Effects of Haematococcus pluvialis Addition on the Sensory Properties of Plant-Based Meat Analogues
Abstract
:1. Introduction
2. Materials and Methods
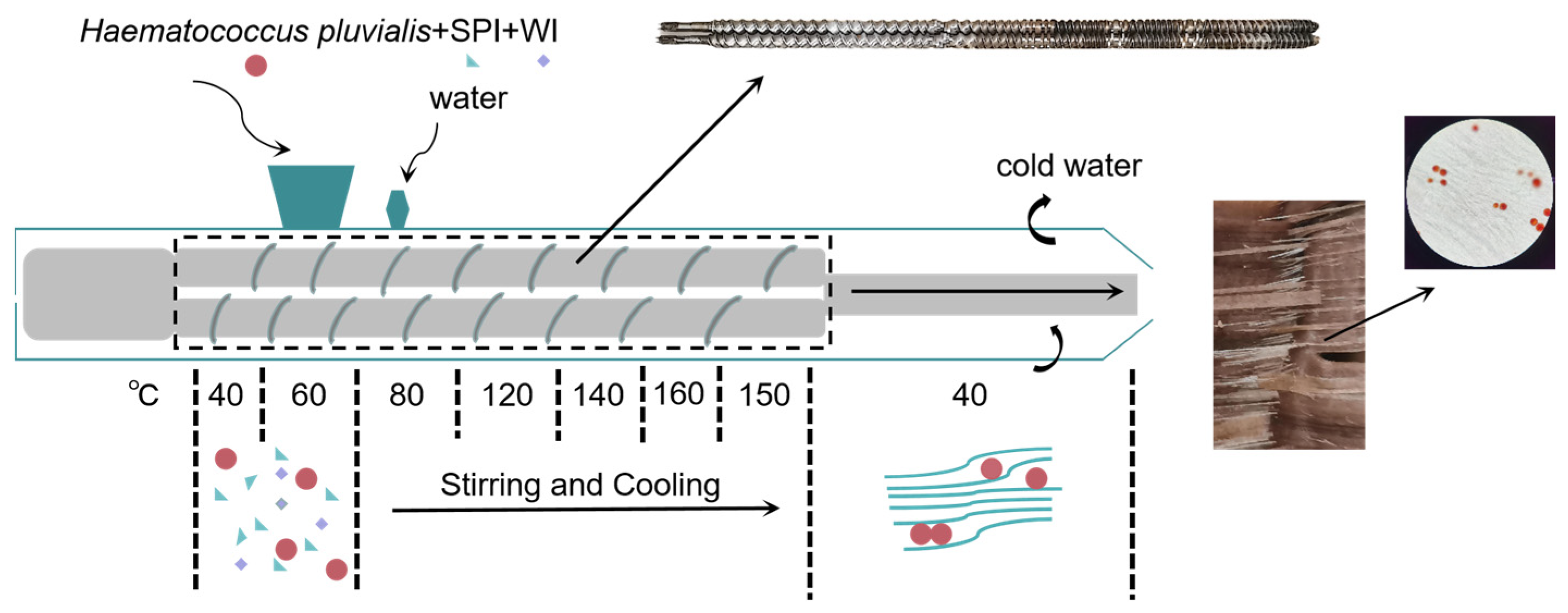
2.1. The Preparation of High-Moisture Extrusion
2.2. Tensile and Water Absorption Analysis
2.3. E-Nose Analysis
2.4. Electron Microscope Analysis
2.5. Rheological Analysis
2.6. Color Difference Analysis
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results
3.1. Appearance and Color
3.2. Texture Analysis
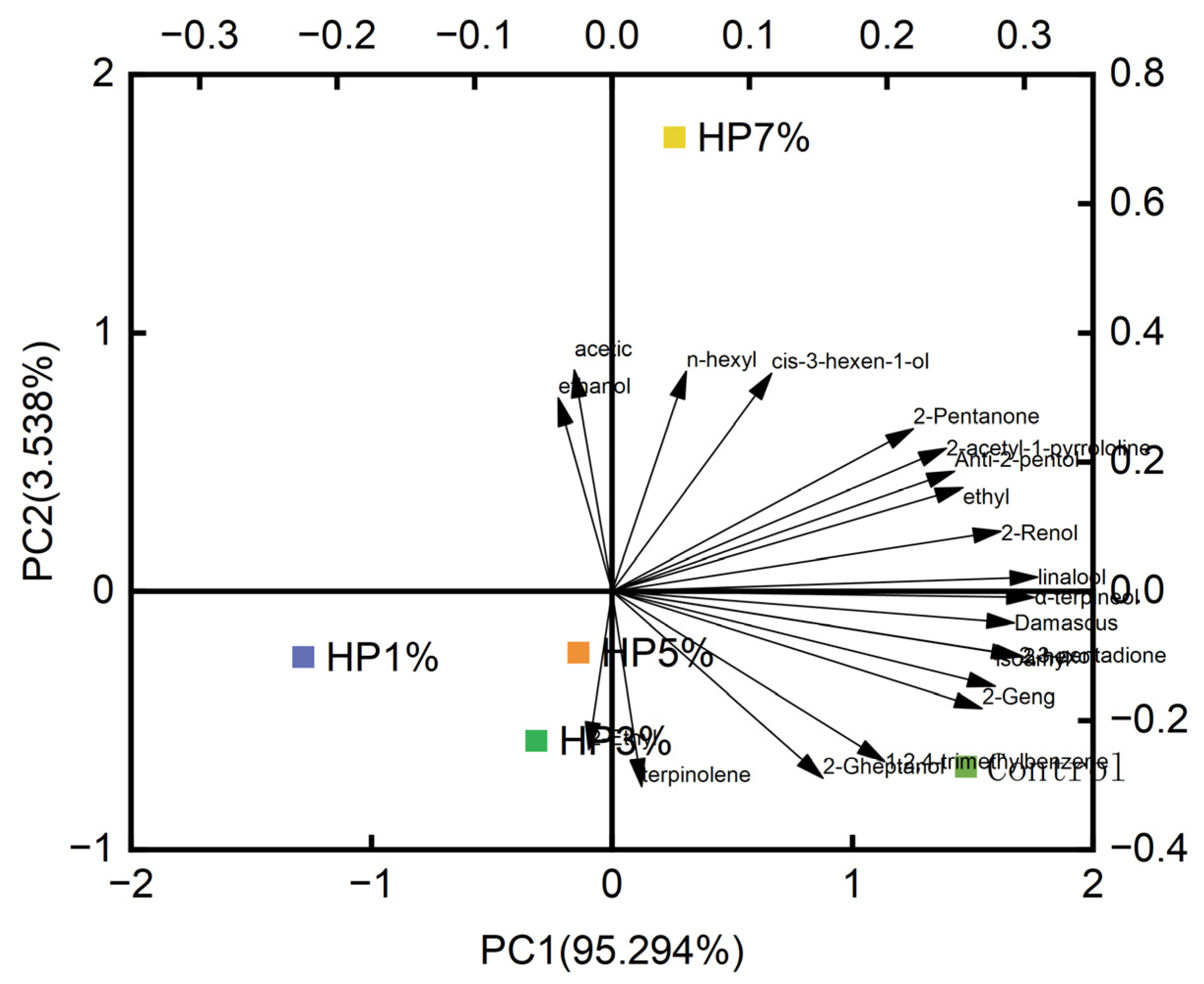
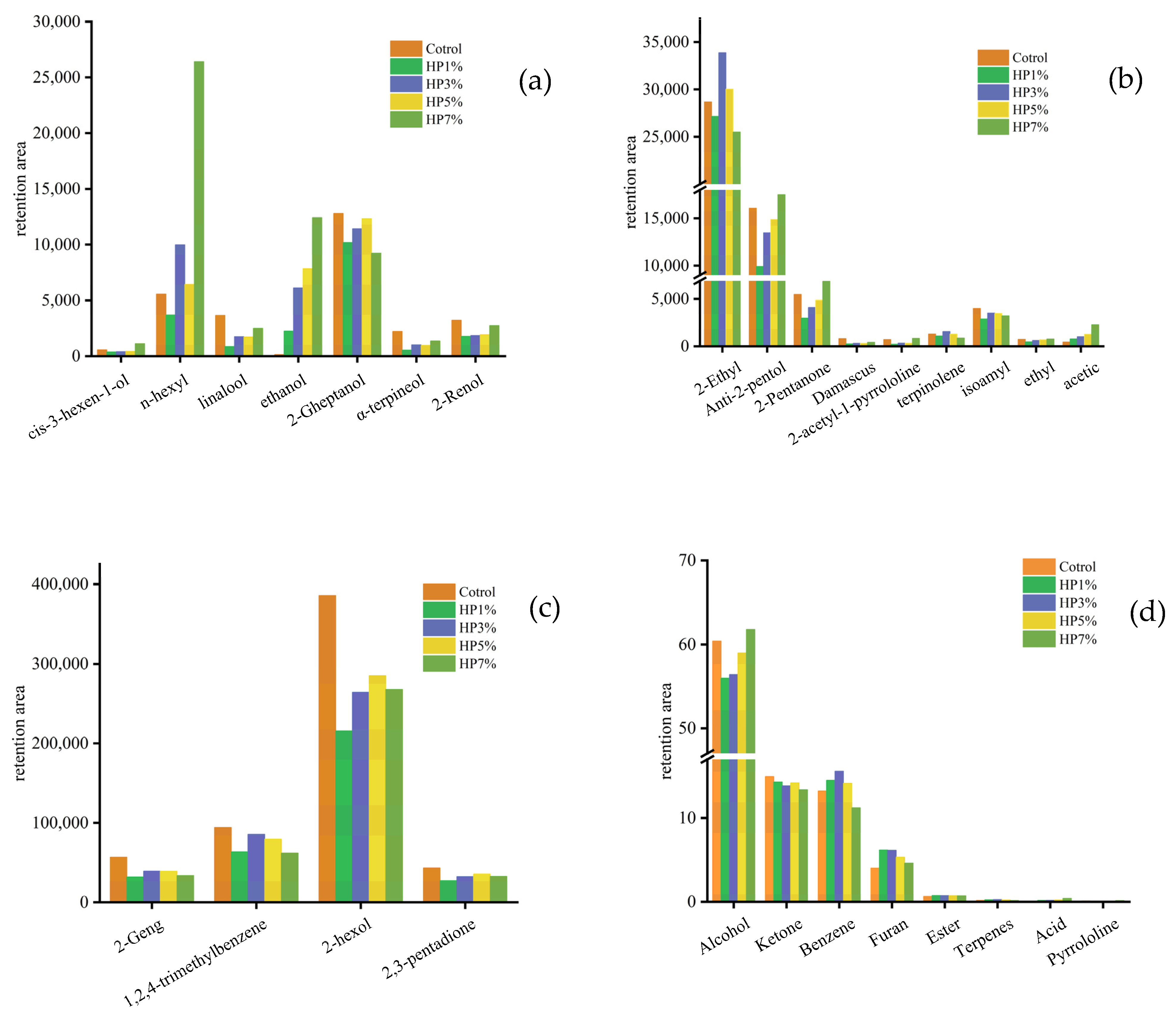
3.3. The Content and Proportion of Volatile Compounds Analysis
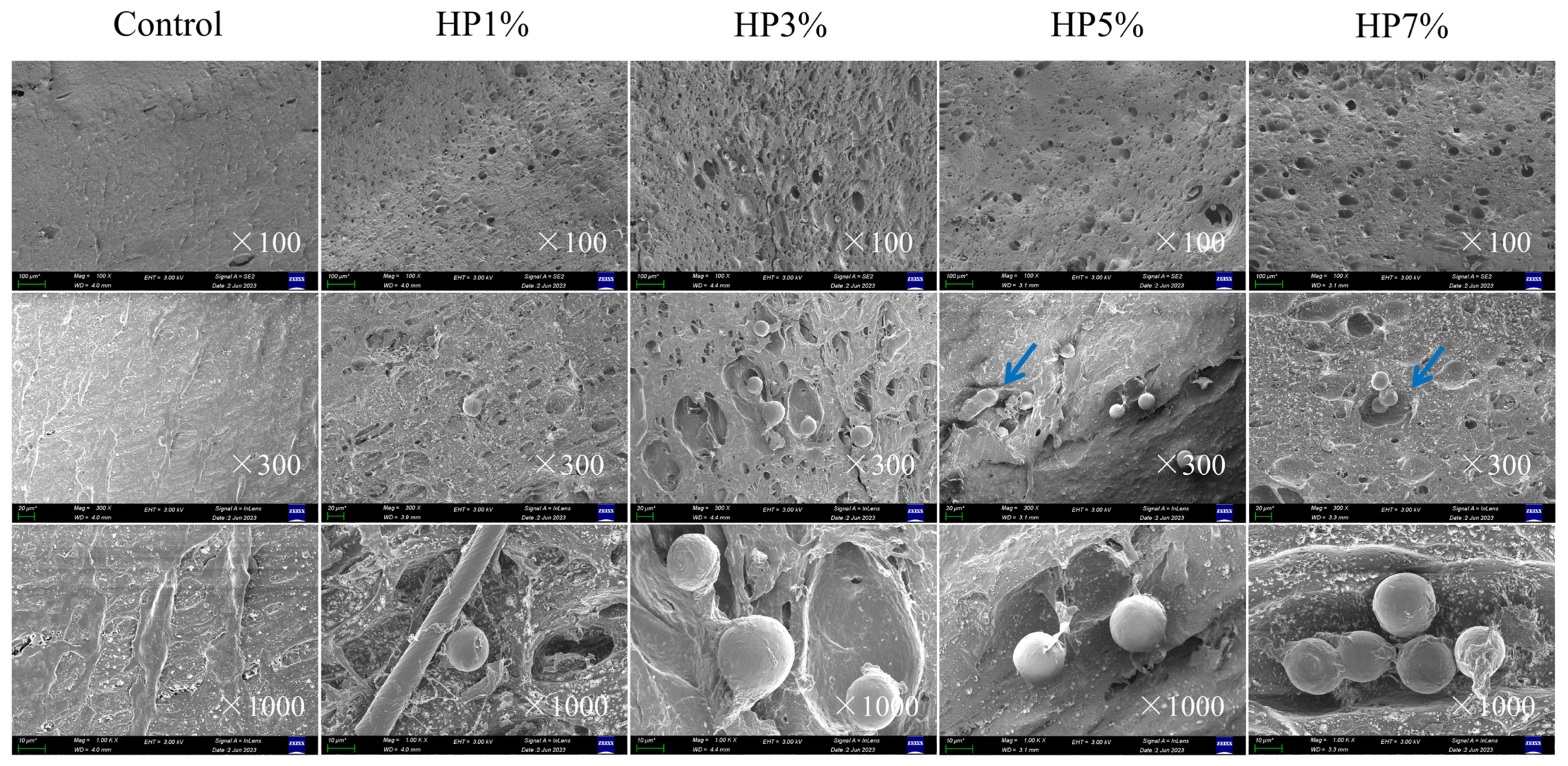
3.4. Microstructure
3.5. Rheological Analysis
3.6. Analysis of Color Difference Value
3.7. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sha, L.; Xiong, Y.L. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Köllmann, N.; Schreuders, F.K.G.; Zhang, L.; van der Goot, A.J. On the importance of cooling in structuring processes for meat analogues. J. Food Eng. 2023, 350, 111490. [Google Scholar] [CrossRef]
- Chen, Y.P.; Feng, X.; Blank, I.; Liu, Y. Strategies to improve meat-like properties of meat analogs meeting consumers’ expectations. Biomaterials 2022, 287, 121648. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.K.; Kang, Y.K.; Jeong, E.W.; Baek, Y.; Lee, K.Y.; Lee, H.G. Applications of various natural pigments to a plant-based meat analog. LWT 2023, 174, 114431. [Google Scholar] [CrossRef]
- Rai, A.; Sharma, V.K.; Sharma, M.; Singh, S.M.; Singh, B.N.; Pandey, A.; Nguyen, Q.D.; Gupta, V.K. A global perspective on a new paradigm shift in bio-based meat alternatives for healthy diet. Food Res. Int. 2023, 169, 112935. [Google Scholar] [CrossRef]
- Sun, L.; Xin, F.; Alper, H.S. Bio-synthesis of food additives and colorants-a growing trend in future food. Biotechnol. Adv. 2021, 47, 107694. [Google Scholar] [CrossRef]
- Singh, U.B.; Ahluwalia, A.S. Microalgae: A promising tool for carbon sequestration. Mitig. Adapt. Strateg. Glob. Change 2013, 18, 73–95. [Google Scholar] [CrossRef]
- Chen, C.; Tang, T.; Shi, Q.; Zhou, Z.; Fan, J. The potential and challenge of microalgae as promising future food sources. Trends Food Sci. Technol. 2022, 126, 99–112. [Google Scholar] [CrossRef]
- Gohara-Beirigo, A.K.; Matsudo, M.C.; Cezare-Gomes, E.A.; Carvalho, J.C.M.d.; Danesi, E.D.G. Microalgae trends toward functional staple food incorporation: Sustainable alternative for human health improvement. Trends Food Sci. Technol. 2022, 125, 185–199. [Google Scholar] [CrossRef]
- Kumar, R.; Hegde, A.S.; Sharma, K.; Parmar, P.; Srivatsan, V. Microalgae as a sustainable source of edible proteins and bioactive peptides—Current trends and future prospects. Food Res. Int. 2022, 157, 111338. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Verma, P. Microalgae Dunaliella as biofuel feedstock and β-carotene production: An influential step towards environmental sustainability. Energy Convers. Manag. X 2022, 13, 100154. [Google Scholar] [CrossRef]
- Aye Myint, A.; Hariyanto, P.; Irshad, M.; Ruqian, C.; Wulandari, S.; Eui Hong, M.; Jun Sim, S.; Kim, J. Strategy for high-yield astaxanthin recovery directly from wet Haematococcus pluvialis without pretreatment. Bioresour. Technol. 2022, 346, 126616. [Google Scholar] [CrossRef] [PubMed]
- Zinnai, A.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Venturi, F. Supercritical fluid extraction from microalgae with high content of LC-PUFAs. A case of study: Sc-CO2 oil extraction from Schizochytrium sp. J. Supercrit. Fluids 2016, 116, 126–131. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Nunes, M.C.; Graça, C.; Vlaisavljević, S.; Tenreiro, A.; Sousa, I.; Raymundo, A. Microalgal cell disruption: Effect on the bioactivity and rheology of wheat bread. Algal Res. 2020, 45, 101749. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Duan, C.; Yi, S.; Gao, Z.; Xiao, C.; Agathos, S.N.; Wang, G.; Li, J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602. [Google Scholar] [CrossRef]
- Sun, J.; Yan, J.; Dong, H.; Gao, K.; Yu, K.; He, C.; Mao, X. Astaxanthin with different configurations: Sources, activity, post modification, and application in foods. Curr. Opin. Food Sci. 2023, 49, 100955. [Google Scholar] [CrossRef]
- Vardanega, R.; Cerezal-Mezquita, P.; Veggi, P.C. Supercritical fluid extraction of astaxanthin-rich extracts from Haematococcus pluvialis: Economic assessment. Bioresour. Technol. 2022, 361, 127706. [Google Scholar] [CrossRef]
- Hossain, A.; Brennan, M.A.; Mason, S.L.; Guo, X.; Zeng, X.A.; Brennan, C.S. The Effect of Astaxanthin-Rich Microalgae “Haematococcus pluvialis” and Wholemeal Flours Incorporation in Improving the Physical and Functional Properties of Cookies. Foods 2017, 6, 57. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, T.; Chen, S.H.Y.; Liu, B.; Sun, P.; Sun, H.; Chen, F. The potentials and challenges of using microalgae as an ingredient to produce meat analogues. Trends Food Sci. Technol. 2021, 112, 188–200. [Google Scholar] [CrossRef]
- Barkallah, M.; Ben Atitallah, A.; Hentati, F.; Dammak, M.; Hadrich, B.; Fendri, I.; Ayadi, M.A.; Michaud, P.; Abdelkafi, S. Effect of Spirulina platensis Biomass with High Polysaccharides Content on Quality Attributes of Common Carp (Cyprinus carpio) and Common Barbel (Barbus barbus) Fish Burgers. Appl. Sci. 2019, 9, 2197. [Google Scholar] [CrossRef]
- Grahl, S.; Palanisamy, M.; Strack, M.; Meier-Dinkel, L.; Toepfl, S.; Mörlein, D. Towards more sustainable meat alternatives: How technical parameters affect the sensory properties of extrusion products derived from soy and algae. J. Clean. Prod. 2018, 198, 962–971. [Google Scholar] [CrossRef]
- Sun, C.; Fu, J.; Chang, Y.; Li, S.; Fang, Y. Structure Design for Improving the Characteristic Attributes of Extruded Plant-Based Meat Analogues. Food Biophys. 2022, 17, 137–149. [Google Scholar] [CrossRef]
- Ran, X.; Lou, X.; Zheng, H.; Gu, Q.; Yang, H. Improving the texture and rheological qualities of a plant-based fishball analogue by using konjac glucomannan to enhance crosslinks with soy protein. Innov. Food Sci. Emerg. Technol. 2022, 75, 102910. [Google Scholar] [CrossRef]
- Rizvi, N.B.; Aleem, S.; Khan, M.R.; Ashraf, S.; Busquets, R. Quantitative Estimation of Protein in Sprouts of Vigna radiate (Mung Beans), Lens culinaris (Lentils), and Cicer arietinum (Chickpeas) by Kjeldahl and Lowry Methods. Molecules 2022, 27, 814. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Kaplan, D.L.; Wang, Q. Protein-amylose/amylopectin molecular interactions during high-moisture extruded texturization toward plant-based meat substitutes applications. Food Hydrocoll. 2022, 127, 107559. [Google Scholar] [CrossRef]
- Jiang, R.; Xiao, Z.; Huo, J.; Wang, H.; Li, H.; Su, S.; Duan, Y.; Gao, Y. Effects of rice bran content on plant-based simulated meat: From the aspects of apparent properties and structural characteristics. Food Chem. 2022, 380, 131842. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Meng, S.; Wang, Q. Rheological properties of pea protein isolate-amylose/amylopectin mixtures and the application in the high-moisture extruded meat substitutes. Food Hydrocoll. 2021, 117, 106732. [Google Scholar] [CrossRef]
- Wen, Y.; Kim, H.W.; Park, H.J. Effect of xylose on rheological, printing, color, texture, and microstructure characteristics of 3D-printable colorant-containing meat analogs based on mung bean protein. Food Res. Int. 2022, 160, 111704. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Böcker, L.; Müssner, C.; Stirnemann, E.; Haberkorn, I.; Adelmann, H.; Handschin, S.; Windhab, E.J.; Mathys, A. Extruded meat analogues based on yellow, heterotrophically cultivated Auxenochlorella protothecoides microalgae. Innov. Food Sci. Emerg. Technol. 2020, 59, 102275. [Google Scholar] [CrossRef]
- Bai, T.; Wan, Q.; Liu, X.; Ke, R.; Xie, Y.; Zhang, T.; Huang, M.; Zhang, J. Drying kinetics and attributes of fructus aurantii processed by hot air thin-layer drying at different temperatures. Heliyon 2023, 9, e15554. [Google Scholar] [CrossRef] [PubMed]
- Isleten Hosoglu, M. Aroma characterization of five microalgae species using solid-phase microextraction and gas chromatography–mass spectrometry/olfactometry. Food Chem. 2018, 240, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ying, Z.; Li, H.; Li, J.; Zhang, T.; Song, Y.; Liu, X. Extrusion production of textured soybean protein: The effect of energy input on structure and volatile beany flavor substances. Food Chem. 2023, 405, 134728. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Sun, Q.; Methven, L.; Elmore, J.S. Comparison of the sensory properties of fragrant and non-fragrant rice (Oryza sativa), focusing on the role of the popcorn-like aroma compound 2-acetyl-1-pyrroline. Food Chem. 2021, 339, 128077. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, X.; Hayat, K.; Huang, M.; Liu, P.; Karangwa, E.; Gu, F.; Jia, C.; Xia, S.; Xiao, Z.; et al. Contribution of beef base to aroma characteristics of beeflike process flavour assessed by descriptive sensory analysis and gas chromatography olfactometry and partial least squares regression. J. Chromatogr. A 2010, 1217, 7788–7799. [Google Scholar] [CrossRef]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Cacho, J.; Ferreira, V.; Escudero, A. Gas chromatographic–olfactometric characterisation of headspace and mouthspace key aroma compounds in fresh and frozen lamb meat. Food Chem. 2011, 129, 1909–1918. [Google Scholar] [CrossRef]

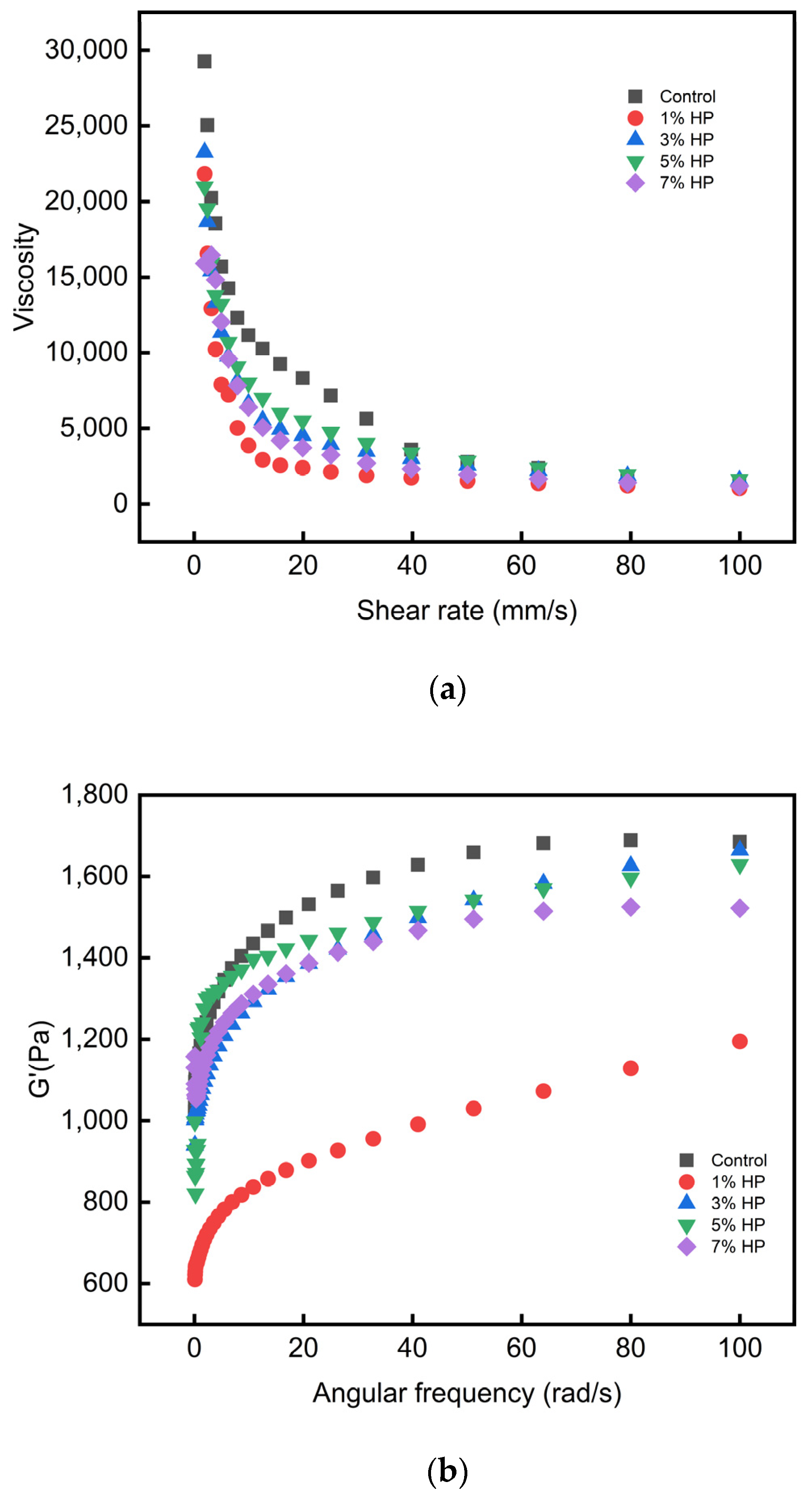
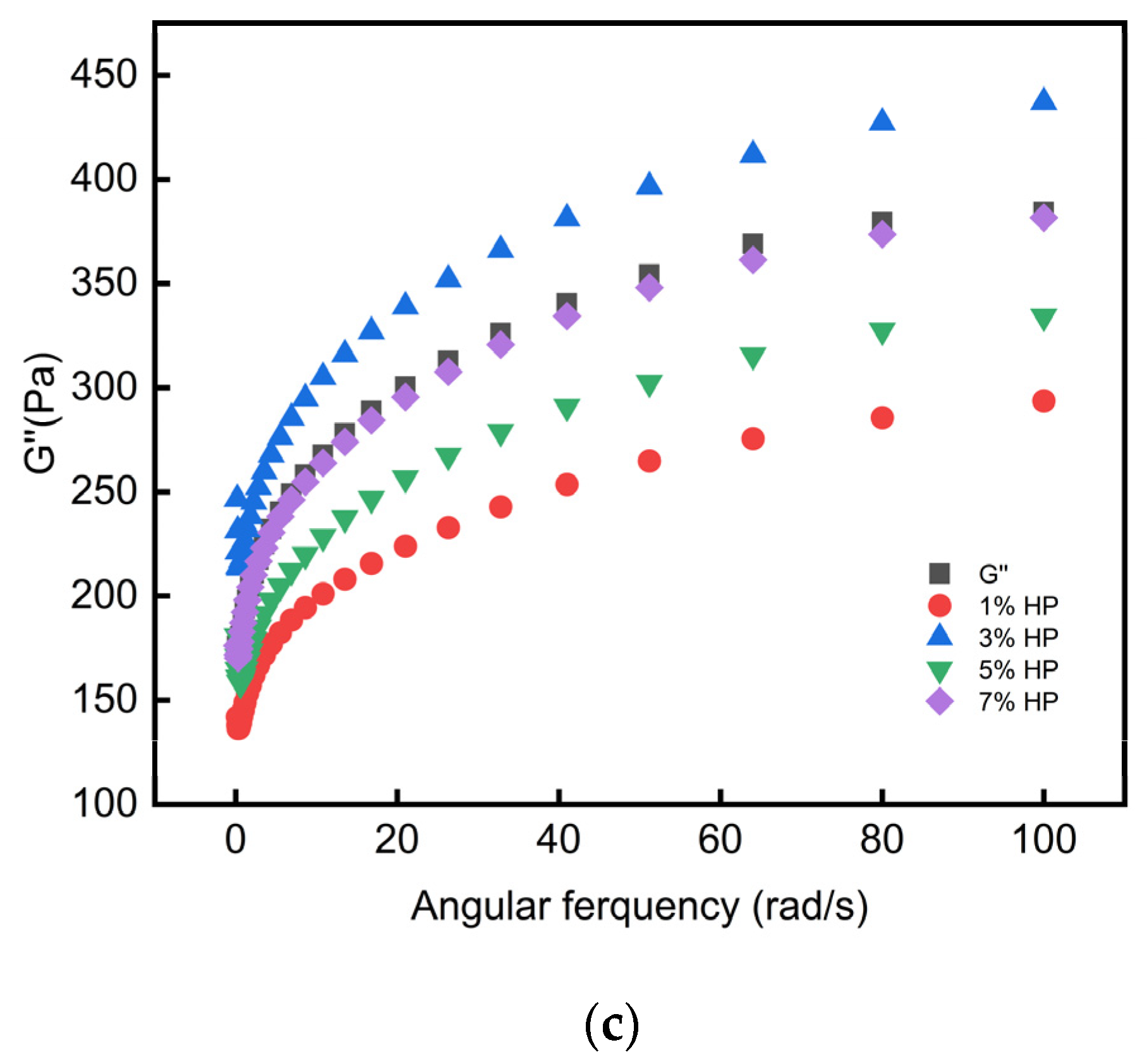
| Mass Structure | HP Content (%) | ||||
|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | |
| Hardness (gf) | 11,976.62 ± 25.32 a | 10,078.48 ± 8.99 b | 9952.64 ± 22.30 c | 8465.25 ± 15.80 d | 8367.95 ± 20.68 e |
| Flexible | 0.92 ± 0.01 b | 0.96 ± 0.02 a | 0.97 ± 0.08 a | 0.97 ± 0.02 a | 0.98 ± 0.01 a |
| Chewiness (gf) | 9774.10 ± 104.28 a | 8398.90 ± 279.63 b | 4254.26 ± 17.17 c | 4270.79 ± 20.80 c | 4252.36 ± 17.45 c |
| Cohesiveness | 0.88 ± 0.01 a | 0.86 ± 0.02 a | 0.55 ± 0.06 b | 0.46 ± 0.01 c | 0.47 ± 0.02 c |
| Longitudinal tensile force (N) | 40.06 ± 2.11 b | 45.93 ± 2.01 a | 34.82 ± 2.94 c | 27.28 ± 2.31 d | 34.31 ± 2.70 c |
| Transverse tensile force (N) | 24.72 ± 2.94 a,b | 27.98 ± 1.80 a | 22.99 ± 1.86 b | 15.21 ± 1.54 c | 24.25 ± 2.72 a,b |
| Organizational degree | 1.63 ± 0.17 a,b | 1.64 ± 0.07 a,b | 1.61 ± 0.19 a,b | 1.80 ± 0.05 a | 1.42 ± 0.10 b |
| Protein content (%) | 33.07 ± 0.46 b | 33.59 ± 0.25 b | 35.27 ± 1.03 a | 33.02 ± 0.61 b | 31.32 ± 0.16 c |
| Categories | CAS Number | Volatile Compounds | RI-5 | RI-1701 | Molecular Formula | HP Content (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | ||||||
| Alcohol | 626-93-7 | 2-hexol | 801 | 900 | C6H14O | 54.20 | 49.24 | 48.06 | 50.72 | 48.49 |
| 928-96-1 | cis-3-hexen-1-ol | 861 | 976 | C6Hl2O | 0.08 | 0.08 | 0.07 | 0.07 | 0.20 | |
| 111-27-3 | n-hexyl alcohol | 870 | 980 | C6H14O | 0.78 | 0.84 | 1.81 | 1.15 | 4.78 | |
| 78-70-6 | linalool | 1099 | 1095 | C10H18O | 0.51 | 0.20 | 0.32 | 0.30 | 0.45 | |
| 64-17-5 | ethanol | 437 | 564 | C2H5OH | 0.02 | 0.51 | 1.11 | 1.39 | 2.25 | |
| 543-49-7 | 2-Gheptanol | 901 | 1000 | C7H16O | 1.80 | 2.33 | 2.08 | 2.20 | 1.67 | |
| 98-55-5 | α-terpineol | 1189 | 1300 | C10H18O | 0.31 | 0.12 | 0.18 | 0.17 | 0.25 | |
| 628-99-9 | 2-Renol | 1102 | 1200 | C9H20O | 0.45 | 0.40 | 0.33 | 0.34 | 0.50 | |
| 1576-96-1 | Anti-2-pentol | 769 | 888 | C5H12O | 2.26 | 2.26 | 2.45 | 2.65 | 3.17 | |
| 6032-29-7 | 2-amyl alcohol | 691 | 795 | C5H10O | 0.77 | 0.68 | 0.74 | 0.86 | 1.24 | |
| Ketone | 23726-93-4 | Damascus ketone | 1386 | 1496 | C13H18O | 0.11 | 0.05 | 0.06 | 0.05 | 0.08 |
| 110-43-0 | 2-Geng ketone | 891 | 984 | C7H14O | 7.98 | 7.31 | 7.13 | 6.94 | 6.14 | |
| 600-14-6 | 2,3-pentadione | 698 | 788 | C5H8O2 | 6.09 | 6.26 | 5.91 | 6.34 | 5.94 | |
| Pyrrolidine | 85213-22-5 | 2-acetyl-1-pyrrolidine | 932 | 1032 | C6H9NO | 0.10 | 0.04 | 0.06 | 0.06 | 0.15 |
| Aromatic hydrocarbon | 95-63-6 | 1,2,4-trimethylbenzene | 993 | 1039 | C9H12 | 13.24 | 14.51 | 15.58 | 14.15 | 11.23 |
| Terpenes | 586-62-9 | terpinolene | 1088 | 1112 | C10H16 | 0.18 | 0.25 | 0.28 | 0.23 | 0.16 |
| Ester | 123-92-2 | isoamyl acetate | 878 | 945 | C7H14O2 | 0.56 | 0.66 | 0.63 | 0.61 | 0.58 |
| 623-70-1 | ethyl crotonate | 835 | 923 | C6H10O2 | 0.10 | 0.10 | 0.11 | 0.12 | 0.14 | |
| Acid | 64-19-7 | acetic | 619 | 773 | CH3COOH | 0.06 | 0.18 | 0.18 | 0.23 | 0.41 |
| Furan | 3208-16-0 | 2-Ethyl furan | 703 | 735 | C6H8O | 4.03 | 6.20 | 6.16 | 5.34 | 4.62 |
| Color Attribute | HP Content (%) | Monascus Red | Beef | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | |||
| Extrudates/Cook thoroughly | |||||||
| L* | 51.50 ± 0.44 b | 51.42 ± 0.79 b | 45.84 ± 0.20 c | 39.48 ± 1.76 d | 36.34 ± 2.41 e | 58.31 ± 1.40 a | 57.15 ± 1.23 a |
| a* | 4.67 ± 0.04 d | 8.35 ± 0.45 c | 10.07 ± 0.79 b,c | 11.60 ± 1.02 b | 19.25 ± 2.14 a | 5.16 ± 0.11 d | 9.20 ± 0.48 c |
| b* | 16.70 ± 0.59 a,b | 15.42 ± 0.33 a,b | 14.59 ± 1.76 b,c | 12.89 ± 1.50 c,d | 15.48 ± 1.60 a,b | 17.32 ± 0.09 a | 11.73 ± 0.28 d |
| ΔE | 47.01 ± 0.33 d | 46.47 ± 0.74 d | 53.80 ± 0.54 c | 57.77 ± 2.18 b | 62.02 ± 1.64 a | 40.30 ± 1.32 e | 41.16 ± 1.18 e |
| Raw material | |||||||
| L* | 88.40 ± 3.29 a | 75.48 ± 2.30 b | 73.44 ± 1.99 b,c | 65.74 ± 4.67 d | 68.62 ± 0.36 c,d | 87.57 ± 1.46 a | 38.06 ± 0.68 e |
| a* | 4.60 ± 0.18 f | 5.33 ± 0.16 d | 5.17 ± 0.13 d | 5.80 ± 0.15 c | 6.63 ± 0.02 b | 4.88 ± 0.09 e | 15.64 ± 0.14 a |
| b* | 19.16 ± 0.24 a | 16.12 ± 0.28 b | 13.15 ± 0.44 c | 11.87 ± 1.09 d | 12.55 ± 0.29 c,d | 19.70 ± 0.36 a | 10.20 ± 0.12 e |
| ΔE | 21.09 ± 0.89 e | 26.14 ± 1.63 d | 27.81 ± 2.44 c,d | 32.93 ± 3.99 b | 30.63 ± 0.27 b,c | 20.88 ± 0.34 e | 60.43 ± 0.62 a |
| Sensory Properties | HP Content (%) | ||||
|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | |
| A group | |||||
| color | 4.71 ± 2.36 a,b | 6.86 ± 2.12 a | 6.71 ± 2.06 a | 4.43 ± 2.51 a,b | 3.43 ± 2.57 b |
| odor | 2.57 ± 1.13 b | 4.29 ± 2.06 a,b | 3.86 ± 2.41 a,b | 4.57 ± 1.81 a,b | 6.14 ± 2.97 a |
| E group | |||||
| color | 2.71 ± 1.38 b | 5.71 ± 3.04 a | 5.43 ± 2.07 a,b | 3.57 ± 1.81 a,b | 3.29 ± 1.98 a,b |
| odor | 2.29 ± 1.11 b | 4.14 ± 1.46 a,b | 3.86 ± 1.68 a,b | 4.86 ± 2.79 a,b | 5.29 ± 3.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Wang, Y.; Zhu, L.; Zhao, X. Effects of Haematococcus pluvialis Addition on the Sensory Properties of Plant-Based Meat Analogues. Foods 2023, 12, 3435. https://doi.org/10.3390/foods12183435
Liu M, Wang Y, Zhu L, Zhao X. Effects of Haematococcus pluvialis Addition on the Sensory Properties of Plant-Based Meat Analogues. Foods. 2023; 12(18):3435. https://doi.org/10.3390/foods12183435
Chicago/Turabian StyleLiu, Meng, Yanli Wang, Laijing Zhu, and Xiangzhong Zhao. 2023. "Effects of Haematococcus pluvialis Addition on the Sensory Properties of Plant-Based Meat Analogues" Foods 12, no. 18: 3435. https://doi.org/10.3390/foods12183435
APA StyleLiu, M., Wang, Y., Zhu, L., & Zhao, X. (2023). Effects of Haematococcus pluvialis Addition on the Sensory Properties of Plant-Based Meat Analogues. Foods, 12(18), 3435. https://doi.org/10.3390/foods12183435







