Stabilization of Hydroxy-α-Sanshool by Antioxidants Present in the Genus Zanthoxylum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sansho and Chemicals
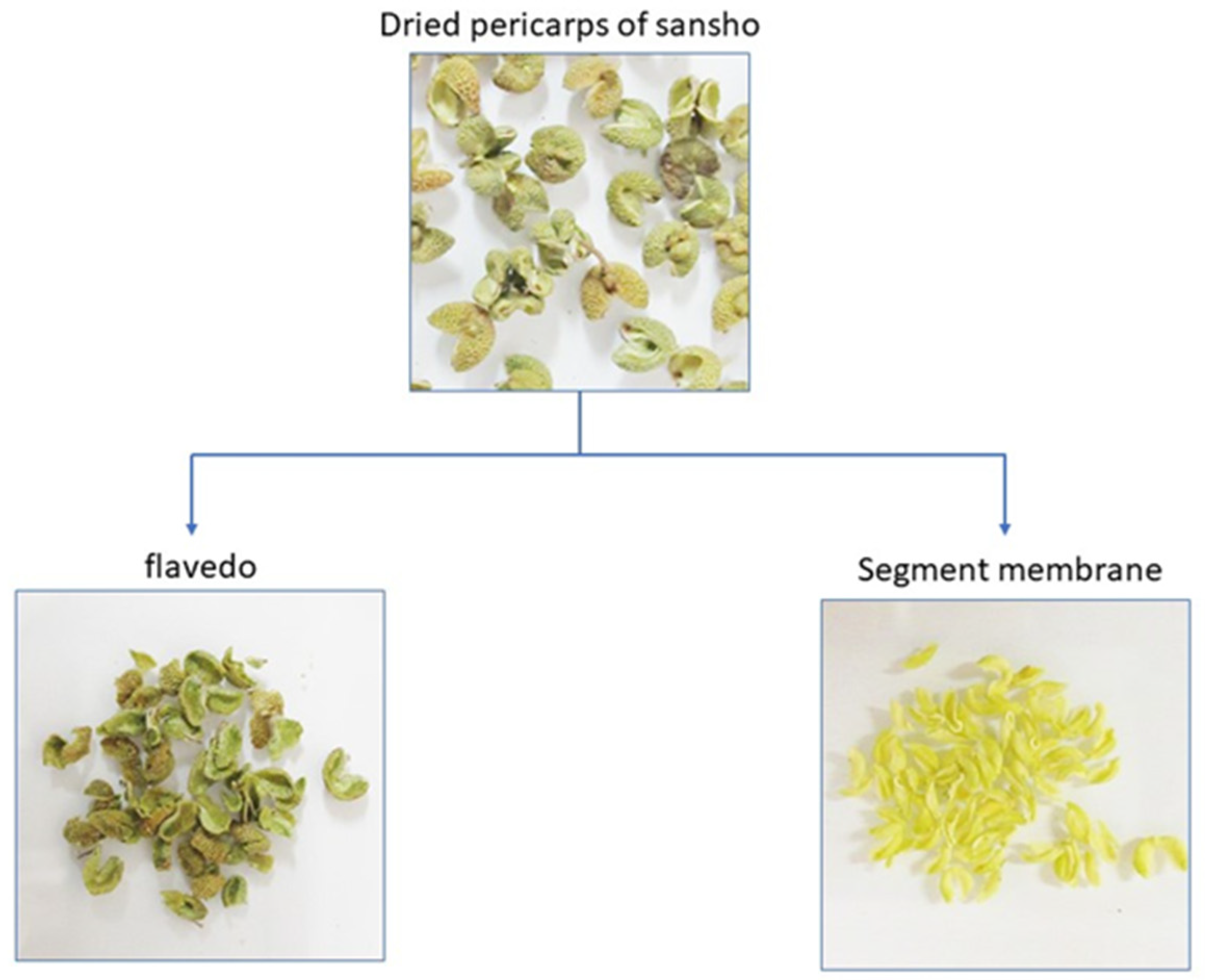
2.2. Preparation of Segment Membrane
2.3. Accelerated Test of HαS-Stabilizing Activity
2.4. HPLC and LC-MS
2.5. Quantitation of HαS
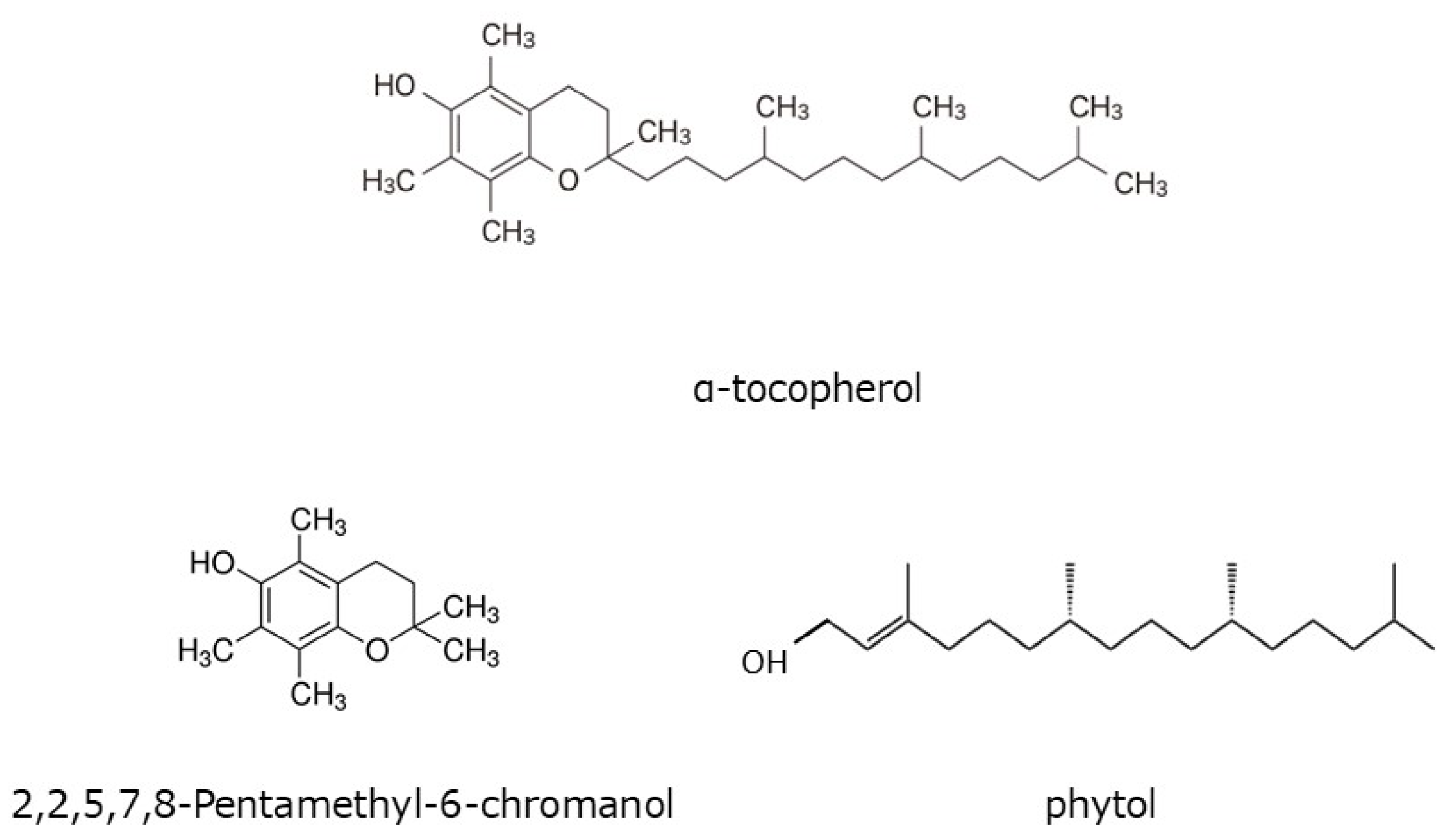
2.6. Quantitation of Tocopherol
2.7. Statistical Analysis
3. Results
3.1. Sansho Harvest Season
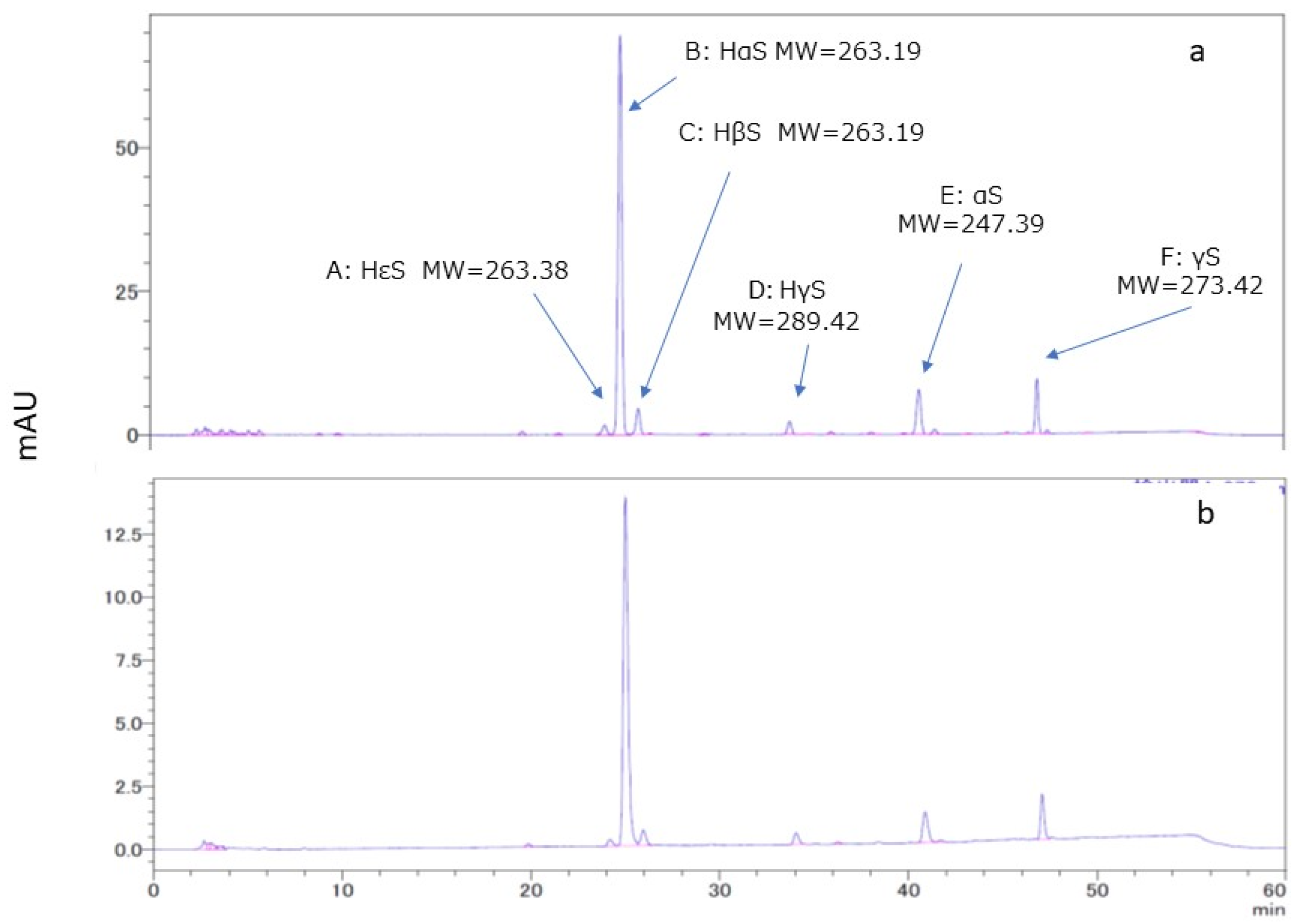
3.2. HPLC and LC/MS of Ethanol Extracts of Pericarps
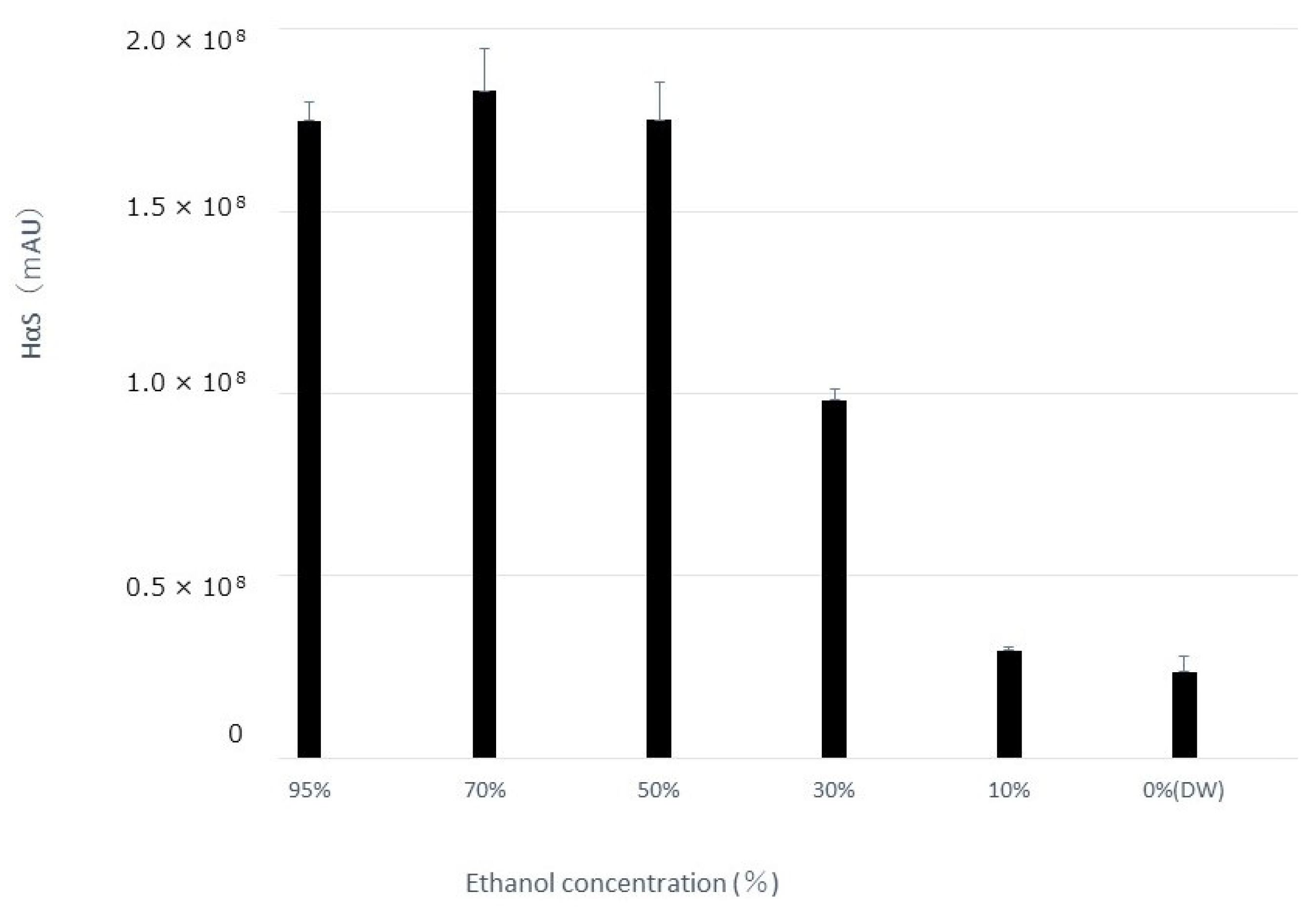
3.3. Effect of Alcohol Concentration on Extraction Amount of HαS and Its Stability
3.4. Comparison of the Stability of HαS in Ethanolic Extracts of Pericarp and Segment Membranes
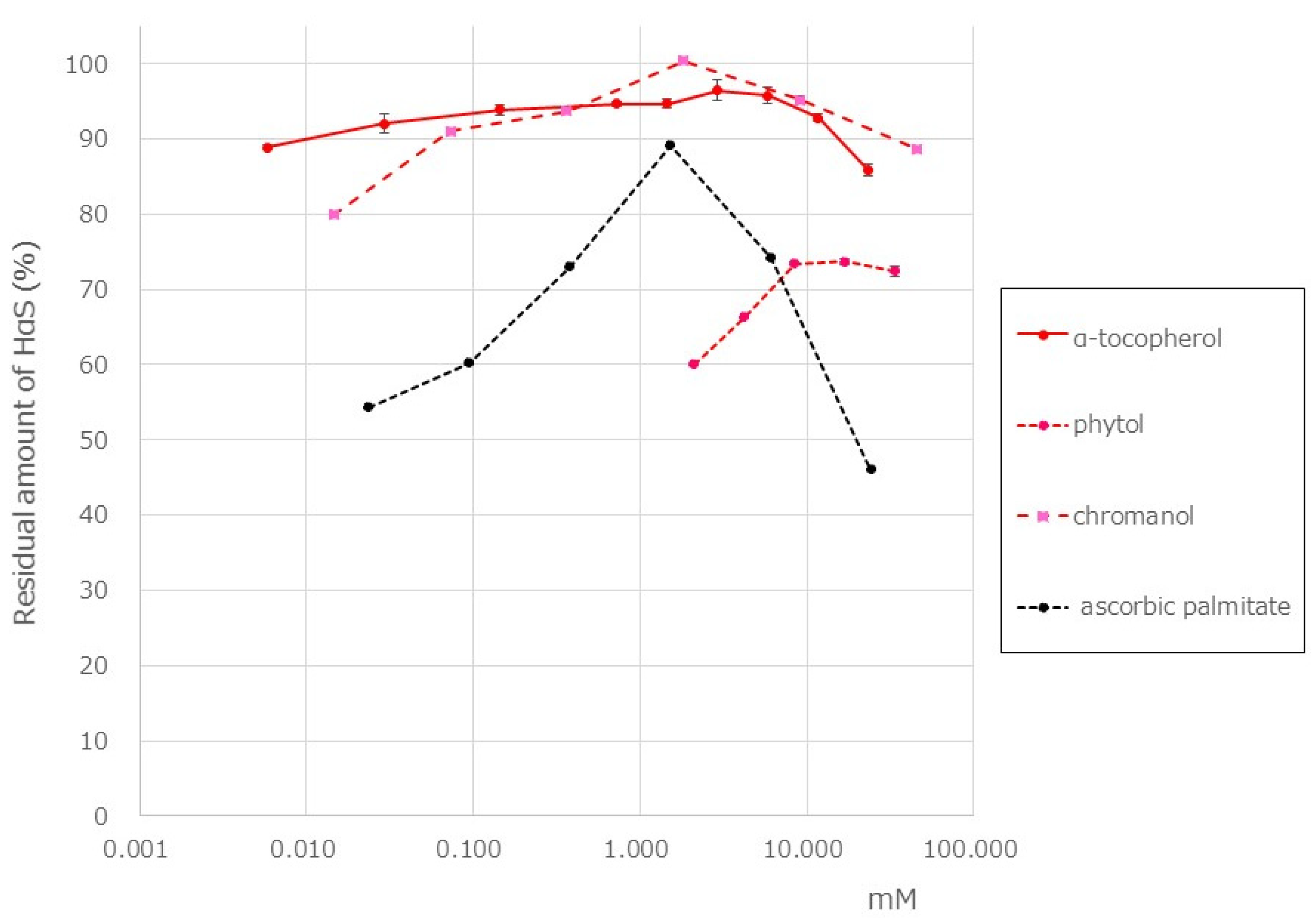
3.5. HαS-Stabilizing Activity of Antioxidant Vitamin
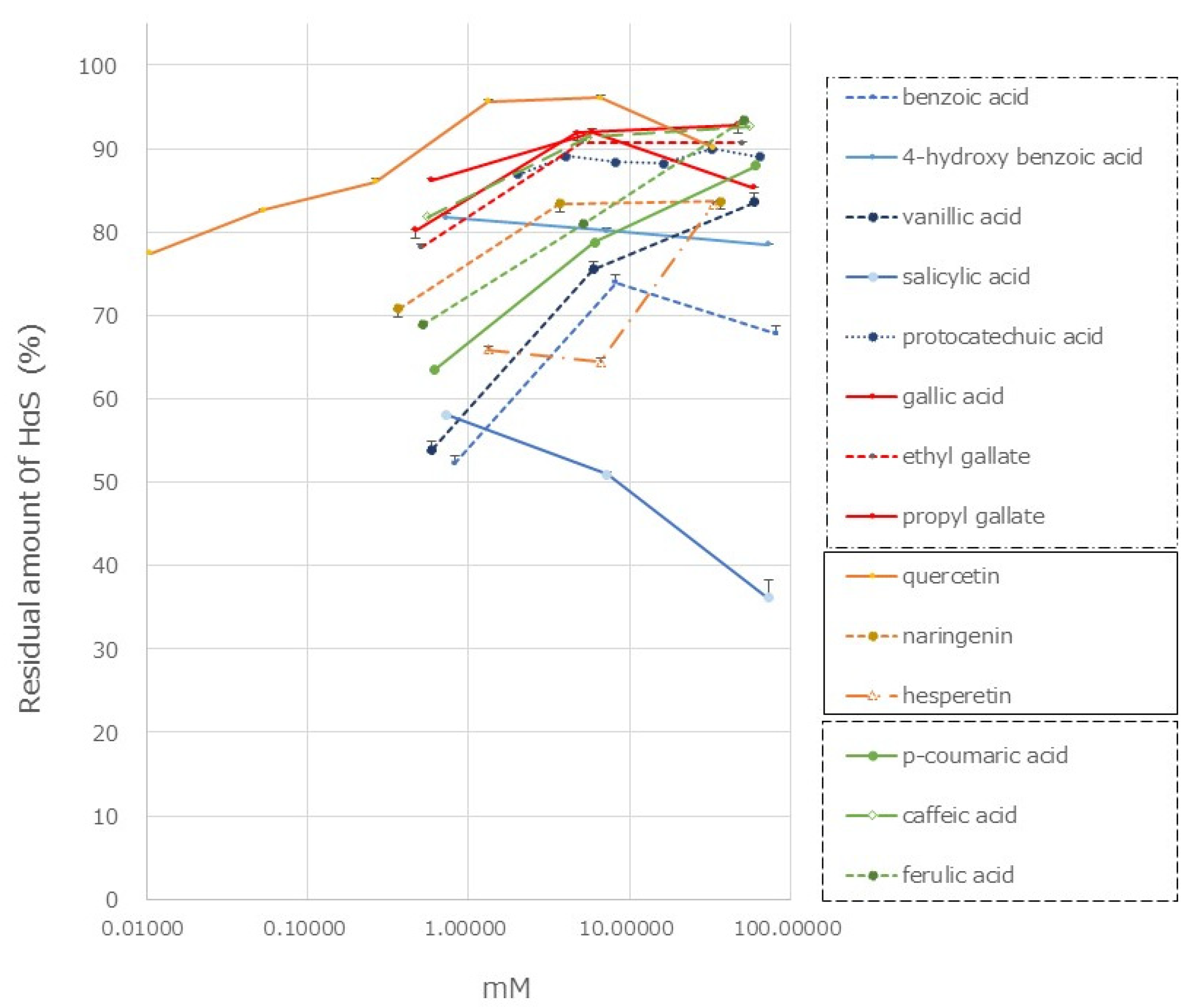
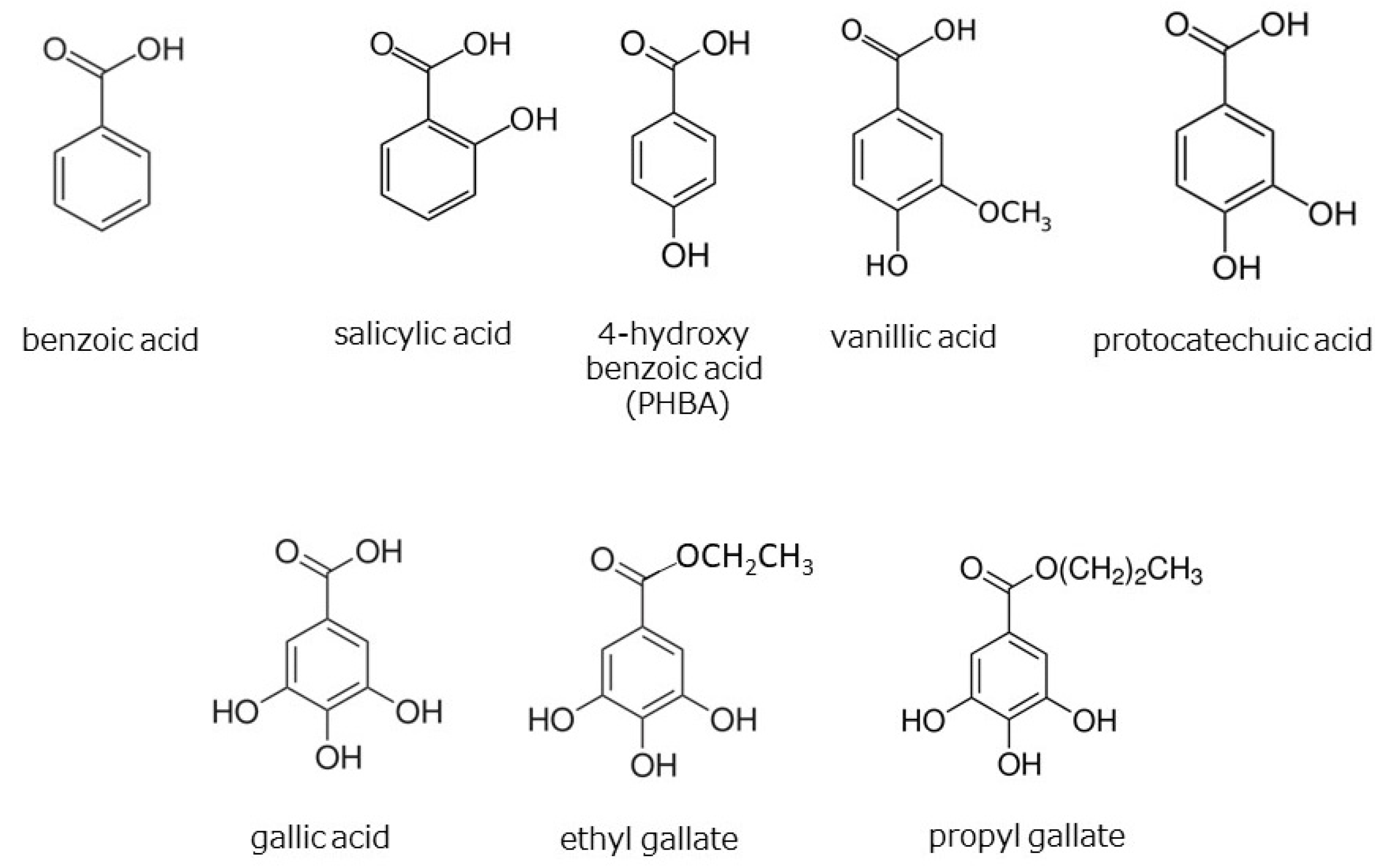
3.6. HαS-Stabilizing Activity of Phenolics
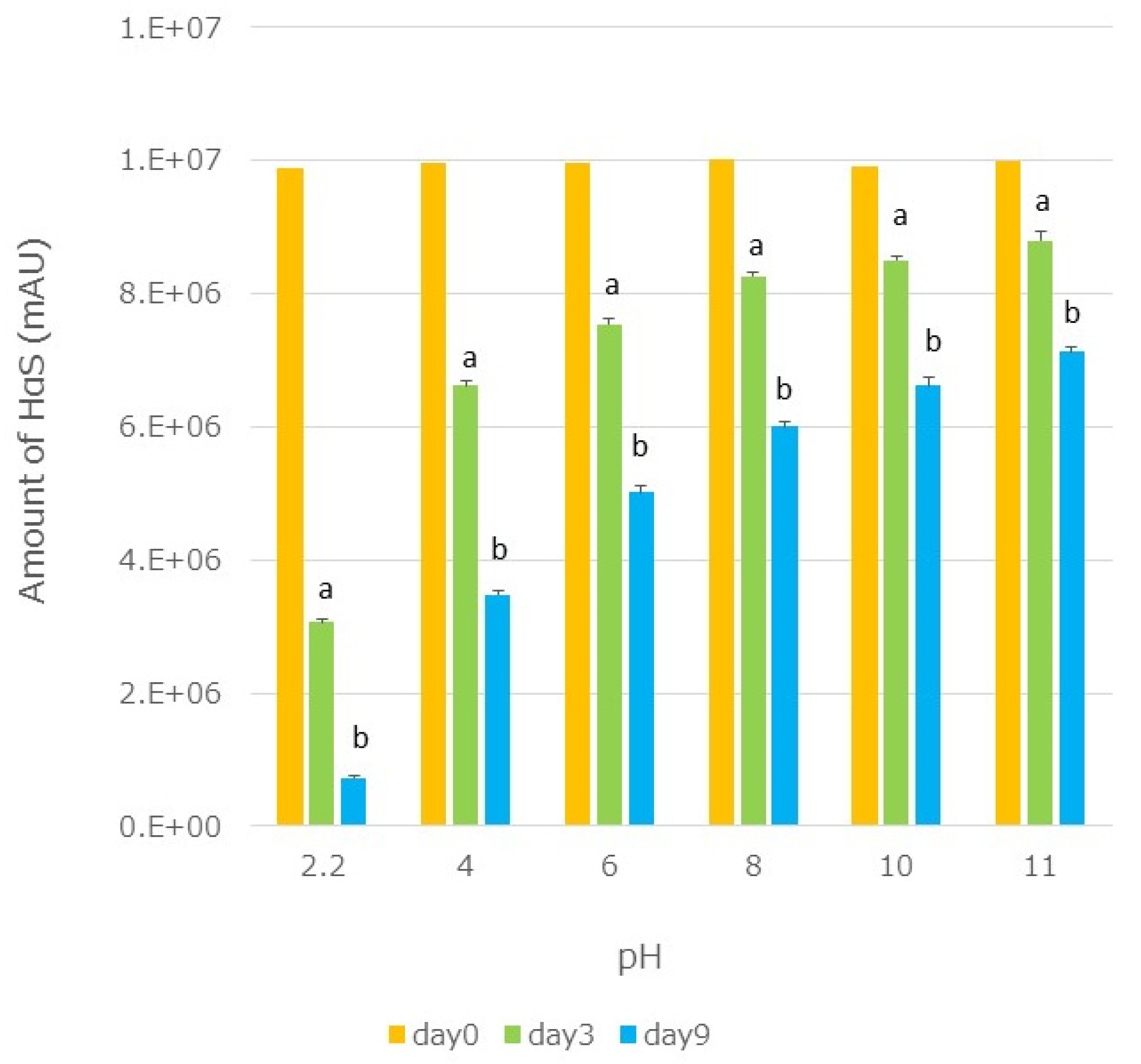
3.7. Effect of pH on the Stability of HαS
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, R.; Fujii, I.; Tsuji, K. Sansho production areas in Wakayama prefecture (in Japanese). In History of Agricultural Development in Wakayama Prefecture Ⅱ; Research Center for Food and Agriculture, Wakayama University: Wakayama, Japan, 2020; pp. 257–282. [Google Scholar]
- Chruma, J.J.; Cullen, D.J.; Bowman, L.; Toy, P.H. Polyunsaturated fatty acid amides from the Zanthoxylum genus—From culinary curiosities to probes for chemical biology. Nat. Prod. Rep. 2018, 35, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, I.; Takeya, K.; Itokawa, H. Distribution of unsaturated aliphatic acid amides in Japanese Zanthoxylum species. Phytochemistry 1982, 21, 1295–1298. [Google Scholar] [CrossRef]
- Mizutani, K.; Fukunaga, Y.; Tanaka, O.; Takasugi, N.; Saruwatari, Y.-I.; Fuwa, T.; Yamauchi, T.; Wang, J.; Jia, M.-R.; Li, F.-Y.; et al. Amides from huajiao, pericarps of Zanthoxylum bungeanum MAXIM. Chem. Pharm. Bull. 1988, 36, 2362–2365. [Google Scholar] [CrossRef]
- Sugai, E.; Morimitsu, Y.; Kubota, K. Quantitative analysis of sanshool compounds in Japanese pepper (Xanthoxylum piperitum DC.) and their pungent characteristics bioscience. Biosci. Biotechnol. Biochem. 2005, 69, 1958–1962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiaolong, W.; Zhu, L.; Li, T.; Jiang, W.; Zhou, J.; Peng, W.; Wu, C. Zanthoxylum bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology. Int. J. Mol. Sci. 2017, 18, 2172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kubota, K. Differences in the volatile components and their odor characteristics of green and ripe fruits and dried pericarp of Japanese pepper (Xanthoxylum piperitum DC.). J. Agric. Food Chem. 2004, 52, 4197–4203. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Fukutome, N.; Hayakawa, F.; Ibaragi, N.; Nagano, Y. Classification of Japanese Pepper (Zanthoxylum piperitum DC.) from Different Growing Regions Based on Analysis of Volatile Compounds and Sensory Evaluation. Molecules 2022, 27, 4946. [Google Scholar] [CrossRef] [PubMed]
- Yang, X. Aroma constituents and alkylamides of red and green huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J. Agric. Food Chem. 2008, 56, 1689–1696. [Google Scholar] [CrossRef]
- Tan, F.; Xu, L.; Liu, Y.; Li, H.; Dahan, Z.; Qin, C.; Han, Y.; Han, J. Design of hydroxy-a-sanshool loaded nanostructured lipid carriers as a potential local anesthetic. Drug Deliv. 2022, 29, 743–753. [Google Scholar] [CrossRef]
- Kono, T.; Shimada, M.; Yamamoto, M.; Kaneko, A.; Oomiya, Y.; Kubota, K.; Kase, Y.; Lee, K.; Uezono, Y. Complementary and synergistic therapeutic effects of compounds found in Kampo medicine: Analysis of daikenchuto. Front. Pharmacol. 2015, 6, 159. [Google Scholar] [CrossRef]
- Saroh, K.; Kase, Y.; Hayakawa, T.; Murata, P.; Ishige, A.; Sasaki, H. Dai-kenchu-to accelerated small intestinal movement. Biol. Pharm. Bull. 2001, 24, 1122–1126. [Google Scholar] [CrossRef]
- Nagano, T.; Itoh, H.; Takeyama, M. Effect of Dai-kenchu-to on levels of 3 brain-gut peptides (motilin, gastrin and somatostatin) in human plasma. Biol. Pharm. Bull. 1999, 22, 1131–1133. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.; Stark, T.D.; Dawid, C.; Lösch, S.; Hofmann, T. All-trans-configuration in Zanthoxylum alkylamides swaps the tingling with a numbing sensation and diminishes salivation. J. Agric. Food Chem. 2014, 62, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.Y.; Jang, Y.; Cho, H.; Lee, C.-H.; Jang, K.H.; Ha Chang, Y.H.; Shin, J.; Oh, U. Hydroxy-alpha-sanshool activates TRPV1 and TRPA1 in sensory neurons. Eur. J. Neurosci. 2007, 26, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Sigal, Y.M.; Milstein, A.D.; Garrison, J.L.; Zorn, J.A.; Tsuruda, P.R.; Nicoll, R.A.; Julius, D. Pungent agents from Szechuan peppers excitesensory neurons by inhibiting two-pore potassium channels. Nat. Neurosci. 2008, 11, 772. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Ohtake, N.; Ohbuchi, K.; Mase, A.; Imamura, S.; Sudo, Y.; Miyano, K.; Yamamoto, M.; Kono, T.; Uezono, Y. Hydroxy-α sanshool induces colonic motor activity in rat proximal colon: A possible involvement of KCNK9. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G579–G590. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fan, W.; Zhang, M.; Zhang, Q.; Li, L.; Wang, J.; Zhu, L.; Wei, D.; Peng, W.; Wu, C. Antiobesity, regulation of lipid metabolism, and attenuation of liver oxidative stress effects of hydroxy-α-sanshool isolated from Zanthoxylum bungeanum on high-fat diet-induced hyperlipidemic rats. Oxid. Med. Cell. Longev. 2019, 27, 5852494. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhu, Y.; Lu, M.; Qin, L.; Zhao, D.; Ren, T. Effects of Hydroxy-Alpha-Sanshool on Intestinal Metabolism in Insulin-Resistant Mice in Insulin-Resistant Mice. Foods 2022, 11, 2040. [Google Scholar] [CrossRef] [PubMed]
- Kumagay, M. Shin-Shokuhinbunsekiho; The Japanese Society for Food Science and Technology: Tokyo, Japan, 1996; pp. 336–340. (In Japanese) [Google Scholar]
- Iseli, V.; Potterat, O.; Hagmann, L.; Egli, J.; Hamburger, M. Characterization of the pungent principles and the essential oil of Zanthoxylum schinifolium pericarp. Pharmazie 2007, 62, 396–400. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Zhu, R.-X.; Zhong, K.; He, Q.; Luo, A.-M.; Gao, H. Characterization and comparison of the pungent components in commercial Zanthoxylum bungeanum oil and Zanthoxylum schinifolium oil. J. Food Sci. 2013, 78, C1516–C1522. [Google Scholar] [CrossRef]
- Hisatomi, E.; Matsui, M.; Kubota, K.; Kobayashi, A. Antioxidative activity in the pericarp and seed of Japanese pepper (Xanthoxylum piperitum DC). J. Agric. Food Chem. 2000, 48, 4924–4928. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, E.; Inagaki, M.; Kurita, O.; Inoue, T. Antioxidant activity of Japanese pepper (Zanthoxylum piperitum DC.) fruit. Food Chem. 2007, 100, 171–177. [Google Scholar] [CrossRef]
- Hur, J.M.; Park, J.G.; Hwang, Y.H. Aromatic acid and flavonoids from leaves of Zanthoxylum piperitum. Nat. Prod. Sci. 2001, 7, 23–26. [Google Scholar]
- Yang, L.-C.; Li, R.; Tan, J.; Jiang, Z.T. Polyphenolics composition of the leaves of Zanthoxylum bungeanum Maxim. Grown in Hebei, China, and their radical scavenging activities. J. Agric. Food Chem. 2013, 61, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Yang, L.; Zhou, D.; Zhang, J. Purification and characterization of flavonoids from the leaves of Zanthoxylum bungeanum and correlation between their structure and antioxidant activity research. PLoS ONE 2014, 9, e105725. [Google Scholar] [CrossRef]
- Zhong, K.; Li, X.-J.; Gou, A.-N.; Huang, Y.-N.; Bu, Q.; Gao, H. Antioxidant and cytoprotective activities of flavonoid glycosides-rich extract from the leaves of Zanthoxylum bungeanum. J. Food Nutr. Res. 2014, 2, 349–356. [Google Scholar] [CrossRef]
- Britton, H.T.S.; Robinson, R.A. CXCVIII. Universal buffer solutions and the dissociation constant of veronal. J. Chem. Soc. 1931, 1456–1462. [Google Scholar] [CrossRef]
- Soll, J.; Schultz, G. 2-Methyl-6-phytylquinol and 2,3-dimethyl-5-phytylquinol as precursors of tocopherol synthesis in spinach chloroplasts. Phytochemistry 1980, 19, 215–218. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. The Function of Tocopherols and Tocotrienols in Plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Marquardt, D.; Williams, J.A.; Kučerka, N.; Atkinson, J.; Wassall, S.R.; Katsaras, J.; Harroun, T.A. Tocopherol activity correlates with its location in a membrane: A new perspective on the antioxidant vitamin E. J. Am. Chem. Soc. 2013, 135, 7523–7533. [Google Scholar] [CrossRef]
- Marquardt, D.; Kučerka, N.; Katsaras, J.; Harroun, T.A. α-Tocopherol’s Location in Membranes Is Not Affected by Their Composition. Langmuir 2015, 31, 4464–4472. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Aoki, K.; Nishimura, H.; Morishita, I.; Usui, K. Total synthesis of hydroxy-α- and hydroxy-β-sanshool using Suzuki-Miyaura coupling. Chem. Pharm. Bull. 2012, 60, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiao, Y.; Chen, T.; Jiyu, G.; Huang, W.; Li, Z. Synthesis of hydroxy-α-sanshool. J. Chem. Res. 2021, 45, 310–314. [Google Scholar] [CrossRef]
- Nakamura, A.; Mimaki, K.; Tanigami, K.I.; Maegawa, T. An improved and practical method for synthesizing of α-sanshools and spilanthol. Front. Chem. 2020, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R. Chemistry, pharmacology and clinical applications of Echinacea products. In Herbs, Botanicals & Teas; Mazza, G.O., Ed.; Technomic Publishing: Lancaster, UK, 2000; pp. 45–73. [Google Scholar]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Murphy, P.A. Alkamide stability in Echinacea purpurea extracts with and without phenolic acids in dry films and in solution. J. Agric. Food Chem. 2007, 55, 120–126. [Google Scholar] [CrossRef]



| Stage | Harvest Time | Color of the Pericarp | Seed |
|---|---|---|---|
| Unripe fruits | May | Green | Unmatured, soft, pale green |
| Ripe fruits | Late July to Early August | Pale green | Mature, black |
| Fully ripened fruits | September onward | Pale red to red | Mature, black |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitani, T.; Yawata, Y.; Yamamoto, N.; Okuno, Y.; Sakamoto, H.; Nishide, M.; Kayano, S.-i. Stabilization of Hydroxy-α-Sanshool by Antioxidants Present in the Genus Zanthoxylum. Foods 2023, 12, 3444. https://doi.org/10.3390/foods12183444
Mitani T, Yawata Y, Yamamoto N, Okuno Y, Sakamoto H, Nishide M, Kayano S-i. Stabilization of Hydroxy-α-Sanshool by Antioxidants Present in the Genus Zanthoxylum. Foods. 2023; 12(18):3444. https://doi.org/10.3390/foods12183444
Chicago/Turabian StyleMitani, Takahiko, Yasuko Yawata, Nami Yamamoto, Yoshiharu Okuno, Hidefumi Sakamoto, Mitsunori Nishide, and Shin-ichi Kayano. 2023. "Stabilization of Hydroxy-α-Sanshool by Antioxidants Present in the Genus Zanthoxylum" Foods 12, no. 18: 3444. https://doi.org/10.3390/foods12183444
APA StyleMitani, T., Yawata, Y., Yamamoto, N., Okuno, Y., Sakamoto, H., Nishide, M., & Kayano, S.-i. (2023). Stabilization of Hydroxy-α-Sanshool by Antioxidants Present in the Genus Zanthoxylum. Foods, 12(18), 3444. https://doi.org/10.3390/foods12183444






