hypocholesterolemic and anti-obesity effects of radish sprouts (Raphanus sativus) in adult females. Egypt. J. Food Sci. 2021, 49, 19–34. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, R. Health promoting phytochemicals in vegetables: A mini review. Int. J. Food Ferment. Technol. 2018, 8, 107–117. [Google Scholar] [CrossRef]
- Ngamsamer, C.; Sirivarasai, J.; Sutjarit, N. The benefits of anthocyanins against obesity-induced inflammation. Biomolecules 2022, 12, 852. [Google Scholar] [CrossRef]
- Badshah, H.; Ullah, I.; Kim, S.E.; Kim, T.H.; Lee, H.Y.; Kim, M.O. Anthocyanins attenuate body weight gain via modulating neuropeptide Y and GABAB1 receptor in rats hypothalamus. Neuropeptides 2013, 47, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Bakuradze, T.; Tausend, A.; Galan, J.; Groh, I.A.M.; Berry, D.; Tur, J.A.; Marko, D.; Richling, E. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free. Radic. Res. 2019, 53, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xia, M.; Zou, T.; Ling, W.; Zhong, R.; Zhang, W. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J. Nutr. Biochem. 2012, 23, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Dey, P. Gut Microbiota in Phytopharmacology: A Comprehensive Overview of Concepts, Reciprocal Interactions, Biotransformations and Mode of Actions. Pharmacol. Res. 2019, 147, 104367. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, M.; Lee, M. Effects of anthocyanin supplementation on reduction of obesity criteria: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2021, 13, 2121. [Google Scholar] [CrossRef]
- Park, S.; Kang, S.; Jeong, D.Y.; Jeong, S.Y.; Park, J.J.; Yun, H.S. Cyanidin and malvidin in aqueous extracts of black carrots fermented with Aspergillus oryzae prevent the impairment of energy, lipid and glucose metabolism in estrogen-deficient rats by AMPK activation. Genes Nutr. 2015, 10, 6. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Yue, Y.; Shen, P.; Park, Y. Epigallocatechin-3-gallate reduces fat accumulation in Caenorhabditis elegans. Prev. Nutr. Food Sci. 2018, 23, 214–219. [Google Scholar] [CrossRef]
- Peng, C.H.; Cheng, J.J.; Yu, M.H.; Chung, D.J.; Huang, C.N.; Wang, C.J. Solanum nigrum polyphenols reduce body weight and body fat by affecting adipocyte and lipid metabolism. Food Funct. 2020, 11, 483–492. [Google Scholar] [CrossRef]
- Roberts, A.T. Gallic Acid: Inhibiting Angiogenesis in Adipose Tissue; Louisiana State University and Agricultural & Mechanical College: Baton Rouge, LA, USA, 2006. [Google Scholar]
- Guo, X.; Zhang, T.; Shi, L.; Gong, M.; Jin, J.; Zhang, Y.; Liu, R.; Chang, M.; Jin, Q.; Wang, X. The relationship between lipid phytochemicals, obesity and its related chronic diseases. Food Funct. 2018, 9, 6048–6062. [Google Scholar] [CrossRef]
- Lai, C.S.; Wu, J.C.; Pan, M.H. Molecular mechanism on functional food bioactives for anti-obesity. Curr. Opin. Food Sci. 2015, 2, 9–13. [Google Scholar] [CrossRef]
- Trigueros, L.; Peña, S.; Ugidos, A.V.; Sayas-Barberá, E.; Pérez-Álvarez, J.A.; Sendra, E. Food ingredients as anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Carnauba, R.A.; Chaves, D.F.; Baptistella, A.B.; Paschoal, V.; Naves, A.; Buehler, A.M. Association between high consumption of phytochemical-rich foods and anthropometric measures: A systematic review. Int. J. Food Sci. Nutr. 2017, 68, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Abshirini, M.; Mahaki, B.; Bagheri, F.; Siassi, F.; Koohdani, F.; Sotoudeh, G. Higher intake of phytochemical-rich foods is inversely related to prediabetes: A case-control study. Int. J. Prev. Med. 2018, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Zengin, G.; Stefanucci, A.; Ferrante, C.; Menghini, L.; Orlando, G.; Brunetti, L.; Locatelli, M.; Dimmita, M.P.; Novellino, E.; et al. Nutraceutical potential of Corylus avellana daily supplements for obesity and related dysmetabolism. J. Funct. Foods 2018, 47, 562–574. [Google Scholar] [CrossRef]
- Samad, A.; Azlan, A.; Ismail, A. Therapeutic effects of vinegar: A review. Curr. Opin. Food Sci. 2016, 8, 56–61. [Google Scholar] [CrossRef]
- Kim, A.Y.; Jeong, Y.J.; Park, Y.B.; Lee, M.K.; Jeon, S.M.; McGregor, R.A.; Choi, M.S. Dose dependent effects of lycopene enriched tomato-wine on liver and adipose tissue in high-fat diet fed rats. Food Chem. 2012, 130, 42–48. [Google Scholar] [CrossRef]
- Benbouguerra, N.; Hornedo-Ortega, R.; Garcia, F.; El Khawand, T.; Saucier, C.; Richard, T. Stilbenes in grape berries and wine and their potential role as anti-obesity agents: A review. Trends Food Sci. Technol. 2021, 112, 362–381. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Kim, S.H.; Kim, D.S.; Park, S.H.; Yoo, B.W.; Kim, H.K. Nutritional composition and anti-obesity effects of cereal bar containing Allium fistulosum (welsh onion) extract. J. Funct. Foods 2014, 6, 428–437. [Google Scholar] [CrossRef]
- Aljutaily, T.; Elbeltagy, A.; Ali, A.A.; Gadallah, M.G.; Khalil, N.A. Anti-Obesity Effects of Formulated Biscuits Supplemented with Date’s Fiber; Agro-Waste Products Used as a Potent Functional Food. Nutrients 2022, 14, 5315. [Google Scholar] [CrossRef]
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanović, S. Evaluation of apple pomace flour obtained industrially by dehydration as a source of biomolecules with antioxidant, antidiabetic and antiobesity effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef]
- Cho, Y.J.; Lee, H.G.; Seo, K.H.; Yokoyama, W.; Kim, H. Antiobesity effect of prebiotic polyphenol-rich grape seed flour supplemented with probiotic kefir-derived lactic acid bacteria. J. Agric. Food Chem. 2018, 66, 12498–12511. [Google Scholar] [CrossRef]
- Zhou, F.; Yin, M.; Liu, Y.; Han, X.; Guo, J.; Ren, C.; Wang, W.; Huang, W.; Zhan, J.; You, Y. Grape seed flour intake decreases adiposity gain in high-fat-diet induced obese mice by activating thermogenesis. J. Funct. Foods 2019, 62, 103509. [Google Scholar] [CrossRef]
- Lim, W.C.; Ho, J.N.; Lee, H.S.; Cho, H.Y. Germinated waxy black rice extract inhibits lipid accumulation with regulation of multiple gene expression in 3T3-L1 adipocytes. Food Sci. Biotechnol. 2016, 25, 821–827. [Google Scholar] [CrossRef]
- Koh, S.P.; Sew, Y.S.; Sabidi, S.; Maarof, S.; Sharifudin, S.A.; Abdullah, R. Anti-obesity Effects of SCOBY Jackfruit Beverages and Their Influence on Gut Microbiota. Explor. Res. Hypothesis Med. 2023, 8, 14–24. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, Z.; Yin, J.; Long, H.; Zheng, X. Anti-obesity effects of artificial planting blueberry (Vaccinium ashei) anthocyanin in high-fat diet-treated mice. Int. J. Food Sci. Nutr. 2016, 67, 257–264. [Google Scholar] [CrossRef]
- Ali, Z.; Ma, H.; Rashid, M.T.; Ayim, I.; Wali, A. Reduction of body weight, body fat mass, and serum leptin levels by addition of new beverage in normal diet of obese subjects. J. Food Biochem. 2018, 42, e12554. [Google Scholar] [CrossRef]
- Sheng, Z.; Yu, L.; Li, X.; Zhao, Y.; Dai, W.; Chang, S.K.; Liu, J. The anti-obesity effect of fermented tremella/blueberry and its potential mechanisms in metabolically healthy obese rats. J. Funct. Foods 2021, 86, 104670. [Google Scholar] [CrossRef]
- Beh, B.K.; Mohamad, N.E.; Yeap, S.K.; Ky, H.; Boo, S.Y.; Chua, J.Y.H.; Tan, S.W.; Ho, W.Y.; Sharifuddin, S.A.; Long, K.; et al. Anti-obesity and anti-inflammatory effects of synthetic acetic acid vinegar and Nipa vinegar on high-fat-diet-induced obese mice. Sci. Rep. 2017, 7, 6664. [Google Scholar] [CrossRef]
- Saravanan, G.; Ponmurugan, P.; Deepa, M.A.; Senthilkumar, B. Anti-obesity action of gingerol: Effect on lipid profile, insulin, leptin, amylase and lipase in male obese rats induced by a high-fat diet. J. Sci. Food Agric. 2014, 94, 2972–2977. [Google Scholar] [CrossRef]
- Zhou, X.; Pak, S.; Li, D.; Dong, L.; Chen, F.; Hu, X.; Ma, L. Bamboo Shoots Modulate Gut Microbiota, Eliminate Obesity in High-Fat-Diet-Fed Mice and Improve Lipid Metabolism. Foods 2023, 12, 1380. [Google Scholar] [CrossRef]
- Li, X.; Tian, S.; Wang, Y.; Liu, J.; Wang, J.; Lu, Y. Broccoli microgreens juice reduces body weight by enhancing insulin sensitivity and modulating gut microbiota in high-fat diet-induced C57BL/6J obese mice. Eur. J. Nutr. 2021, 60, 3829–3839. [Google Scholar] [CrossRef]
- Kuesten, C.; Dang, J.; Nakagawa, M.; Bi, J.; Meiselman, H.L. Japanese consumer segmentation based on general self-efficacy psychographics data collected in a phytonutrient supplement study: Influence on health behaviors, well-being, product involvement and liking. Food Qual. Prefer. 2022, 99, 104545. [Google Scholar] [CrossRef]
- Liudvytska, O.; Kolodziejczyk-Czepas, J. A review on rhubarb-derived substances as modulators of cardiovascular risk factors—A special emphasis on anti-obesity action. Nutrients 2022, 14, 2053. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Hung, H.Y. Recent advances in natural anti-obesity compounds and derivatives based on in vivo evidence: A mini-review. Eur. J. Med. Chem. 2022, 237, 114405. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, D.D.; Lakhawat, S.S.; Yasmeen, N.; Pandey, A.; Singla, R.K. Biogenic phytochemicals modulating obesity: From molecular mechanism to preventive and therapeutic approaches. Evid. Based Complement. Altern. Med. 2022, 2022, 68522. [Google Scholar] [CrossRef]
- Kazemipoor, M.; Cordell, G.A.; Sarker, M.M.R.; Radzi, C.W.J.B.W.M.; Hajifaraji, M.; En Kiat, P. Alternative treatments for weight loss: Safety/risks and effectiveness of anti-obesity medicinal plants. Int. J. Food Prop. 2015, 18, 1942–1963. [Google Scholar] [CrossRef]
- Sun, N.N.; Wu, T.Y.; Chau, C.F. Natural dietary and herbal products in anti-obesity treatment. Molecules 2016, 21, 1351. [Google Scholar] [CrossRef]
- Patil, B.S.; Uckoo, R.M.; Jayaprakasha, G.K.; Palma, M.A. Consumers’ changing perceptions of quality: Revisiting the science of fruit and vegetable cultivation for improved health benefits. In XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014); International Society for Horticultural Science: Leuven, Belgium, 2016; Volume 1120, pp. 459–468. [Google Scholar] [CrossRef]
- Paraschivu, M.; Cotuna, O.; Sărățeanu, V.; Durău, C.C.; Păunescu, R.A. Microgreens-current status, global market trends and forward statements. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural. Dev. 2021, 21, 633–639. [Google Scholar]
- Viganó, J.; Zabot, G.L.; Martínez, J. Supercritical fluid and pressurized liquid extractions of phytonutrients from passion fruit by-products: Economic evaluation of sequential multi-stage and single-stage processes. J. Supercrit. Fluids 2017, 122, 88–98. [Google Scholar] [CrossRef]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef]
- Sawant, O.; Khan, T. Standardization of marketed anti-obesity nutraceuticals containing amla and ginseng. J. Food Process. Preserv. 2021, 45, e15693. [Google Scholar] [CrossRef]
- Bessesen, D.H.; Van Gaal, L.F. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018, 6, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Koncz, D.; Tóth, B.; Roza, O.; Csupor, D. A systematic review of the European rapid alert system for food and feed: Tendencies in illegal food supplements for weight loss. Front. Pharmacol. 2021, 11, 611361. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, A.G.; Akhgari, M.; Kamali, A.; Mousavi, Z. Principal component analysis of synthetic adulterants in herbal supplements advertised as weight loss drugs. Complement. Ther. Clin. Pract. 2018, 31, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Kumar, B.K.; Sharma, P.K.; Murugesan, S.; Deepa, P.R. In silico and in vitro analysis of PPAR–α/γ dual agonists: Comparative evaluation of potential phytochemicals with anti-obesity drug orlistat. Comput. Biol. Med. 2022, 147, 105796. [Google Scholar] [CrossRef]
- Castrica, M.; Rebucci, R.; Giromini, C.; Tretola, M.; Cattaneo, D.; Baldi, A. Total phenolic content and antioxidant capacity of agri-food waste and by-products. Ital. J. Anim. Sci. 2019, 18, 336–341. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).