Advanced Insights into Walnut Protein: Structure, Physiochemical Properties and Applications
Abstract
:1. Introduction
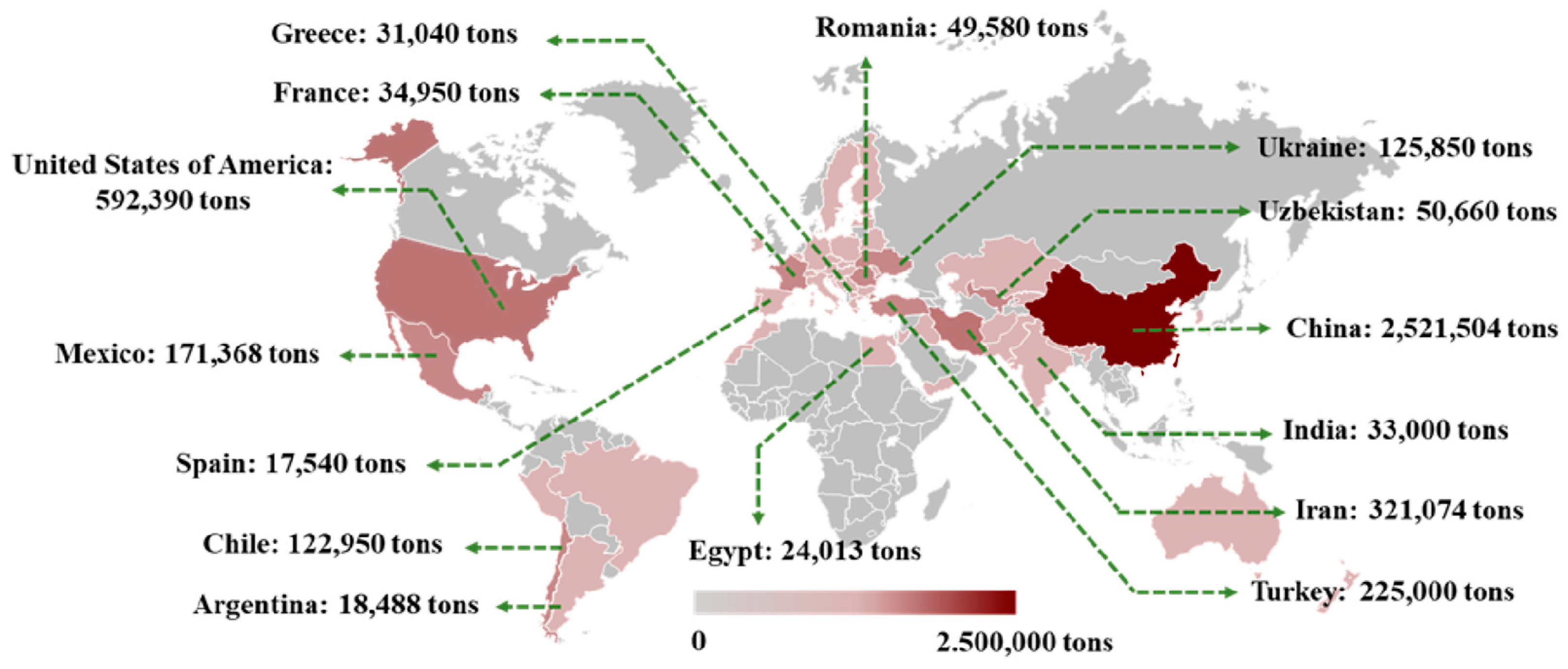
2. Global Distribution and Characteristics of Different Species
2.1. Asia
2.2. Europe
2.3. America
3. Structural and Physiochemical Properties of Walnut Proteins
3.1. Composition and Molecular Structure of Walnut Protein
3.2. Properties of Walnut Protein
3.2.1. Solubility
3.2.2. Emulsification
3.2.3. Water and Oil Retention
4. Functional Attributes of Walnut Proteins
4.1. Walnut Peptide
4.2. Encapsulation
4.3. Product
5. Concerns and Discussion
5.1. Allergy
5.2. Physicochemical Limitations
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mexis, S.F.; Kontominas, M.G. Effect of γ-irradiation on the physicochemical and sensory properties of walnuts (Juglans regia L.). Eur. Food Res. Technol. 2009, 228, 823–831. [Google Scholar] [CrossRef]
- Bakkalbasi, E.; Meral, R.; Dogan, I. Bioactive compounds, physical and sensory properties of cake made with walnut press-cake. J. Food Qual. 2015, 38, 422–430. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, W.; Yi, J.; Liu, N.; Cao, Y.; Lu, J.; Decker, E.A.; McClements, D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018, 106, 853–861. [Google Scholar] [CrossRef]
- FAO. FAO Statistical Data; FAO: Rome, Italy, 2018; Available online: http://www.fao.org/faostat/en/ (accessed on 4 June 2020).
- Ebru, K.; Sule, H.A.; Muhammet, A.G.; Akide, O.; Muharrem, E. Phenolic and fatty acid profile, and protein content of different walnut cultivars and genotypes (Juglans regia L.) grown in the USA. Int. J. Fruit Sci. 2020, 20, 1711–1720. [Google Scholar] [CrossRef]
- Darab, H.; Saadat, S.; Raana, D.; Razie, M.; Asghar, S.; Kourosh, V. Situation and recent trends on cultivation and breeding of Persian walnut in Iran. Sci. Hortic. 2020, 270, 109369. [Google Scholar] [CrossRef]
- FAO. FAO Statistical Data; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023; Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 24 March 2023).
- Wang, G.P.; Tekintas, F.E. Walnut Footprints in Turkey. Shanxi Fruits 2016, 4, 52–53. [Google Scholar] [CrossRef]
- Surhone, L.M.; Tennoe, M.T.; Henssonow, S.F. Richardson-Lucy Deconvolution: Deconvolution, Iterative Procedure, Convolution. Point Spread Function, Poisson Distribution, Photon Noise, Maximun Likelihood; National Institute of Statistics: Bucharest, Romania, 2010. [Google Scholar]
- Zhao, P.; Liu, J.F. Selection of California walnut varieties. Agric. Dev. Equip. 2014, 6, 135–136. [Google Scholar]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef]
- Downs, M.L.; Simpson, A.; Custovic, A.; Semic-Jusufagic, A.; Bartra, J.; Fernandez-Rivas, M.; Taylor, S.L.; Baumert, J.L.; Mills, E.C. Insoluble and soluble roasted walnut proteins retain antibody reactivity. Food Chem. 2016, 194, 1013–1021. [Google Scholar] [CrossRef]
- Delcour, J.A.; Joye, I.J.; Pareyt, B.; Wilderjans, E.; Brijs, K.; Lagrain, B. Wheat gluten functionality as a quality determinant in cereal-based food products. Annu. Rev. Food Sci. Technol. 2012, 3, 469–492. [Google Scholar] [CrossRef]
- Ortolan, F.; Steel, C.J. Protein characteristics that affect the quality of vital wheat gluten to be used in baking: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Sze-Tao, K.W.C.; Sathe, S.K. Walnuts (Juglans regia L): Proximate composition, protein solubility, protein amino acid composition and protein in vitro digestibility. J. Sci. Food Agric. 2000, 80, 1393–1401. [Google Scholar] [CrossRef]
- Mao, X.Y.; Hua, Y.F.; Chen, G.G. Amino acid composition, molecular weight distribution and gel electrophoresis of Walnut (Juglans regia L.) Proteins and Protein Fractionations. Int. J. Mol. Sci. 2014, 15, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.F.; Sun, Y.L.; Zhao, X.Y. Functional and conformational characterisation of walnut protein obtained through AOT reverse micelles. Int. J. Food Sci. Technol. 2015, 50, 2351–2359. [Google Scholar] [CrossRef]
- Zhang, M.L.; Gao, J.L.; Yang, H.X. Functional Properties of 7s Globulin Extracted from Cowpea Vicilins. Cereal Chem. 2009, 86, 261–266. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Lawal, O.S. Functionality of African locust bean (Parkia biglobossa) protein isolate: Effects of pH, ionic strength and various protein concentrations. Food Chem. 2004, 86, 345–355. [Google Scholar] [CrossRef]
- Diana, L.; Damian, M.; Alicia, L. Molecular characterization, antioxidant and protein solubility-related properties of polyphenolic compounds from walnut (Juglans regia). Nat. Prod. Commun. 2016, 11, 637–640. [Google Scholar] [CrossRef]
- Prasad, R.B.N. Walnuts and pecans. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 6071–6079. [Google Scholar]
- Moure, A.; Sineiro, J.; Dominguez, H.; Parajo, J.C. Functionality of oilseed protein products: A review. Food Res. Int. 2007, 39, 945–963. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, L.; Lu, X.; Zhang, C.; Hua, Y.; Chen, Y. Effect of high-speed shearing treatment on dehulled walnut proteins. LWT-Food Sci. Technol. 2019, 116, 108500. [Google Scholar] [CrossRef]
- Madadlou, A.; Emam-Djomeh, Z.; Mousavi, M.E.; Mohamadifar, M.; Ehsani, M. Acid-induced gelation behavior of sonicated casein solutions. Ultrason. Sonochem. 2010, 17, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Madadlou, A.; Mousavi, M.E.; Emam-Djomeh, Z.; Ehsani, M.; Sheehan, D. Comparison of pH-dependent sonodisruption of re-assembled casein micelles by 35 and 130 kHz ultrasounds. J. Food Eng. 2009, 95, 505–509. [Google Scholar] [CrossRef]
- Li, C.; Enomoto, H.; Hayashi, Y.; Zhao, H.; Aoki, T. Recent advances in phosphorylation of food proteins: A review. LWT-Food Sci. Technol. 2010, 43, 1295–1300. [Google Scholar] [CrossRef]
- Matheis, G. Phosphorylation of food proteins with phosphorus oxychloride improvement of functional and nutritional properties: A review. Food Chem. 1991, 39, 13–26. [Google Scholar] [CrossRef]
- Hu, Z.; Qiu, L.; Sun, Y.; Xiong, H.; Ogra, Y. Improvement of the solubility and emulsifying properties of rice bran protein by phosphorylation with sodium trimetaphosphate. Food Hydrocoll. 2019, 96, 288–299. [Google Scholar] [CrossRef]
- Deng, X.L.; Zhao, Q.Z. Study on conformation and functional properties of walnut protein and its components. Mod. Food Sci. Technol. 2017, 33, 48–61. [Google Scholar] [CrossRef]
- Luo, Q.G.; Xin, L.F. Study on stability of walnut sauce in production process. Acad. Period. Farm Prod. Process. 2004, 12, 38–39. [Google Scholar]
- Taha, A.; Ahmed, E.; Hu, T.; Xu, X.; Pan, S.; Hu, H. Effects of different ionic strengths on the physicochemical properties of plant and animal proteins-stabilized emulsions fabricated using ultrasound emulsification. Ultrason. Sonochem. 2019, 58, 104627. [Google Scholar] [CrossRef]
- Taha, A.; Hu, T.; Zhang, Z.; Bakry, A.M.; Khalifa, I.; Pan, S.Y.; Hu, H. Effect of different oils and ultrasound emulsification conditions on the physicochemical properties of emulsions stabilized by soy protein isolate. Ultrason. Sonochem. 2018, 49, 283–293. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. Process Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Goula, A.M. Ultrasound-assisted extraction of pomegranate seed oil-Kinetic modeling. J. Food Eng. 2013, 117, 492–498. [Google Scholar] [CrossRef]
- Balachandran, S.; Kentish, S.; Mawson, R.; Ashokkumar, M. Ultrasonic enhancement of the supercritical extraction from ginger. Ultrason. Sonochem. 2006, 13, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Viljanen, K.; Kivikari, R.; Heinonen, M. Protein-lipid interactions during liposome oxidation with added anthocyanin and other phenolic compounds. J. Agric. Food Chem. 2004, 52, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Mestdagh, F.; Kerkaert, B.; Cucu, T.; Meulenaer, B.D. Interaction between whey proteins and lipids during light-induced oxidation. Food Chem. 2010, 126, 1190–1197. [Google Scholar] [CrossRef]
- Acharya, D.P.; Sanguansri, L.; Augustin, M.A. Binding of resveratrol with sodium caseinate in aqueous solutions. Food Chem. 2013, 141, 1050–1054. [Google Scholar] [CrossRef]
- Jauregi, P.; Guo, Y.; Adeloye, J.B. Whey proteins-polyphenols interactions can be exploited to reduce astringency or increase solubility and stability of bioactives in foods. Food Res. Int. 2021, 141, 110019. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein-phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Gu, L.; Su, Y.; Zhang, M.; Chang, C.; Li, J.; McClements, D.J.; Yang, Y. Protection of β-carotene from chemical degradation in emulsion-based delivery systems using antioxidant interfacial complexes: Catechin-egg white protein conjugates. Food Res. Int. 2017, 96, 84–93. [Google Scholar] [CrossRef]
- Hu, H.; Fan, T.; Zhao, X.; Zhang, X.; Sun, Y.; Liu, H. Influence of pH and salt concentration on functional properties of walnut protein from different extraction methods. J. Food Sci. Technol. 2017, 54, 2833–2841. [Google Scholar] [CrossRef]
- Ding, X.W.; Zhang, H.; Yao, S.; Li, G.J. Study on the functional properties of walnut protein and their affecting factors. J. Southwest Agric. Univ. (Nat. Sci.) 2005, 27, 766–780. [Google Scholar]
- Sun, Q.; Ma, Z.F.; Zhang, H.X.; Ma, S.J.; Kong, L.M. Structural characteristics and functional properties of walnut glutelin as hydrolyzed: Effect of enzymatic modification. Int. J. Food Prop. 2019, 22, 265–279. [Google Scholar] [CrossRef]
- Ni, Z.J.; Zhang, Y.G.; Chen, S.X.; Thakur, K.; Wang, S.; Zhang, J.G.; Shang, Y.F.; Wei, Z.J. Exploration of walnut components and their association with health effects. Crit. Rev. Food Sci. Nutr. 2021, 62, 5113–5129. [Google Scholar] [CrossRef] [PubMed]
- Ulug, S.K.; Jahandideh, F.; Wu, J. Novel technologies for the production of bioactive peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Vo, T.S.; Ryu, B.; Kim, S.K. Purification of novel anti-inflammatory peptides from enzymatic hydrolysate of the edible microalgal Spirulina maxima. J. Funct. Foods 2013, 5, 1336–1346. [Google Scholar] [CrossRef]
- Zablocka, A.; Ogorzalek, A.; Macala, J. A proline-rich polypeptide complex (PRP) influences inducible nitric oxide synthase in mice at the protein level. Nitric Oxide-Biol. Chem. 2010, 23, 20–25. [Google Scholar] [CrossRef]
- Daroit, D.J.; Brandelli, A. In Vivo bioactivities of food protein-derived peptides—A current review. Curr. Opin. Food Sci. 2021, 39, 120–129. [Google Scholar] [CrossRef]
- Gu, M.; Chen, H.P.; Zhao, M.M.; Wang, X.; Yang, B.; Ren, J.Y.; Su, G.W. Identification of antioxidant peptides released from defatted walnut (Juglans Sigillata Dode) meal proteins with pancreatin. LWT-Food Sci. Technol. 2015, 60, 213–220. [Google Scholar] [CrossRef]
- Jahanbani, R.; Ghaffari, S.M.; Salami, M.; Vahdati, K.; Sepehri, H.; Sarvestani, N.N.; Sheibani, N.; Moosavi-Movahedi, A.A. Antioxidant and anticancer activities of walnut (Juglans regia L.) protein hydrolysates using different proteases. Plant Foods Hum. Nutr. 2016, 71, 402–409. [Google Scholar] [CrossRef]
- Zou, J.; Cai, P.S.; Xiong, C.M.; Ruan, J.L. Neuroprotective effect of peptides extracted from walnut (Juglans Sigilata Dode) proteins on Aβ25-35-induced memory impairment in mice. J. Huazhong Univ. Sci. Technol. 2016, 36, 21–30. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, M.; Lin, L.; Wang, J.; Sun-Waterhouse, D.; Dong, Y.; Zhuang, M.; Su, G. Identification of antioxidative peptides from defatted walnut meal hydrolysate with potential for improving learning and memory. Food Res. Int. 2015, 78, 216–223. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Zhao, F.; Qin, H.; Lu, H.; Fang, L.; Wang, J.; Min, W. Potential mechanisms mediating the protective effects of a peptide from walnut (Juglans mandshurica Maxim.) against hydrogen peroxide induced neurotoxicity in PC12 cells. Food Funct. 2019, 10, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, M.; Zhang, Y.; Xu, W.; Wang, C.; Wang, K.; Zhang, L. Purification and identification of an ACE inhibitory peptide from walnut protein. J. Agric. Food Chem. 2013, 61, 4097–4100. [Google Scholar] [CrossRef]
- Wang, C.; Tu, M.L.; Wu, D.; Chen, H.; Chen, C.; Wang, Z.Y.; Jiang, L.Z. Identification of an ACE-inhibitory peptide from walnut protein and its evaluation of the inhibitory mechanism. Int. J. Mol. Sci. 2018, 19, 1156. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Li, W.; Ullah, K.; Hasan, M.; Linna, G.; Awan, U.; Zhang, Y.; Batool, S.; Qing, H.; Deng, Y. Study of rat hypothalamic proteome by HPLC/ESI-Ion trap and HPLC/ESI-Q-TOF mass spectrometry. Proteomics 2013, 13, 2455–2468. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.F.; Qi, W.; Li, T.H.; Lu, D.; Su, R.X.; He, Z.M. Simultaneous production of multi-functional peptides by pancreatic hydrolysis of bovine casein in an enzymatic membrane reactor via combinational chromatography. Food Chem. 2013, 141, 2944–2951. [Google Scholar] [CrossRef]
- Golly, M.K.; Ma, H.; Duan, Y.; Liu, D.; Quaisie, J.; Tuli, J.A.; Mintah, B.K.; Dzah, C.S.; Agordoh, P.D. Effect of multi-frequency countercurrent ultrasound treatment on extraction optimization, functional and structural properties of protein isolates from Walnut (Juglans regia L.) meal. J. Food Biochem. 2020, 44, e13210. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef]
- Malekzad, H.; Mirshekari, H.; Sahandi Zangabad, P.; Sahandi Zangabad, P.; Moosavi Basri, S.M.; Baniasadi, F.; Sharifi Aghdam, M.; Karimi, M.; Hamblin, M.R. Plant protein-based hydrophobic fine and ultrafine carrier particles in drug delivery systems. Crit. Rev. Biotechnol. 2018, 38, 47–67. [Google Scholar] [CrossRef]
- Asadi, M.; Salami, M.; Hajikhani, M.; Emam-Djomeh, Z.; Aghakhani, A.; Ghasemi, A. Electrospray production of curcumin-walnut protein nanoparticles. Food Biophys. 2021, 16, 15–26. [Google Scholar] [CrossRef]
- Lv, S.Y.; Lu, Q.; Pan, S.Y. Stability and In Vitro Digestion of Pectin-Walnut Proteins Stabilized Emulsions Encapsulating Curcumin. J. Food Sci. 2021, 8, 1–9. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=cjfd2021&filename=spkx202108001&dbcode=cjfq (accessed on 6 April 2020).
- Mohamad-Aziz, S.N.; Mishra, P.; Zularisam, A.W.; Sakinah, A.M. Isooctane-based anionic and zwitterionic surfactant: Synergistic interaction of mixed reverse micelle and solubilisation of erythromycin. J. Mol. Liq. 2019, 286, 110882. [Google Scholar] [CrossRef]
- Lei, Y.; Gao, S.; Xiang, X.; Li, X.; Yu, X.; Li, S. Physicochemical, structural and adhesion properties of walnut protein isolate-xanthan gum composite adhesives using walnut protein modified by ethanol. Int. J. Biol. Macromol. 2021, 192, 644–653. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, Y.; Zhang, T.; Zang, J. Microencapsulation of Carvacrol by Complex Coacervation of Walnut Meal Protein Isolate and Gum Arabic: Preparation, Characterization and Bio-Functional Activity. Foods 2022, 11, 3382. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Sabharanjak, S.M.; Zengin, G.; Mollica, A.; Szostak, A.; Simirgiotis, M.; Huminiecki, L.; Horbanczuk, O.K.; Nabavi, S.M.; Mocan, A. Pecan nuts: A review of reported bioactivities and health effects. Trends Food Sci. Technol. 2018, 71, 246–257. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Mousavi, S.M.; Khodaiyan, F.; Hamedi, M. Optimization and characterization of walnut beverage emulsions in relation to their composition and structure. Int. J. Biol. Macromol. 2012, 50, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, C.X.; Xue, Y.H.; Gao, Y.X. Impact of pH, freeze–thaw and thermal sterilization on physicochemical stability of walnut beverage emulsion. Food Chem. 2016, 196, 475–485. [Google Scholar] [CrossRef]
- Cui, X.H.; Chen, S.J.; Wang, Y.; Han, J.R. Fermentation conditions of walnut milk beverage inoculated with kefir grains. LWT-Food Sci. Technol. 2013, 50, 349–352. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, G.; Huang, L.; Chen, C.; Sun, J.; Zhu, C. DNA barcoding coupled with high-resolution melting analysis for nut species and walnut milk beverage authentication. J. Sci. Food Agric. 2020, 100, 2372–2379. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Serrano, A.; Ayo, J.; Solas, M.T.; Cofrades, S.; Carballo, J. Physicochemical and sensory characteristics of restructured beef steak with added walnuts. Meat Sci. 2003, 65, 1391–1397. [Google Scholar] [CrossRef]
- Serrano, A.; Cofrades, S.; Jimnez-Colmenero, F. Characteristics of restructured beef steak with different proportions of walnut during frozen storage. Meat Sci. 2006, 72, 108–115. [Google Scholar] [CrossRef]
- Canales, A.; Benedí, J.; Nus, M.; Librelotto, J.; Sánchez-Montero, J.M.; Sánchez-Muniz, F.J. Effect of walnut-enriched restructured meat in the antioxidant status of overweight/obese senior subjects with at least one extra CHD-risk factor. J. Am. Coll. Nutr. 2007, 26, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Muñoz-Furlong, A.; Godbold, J.H.; Sampson, H.A. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J. Allergy Clin. Immunol. 2010, 125, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Furlong, T.J.; Muoz-Furlong, A.; Burks, A.W.; Sampson, H.A. A voluntary registry for peanut and tree nut allergy: Characteristics of the first 5149 registrants. J. Allergy Clin. Immunol. 2001, 108, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.A.; Muñoz-Furlong, A.; Sampson, H.A. Fatalities due to anaphylactic reactions to foods. J. Allergy Clin. Immunol. 2001, 107, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Mendelson, L.; Rosen, J.P. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N. Engl. J. Med. 1992, 327, 380–384. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Burks, A.W.; Sampson, H.A. Electronic Article: Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics 1998, 102, e6. [Google Scholar] [CrossRef]
- Teuber, S.S.; Dandekar, A.M.; Peterson, W.R.; Sellers, C.L. Cloning and sequencing of a gene encoding a 2S albumin seed storage protein precursor from English walnut (Juglans regia), a major food allergen. J. Allergy Clin. Immunol. 1998, 101, 807–814. [Google Scholar] [CrossRef]
- Teuber, S.S.; Jarvis, K.C.; Dandekar, A.M.; Peterson, W.R.; Ansari, A.A. Identification and cloning of a complementary DNA encoding a vicilin-like proprotein, Jug r 2, from English walnut kernel (Juglans regia), a major food allergen. J. Allergy Clin. Immunol. 1999, 104, 1311–1320. [Google Scholar] [CrossRef]
- Pastorello, E.A.; Farioli, L.; Pravettoni, V.; Robino, A.M.; Scibilia, J.; Fortunato, D.; Conti, A.; Borgonovo, L.; Bengtsson, A.; Ortolani, C. Lipid transfer protein and vicilin are important walnut allergens in patients not allergic to pollen. J. Allergy Clin. Immunol. 2004, 114, 908–914. [Google Scholar] [CrossRef]
- Wallowitz, M.; Peterson, W.R.; Uratsu, S.; Comstock, S.S.; Dandekar, A.M.; Teuber, S.S. Jug r 4, a legumin group food allergen from walnut (Juglans regia Cv. Chandler). J. Agric. Food Chem. 2006, 54, 8369–8375. [Google Scholar] [CrossRef]
- Sordet, C.; Culerrier, R.; Granier, C.; Rancé, F.; Didier, A.; Barre, A.; Rougé, P. Expression of Jug r 1, the 2S albumin allergen from walnut (Juglans regia), as a correctly folded and functional recombinant protein. Peptides 2009, 30, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Sordet, C.; Culerrier, R.; Rancé, F.; Didier, A.; Rougé, P. Vicilin allergens of peanut and tree nuts (walnut, hazelnut and cashew nut) share structurally related IgE-binding epitopes. Mol. Immunol. 2008, 45, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Downs, M.L.; Semic-Jusufagic, A.; Simpson, A.; Bartra, J.; Fernandez-Rivas, M.; Rigby, N.M.; Taylor, S.L.; Baumert, J.L.; Mills, E.N.C. Characterization of low molecular weight allergens from English walnut (Juglans regia). J. Agric. Food Chem. 2014, 62, 11767–11775. [Google Scholar] [CrossRef] [PubMed]
- Suet, M.; Venkatachalam, M.; Teuber, S.S.; Roux, K.H.; Sathe, S.K. Impact of g-irradiation and thermal processing on the antigenicity of almond, cashew nut and walnut protein. J. Sci. Food Agric. 2004, 84, 1119–1125. [Google Scholar] [CrossRef]
- Jiang, S.S.; Zhao, B.; Han, S.W.; Che, H.L. Effect of Different Thermal Processing Treatments on Allergenicity of Walnut Proteins. J. Food Sci. 2018, 39, 94–99. [Google Scholar]
- Costa, J.; Oliveira, M.B.P.P.; Mafra, I. Effect of thermal processing on the performance of the novel single-tube nested real-time PCR for the detection of walnut allergens in sponge cakes. Food Res. Int. 2013, 54, 1722–1729. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Herceg, Z.; Herceg, I.L. Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J. Food Eng. 2008, 86, 281–287. [Google Scholar] [CrossRef]
- Hu, H.; Wu, J.; Li-Chan, E.C.Y.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Wang, J.Y.; Yang, Y.L.; Tang, X.Z.; Ni, W.X.; Zhou, L. Effects of pulsed ultrasound on rheological and structural properties of chicken myofibrillar protein. Ultrason. Sonochem. 2017, 38, 225–233. [Google Scholar] [CrossRef]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Morel, M.H. Effects of temperature, sonication time, and power settings on size distribution and extractability of total wheat flour proteins as determined by size-exclusion high-performance liquid chromatography. Cereal Chem. 2000, 77, 685–691. [Google Scholar] [CrossRef]
- Prabhu, A.A.; Purkayastha, A.; Mandal, B.; Kumar, J.P.; Mandal, B.B.; Veeranki, V.D. A novel reverse micellar purification strategy for histidine tagged human interferon gamma (hIFN-gamma) protein from Pichia pastoris. Int. J. Biol. Macromol. 2018, 107, 2512–2524. [Google Scholar] [CrossRef] [PubMed]
- Hasan-Beikdashti, M.; Forootanfar, H.; Safiarian, M.S.; Ameri, A.; Ghahremani, M.H.; Khoshayand, M.R.; Faramarzi, M.A. Optimization of culture conditions for production of lipase by a newly isolated bacterium Stenotrophomonas maltophilia. J. Taiwan Inst. Chem. Eng. 2012, 43, 670–677. [Google Scholar] [CrossRef]
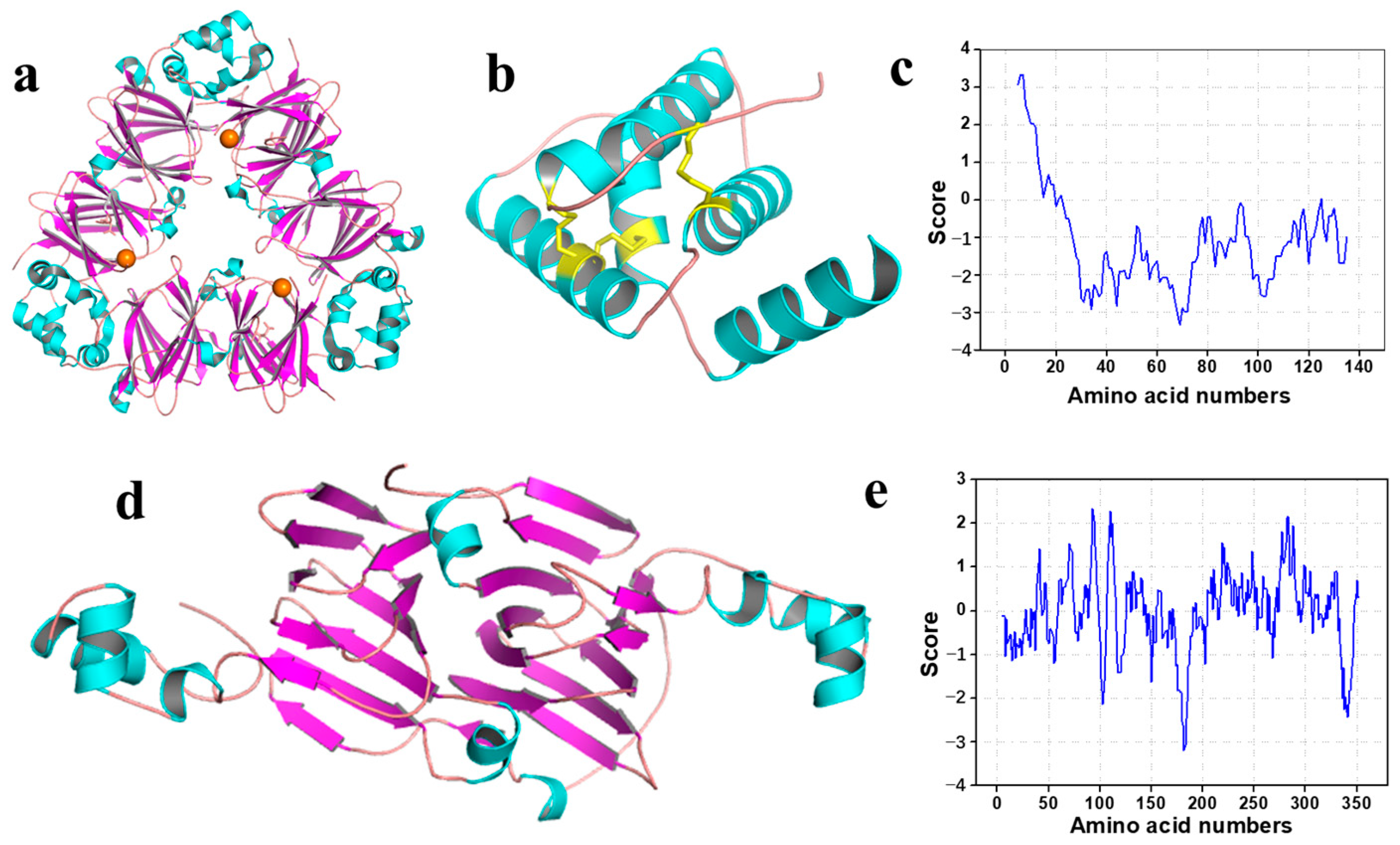


| Amino Acids | Walnut Protein | Albumin | Globulin | Prolamin | Glutelin | FAO/WHO (1990) | |
|---|---|---|---|---|---|---|---|
| Acidic amino acid | Asp | 10.04 ± 0.43 | 8.02 ± 0.57 | 7.13 ± 0.51 | 18.08 ± 0.42 | 10.51 ± 0.44 | |
| Glu | 22.16 ± 0.4 | 28.7 ± 3.36 | 28.8 ± 1.26 | 33.03 ± 1.06 | 22.7 ± 2.05 | ||
| Alkaline amino acid | Arg | 14.73 ± 0.42 | 15.67 ± 0.34 | 16.01 ± 0.33 | 17.52 ± 0.43 | 13.47 ± 0.33 | |
| Lys | 2.58 ± 0.12 | 3.31 ± 0.16 | 2.52 ± 0.16 | 0.83 ± 0.10 | 1.7 ± 0.17 | 5.8 (1.6) | |
| Aromatic amino acid | Phe | 4.94 ± 0.23 | 3.89 ± 0.15 | 3.78 ± 0.08 | 1.92 ± 0.10 | 5.11 ± 0.11 | 6.3 (1.9) |
| His | 2.38 ± 0.26 | 2.23 ± 0.14 | 2.01 ± 0.05 | 1.4 ± 0.35 | 2.19 ± 0.16 | 1.9 (1.6) | |
| Tyr | 2.76 ± 0.11 | 2.53 ± 0.06 | 0.76 ± 0.07 | 3.72 ± 0.09 | 2.83 ± 0.09 | ||
| Pro | 4.22 ± 0.29 | 4.03 ± 0.10 | 4.27 ± 0.13 | 1.64 ± 0.11 | 5.3 ± 0.24 | ||
| Sulfur-containing amino acid | Met | 1.16 ± 0.12 | 1.7 ± 0.10 | 2.32 ± 0.08 | 0.84 ± 0.14 | 1.55 ± 0.11 | 2.5 (1.7) |
| Cys | 0.84 ± 0.08 | 2.21 ± 0.10 | 1.97 ± 0.09 | 2 ± 0.04 | 0.56 ± 0.09 | ||
| Neutral amino acid | Gly | 5.43 ± 0.07 | 5.89 ± 0.17 | 8.73 ± 0.17 | 7.68 ± 0.27 | 5.28 ± 0.25 | |
| Ala | 4.74 ± 0.19 | 3.29 ± 0.24 | 2.62 ± 0.34 | 2.57 ± 0.18 | 4.73 ± 0.27 | ||
| Val | 4.18 ± 0.14 | 3.24 ± 0.11 | 4.05 ± 0.16 | 1.49 ± 0.16 | 4.15 ± 0.16 | 3.5 (1.3) | |
| Leu | 7.13 ± 0.12 | 5.21 ± 0.11 | 5.48 ± 0.16 | 1.51 ± 0.13 | 7.31 ± 0.26 | 6.6 (1.9) | |
| IIe | 3.28 ± 0.15 | 2.66 ± 0.16 | 2.79 ± 0.13 | 0.94 ± 0.07 | 3.32 ± 0.17 | 2.8 (1.3) | |
| Hydroxy amino acid | Ser | 5.84 ± 0.12 | 4.8 ± 0.36 | 5.75 ± 0.23 | 3.22 ± 0.12 | 5.81 ± 0.20 | |
| Thr | 3.58 ± 0.20 | 2.64 ± 0.07 | 2.02 ± 0.07 | 1.59 ± 0.13 | 3.49 ± 0.04 | 3.4 (0.9) | |
| Allergen | Molecular Weight/kD | Subtype | Biochemical Classification | Nucleotide Sequence (NCBI) | Protein Sequence (NCBI) | Protein Sequence (UniProt) |
|---|---|---|---|---|---|---|
| Jug r 1 | 16.4 | Jug r 1.01 | 2S albumin (Prolamin superfamily) | U66866.1 | AAB41308.1 | P93198 |
| Jug r 2 | 44 | Jug r 2.01 | 7S globulin (Cupin superfamily) | AF066055.1 | AAF18269.1 | Q9SEW4 |
| Jug r 3 | 11.8 | Jug r 3.01 | Nonspecific lipid transfer Protein (nsLTP) (Prolamin superfamily) | EU780670.1 | ACI47547.1 | C5H617 |
| Jug r 4 | 58 | Jug r 4.01 | 11S globulin (Cupin superfamily) | AY692446.1 | AAW29810.1 | Q2TPW5 |
| Jug n 1 | 18.9 | Jug n 1.01 | 2S albumin (Prolamin superfamily) | AY102930.1 | AAW29810.1 | Q7Y1C2 |
| Jug n 2 | 55.7 | Jug n 2.01 | Vicilin (Cupin superfamily) | AY102931.1 | AAM54366.1 | Q7Y1C1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; He, W.; Zhao, S.; Jiao, T.; Hu, H.; Li, J.; Zhang, L.; Zang, J. Advanced Insights into Walnut Protein: Structure, Physiochemical Properties and Applications. Foods 2023, 12, 3603. https://doi.org/10.3390/foods12193603
Zhao Y, He W, Zhao S, Jiao T, Hu H, Li J, Zhang L, Zang J. Advanced Insights into Walnut Protein: Structure, Physiochemical Properties and Applications. Foods. 2023; 12(19):3603. https://doi.org/10.3390/foods12193603
Chicago/Turabian StyleZhao, Yuxuan, Weiheng He, Sihan Zhao, Teng Jiao, Haifang Hu, Jingming Li, Lei Zhang, and Jiachen Zang. 2023. "Advanced Insights into Walnut Protein: Structure, Physiochemical Properties and Applications" Foods 12, no. 19: 3603. https://doi.org/10.3390/foods12193603
APA StyleZhao, Y., He, W., Zhao, S., Jiao, T., Hu, H., Li, J., Zhang, L., & Zang, J. (2023). Advanced Insights into Walnut Protein: Structure, Physiochemical Properties and Applications. Foods, 12(19), 3603. https://doi.org/10.3390/foods12193603





