Chemical and Functional Characterization of Propolis Collected from Different Areas of South Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
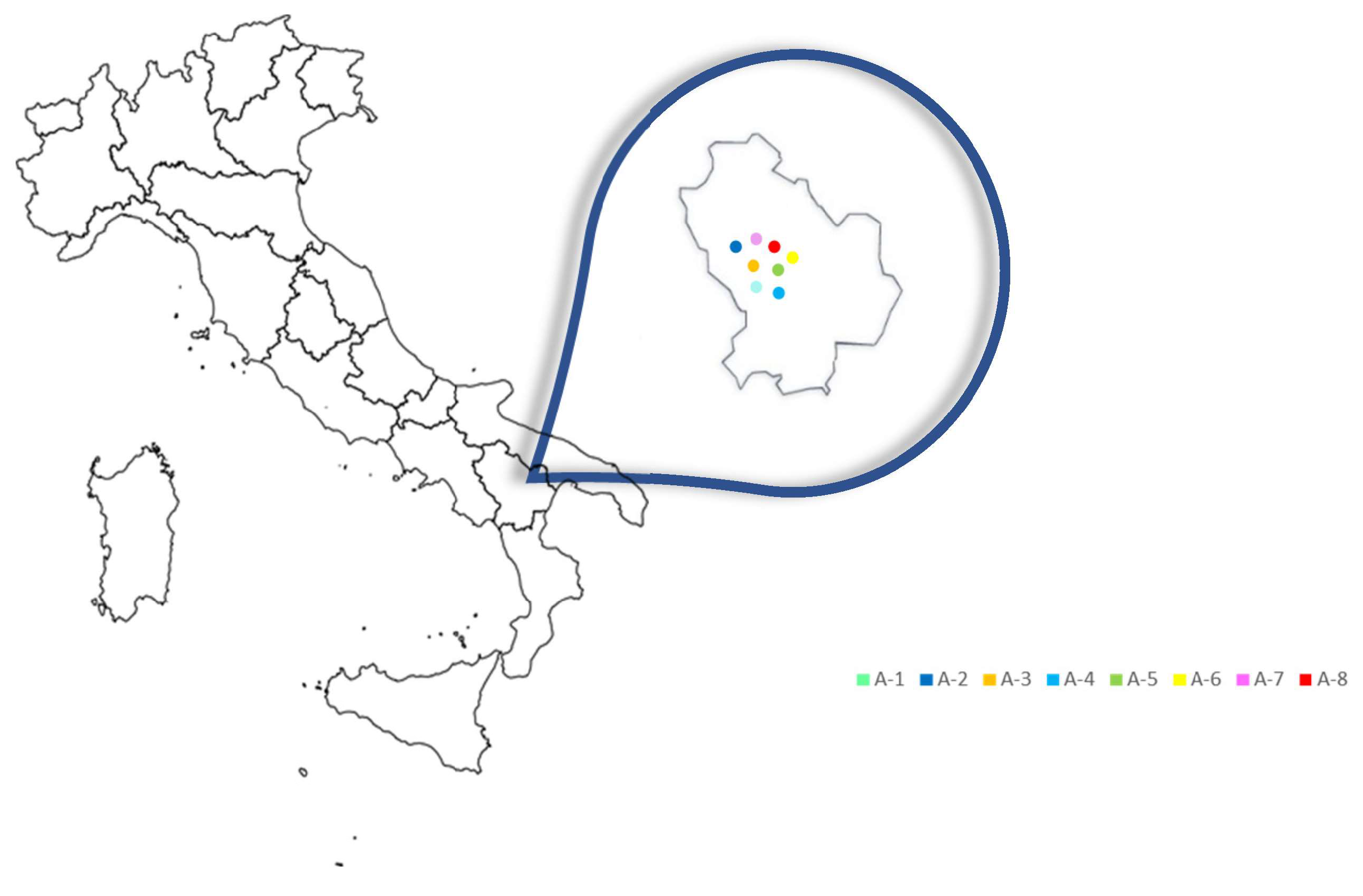
2.2. Propolis Samples
2.3. Water Content
2.4. Ash Content
2.5. Wax Extraction
2.6. Balsam Extraction and Quantification
2.7. Resin Extraction
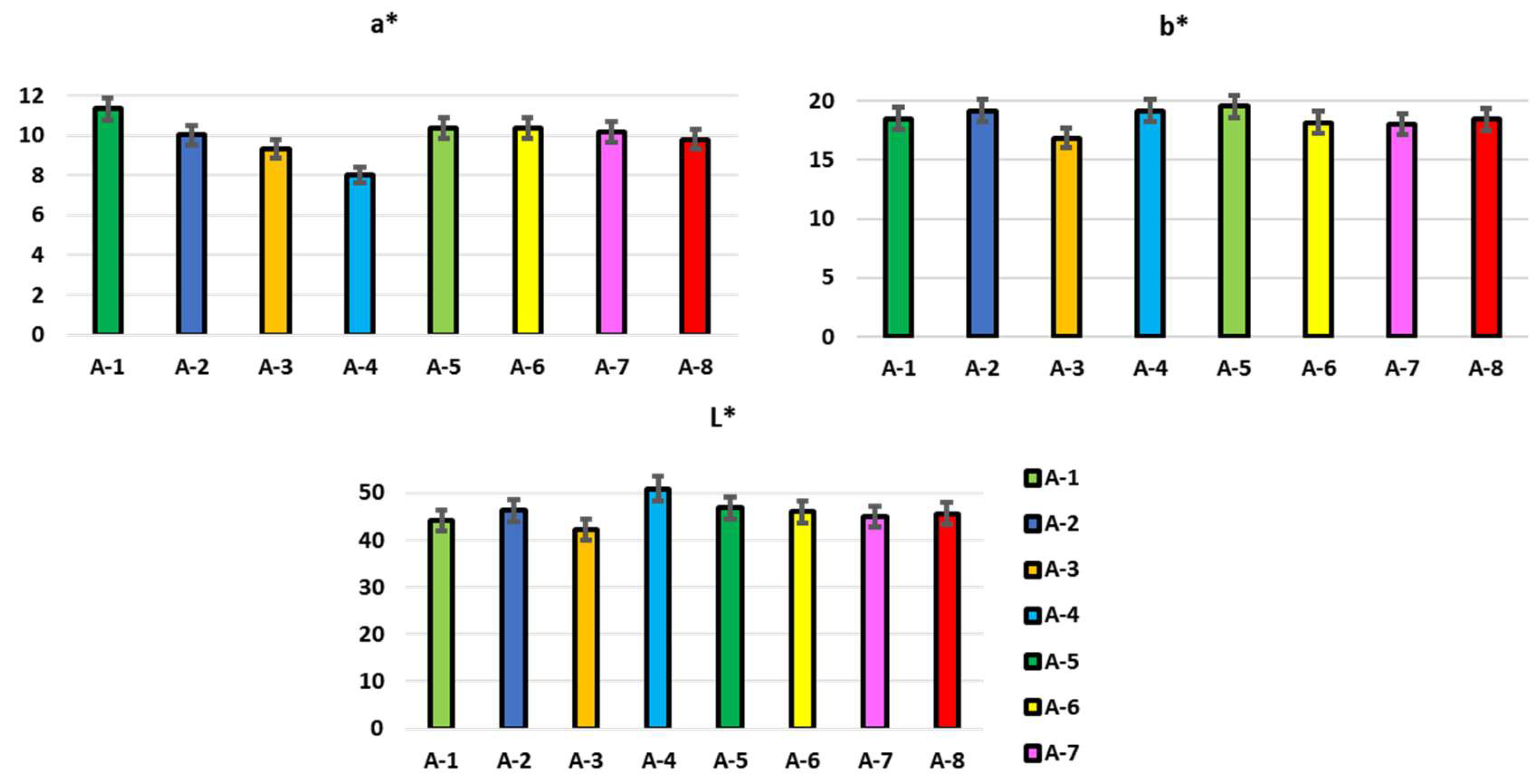
2.8. Colorimetric Analysis
2.9. Extraction of Phenolic Compounds
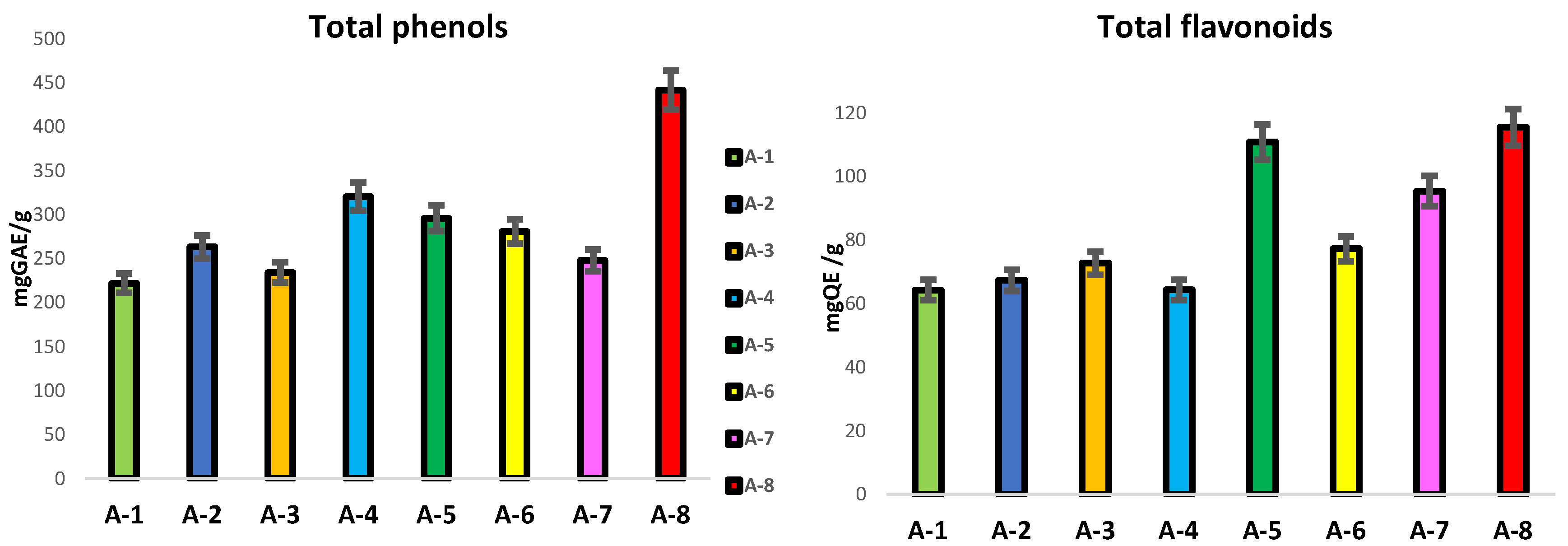
2.10. Total Phenols Content (TPC)
2.11. Total Flavonoid Content (TFC)
2.12. Antioxidant Power (FRAP) Method
2.13. ABTS Free Radical Scavenging
2.14. Statistical Analysis
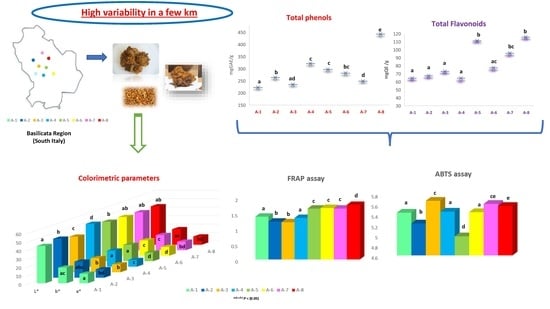
3. Results
3.1. Physico-Chemical Composition in Propolis Samples
3.2. Content in Total Phenols and Flavonoids
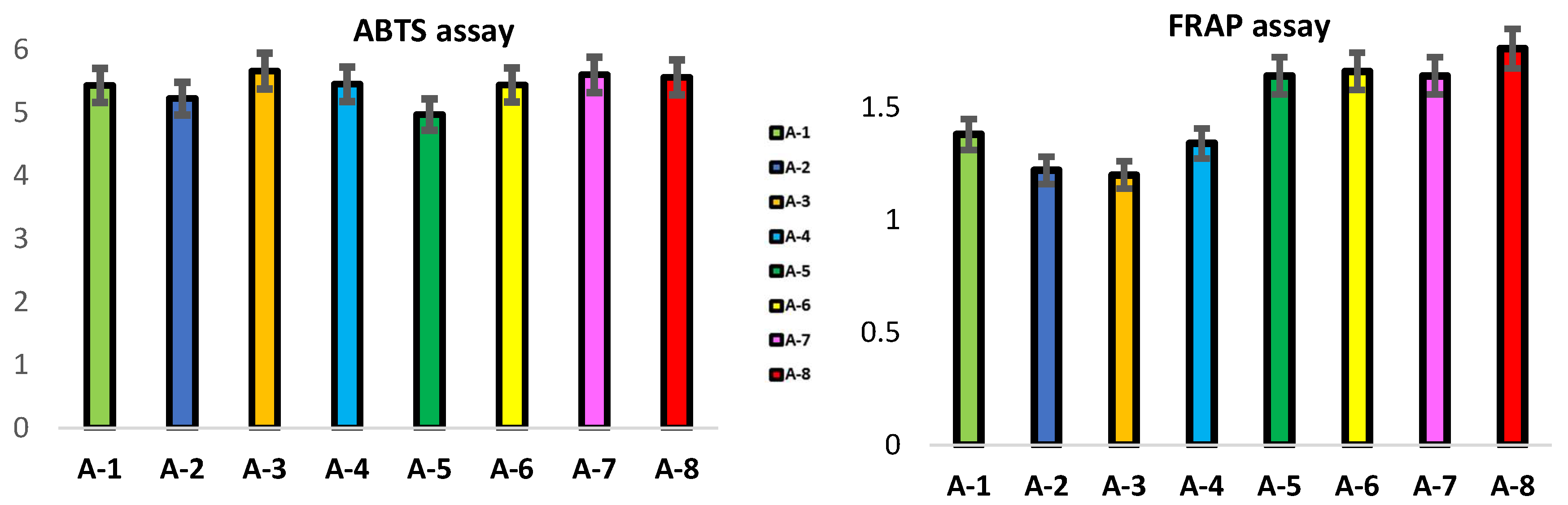
3.3. Antioxidant Activity in Propolis Samples: ABTS and FRAP
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pahlavani, N.; Malekahmadi, M.; Firouzi, S.; Rostami, D.; Sedaghat, A.; Moghaddam, A.B.; Ferns, G.A.; Navashenaq, J.G.; Reazvani, R.; Safarian, M.; et al. Molecular and Cellular Mechanisms of the Effects of Propolis in Inflammation, Oxidative Stress and Glycemic Control in Chronic Diseases. Nutr. Metab. 2020, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Thakur, M.; Chopra, H.K.; Dar, B.N.; Nanda, V. Assessment of Fatty Acids, Amino Acids, Minerals, and Thermal Properties of Bee Propolis from Northern India Using a Multivariate Approach. J. Food Compos. Anal. 2022, 111, 104624. [Google Scholar] [CrossRef]
- Drescher, N.; Klein, A.-M.; Neumann, P.; Yañez, O.; Leonhardt, S. Inside Honeybee Hives: Impact of Natural Propolis on the Ectoparasitic Mite Varroa Destructor and Viruses. Insects 2017, 8, 15. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Borba, R.S.; Wilson, M.; Spivak, M. Propolis Counteracts Some Threats to Honey Bee Health. Insects 2017, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.; Bankova, V.; Viljoen, A.M. Propolis: Chemical Diversity and Challenges in Quality Control. Phytochem. Rev. 2022, 21, 1887–1911. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, C.; Bueno, I.L.; Quaresma, A.C.M.; Longato, G.B. Healing Potential of Propolis in Skin Wounds Evidenced by Clinical Studies. Pharmaceuticals 2022, 15, 1143. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, S.; Alenzi, N.; Fearnley, J.; Harnett, W.; Watson, D.G. Temperate Propolis Has Anti-Inflammatory Effects and Is a Potent Inhibitor of Nitric Oxide Formation in Macrophages. Metabolites 2020, 10, 413. [Google Scholar] [CrossRef]
- Santos, L.A.; Rosalen, P.L.; Dias, N.A.; Grisolia, J.C.; Nascimento Gomes, B.J.; Blosfeld-Lopes, L.; Ikegaki, M.; de Alencar, S.M.; Burger, E. Brazilian Red Propolis Shows Antifungal and Immunomodulatory Activities against Paracoccidioides brasiliensis. J. Ethnopharmacol. 2021, 277, 114181. [Google Scholar] [CrossRef]
- Forma, E.; Bryś, M. Anticancer Activity of Propolis and Its Compounds. Nutrients 2021, 13, 2594. [Google Scholar] [CrossRef]
- Graikou, K.; Popova, M.; Gortzi, O.; Bankova, V.; Chinou, I. Characterization and Biological Evaluation of Selected Mediterranean Propolis Samples. Is It a New Type? LWT-Food Sci. Technol. 2016, 65, 261–267. [Google Scholar] [CrossRef]
- Gardini, S.; Bertelli, D.; Marchetti, L.; Graziosi, R.; Pinetti, D.; Plessi, M.; Marcazzan, G.L. Chemical Composition of Italian Propolis of Different Ecoregional Origin. J. Apic. Res. 2018, 57, 639–647. [Google Scholar] [CrossRef]
- Blasi, C.; Capotorti, G.; Copiz, R.; Guida, D.; Mollo, B.; Smiraglia, D.; Zavattero, L. Classification and Mapping of the Ecoregions of Italy. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2014, 148, 1255–1345. [Google Scholar] [CrossRef]
- Funari, C.S.; Ferro, V.O. Análise de Própolis. Ciênc. Tecnol. Aliment. 2006, 26, 171–178. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis Program. Available online: https://www.aoac.org/scientific-solutions/standards-and-official-methods/ (accessed on 23 February 2023).
- Papotti, G.; Bertelli, D.; Bortolotti, L.; Plessi, M. Chemical and Functional Characterization of Italian Propolis Obtained by Different Harvesting Methods. J. Agric. Food Chem. 2012, 60, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Özkök, A.; Keskin, M.; Tanuğur Samancı, A.E.; Yorulmaz Önder, E.; Takma, Ç. Determination of Antioxidant Activity and Phenolic Compounds for Basic Standardization of Turkish Propolis. Appl. Biol. Chem. 2021, 64, 37. [Google Scholar] [CrossRef] [PubMed]
- Escriche, I.; Juan-Borrás, M. Standardizing the Analysis of Phenolic Profile in Propolis. Food Res. Int. 2018, 106, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- SAS Institute. SAS User’s Guide: Statistics, 7th ed.; SAS Institute Inc.: Cary, NC, USA, 1996. [Google Scholar]
- El Menyiy, N.; Bakour, M.; El Ghouizi, A.; El Guendouz, S.; Lyoussi, B. Influence of Geographic Origin and Plant Source on Physicochemical Properties, Mineral Content, and Antioxidant and Antibacterial Activities of Moroccan Propolis. Int. J. Food Sci. 2021, 2021, e5570224. [Google Scholar] [CrossRef]
- Park, Y.K.; Alencar, S.M.; Aguiar, C.L. Botanical Origin and Chemical Composition of Brazilian Propolis. J. Agric. Food Chem. 2002, 50, 2502–2506. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Lyoussi, B.; Miguel, M.G.; Figueiredo, A.C. Characterization of Volatiles from Moroccan Propolis Samples. J. Essent. Oil Res. 2019, 31, 27–33. [Google Scholar] [CrossRef]
- Sauri-Duch, E.; Gutiérrez-Canul, C.; Cuevas-Glory, L.F.; Ramón-Canul, L.; Pérez-Pacheco, E.; Moo-Huchin, V.M. Determination of Quality Characteristics, Phenolic Compounds and Antioxidant Activity of Propolis from Southeastern Mexico. J. Apic. Sci. 2021, 65, 109–122. [Google Scholar] [CrossRef]
- Gomes, S.; Dias, L.G.; Moreira, L.L.; Rodrigues, P.; Estevinho, L. Physicochemical, Microbiological and Antimicrobial Properties of Commercial Honeys from Portugal. Food Chem. Toxicol. 2010, 48, 544–548. [Google Scholar] [CrossRef]
- da Silva, J.F.M.; de Souza, M.C.; Matta, S.R.; de Andrade, M.R.; Vidal, F.V.N. Correlation Analysis between Phenolic Levels of Brazilian Propolis Extracts and Their Antimicrobial and Antioxidant Activities. Food Chem. 2006, 99, 431–435. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Gutiérrez, A.L. The Antimicrobial Effects of Propolis Collected in Different Regions in the Basque Country (Northern Spain). World J. Microbiol. Biotechnol. 2012, 28, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Özkırım, A.; Çelemli, Ö.G.; Schiesser, A.; Charistos, L.; Hatjina, F. A Comparison of the Activities of Greek and Turkish Propolis against Paenibacillus larvae. J. Apic. Res. 2014, 53, 528–536. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant Activity of Propolis of Various Geographic Origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Martín, I.; Revilla, I.; Vivar-Quintana, A.; Betances Salcedo, E. Pesticide Residues in Propolis from Spain and Chile. An Approach Using near Infrared Spectroscopy. Talanta 2017, 165, 533–539. [Google Scholar] [CrossRef]
- Kasote, D.M.; Pawar, M.V.; Bhatia, R.S.; Nandre, V.S.; Gundu, S.S.; Jagtap, S.D.; Kulkarni, M.V. HPLC, NMR Based Chemical Profiling and Biological Characterisation of Indian Propolis. Fitoterapia 2017, 122, 52–60. [Google Scholar] [CrossRef]
- Sahlan, M.; Rizka Alia Hapsari, N.; Diah Pratami, K.; Cahya Khayrani, A.; Lischer, K.; Alhazmi, A.; Mohammedsaleh, Z.M.; Shater, A.F.; Saleh, F.M.; Alsanie, W.F.; et al. Potential Hepatoprotective Effects of Flavonoids Contained in Propolis from South Sulawesi against Chemotherapy Agents. Saudi J. Biol. Sci. 2021, 28, 5461–5468. [Google Scholar] [CrossRef]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic Composition and Antioxidant Activity of Propolis from Various Regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef]
| Dry Matter | Ash | Wax | Resin | Balsam | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| µ | 1 SD | µ | 1 SD | µ | 1 SD | µ | 1 SD | µ | 1 SD | |
| A-1 | 3.72 ab | 0.31 | 1.75 a | 0.09 | 31.55 a | 1.82 | 48.67 a | 1.67 | 4.33 a | 0.2 |
| A-2 | 3.23 a | 0.30 | 1.01 b | 0.07 | 30.4 ab | 1.47 | 56.49 b | 2.25 | 6.39 b | 0.23 |
| A-3 | 3.39 ab | 0.18 | 0.89 c | 0.05 | 29.08 bc | 1.01 | 59.81 c | 1.23 | 6.4 b | 0.26 |
| A-4 | 3.79 b | 0.28 | 1.84 d | 0.03 | 27.88 c | 1.97 | 64.3 d | 1.36 | 7.82 c | 0.51 |
| A-5 | 3.78 ab | 0.32 | 0.77 e | 0.03 | 27.55 c | 1.61 | 63.4 d | 1.46 | 4.42 a | 0.42 |
| A-6 | 3.31 a | 0.28 | 2.06 f | 0.15 | 24.47 d | 1.57 | 71.78 e | 1.51 | 9.63 d | 0.73 |
| A-7 | 3.53 ab | 0.25 | 0.7 g | 0.03 | 30.9 a | 1.45 | 58.38 bc | 1.75 | 5.41 e | 0.52 |
| A-8 | 3.82 ab | 0.27 | 0.89 c | 0.05 | 24.4 d | 1.15 | 68.39 f | 1.38 | 4.54 a | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassi, G.; Capasso, G.; Gambacorta, E.; Perna, A.M. Chemical and Functional Characterization of Propolis Collected from Different Areas of South Italy. Foods 2023, 12, 3481. https://doi.org/10.3390/foods12183481
Grassi G, Capasso G, Gambacorta E, Perna AM. Chemical and Functional Characterization of Propolis Collected from Different Areas of South Italy. Foods. 2023; 12(18):3481. https://doi.org/10.3390/foods12183481
Chicago/Turabian StyleGrassi, Giulia, Giambattista Capasso, Emilio Gambacorta, and Anna Maria Perna. 2023. "Chemical and Functional Characterization of Propolis Collected from Different Areas of South Italy" Foods 12, no. 18: 3481. https://doi.org/10.3390/foods12183481
APA StyleGrassi, G., Capasso, G., Gambacorta, E., & Perna, A. M. (2023). Chemical and Functional Characterization of Propolis Collected from Different Areas of South Italy. Foods, 12(18), 3481. https://doi.org/10.3390/foods12183481