Characterization of Molecular Chaperone GroEL as a Potential Virulence Factor in Cronobacter sakazakii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Bacterial Fractionation
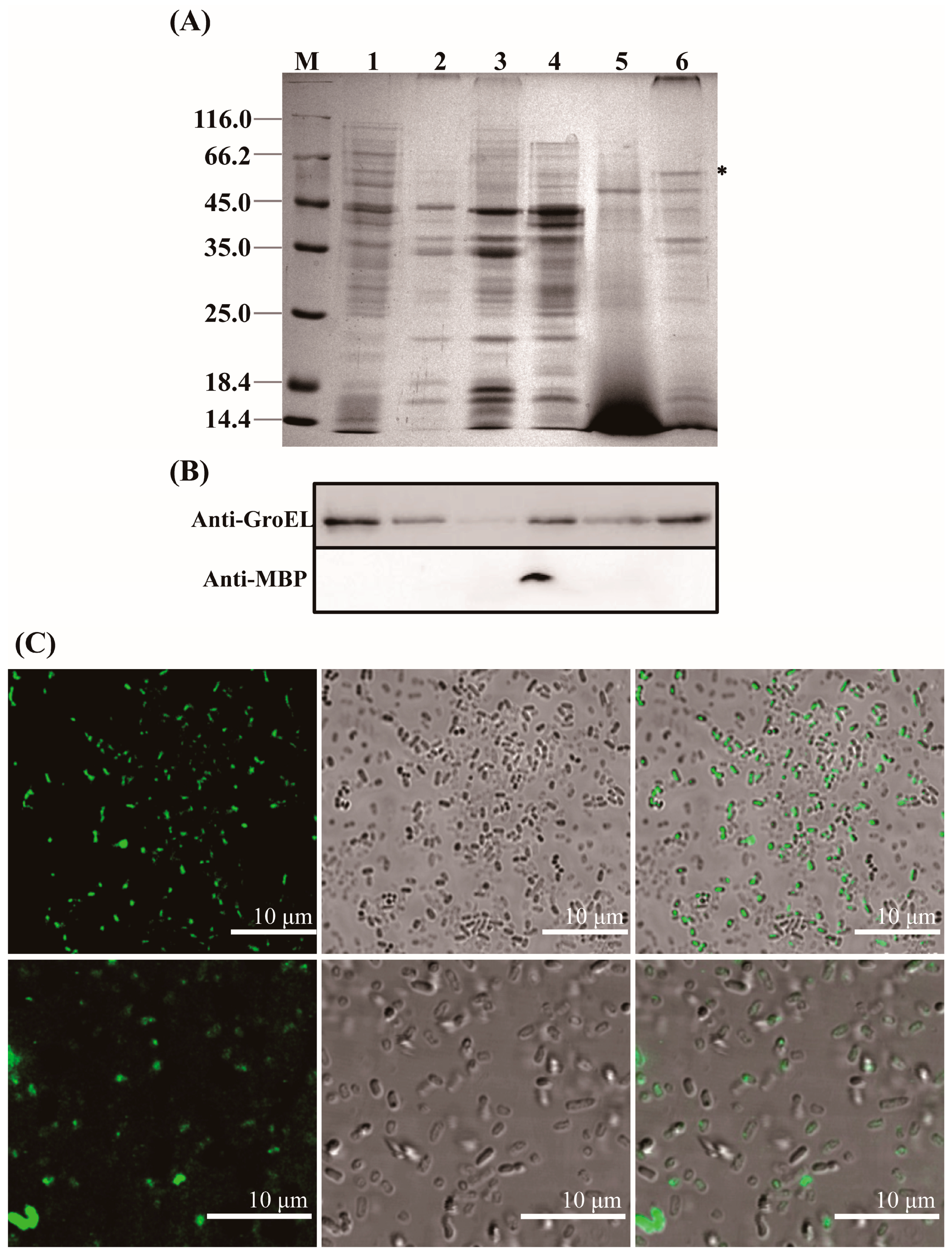
2.3. Identification of GroEL and Its Forms in Different Bacterial Fractions
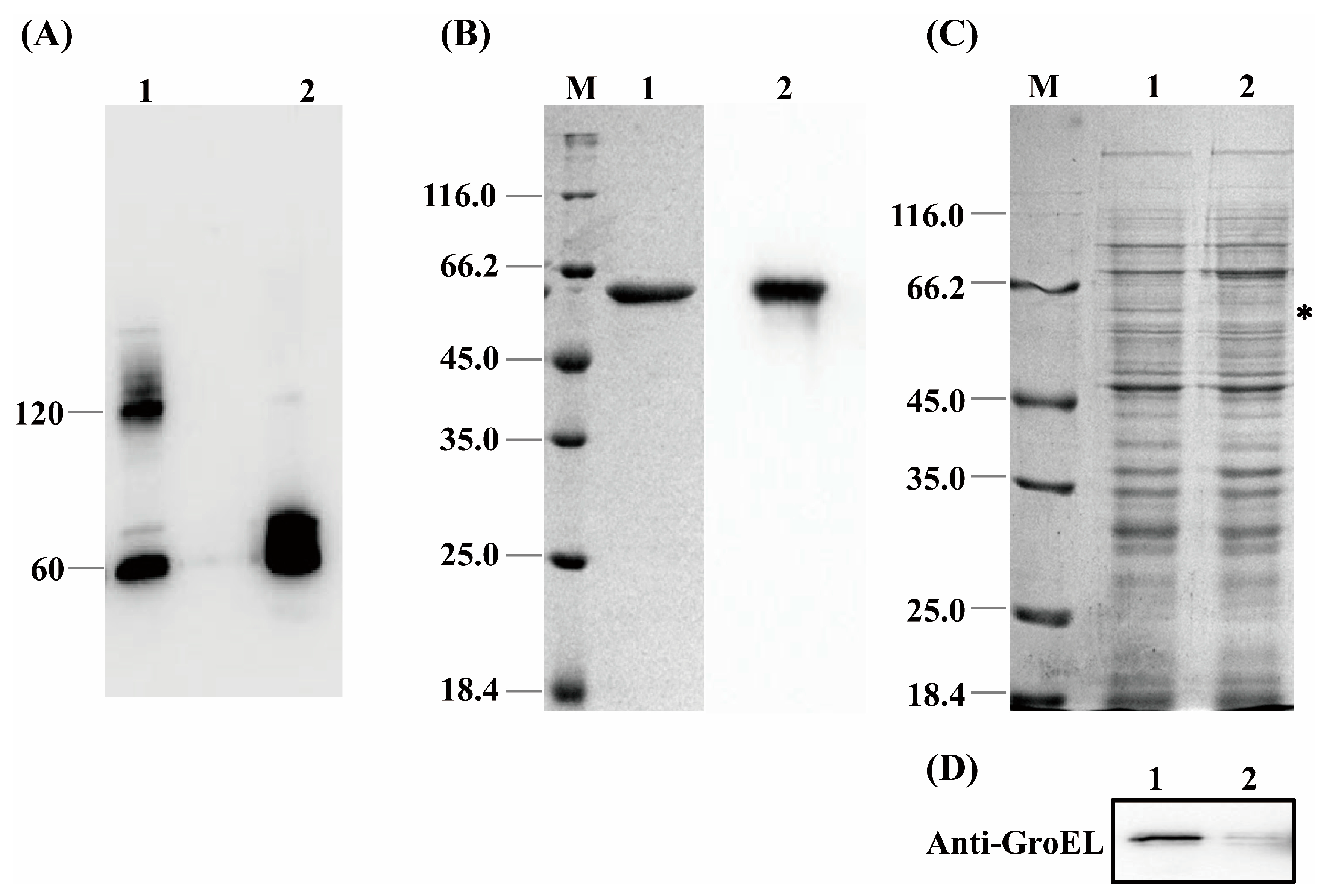
2.4. Determination of the Bacterial Surface GroEL
2.5. Adhesive Ability of GroEL to HCT-8 Cells
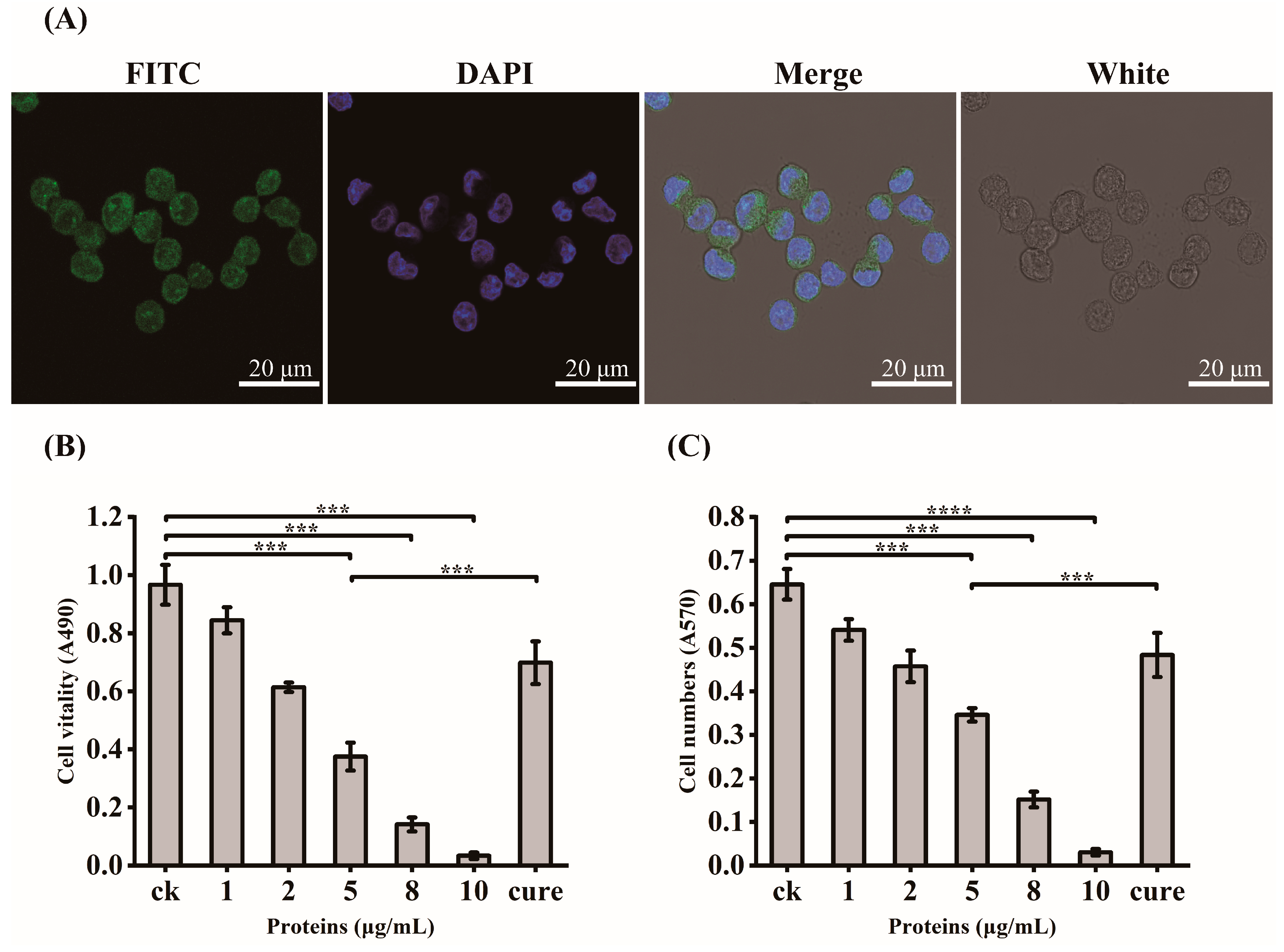
2.6. Determination of GroEL Protein Virulent Effects
2.7. Detection of Cytokines
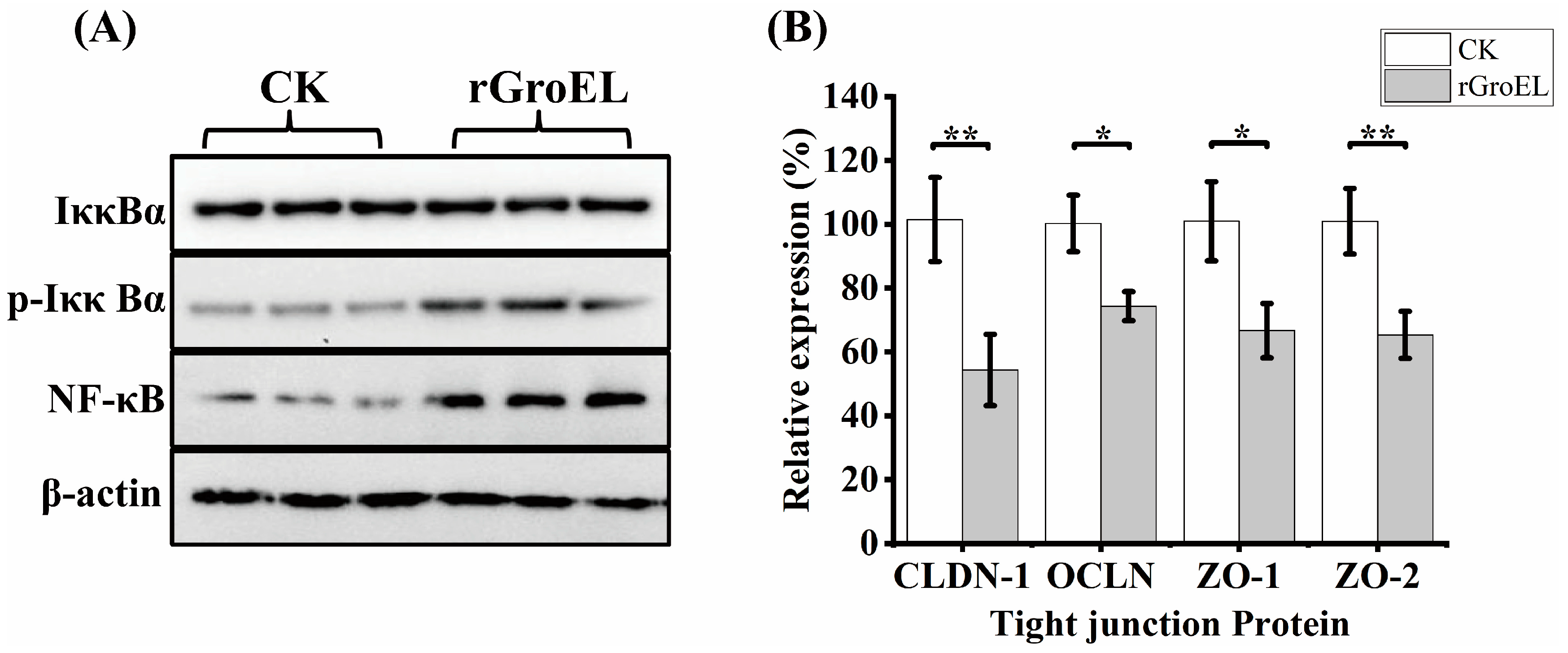
2.8. Signaling Pathway Analysis
2.9. C. sakazakii grol Gene Silencing
2.10. Bacterial Viability Assay
2.11. Bacterial Adhesion and Invasion
2.12. Biofilm Formation Experiment
2.13. Bacterial Motility and Morphology
2.14. Statistical Analysis
3. Results
3.1. Subcellular Localization and Quaternary Structure of GroEL
3.2. Virulent Effects of rGroEL Protein on HCT-8 Cells
3.3. Nuclear Factor Kappa-B (NF-κB) Activization and Downregulation of Tight-Junction Proteins in HCT-8 Cells under rGroEL Stimulation
3.4. Cytokine Release from HCT-8 Cells under rGroEL Stimulation
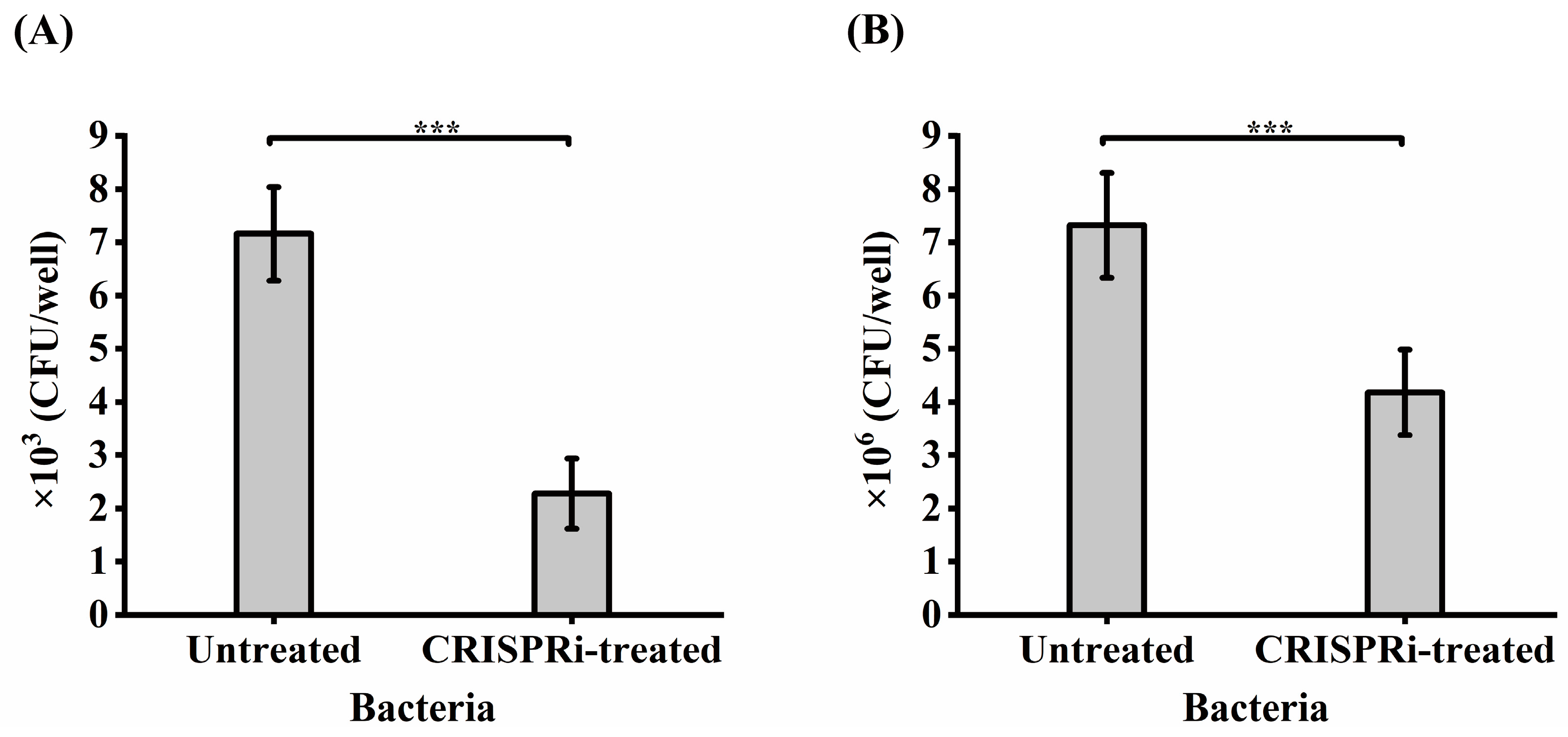
3.5. CRISPRi-Mediated grol Gene Silencing and Bacterial Viability
3.6. Effects of GroEL on C. sakazakii Adhesion and Invasion
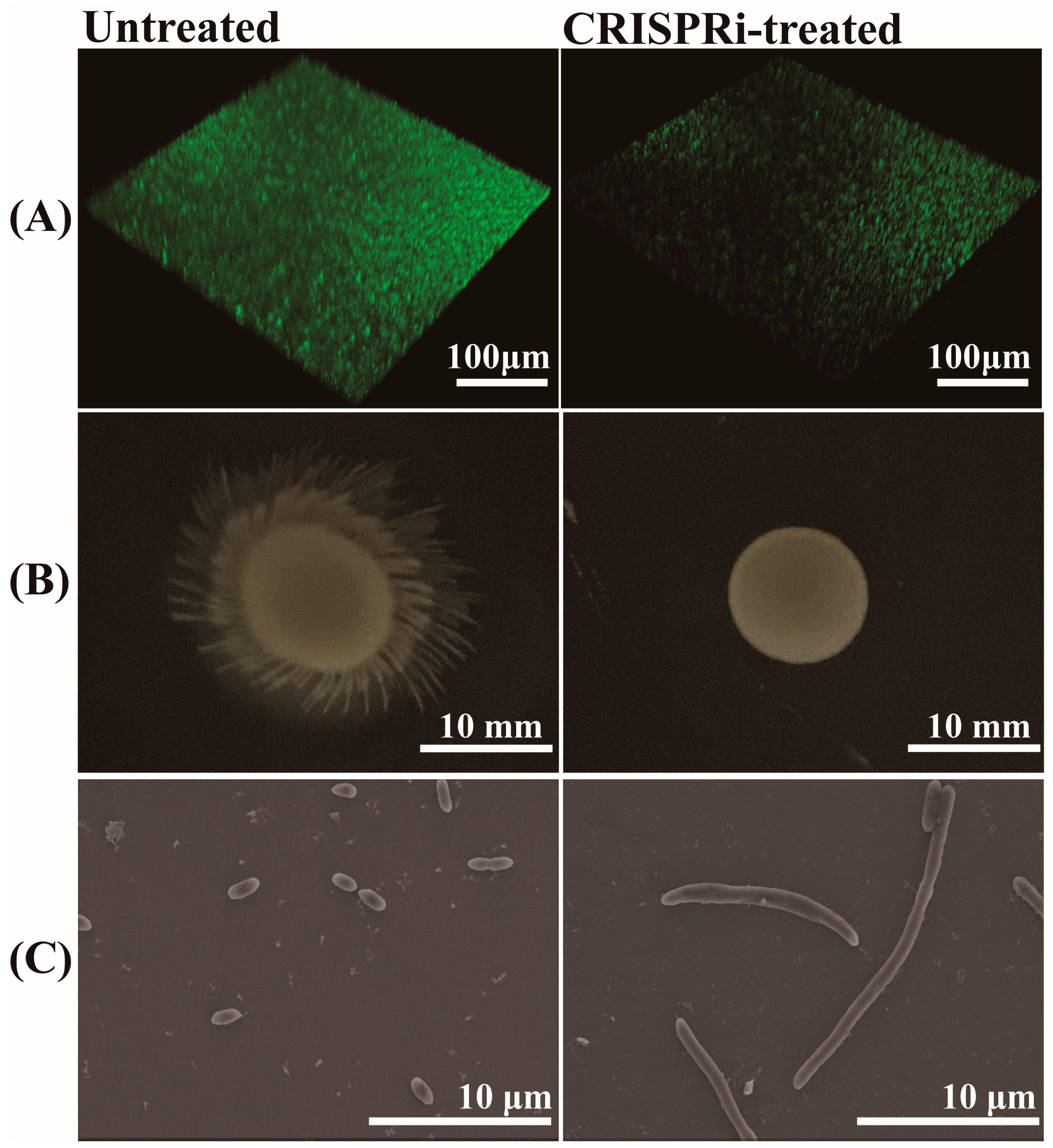
3.7. Effects of GroEL on Biofilm Formation, Motility and Morphology of C. sakazakii
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Henry, M.; Fouladkhah, A. Outbreak history, biofilm formation, and preventive measures for control of Cronobacter sakazakii in infant formula and infant care settings. Microorganisms 2019, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.K. Enterobacter sakazakii infections among neonates, infants, children, and adults. Medicine 2001, 80, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.E.; Flores, J.; Fernández-Escartín, E.P.; Forsythe, S.J. Reevaluation of a suspected Cronobacter sakazakii outbreak in Mexico. J. Food Prot. 2015, 78, 1191–1196. [Google Scholar] [CrossRef]
- Bowen, A.; Wiesenfeld, H.C.; Kloesz, J.L.; Pasculle, A.W.; Nowalk, A.J.; Brink, L.; Elliot, E.; Martin, H.; Tarr, C.L. Notes from the field: Cronobacter sakazakii infection associated with feeding extrinsically contaminated expressed human milk to a premature infant—Pennsylvania, 2016. MMWR. Morb. Mortal. Wkly. Rep 2017, 66, 761–762. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Kornacki, J.L.; Beuchat, L.R. Enterobacter sakazakii: A coliform of increased concern to infant health. Int. J. Food. Microbiol. 2005, 104, 1–34. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Chen, L.; Cai, Z.; Wu, S.; Gu, Q.; Zhang, Y.; Wei, X.; Zhang, J.; Yang, X.; et al. Cronobacter spp. isolated from quick-frozen foods in china: Incidence, genetic characteristics, and antibiotic resistance. Foods 2023, 12, 3019. [Google Scholar] [CrossRef]
- Haston, J.C.; Miko, S.; Cope, J.R.; McKeel, H.; Walters, C.; Joseph, L.A.; Griswold, T.; Katz, L.S.; Andújar, A.A.; Tourdot, L.; et al. Cronobacter sakazakii infections in two infants linked to powdered infant formula and breast pump equipment—United States, 2021 and 2022. MMWR. Morb. Mortal. Wkly. Rep. 2023, 72, 223–226. [Google Scholar] [CrossRef]
- Chandrapala, D.; Kim, K.; Choi, Y.; Senevirathne, A.; Kang, D.H.; Ryu, S.; Kim, K.-P. Putative Inv is essential for basolateral invasion of Caco-2 cells and acts synergistically with OmpA to affect in vitro and in vivo virulence of Cronobacter sakazakii ATCC 29544. Infect. Immun. 2014, 82, 1755–1765. [Google Scholar] [CrossRef]
- Pagotto, F.J.; Nazarowec-White, M.; Bidawid, S.; Farber, J.M. Enterobacter sakazakii: Infectivity and enterotoxin production in vitro and in vivo. J. Food. Prot. 2003, 66, 370–375. [Google Scholar] [CrossRef]
- Cruz-Córdova, A.; Rocha-Ramírez, L.M.; Ochoa, S.A.; González-Pedrajo, B.; Espinosa, N. and Eslava, C. Flagella from five Cronobacter species induce pro-inflammatory cytokines in macrophage derivatives from human monocytes. PLoS ONE 2012, 7, e52091. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, K.P.; Choi, J. Outer membrane proteins A (OmpA) and X (OmpX) are essential for basolateral invasion of Cronobacter sakazakii. Appl. Environ. Microbiol. 2010, 76, 5188–5198. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Grim, C.J.; Franco, A.A.; Jarvis, K.G.; Sathyamoorthy, V.; Kothary, M.H.; McCardell, B.A.; Tall, B.D. Analysis of the cellulose synthase operon genes, bcsA, bcsB, and bcsC in Cronobacter species: Prevalence among species and their roles in biofilm formation and cell-cell aggregation. Food. Microbiol. 2015, 52, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, S.; Hwang, H.; Kim, K.P.; Kang, D.H.; Ryu, S. Plasmid-encoded MCP is involved in virulence, motility, and biofilm formation of Cronobacter sakazakii ATCC 29544. Infect. Immun. 2015, 83, 197–204. [Google Scholar] [CrossRef]
- Kim, S.; Yoon, H.; Ryu, S. New virulence factor CSK29544_02616 as LpxA binding partner in Cronobacter sakazakii. Sci. Rep. 2018, 8, 835. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Rosenshine, I.; Tung, S.L.; Wang, X.H.; Friedberg, D.; Hew, C.L. Comparative proteomic analysis of extracellular proteins of enterohemorrhagic and enteropathogenic Escherichia coli strains and their ihf and ler mutants. Appl. Environ. Microbiol. 2004, 70, 5274–5282. [Google Scholar] [CrossRef]
- Aguilera, L.; Ferreira, E.; Giménez, R.; Fernández, F.J.; Taulés, M.; Aguilar, J.; Vega, M.C.; Badia, J.; Baldomà, L. Secretion of the housekeeping protein glyceraldehyde-3-phosphate dehydrogenase by the LEE-encoded type III secretion system in enteropathogenic Escherichia coli. Int. J. Biochem. Cell Biol. 2012, 44, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Terrasse, R.; Amoroso, A.; Vernet, T.; Di Guilmi, A.M. Streptococcus pneumoniae GAPDH is released by cell lysis and interacts with peptidoglycan. PLoS ONE 2015, 10, e0125377. [Google Scholar] [CrossRef]
- Ebner, P.; Luqman, A.; Reichert, S.; Hauf, K.; Popella, P.; Forchhammer, K.; Otto, M.; Götz, F. Non-classical protein excretion is boosted by PSMα-induced cell leakage. Cell Rep. 2017, 20, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Daubenspeck, J.M.; Liu, R.; Dybvig, K. Rhamnose links moonlighting proteins to membrane phospholipid in mycoplasmas. PLoS ONE 2016, 11, e0162505. [Google Scholar] [CrossRef]
- Wang, J.; Du, X.J.; Lu, X.N.; Wang, S. Immunoproteomic identification of immunogenic proteins in Cronobacter sakazakii strain BAA-894. Appl. Microbiol. Biotechnol. 2013, 97, 2077–2091. [Google Scholar] [CrossRef]
- Yan, X.; Shi, Q.; Bracher, A.; Miličić, G.; Singh, A.K.; Hartl, F.U. GroEL ring separation and exchange in the chaperonin reaction. Cell 2018, 172, 605–617. [Google Scholar] [CrossRef]
- Chapman, E.; Farr, G.W.; Usaite, R.; Furtak, K.; Fenton, W.A.; Chaudhuri, T.K.; Hondorp, E.R.; Matthews, R.G.; Wolf, S.G.; Yates, J.R.; et al. Global aggregation of newly translated proteins in an Escherichia coli strain deficient of the chaperonin GroEL. Proc. Natl. Acad. Sci. USA 2006, 103, 15800–15805. [Google Scholar] [CrossRef]
- Pantzar, M.; Teneberg, S.; Lagergård, T. Binding of Haemophilus ducreyi to carbohydrate receptors is mediated by the 58.5-kDa GroEL heat shock protein. Microbes. Infect. 2006, 8, 2452–2458. [Google Scholar] [CrossRef]
- Nalbant, A.; Kant, M. Bacterial heat shock protein GroEL (Hsp64) exerts immunoregulatory effects on T Cells by utilizing apoptosis. PLoS ONE 2016, 11, e0164380. [Google Scholar] [CrossRef]
- Hagiwara, M.; Kurita-Ochiai, T.; Kobayashi, R.; Hashizume-Takizawa, T.; Yamazaki, K.; Yamamoto, M. Sublingual vaccine with GroEL attenuates atherosclerosis. J. Dent. Res. 2014, 93, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Goulhen, F.; Hafezi, A.; Uitto, V.J.; Hinode, D.; Nakamura, R.; Grenier, D.; Mayrand, D. Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans. Infect. Immun. 1998, 66, 5307–5313. [Google Scholar] [CrossRef] [PubMed]
- Qamra, R.; Srinivas, V.; Mande, S.C. Mycobacterium tuberculosis GroEL homologues unusually exist as lower oligomers and retain the ability to suppress aggregation of substrate proteins. J. Mol. Biol. 2004, 342, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Song, J.R.; Fu, Y.W.; Li, P.; Du, T.; Du, X.J.; Wang, S. Cronobacter sakazakii protective effect of recombinant proteins of during pregnancy on the offspring. Front. Cell Infect. Microbiol. 2020, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, H.; Wang, F.; Wei, D.; Wang, X. An improved 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction assay for evaluating the viability of Escherichia coli cells. J. Microbiol. Methods 2010, 82, 330–333. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Sun, J.; Zhang, D. Multimer recognition and secretion by the non-classical secretion pathway in Bacillus subtilis. Sci. Rep. 2017, 7, 44023. [Google Scholar] [CrossRef]
- Ybarra, J.; Horowitz, P.M. Refolding and reassembly of active chaperonin GroEL after denaturation. J. Biol. Chem 1995, 270, 22113–22115. [Google Scholar] [CrossRef]
- Bochkareva, E.S.; Solovieva, M.E.; Girshovich, A.S. Targeting of GroEL to SecA on the cytoplasmic membrane of Escherichia coli. Proc. Natl. Acad. Sci. USA 1998, 95, 478–483. [Google Scholar] [CrossRef]
- Lin, F.Y.; Hsiao, F.P.; Huang, C.Y.; Shih, C.M.; Tsao, N.W.; Yang, S.F.; Chang, N.C.; Hung, S.L.; Lin, Y.-W.; Tsai, C.S. Porphyromonas gingivalis GroEL induces osteoclastogenesis of periodontal ligament cells and enhances alveolar bone resorption in rats. PLoS ONE 2014, 9, e102450. [Google Scholar] [CrossRef]
- Hu, Y.; Henderson, B.; Lund, P.A.; Tormay, P.; Ahmed, M.T.; Gurcha, S.S.; Besra, G.S.; Anthony, R.M. Mycobacterium tuberculosis mutant lacking the groEL homologue cpn60.1 is viable but fails to induce an inflammatory response in animal models of infection. Infect. Immun. 2008, 76, 1535–1546. [Google Scholar] [CrossRef]
- Dann, S.M.; Spehlmann, M.E.; Hammond, D.C.; Iimura, M.; Hase, K.; Choi, L.J.; Hanson, E.; Eckmann, L. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J. Immunol. 2008, 180, 6816–6826. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Alizadeh, A.; Verheijden, K.A.; Schoterman, M.H.; Kraneveld, A.D.; Fink-Gremmels, J. Galacto-oligosaccharides Protect the intestinal barrier by maintaining the tight junction network and modulating the inflammatory responses after a challenge with the mycotoxin deoxynivalenol in human Caco-2 cell monolayers and B6C3F1 mice. J. Nutr. 2015, 145, 1604–1613. [Google Scholar] [CrossRef]
- Grabinger, T.; Bode, K.J.; Demgenski, J.; Seitz, C.; Delgado, M.E.; Kostadinova, F.; Reinhold, C.; Etemadi, N.; Wilhelm, S.; Schweinlin, M.; et al. Inhibitor of apoptosis protein-1 regulates tumor necrosis factor-mediated destruction of intestinal epithelial cells. Gastroenterology 2017, 152, 867–879. [Google Scholar] [CrossRef]
- Marchiando, A.M.; Shen, L.; Graham, W.V.; Edelblum, K.L.; Duckworth, C.A.; Guan, Y.; Montrose, M.H.; Turner Jerrold, R.; Watson, A.J.M. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology 2011, 140, 1208–1218. [Google Scholar] [CrossRef]
- Drolia, R.; Tenguria, S.; Durkes, A.C.; Turner, J.R.; Bhunia, A.K. Listeria adhesion protein induces intestinal epithelial barrier dysfunction for bacterial translocation. Cell Host. Microbe 2018, 23, 470–484. [Google Scholar] [CrossRef]
- Noth, R.; Lange-Grumfeld, J.; Stuber, E.; Kruse, M.L.; Ellrichmann, M.; Hasler, R.; Hampe, J.; Bewig, B.; Rosenstiel, P.; Schreiber, S.; et al. Increased intestinal permeability and tight junction disruption by altered expression and localization of occludin in a murine graft versus host disease model. BMC. Gastroenterol. 2011, 11, 109. [Google Scholar] [CrossRef]
- Kerner, M.J.; Naylor, D.J.; Ishihama, Y.; Maier, T.; Chang, H.C.; Stines, A.P.; Georgopoulos, C.; Frishman, D.; Hayer-Hartl, M.; Mann, M.; et al. Proteome-wide analysis of chaperonin-dependent protein folding in Escherichia coli. Cell 2005, 122, 209–220. [Google Scholar] [CrossRef]
- Arora, G.; Sajid, A.; Virmani, R.; Singhal, A.; Kumar, C.M.S.; Dhasmana, N.; Khanna, T.; Maji, A.; Misra, R.; Molle, V.; et al. Ser/Thr protein kinase PrkC-mediated regulation of GroEL is critical for biofilm formation in Bacillus anthracis. NPJ. Biofilms. Microbiomes 2017, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Singh, Y.; Hasija, Y. Bacillus anthracis GroEL Mediates Folding of Serine/Threonine Protein Kinase, PrkC. Indian. J. Microbiol 2018, 58, 520–524. [Google Scholar] [CrossRef]
- Calloni, G.; Chen, T.; Schermann, S.M.; Chang, H.C.; Genevaux, P.; Agostini, F.; Tartaglia, G.G.; Hayer-Hartl, M.; Hartl, F.U. DnaK functions as a central hub in the E. coli chaperone network. Cell Rep. 2012, 1, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Smyth, D.; O’Hagan, B.M.; Heap, J.T.; McMullan, G.; Minton, N.P.; Ternan, N.G. Inactivation of the dnaK gene in Clostridium difficile 630 Δerm yields a temperature-sensitive phenotype and increases biofilm-forming ability. Sci. Rep. 2017, 7, 17522. [Google Scholar] [CrossRef]
- Fujiwara, K.; Taguchi, H. Filamentous morphology in GroE-Depleted Escherichia coli induced by impaired folding of FtsE. J. Bacteriol. 2007, 189, 5860–5866. [Google Scholar] [CrossRef]
| Pro-Inflammatory Cytokines (pg/mL) | Control | rGroEL | rGroEL + Anti-GroEL |
|---|---|---|---|
| TNF-α | 328.66 ± 21.36 | 439.35 ± 14.71 | 344.97 ± 28.47 |
| IL-6 | 10.61 ± 0.38 | 13.29 ± 0.57 | 10.89 ± 1.32 |
| IL-8 | 5.33 ± 0.55 | 11.23 ± 0.64 | 6.23 ± 1.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Fan, Y.; Wang, X.; Li, P.; Huang, Y.; Jiao, J.; Zhao, C.; Li, Y.; Wang, S.; Du, X. Characterization of Molecular Chaperone GroEL as a Potential Virulence Factor in Cronobacter sakazakii. Foods 2023, 12, 3404. https://doi.org/10.3390/foods12183404
Zhu D, Fan Y, Wang X, Li P, Huang Y, Jiao J, Zhao C, Li Y, Wang S, Du X. Characterization of Molecular Chaperone GroEL as a Potential Virulence Factor in Cronobacter sakazakii. Foods. 2023; 12(18):3404. https://doi.org/10.3390/foods12183404
Chicago/Turabian StyleZhu, Dongdong, Yufei Fan, Xiaoyi Wang, Ping Li, Yaping Huang, Jingbo Jiao, Chumin Zhao, Yue Li, Shuo Wang, and Xinjun Du. 2023. "Characterization of Molecular Chaperone GroEL as a Potential Virulence Factor in Cronobacter sakazakii" Foods 12, no. 18: 3404. https://doi.org/10.3390/foods12183404
APA StyleZhu, D., Fan, Y., Wang, X., Li, P., Huang, Y., Jiao, J., Zhao, C., Li, Y., Wang, S., & Du, X. (2023). Characterization of Molecular Chaperone GroEL as a Potential Virulence Factor in Cronobacter sakazakii. Foods, 12(18), 3404. https://doi.org/10.3390/foods12183404





