Application of Konjac Glucomannan with Chitosan Coating in Yellow Alkaline Noodles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitosan-Coated Konjac Glucomannan (CK) Powder
2.3. Preparation of Yellow Alkaline Noodles (YANs)
2.4. Color
2.5. Texture
2.6. Water Absorption
2.7. Low-Field Nuclear Magnetic Resonance (LF-NMR)
2.8. In Vitro Digestion Properties
2.9. Total Plate Count (TPC)
2.10. Scanning Electron Microscope (SEM)
2.11. Statistics Analysis
3. Results
3.1. Color Analysis
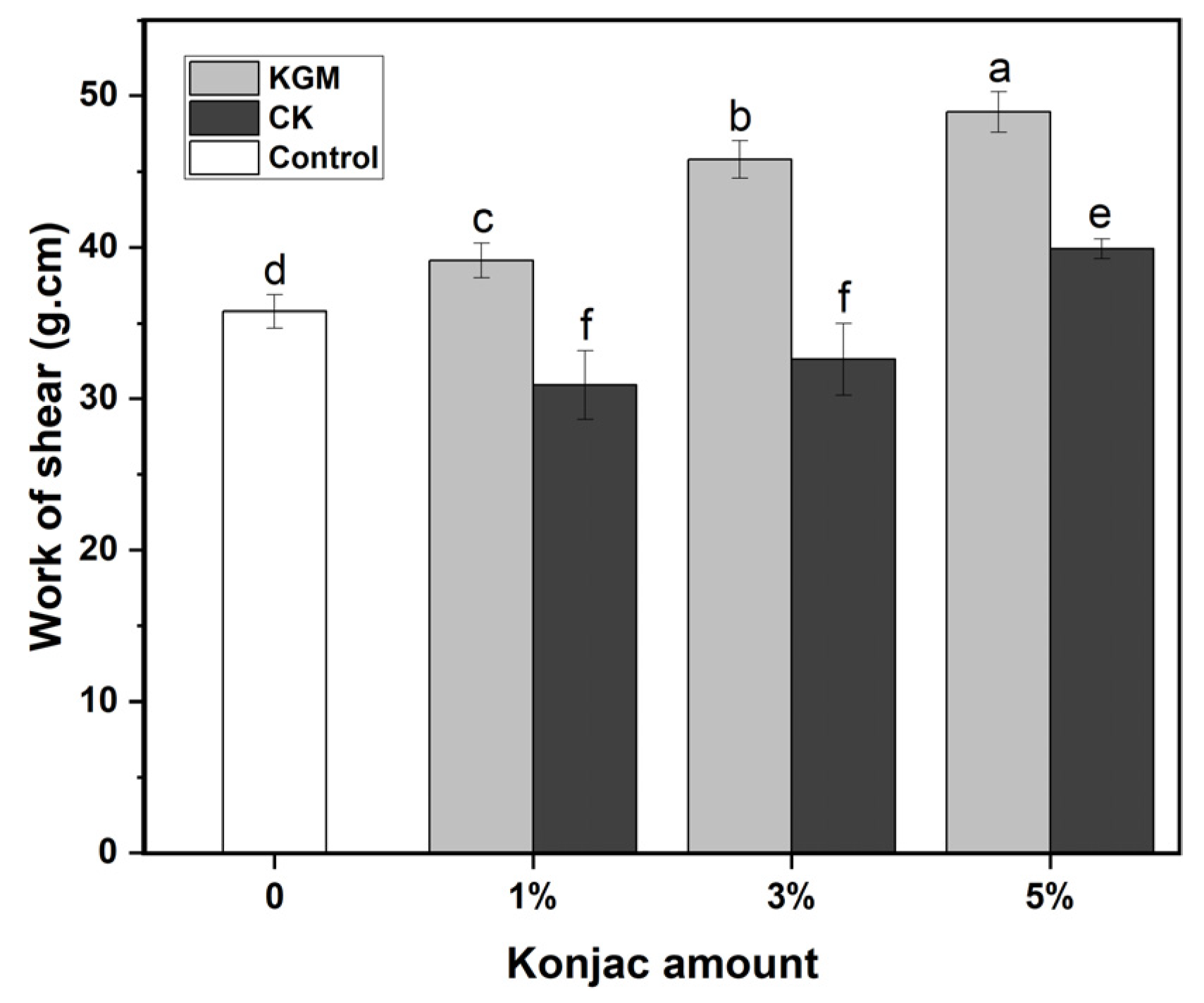
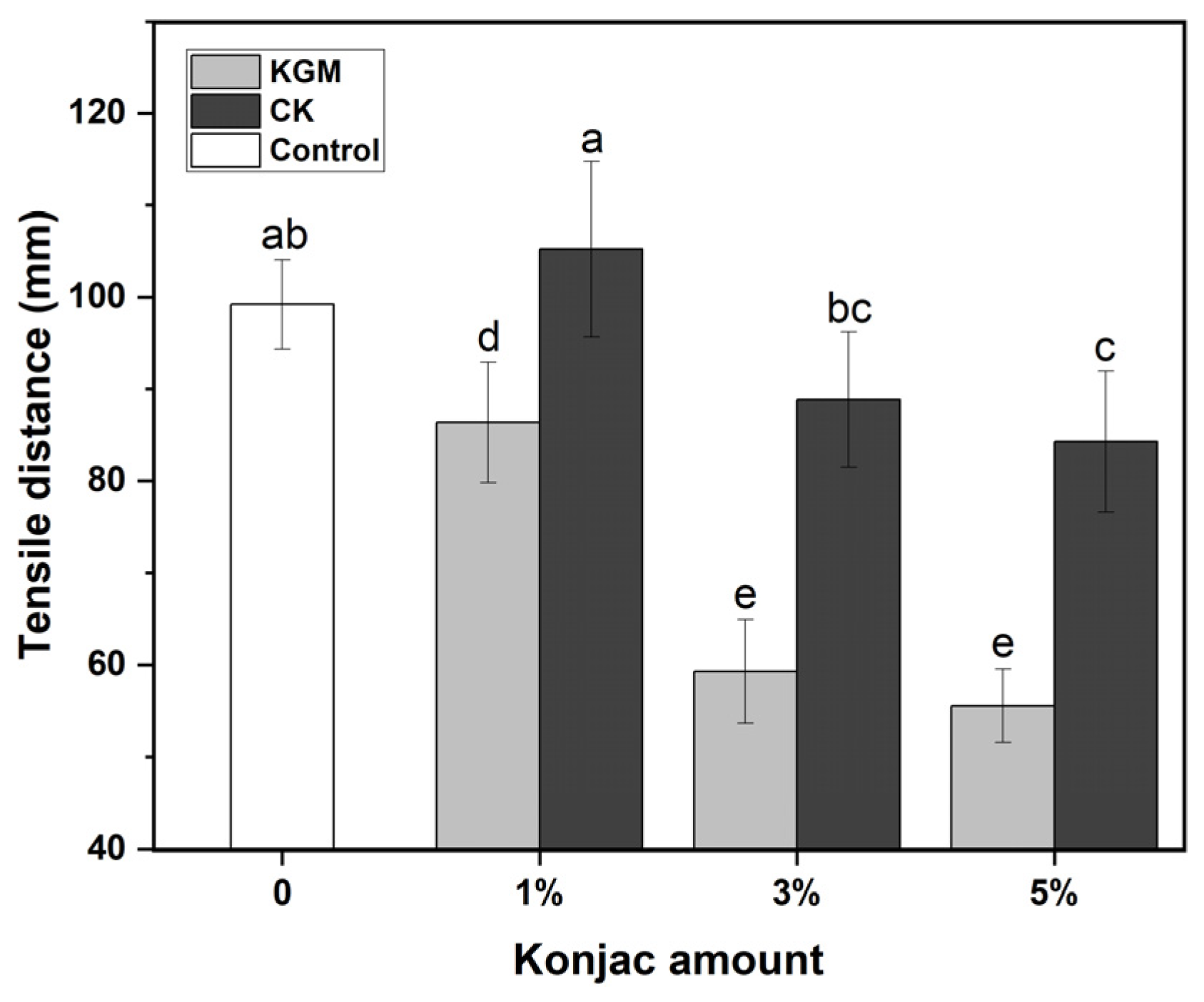
3.2. Texture Analysis
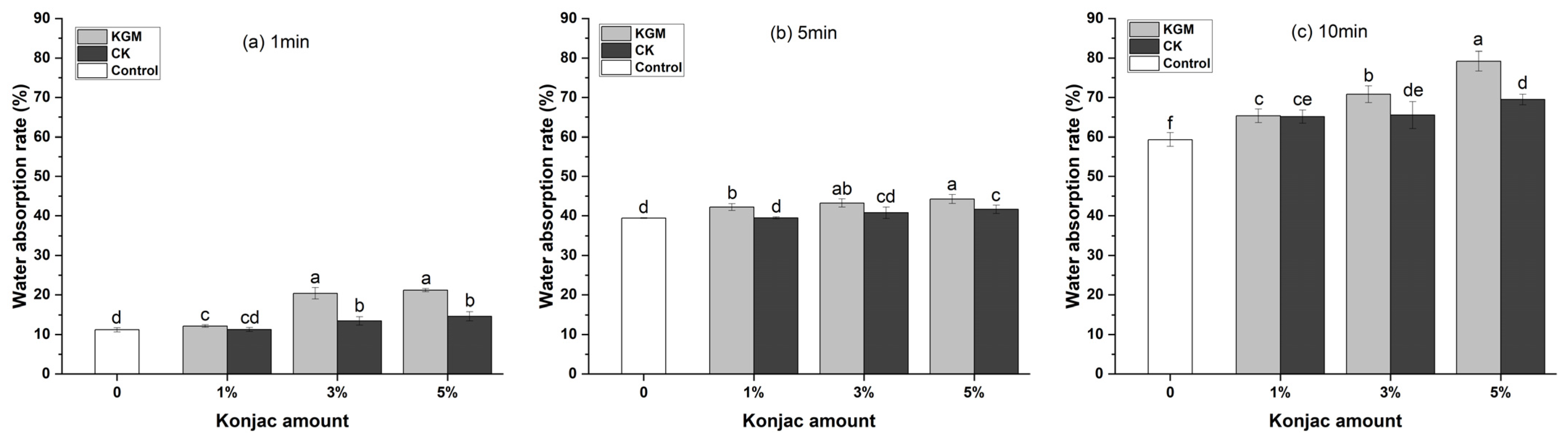
3.3. Water Absorption Analysis
3.4. Water Distribution Analysis
3.5. Starch Digestion Analysis
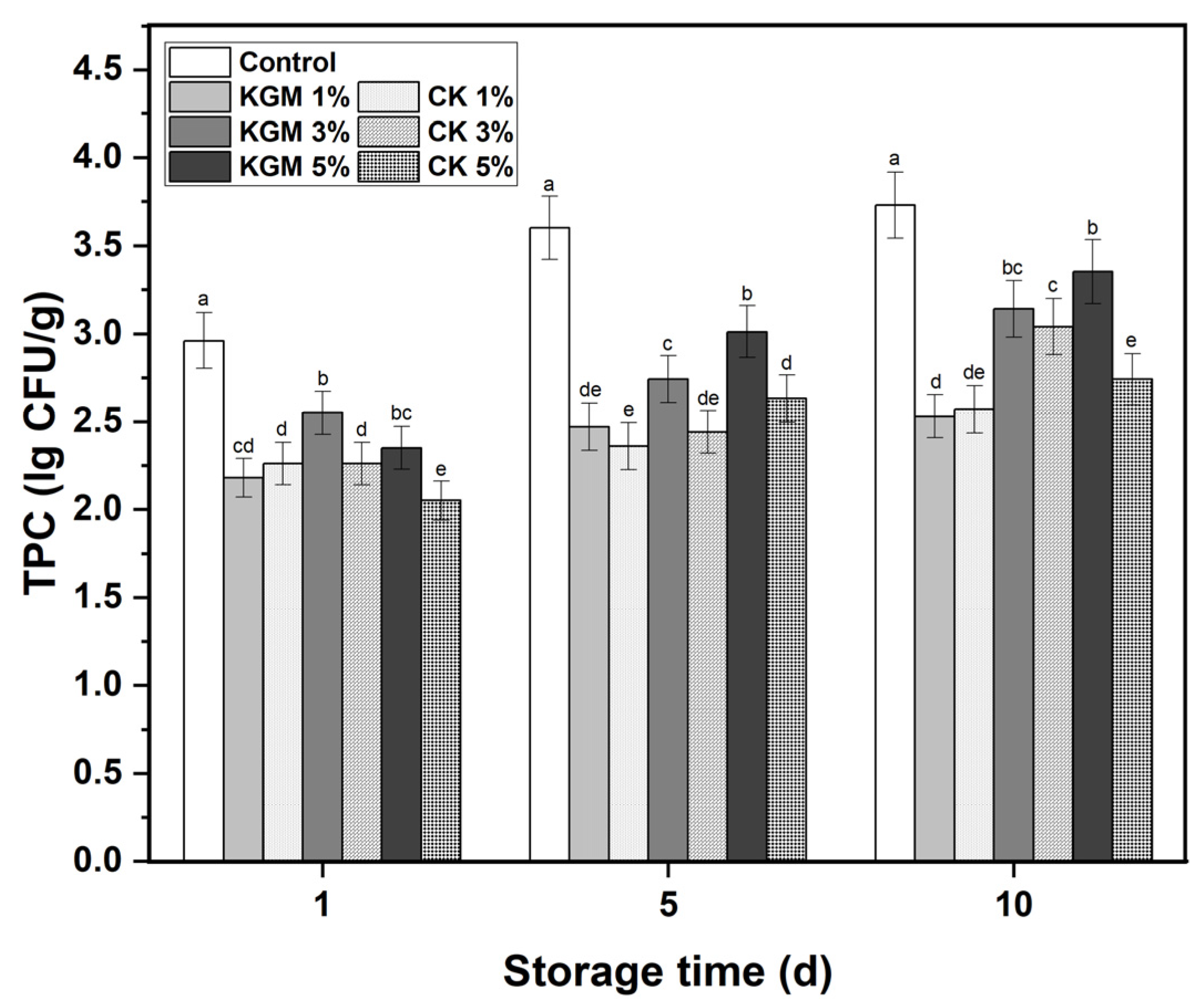
3.6. Total Plate Count (TPC) Analysis
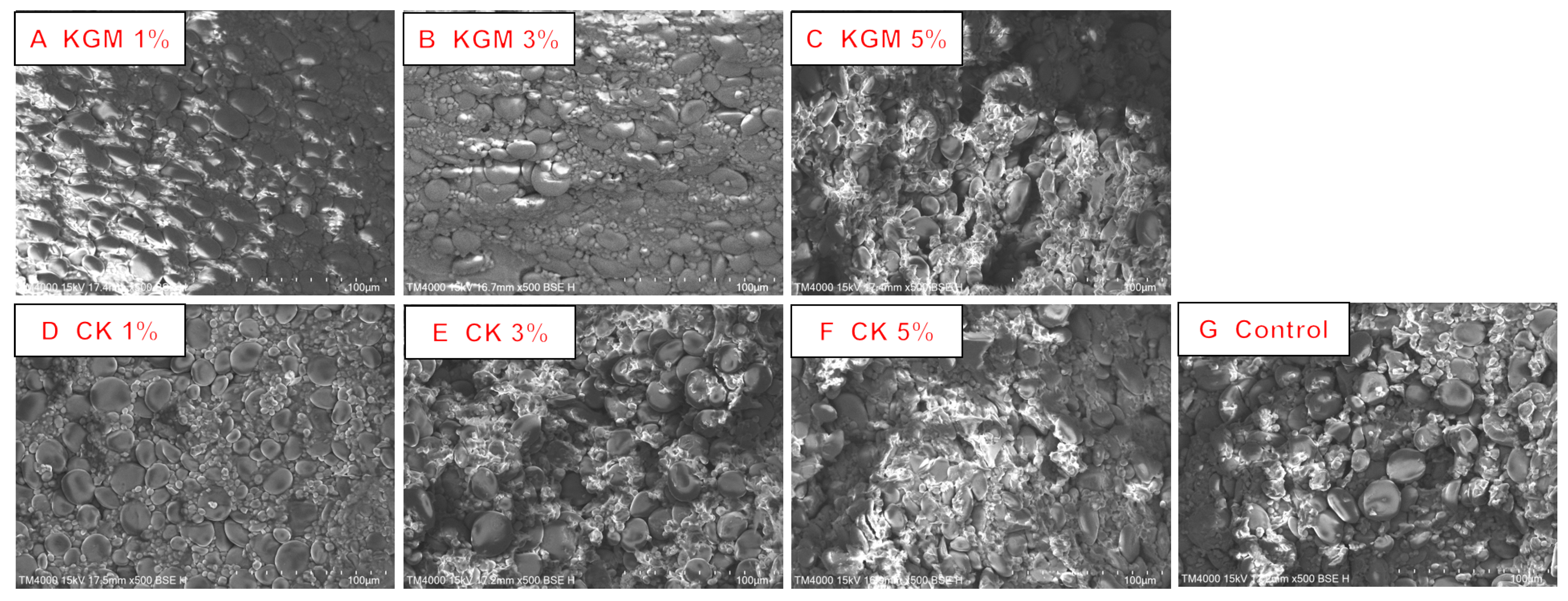
3.7. Microstructure Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, M.; Du, J.J.; Hou, G.G.; Du, X.F. Effect of tea extract on starch gelatinisation, gluten aggregation and quality characteristics of dry yellow alkaline noodle. Int. J. Food Sci. Technol. 2023, 58, 1263–1274. [Google Scholar] [CrossRef]
- Seetapan, N.; Limparyoon, N.; Yooberg, R.; Leelawat, B.; Charunuch, C. Influence of addition of extruded rice flour on preparation and quality of fresh gluten-free yellow alkaline noodles. J. Cereal Sci. 2019, 90, 102828. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Feng, X.; Ren, F.; Wang, J. Effect of hydrocolloids on gluten proteins, dough, and flour products: A review. Food Res. Int. 2023, 164, 112292. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-L.; Tan, T.-C.; Easa, A.M. The use of selected hydrocolloids and salt substitutes on structural integrity, texture, sensory properties, and shelf life of fresh no salt wheat noodles. Food Hydrocoll. 2020, 108, 105996. [Google Scholar] [CrossRef]
- Gasparre, N.; Rosell, C.M. Role of hydrocolloids in gluten free noodles made with tiger nut flour as non-conventional powder. Food Hydrocoll. 2019, 97, 105194. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, J.; Wang, Y.; Wang, Y.; Cheng, Y. Gastrointestinal and metabolic effects of noodles-based konjac glucomannan in rats. Food Nutr. Res. 2019, 63, 1–11. [Google Scholar] [CrossRef]
- Zhao, D.; Zhou, Y.; Liu, H.; Liang, J.; Cheng, Y.; Nirasawa, S. Effects of dough mixing time before adding konjac glucomannan on the quality of noodles. J. Food Sci. Technol. 2017, 54, 3837–3846. [Google Scholar] [CrossRef]
- Li, S.; Qu, Z.; Feng, J.; Chen, Y. Improved physicochemical and structural properties of wheat gluten with konjac glucomannan. J. Cereal Sci. 2020, 95, 103050. [Google Scholar] [CrossRef]
- Cao, G.; Chen, X.; Wang, N.; Tian, J.; Song, S.; Wu, X.; Wang, L.; Wen, C. Effect of konjac glucomannan with different viscosities on the quality of surimi-wheat dough and noodles. Int. J. Biol. Macromol. 2022, 221, 1228–1237. [Google Scholar] [CrossRef]
- Ge, Z.; Wang, W.; Gao, S.; Xu, M.; Liu, M.; Wang, X.; Zhang, L.; Zong, W. Effects of konjac glucomannan on the long-term retrogradation and shelf life of boiled wheat noodles. J. Sci. Food Agric. 2021, 102, 644–652. [Google Scholar] [CrossRef]
- Yu, A.H.M.; Phoon, P.Y.; Ng, G.C.F.; Henry, C.J. Physicochemical characteristics of green banana flour and its use in the development of konjac-green banana noodles. J. Food Sci. 2020, 85, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Kim, H.Y.; Shin, H.Y.; Jeon, Y.I.; Kim, J.M.; Kim, S.; Kim, J.Y. Change in textural properties, starch digestibility, and aroma of nonfried instant noodles by substitution of konjac glucomannan. Cereal Chem. 2019, 96, 784–791. [Google Scholar] [CrossRef]
- He, Y.; Guo, J.; Ren, G.; Cui, G.; Han, S.; Liu, J. Effects of konjac glucomannan on the water distribution of frozen dough and corresponding steamed bread quality. Food Chem. 2020, 330, 127243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cao, H.; Hou, M.; Nirasawa, S.; Tatsumi, E.; Foster, T.J.; Cheng, Y. Effect of konjac glucomannan on physical and sensory properties of noodles made from low-protein wheat flour. Food Res. Int. 2013, 51, 879–885. [Google Scholar] [CrossRef]
- Liu, X.; Liao, W.; Xia, W. Recent advances in chitosan based bioactive materials for food preservation. Food Hydrocoll. 2023, 140, 108612. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Dong, Q.; Xu, C.; Deng, S.; Kang, Y.; Fan, M.; Li, L. Application of functionalized chitosan in food: A review. Int. J. Biol. Macromol. 2023, 235, 123716. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, L.; Rasheed, H.A.; Xia, X.; Tekliye, M.; Li, Z.; Li, J.; Dong, M. Effects of chitosan on the physicochemical properties, in vitro starch digestibility, antimicrobial potentials, and antioxidant activities of purple highland barley noodles. Lwt 2020, 132, 109802. [Google Scholar] [CrossRef]
- Chen, H.; Guo, X.-N.; Zhu, K.-X. The effect of chitosan oligosaccharides on the shelf-life and quality of fresh wet noodles. Carbohydr. Polym. 2023, 309, 120704. [Google Scholar] [CrossRef]
- Tantala, J.; Meethongchai, S.; Suethong, W.; Ratanasumawong, S.; Rachtanapun, C. Mold-free shelf-life extension of fresh rice noodles by synergistic effects of chitosan and common food preservatives. Food Control 2022, 133, 108597. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Jin, W.; Geng, X.; Xu, W.; Ye, T.; Lei, J.; Li, B.; Wang, L. Preparation and characterization of a novel pH-response dietary fiber: Chitosan-coated konjac glucomannan. Carbohydr. Polym. 2015, 117, 1–10. [Google Scholar] [CrossRef]
- Fan, H.P.; Ai, Z.L.; Chen, Y.H.; Fu, F.; Bian, K. Effect of alkaline salts on the quality characteristics of yellow alkaline noodles. J. Cereal Sci. 2018, 84, 159–167. [Google Scholar] [CrossRef]
- Koh, W.Y.; Matanjun, P.; Lim, X.X.; Kobun, R. Sensory, Physicochemical, and Cooking Qualities of Instant Noodles Incorporated with Red Seaweed (Eucheuma denticulatum). Foods 2022, 11, 2669. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Xu, F.; Han, R.; Quan, M. Effect of Tremella fuciformis and Different Hydrocolloids on the Quality Characteristics of Wheat Noodles. Foods 2022, 11, 2617. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gidley, M.J.; Warren, F.J. Tea polyphenols enhance binding of porcine pancreatic α-amylase with starch granules but reduce catalytic activity. Food Chem. 2018, 258, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, G.; Mehta, S. Effect of chitosan on physicochemical and rheological attributes of bread. Food Sci. Technol. Int. 2018, 25, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Asenstorfer, R.E.; Wang, Y.; Mares, D.J. Chemical structure of flavonoid compounds in wheat (Triticum aestivum L.) flour that contribute to the yellow colour of Asian alkaline noodles. J. Cereal Sci. 2006, 43, 108–119. [Google Scholar] [CrossRef]
- Ma, Y.; Hong, T.; Xu, D.; Wu, F.; Xu, X. Inhibition of PPO-related browning in fresh noodles: A combination of chemical and heat treatment. Food Chem. 2023, 404, 134549. [Google Scholar] [CrossRef]
- Guo, J.; Liu, F.; Gan, C.; Wang, Y.; Wang, P.; Li, X.; Hao, J. Effects of Konjac glucomannan with different viscosities on the rheological and microstructural properties of dough and the performance of steamed bread. Food Chem. 2022, 368, 130853. [Google Scholar] [CrossRef]
- Tobin, J.T.; Fitzsimons, S.M.; Chaurin, V.; Kelly, A.L.; Fenelon, M.A. Thermodynamic incompatibility between denatured whey protein and konjac glucomannan. Food Hydrocoll. 2012, 27, 201–207. [Google Scholar] [CrossRef]
- Meng, K.; Gao, H.; Zeng, J.; Zhao, J.; Qin, Y.; Li, G.; Su, T. Rheological and microstructural characterization of wheat dough formulated with konjac glucomannan. J. Sci. Food Agric. 2021, 101, 4373–4379. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhou, Y.; Nirasawa, S.; Tatsumi, E.; Li, X.; Cheng, Y. Effects of konjac glucomannan on heat-induced changes of wheat gluten structure. Food Chem. 2017, 229, 409–416. [Google Scholar] [CrossRef]
- Xiao, W.; Ding, Y.; Cheng, Y.; Xu, S.; Lin, L. Understanding the Changes in Quality of Semi-Dried Rice Noodles during Storage at Room Temperature. Foods 2022, 11, 2130. [Google Scholar] [CrossRef]
- Wang, J.; Si, X.; Shang, W.; Zhou, Z.; Strappe, P.; Blanchard, C. Effect of single or combined administration of resistant starch and chitosan oligosaccharides on insulin resistance in rats fed with a high-fat diet. Starch-Stärke 2017, 69, 1600209. [Google Scholar] [CrossRef]
- Diao, Y.; Si, X.; Shang, W.; Zhou, Z.; Wang, Z.; Zheng, P.; Strappe, P.; Blanchard, C. Effect of interactions between starch and chitosan on waxy maize starch physicochemical and digestion properties. CyTA-J. Food 2017, 15, 327–335. [Google Scholar] [CrossRef]
- Guo, Q.; Cai, J.-h.; Ren, C.-w.; Li, Y.-t.; Farooq, M.A.; Xu, B. A new strategy for the shelf life extension of fresh noodles by accurately targeting specific microbial species. Food Control 2022, 138, 109037. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Kraithong, S.; Theppawong, A.; Lee, S.; Huang, R. Understanding of hydrocolloid functions for enhancing the physicochemical features of rice flour and noodles. Food Hydrocoll. 2023, 142, 108821. [Google Scholar] [CrossRef]
| Sample | Flour | KGM | CK | Water | Soda | Salt |
|---|---|---|---|---|---|---|
| Control | 100.0 | 0 | 0 | 35.0 | 0.6 | 0.8 |
| KGM 1% | 99.0 | 1.0 | 0 | 35.0 | 0.6 | 0.8 |
| KGM 3% | 97.0 | 3.0 | 0 | 35.0 | 0.6 | 0.8 |
| KGM 5% | 95.0 | 5.0 | 0 | 35.0 | 0.6 | 0.8 |
| CK 1% | 99.0 | 0 | 1.0 | 35.0 | 0.6 | 0.8 |
| CK 3% | 97.0 | 0 | 3.0 | 35.0 | 0.6 | 0.8 |
| CK 5% | 95.0 | 0 | 5.0 | 35.0 | 0.6 | 0.8 |
| Sample | L | a | b | ∆E |
|---|---|---|---|---|
| Control | 88.50 ± 0.02 a | −1.40 ± 0.02 b | 20.38 ± 0.23 b | |
| KGM 1% | 86.03 ± 0.17 f | −1.41 ± 0.01 b | 22.55 ± 0.31 a | 3.29 ± 0.17 b |
| KGM 3% | 86.73 ± 0.01 e | −1.50 ± 0.05 a | 19.69 ± 0.21 c | 1.91 ± 0.21 c |
| KGM 5% | 85.47 ± 0.01 g | −1.53 ± 0.03 a | 18.40 ± 0.24 d | 3.63 ± 0.02 a |
| CK 1% | 87.85 ± 0.01 b | −1.38 ± 0.03 b | 20.19 ± 0.26 b | 0.68 ± 0.03 f |
| CK 3% | 87.09 ± 0.11 d | −1.13 ± 0.02 c | 20.38 ± 0.33 b | 1.45 ± 0.13 e |
| CK 5% | 87.41 ± 0.03 c | −0.93 ± 0.01 d | 17.75 ± 0.32 e | 2.89 ± 0.09 d |
| Sample | T21/ms | T22/ms |
|---|---|---|
| Control | 2.58 ± 0.00 a | 44.49 ± 0.02 a |
| KGM 1% | 1.96 ± 0.00 b | 19.34 ± 0.13 b |
| KGM 3% | 1.48 ± 0.01 c | 14.65 ± 0.09 c |
| KGM 5% | 1.29 ± 0.00 d | 11.90 ± 0.40 d |
| CK 1% | 3.18 ± 0.01 e | 31.44 ± 0.23 e |
| CK 3% | 2.25 ± 0.00 f | 27.36 ± 1.00 f |
| CK 5% | 1.59 ± 0.00 g | 16.83 ± 0.10 g |
| Sample | RDS (%) | SDS (%) | RS (%) |
|---|---|---|---|
| Control | 73.64 ± 0.10 a | 9.55 ± 0.10 g | 16.82 ± 0.11 c |
| KGM 1% | 64.98 ± 0.12 d | 16.96 ± 0.10 d | 18.06 ± 0.16 a |
| KGM 3% | 63.70 ± 0.11 f | 18.63 ± 0.09 b | 17.67 ± 0.14 b |
| KGM 5% | 62.66 ± 0.11 g | 19.76 ± 0.11 a | 17.58 ± 0.16 b |
| CK 1% | 69.33 ± 0.12 b | 12.46 ± 0.09 f | 18.21 ± 0.15 a |
| CK 3% | 66.06 ± 0.09 c | 15.86 ± 0.08 e | 18.08 ± 0.12 a |
| CK 5% | 64.08 ± 0.12 e | 17.95 ± 0.08 c | 17.97 ± 0.14 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; He, J.; Huang, S.; Li, B. Application of Konjac Glucomannan with Chitosan Coating in Yellow Alkaline Noodles. Foods 2023, 12, 3569. https://doi.org/10.3390/foods12193569
Wang S, He J, Huang S, Li B. Application of Konjac Glucomannan with Chitosan Coating in Yellow Alkaline Noodles. Foods. 2023; 12(19):3569. https://doi.org/10.3390/foods12193569
Chicago/Turabian StyleWang, Shishuai, Jiaxin He, Shanshan Huang, and Bin Li. 2023. "Application of Konjac Glucomannan with Chitosan Coating in Yellow Alkaline Noodles" Foods 12, no. 19: 3569. https://doi.org/10.3390/foods12193569
APA StyleWang, S., He, J., Huang, S., & Li, B. (2023). Application of Konjac Glucomannan with Chitosan Coating in Yellow Alkaline Noodles. Foods, 12(19), 3569. https://doi.org/10.3390/foods12193569





