Identification and Characterization of Novel Malto-Oligosaccharide-Forming Amylase AmyCf from Cystobacter sp. Strain CF23
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasmid Construction and Gene Expression
2.3. Enzyme Assay
2.4. Biochemical Properties of the α-Amylase AmyCf
2.5. Analysis of the Hydrolyzed Products
2.6. Sequence Analysis
2.7. Statistical Analysis
2.8. The Accession Number of the Nucleotide Sequence
3. Results and Discussion
3.1. Identification of a Novel α-Amylase AmyCf from Cystobacter sp. Strain CF23
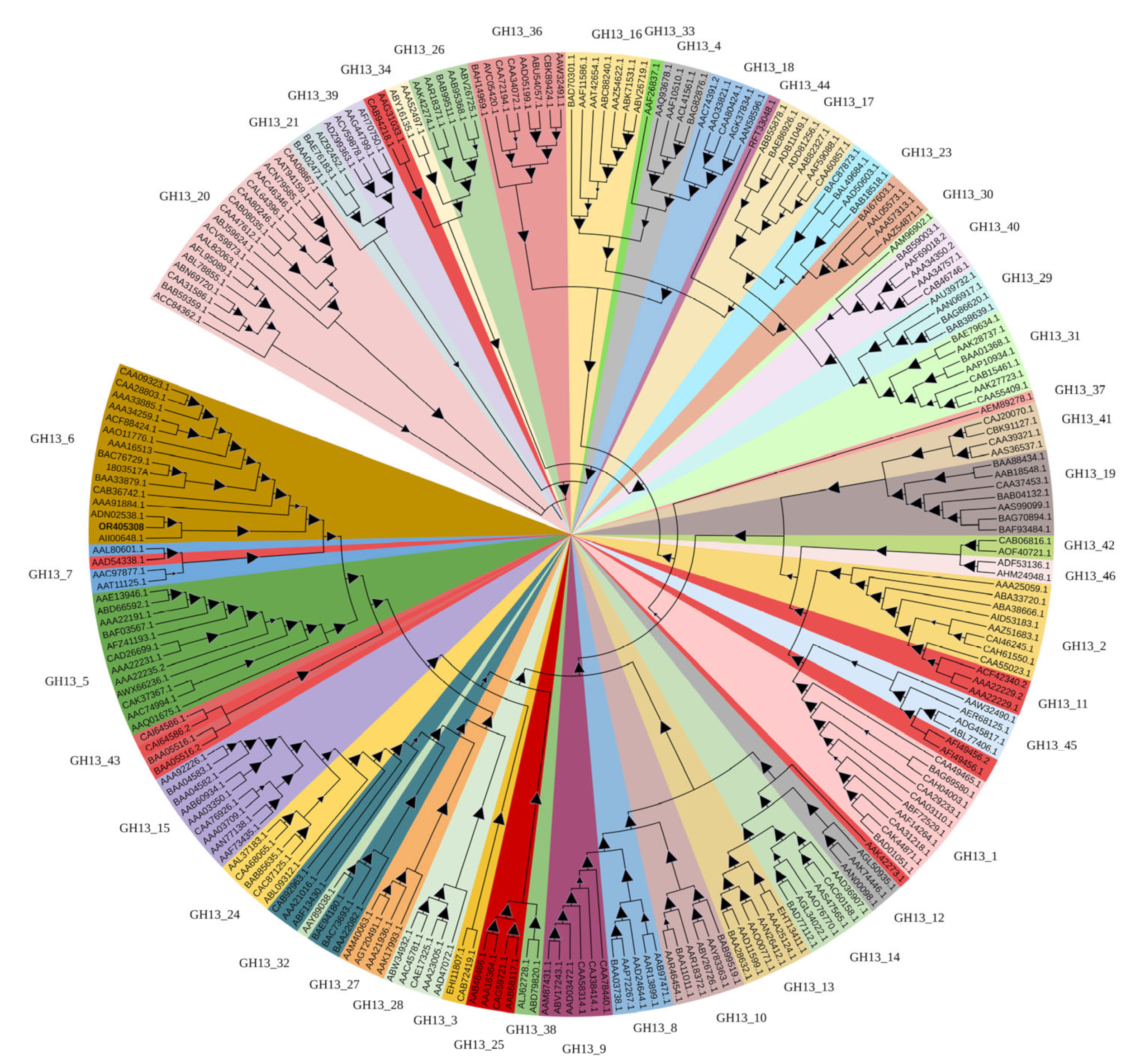
3.2. Expression of the Recombinant α-Amylase AmyCf in P. pastoris
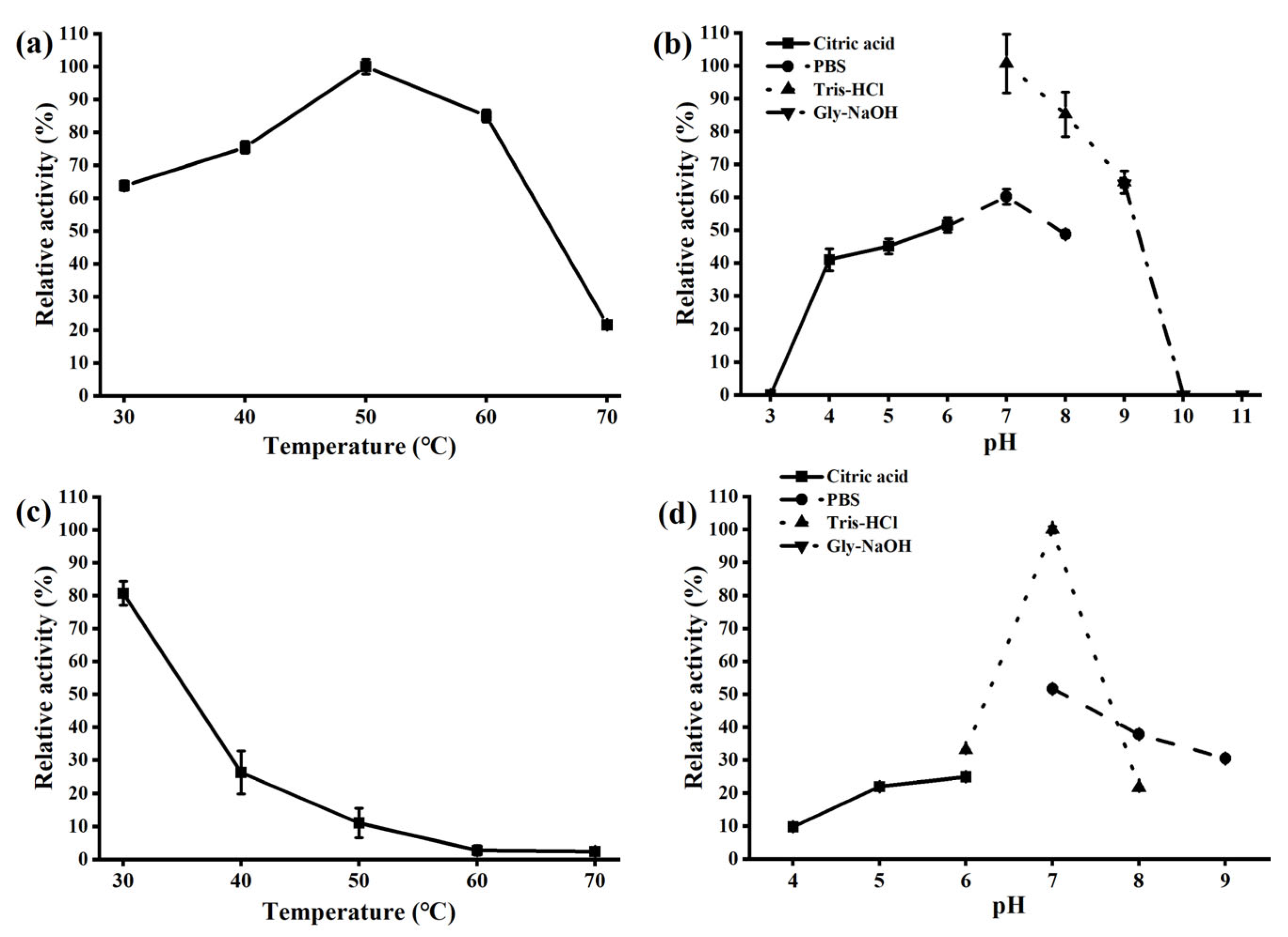
3.3. Characterization of the Recombinant α-Amylase AmyCf
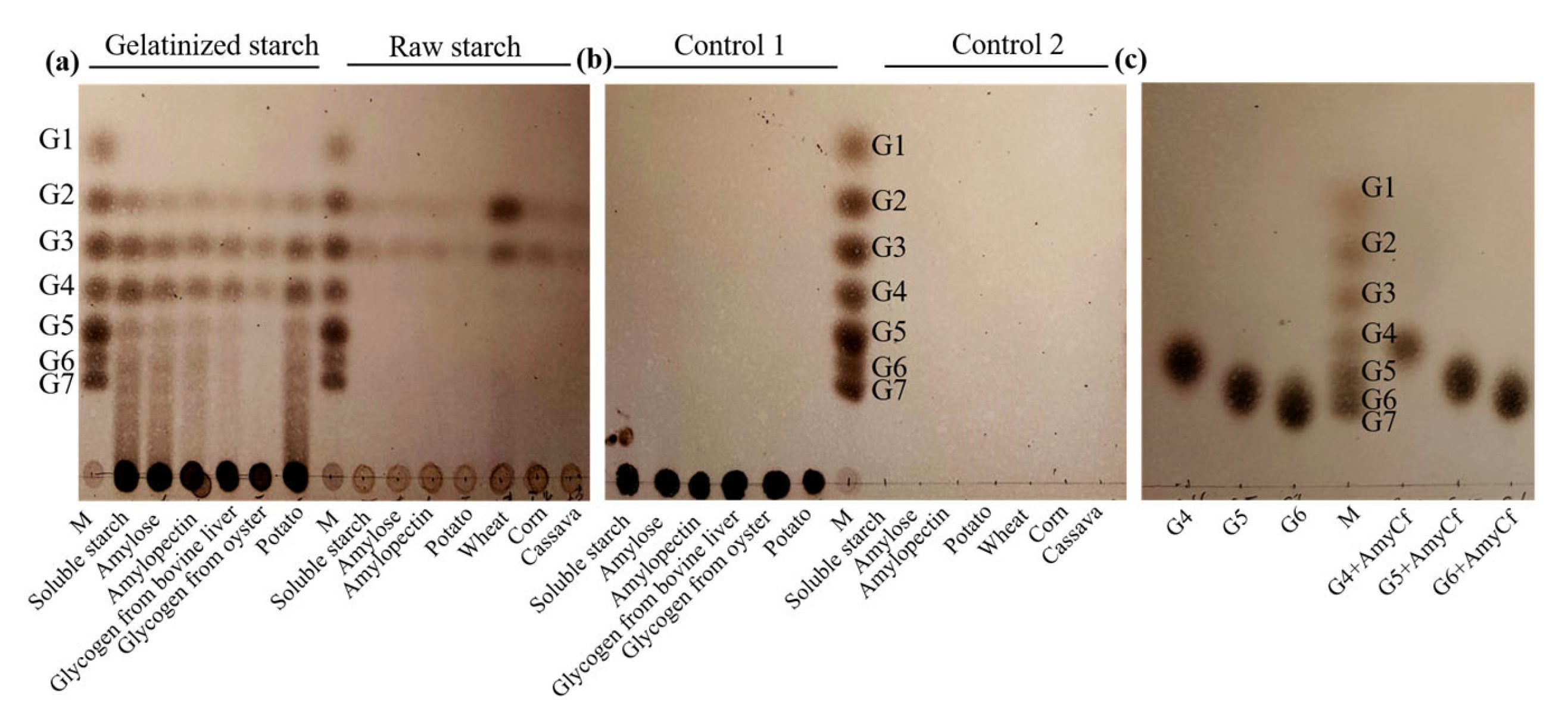
3.4. Substrate Specificity of Recombinant α-Amylase AmyCf
3.5. Action Pattern of Recombinant α-Amylase AmyCf toward Starch and MOSs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ji, H.; Bai, Y.; Liu, Y.; Wang, Y.; Zhan, X.; Long, J.; Chen, L.; Qiu, C.; Jin, Z. Deciphering external chain length and cyclodextrin production with starch catalyzed by cyclodextrin glycosyltransferase. Carbohyd. Polym. 2022, 284, 119156. [Google Scholar] [CrossRef]
- Zhou, D.-N.; Zhang, B.; Chen, B.; Chen, H.-Q. Effects of oligosaccharides on pasting, thermal and rheological properties of sweet potato starch. Food Chem. 2017, 230, 516–523. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Z.; Zhang, H.; Wu, J.; Ye, X.; Dong, W.; Jiang, M.; Huang, Y.; Cui, Z. Novel Maltogenic Amylase CoMA from Corallococcus sp. Strain EGB Catalyzes the Conversion of Malto-oligosaccharides and Soluble Starch to Maltose. Appl. Environ. Microb. 2018, 84, e00152-18. [Google Scholar] [CrossRef]
- Kamon, M.; Sumitani, J.; Tani, S.; Kawaguchi, T. Characterization and gene cloning of a maltotriose-forming exo-amylase from Kitasatospora sp. MK-1785. Appl. Microbiol. Biotechnol. 2015, 99, 4743–4753. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, K.; Kumazawa, M.; Murakami, S.; Takenaka, S.; Aoki, K. Purification, Characterization, and Gene Cloning of Ceriporiopsis sp. Strain MD-1 Peroxidases That Decolorize Human Hair Melanin. Appl. Environ. Microb. 2008, 74, 5106–5112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, D.; Liu, M.; Xia, C.; Fan, Q.; Li, X.; Lan, Z.; Shi, G.; Dong, W.; Li, Z.; et al. Preparation of Active Chitooligosaccharides with a Novel Chitosanase AqCoA and Their Application in Fungal Disease Protection. J. Agric. Food Chem. 2021, 69, 3351–3361. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, J.; Zhang, B.; Wang, F.; Ye, X.; Huang, Y.; Huang, Q.; Cui, Z.; Liu, S.J. AmyM, a Novel Maltohexaose-Forming α-Amylase from Corallococcus sp. Strain EGB. J. Agric. Food Chem. 2015, 81, 1977–1987. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, W.; Zhang, L.; Wang, Y.; Zhang, Y.; Qiao, Y.; Luo, X.; Huang, Y.; Cui, Z. Gene Expression and Biochemical Characterization of a GH77 4-α-Glucanotransferase CcGtase from Corallococcus sp. EGB. Starch-Starke 2019, 71, 1800254. [Google Scholar] [CrossRef]
- Liu, F.; Kerp, H.; Peng, H.; Zhu, H.; Peng, J.J.P. Palynostratigraphy of the Devonian–Carboniferous transition in the Tulong section in South Tibet: A Hangenberg Event sequence analogue in the Himalaya-Tethys zone. Palaeogeogr. Palaeocl. 2018, 531, S0031018217303796. [Google Scholar] [CrossRef]
- Roy, J.K.; Arumugam, N.; Ranjan, B.; Puri, A.K.; Mukherjee, A.K.; Singh, S.; Pillai, S. An Overview of Raw Starch Digesting Enzymes and Their Applications in Biofuel Development. In Bioprospecting of Enzymes in Industry, Healthcare and Sustainable Environment; Thatoi, H., Mohapatra, S., Das, S.K., Eds.; Springer: Singapore, 2021; pp. 49–85. [Google Scholar]
- Sun, H.; Zhao, P.; Ge, X.; Xia, Y.; Hao, Z.; Liu, J.; Peng, M. Recent Advances in Microbial Raw Starch Degrading Enzymes. Appl. Biochem. Biotechnol. 2010, 160, 988–1003. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Janeček, Š.; Svensson, B.; MacGregor, E.A. α-Amylase: An enzyme specificity found in various families of glycoside hydrolases. Cell Mol. Life Sci. 2014, 71, 1149–1170. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sanoja, R.; Oviedo, N.; Sánchez, S. Microbial starch-binding domain. Curr. Opin. Microbiol. 2005, 8, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; An, F.; Tang, M. Effects of arbuscular mycorrhiza fungi on drought resistance of Forsythia suspensa. Mycorrhiza 2022, 32, 396–399. [Google Scholar]
- Janeček, Š.; Mareček, F.; MacGregor, E.A.; Svensson, B. Starch-binding domains as CBM families–history, occurrence, structure, function and evolution. Biotechnol. Adv. 2019, 37, 107451. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Xue, S.; Deng, P.; Zhang, X.; Wang, X.; Xiao, Y.; Fang, Z. AmyZ1: A novel α-amylase from marine bacterium Pontibacillus sp. ZY with high activity toward raw starches. Biotechnol. Biofuels 2019, 12, 95. [Google Scholar] [CrossRef]
- Zhang, L.; Zhong, L.; Wang, J.; Zhao, Y.; Zhang, Y.; Zheng, Y.; Dong, W.; Ye, X.; Huang, Y.; Li, Z.; et al. Efficient hydrolysis of raw starch by a maltohexaose-forming α-amylase from Corallococcus sp. EGB. LWT-Food Sci. Technol. 2021, 152. [Google Scholar] [CrossRef]
- Cripwell, R.A.; Favaro, L.; Viljoen-Bloom, M.; van Zyl, W.H. Consolidated bioprocessing of raw starch to ethanol by Saccharomyces cerevisiae: Achievements and challenges. Biotechnol. Adv. 2020, 42, 107579. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Ubeyitogullari, A. Fabrication of Porous Spherical Beads from Corn Starch by Using a 3D Food Printing System. Foods 2022, 11, 913. [Google Scholar] [CrossRef]
- Zhong, L.; Guo, X.; Xue, H.; Qiao, Y.; Mao, D.; Ye, X.; Cui, Z.; Li, Z.; Hu, G.; Huang, Y. Quality Characteristics of Reduced-Fat Emulsified Sausages Made with Yeast Mannoprotein Enzymatically Prepared with a β-1,6-glucanase. Foods 2023, 12, 2486. [Google Scholar] [CrossRef]
- Huang, Z.; Ni, G.; Wang, F.; Zhao, X.; Chen, Y.; Zhang, L.; Qu, M. Characterization of a Thermostable Lichenase from Bacillus subtilis B110 and Its Effects on β-Glucan Hydrolysis. World J. Microb. Biotechnol. 2022, 32, 484–492. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, Z.; He, J.; Li, Y.; Pan, C.; Wang, C.; Deng, M.R.; Zhu, H. A myxobacterial LPMO10 has oxidizing cellulose activity for promoting biomass enzymatic saccharification of agricultural crop straws. Bioresour. Technol. 2020, 318, 124217. [Google Scholar] [CrossRef]
- Qiao, Y.; Ye, X.; Zhong, L.; Xia, C.; Zhang, L.; Yang, F.; Li, Y.; Fang, X.; Fu, L.; Huang, Y.; et al. Yeast β-1,3-glucan production by an outer membrane β-1,6-glucanase: Process optimization, structural characterization and immunomodulatory activity. Food Funct. 2022, 13, 3917–3930. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Huang, Z.; Qian, M.; Zhang, Q.; Feng, J. Process development of recombinant Aspergillus flavus urate oxidase production in Pichia pastoris intracellularly and its characterization as a potential biosimilar. Process Biochem. 2021, 102, 376–385. [Google Scholar] [CrossRef]
- Yang, M.; Derbyshire, M.K.; Yamashita, R.A.; Marchler-Bauer, A. NCBI’s Conserved Domain Database and Tools for Protein Domain Analysis. Curr. Protoc. Bioinform. 2020, 69, e90. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Tagomori, B.Y.; dos Santos, F.C.; Barbosa-Tessmann, I.P. Recombinant expression, purification, and characterization of an α-amylase from Massilia timonae. 3 Biotech 2021, 11, 13. [Google Scholar] [CrossRef]
- Janeček, Š.; Gabriško, M. Remarkable evolutionary relatedness among the enzymes and proteins from the α-amylase family. Cell Mol. Life Sci. 2016, 73, 2707–2725. [Google Scholar] [CrossRef] [PubMed]
- Janeček, Š.; Lévêque, E.; Belarbi, A.; Haye, B. Close Evolutionary Relatedness of α-Amylases from Archaea and Plants. J. Mol. Evol. 1999, 48, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Mieog, J.C.; Janeček, Š.; Ral, J.-P. New insight in cereal starch degradation: Identification and structural characterization of four α-amylases in bread wheat. Amyla 2017, 1, 35–49. [Google Scholar] [CrossRef]
- Fujita, M.; Torigoe, K.; Nakada, T.; Tsusaki, K.; Kubota, M.; Sakai, S.; Tsujisaka, Y. Cloning and nucleotide sequence of the gene (amyP) for maltotetraose-forming amylase from Pseudomonas stutzeri MO-19. J. Bacteriol. 1989, 171, 1333. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, L.; Dong, C.; Zhong, L.; Fang, X.; Huan, M.; Ye, X.; Huang, Y.; Li, Z.; Cui, Z. Novel Malto-Oligosaccharide-Producing Amylase AmyAc from Archangium sp. Strain AC19 and Its Catalytic Properties. Starch-Starke 2021, 73. [Google Scholar] [CrossRef]
- Robert, X.; Haser, R.; Gottschalk, T.E.; Ratajczak, F.; Driguez, H.; Svensson, B.; Aghajari, N. The Structure of Barley α-Amylase Isozyme 1 Reveals a Novel Role of Domain C in Substrate Recognition and Binding. Structure 2003, 11, 973–984. [Google Scholar] [CrossRef]
- Dworkin, M.; Sadler, W. Induction of Cellular Morphogenesis in Myxococcus xanthus I. General Description. J. Bacteriol. 1966, 91, 1516–1519. [Google Scholar] [CrossRef]
- Thiery, S.; Kaimer, C. The Predation Strategy of Myxococcus xanthus. Front. Microbiol. 2020, 11, 2. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Bretthauer, R.K.; Castellino, F.J. Glycosylation of Pichia pastoris-derived proteins. Biotechnol. Appl. Bioc. 1999, 30, 193–200. [Google Scholar]
- Wang, Y.C.; Yang, W.H.; Yang, C.S.; Hou, M.H.; Tsai, C.L.; Chou, Y.Z.; Hung, M.C.; Chen, Y. Structural basis of SARS-CoV-2 main protease inhibition by a broad-spectrum anti-coronaviral drug. Am. J. Cancer Res. 2020, 10, 2535–2545. [Google Scholar]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat starch production, structure, functionality and applications—A review. Int. J. Food Sci. 2016, 52, 38–58. [Google Scholar] [CrossRef]
- John, H.D. Potato Starch: A Review of Physicochemical, Functional and Nutritional Properties. Am. J. Potato Res. 2019, 96, 127–138. [Google Scholar] [CrossRef]
- Yang, T.; Zhong, L.; Jiang, G.; Liu, L.; Wang, P.; Zhong, Y.; Yue, Q.; Ouyang, L.; Zhang, A.; Li, Z.; et al. Comparative study on bread quality and starch digestibility of normal and waxy wheat (Triticum aestivum L.) modified by maltohexaose producing α-amylases. Food Res. Int. 2022, 162, 112034. [Google Scholar] [CrossRef] [PubMed]
- Ait Kaki El-Hadef El-Okki, A.; Gagaoua, M.; Bourekoua, H.; Hafid, K.; Bennamoun, L.; Djekrif-Dakhmouche, S.; El-Hadef El-Okki, M.; Meraihi, Z. Improving Bread Quality with the Application of a Newly Purified Thermostable α-Amylase from Rhizopus oryzae FSIS4. Foods 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]


| The Type of Metal Ion | Soluble Starch Relative Activity (%) | Raw Wheat Starch Relative Activity (%) | ||
|---|---|---|---|---|
| 1 mM | 5 mM | 1 mM | 5 mM | |
| Control | 100.00 ± 1.27 | 100.00 ± 0.58 | 100.00 ± 2.08 | 100.00 ± 1.21 |
| K+ | 101.31 ± 1.77 | 145.49 ± 1.84 | 93.18 ± 0.59 | 91.24 ± 0.07 |
| Na+ | 101.31 ± 1.81 | 132.19 ± 1.84 | 100.79 ± 6.22 | 102.18 ± 0.31 |
| Ca2+ | 146.43 ± 2.10 | 128.47 ± 2.00 | 104.56 ± 1.05 | 106.83 ± 0.53 |
| Co2+ | 133.29 ± 2.59 | 142.12 ± 1.80 | 116.49 ± 1.18 | 72.99 ± 0.68 |
| Cu2+ | 116.62 ± 2.34 | 86.39 ± 1.51 | 58.79 ± 0.39 | 14.93 ± 1.72 |
| Mn2+ | 115.85 ± 2.42 | 87.24 ± 1.88 | 72.35 ± 0.74 | 47.15 ± 0.01 |
| Zn2+ | 110.53 ± 2.03 | 63.51 ± 1.26 | 61.65 ± 0.69 | 29.78 ± 1.20 |
| Ni2+ | 109.97 ± 0.14 | 109.11 ± 2.18 | 86.69 ± 0.14 | 52.35 ± 0.14 |
| Fe3+ | 104.74 ± 2.30 | 58.37 ± 1.38 | 74.02 ± 0.21 | 40.52 ± 0.96 |
| Cr3+ | 97.35 ± 2.31 | 49.95 ± 1.42 | 59.37 ± 0.58 | 44.77 ± 0.75 |
| Ba2+ | 96.76 ± 2.22 | 63.39 ± 1.51 | 89.76 ± 0.19 | 82.93 ± 0.08 |
| Mg2+ | 87.42 ± 2.30 | 77.31 ± 1.78 | 69.77 ± 0.14 | 61.45 ± 0.69 |
| Soluble Starch | Raw Wheat Starch | ||
|---|---|---|---|
| Substrate | Relatively Activity (%) | Substrate | Relatively Activity (%) |
| Soluble starch | 100.0 ± 0.13 | Wheat | 100.00 ± 0.36 |
| Starch from potato | 95.0 ± 0.13 | Soluble starch | 30.87 ± 0.09 |
| Amylopectin from potato | 86.6 ± 1.70 | Amylose | 27.06 ± 0.08 |
| Amylose from potato | 70.8 ± 0.30 | Corn | 26.56 ± 0.08 |
| Glycogen | 5.8 ± 1.66 | Amylopectin | 22.86 ± 0.07 |
| α-Cyclodextrin | 0.0 | Cassava | 16.76 ± 0.04 |
| Pullulan | 0.0 | Starch from potato | 16.03 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, L.; Wang, P.; Lei, J.; Zhong, L.; Zhan, L.; Ye, X.; Huang, Y.; Luo, X.; Cui, Z.; et al. Identification and Characterization of Novel Malto-Oligosaccharide-Forming Amylase AmyCf from Cystobacter sp. Strain CF23. Foods 2023, 12, 3487. https://doi.org/10.3390/foods12183487
Wang J, Zhang L, Wang P, Lei J, Zhong L, Zhan L, Ye X, Huang Y, Luo X, Cui Z, et al. Identification and Characterization of Novel Malto-Oligosaccharide-Forming Amylase AmyCf from Cystobacter sp. Strain CF23. Foods. 2023; 12(18):3487. https://doi.org/10.3390/foods12183487
Chicago/Turabian StyleWang, Jihong, Lei Zhang, Peiwen Wang, Jinhui Lei, Lingli Zhong, Lei Zhan, Xianfeng Ye, Yan Huang, Xue Luo, Zhongli Cui, and et al. 2023. "Identification and Characterization of Novel Malto-Oligosaccharide-Forming Amylase AmyCf from Cystobacter sp. Strain CF23" Foods 12, no. 18: 3487. https://doi.org/10.3390/foods12183487
APA StyleWang, J., Zhang, L., Wang, P., Lei, J., Zhong, L., Zhan, L., Ye, X., Huang, Y., Luo, X., Cui, Z., & Li, Z. (2023). Identification and Characterization of Novel Malto-Oligosaccharide-Forming Amylase AmyCf from Cystobacter sp. Strain CF23. Foods, 12(18), 3487. https://doi.org/10.3390/foods12183487




