The Chemical Composition of Scaptotrigona mexicana Honey and Propolis Collected in Two Locations: Similarities and Differences
Abstract
:1. Introduction
2. Materials and Methods
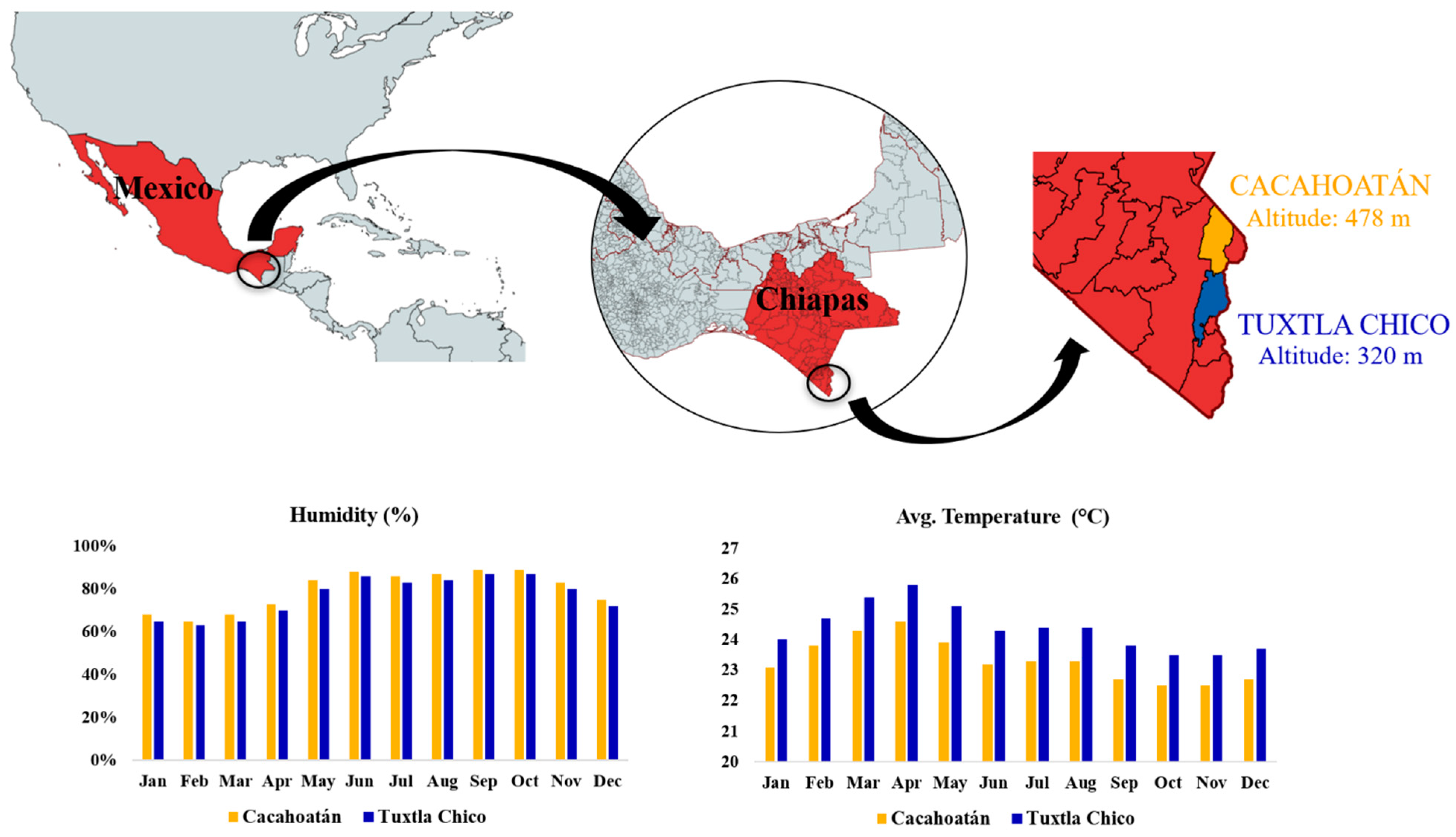
2.1. Bee Species and Study Sites
2.2. Propolis Sample Collection
2.3. Honey Sample Collection
2.4. Honey Sample Preparation
2.5. NMR Spectroscopy
2.6. Propolis Extraction and Sample Preparation
2.7. Total Phenolics
2.8. GC-MS Analysis
2.9. Free Radical Scavenging Activity
2.10. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.11. Statistical Analysis
3. Results and Discussion
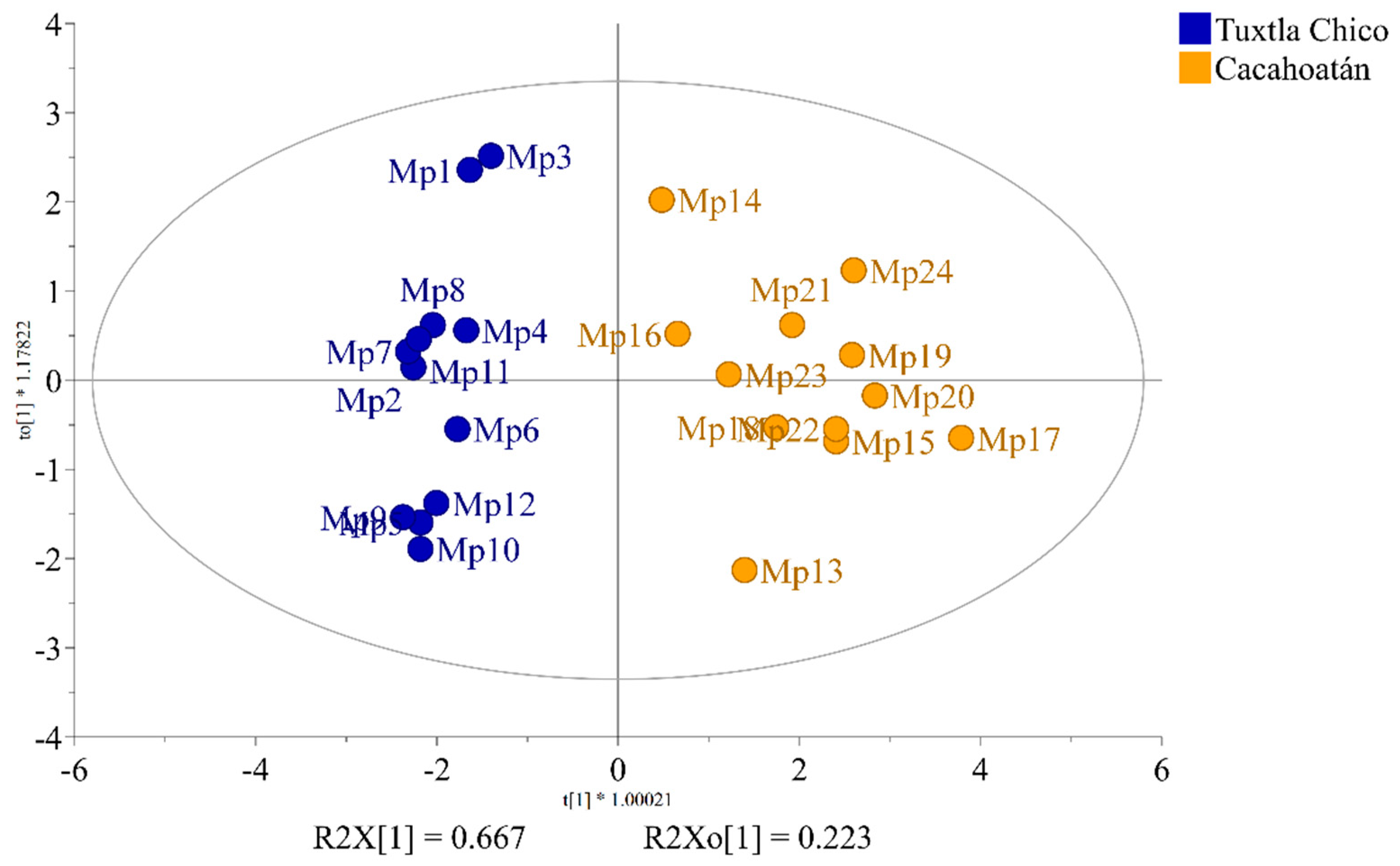
3.1. Chemical Profiles of Honey
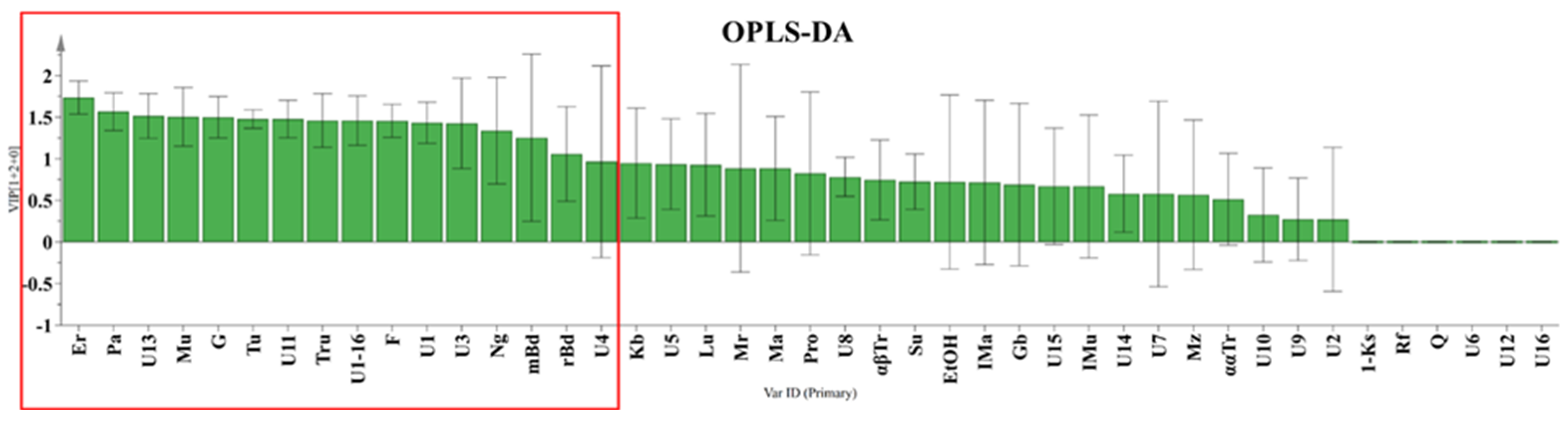
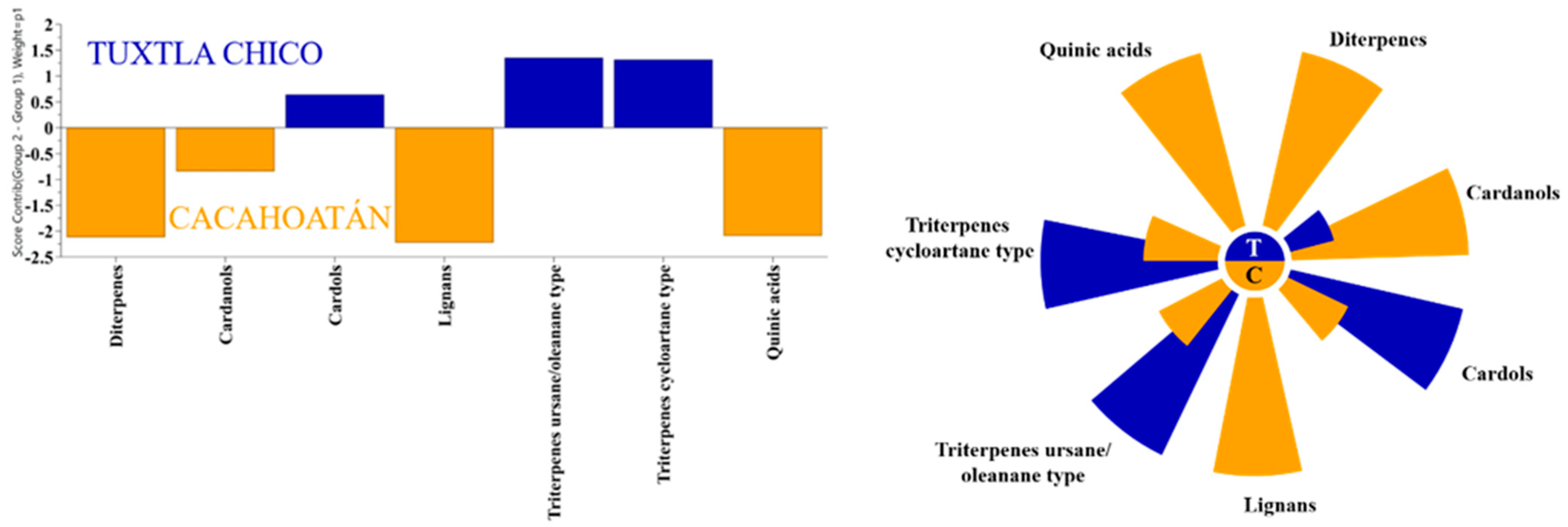
3.2. Chemical Profiles of Propolis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roubik, D.W.; Smith, B.H.; Carlson, R.G. Formic acid in caustic cephalic secretions of stingless bee, Oxytrigona (Hymenoptera: Apidae). J. Chem. Ecol. 1987, 13, 1079–1086. [Google Scholar] [CrossRef]
- Hrncir, M.; Jarau, S.; Barth, F.G.J. Stingless bees (Meliponini): Senses and behavior. J. Comp. Physiol. A 2016, 202, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Roubik, D.W. Ecology and Natural History of Tropical Bees; Cambridge University Press: New York, NY, USA, 1989. [Google Scholar]
- Sanches, M.A.; Pereira, A.M.S.; Serrão, J.E. Pharmacological actions of extracts of propolis of stingless bees (Meliponini). J. Apicult. Res. 2017, 56, 50–57. [Google Scholar] [CrossRef]
- Reyes-González, A.; Camou-Guerrero, A.; Del-Val, E.; Ramírez, M.I.; Porter-Bolland, L. Biocultural diversity loss: The decline of native stingless bees (Apidae: Meliponini) and local ecological knowledge in Michoacán, Western México. Hum. Ecol. 2020, 48, 411–422. [Google Scholar] [CrossRef]
- Arnold, N.; Zepeda, R.; Vásquez, V.D.; Maya, E.M.A. Las Abejas sin Aguijón y su Cultivo en Oaxaca, México; ECOSUR, El Colegio de la Frontera Sur.: Lerma Campeche, Mexico, 2018; ISBN 978-607-8429-53-0. [Google Scholar]
- Gutierrez, A.; Obregon, F.H.; Jones, W.R. Optimum brood size for artificial propagation of the stingless bee, Scaptotrigona mexicana. J. Apic. Res. 2002, 41, 62–63. [Google Scholar] [CrossRef]
- Vit, P.; Medina, M.; Eunice Enríquez, M. Quality standards for medicinal uses of Meliponinae honey in Guatemala, Mexico and Venezuela. Bee World 2004, 85, 2–5. [Google Scholar] [CrossRef]
- Jimenez, M.; Beristain, C.I.; Azuara, E.; Mendoza, M.R.; Pascual, L.A. Physicochemical and antioxidant properties of honey from Scaptotrigona mexicana bee. J. Apic. Res. 2016, 55, 151–160. [Google Scholar] [CrossRef]
- Popova, M.; Gerginova, D.; Trusheva, B.; Simova, S.; Tamfu, A.N.; Ceylan, O.; Clark, K.; Bankova, V. A preliminary study of chemical profiles of honey, cerumen, and propolis of the African stingless bee Meliponula ferruginea. Foods 2021, 10, 997. [Google Scholar] [CrossRef]
- Garay, L.A.L.; Téllez, L.I.T.; Merino, F.C.G.; Oliva, A.C.; Sato, J.A.P.; Ruíz, J.S. Physicochemical properties of Scaptotrigona mexicana honey from the Highlands of Veracruz, Mexico. Ecosistemas y Recursos Agropecuarios 2023, 10, 15. [Google Scholar]
- Grajales-Conesa, J.; Vandame, R.; Santiesteban-Hernández, A.; López-García, A.; Guzmán-Díaz, M. Propiedades fisicoquímicas y antibacterianas de mieles de abejas sin aguijón del Sur de Chiapas, México. IBCiencias 2018, 1, 1–7. [Google Scholar]
- Vit, P.; Rojas, L.B.; Usubillaga, A.; Aparicio, R.; Meccia, G.; Muiño, M.A.F.; Sancho, M.T. Presence of lactic acid and other semivolatil compound in Meliponini honeys. RINHRR 2011, 42, 58–63. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0798-04772011000100008&lng=es&nrm=iso (accessed on 25 April 2023).
- Grajales-Conesa, J.; Elías-Chirino, J.; Lozano-Guzmán, E.; Moreno-Cruz, F.; Albores-Flores, V.; López-García, A. Stingless bees propolis antimicrobial activity in combination with garlic, Allium sativum (Amaryllidaceae). Rev. Biol. Trop. 2021, 69, 22–35. [Google Scholar]
- Greenaway, W.; Scaysbrook, T.; Whatley, F.R. The composition and plant origin of propolis: A report of work at Oxford. Bee World 1990, 71, 107–118. [Google Scholar] [CrossRef]
- Ahn, M.R.; Kumazawa, S.; Usui, Y.; Nakamura, J.; Matsuka, M.; Zhu, F.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 2007, 101, 1383–1392. [Google Scholar] [CrossRef]
- Mohamed, W.A.S.; Ismail, N.Z.; Muhamad, M.; Omar, E.A.; Samad, N.A.; Ooi, J.P.; Mohamad, S. Q-TOF LC-MS compounds evaluation of propolis extract derived from Malaysian stingless bees, Tetrigona apicalis, and their bioactivities in breast cancer cell, MCF7. Saudi J. Biol. Sci. 2022, 29, 103403. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Silici, S.; Kaftanoglu, O.; Bankova, V. Antimicrobial activity of Turkish propolis and its qualitative and quantitative chemical composition. Phytomedicine 2005, 12, 221–228. [Google Scholar] [CrossRef]
- Garzoli, S.; Maggio, F.; Vinciguerra, V.; Rossi, C.; Donadu, M.G.; Serio, A. Chemical Characterization and Antimicrobial Properties of the Hydroalcoholic Solution of Echinacea purpurea (L.) Moench. and Propolis from Northern Italy. Molecules 2023, 28, 1380. [Google Scholar] [CrossRef]
- Souza, B.; Roubik, D.; Barth, O.; Heard, T.; Enríquez, E.; Carvalho, C.; Villas-Bôas, M.L.; Locatelli, J.; Persano-Oddo, L.; Almeida-Muradian, L.; et al. Composition of stingless bee honey: Setting quality standards. Interciencia 2006, 31, 867–875. [Google Scholar]
- Kek, S.P.; Chin, N.L.; Tan, S.W.; Yusof, Y.A.; Chua, L.S. Molecular identification of honey entomological origin based on bee mitochondrial 16S rRNA and COI gene sequences. Food Control 2017, 78, 150–159. [Google Scholar] [CrossRef]
- Sujanto, I.S.R.; Ramly, N.S.; Abd Ghani, A.; Huat, J.T.Y.; Alias, N.; Ngah, N. The composition and functional properties of stingless bee honey: A review. Malaysian, J. Anal. Sci. 2021, 6, 111–127. [Google Scholar]
- Schievano, E.; Mammi, S.; Menegazzo, I. Nuclear Magnetic Resonance as a Method to Predict the Geographical and Entomological Origin of Pot-Honey. In Pot-Honey: A Legacy of Stingless Bees; Vit, P., Pedro, S.R.M., Roubik, D., Eds.; Springer: New York, NY, USA, 2013; pp. 429–445. [Google Scholar] [CrossRef]
- Carneiro, M.J.; López, B.G.; Lancellotti, M.; Franchi, G.C.; Nowill, A.E.; Sawaya, A.C.H.F. Evaluation of the chemical composition and biological activity of extracts of Tetragonisca angustula propolis and Schinus terebinthifolius Raddi (Anacardiaceae). J. Apic. Res. 2016, 55, 315–323. [Google Scholar] [CrossRef]
- Velikova, M.; Bankova, V.; Tsvetkova, I.; Kujumgiev, A.; Marcucci, M.C. Antibacterial ent-kaurene from Brazilian propolis of native stingless bees. Fitoterapia 2000, 71, 693–696. [Google Scholar] [CrossRef]
- Zawawi, N.; Zhang, J.; Hungerford, N.L.; Yates, H.S.; Webber, D.C.; Farrell, M.; Tinggi, U.; Bhandari, B.; Fletcher, M.T. Unique physicochemical properties and rare reducing sugar trehalulose mandate new international regulation for stingless bee honey. Food Chem. 2022, 373, 131566. [Google Scholar] [CrossRef] [PubMed]
- Gerginova, D.; Dimova, D.; Simova, S. Preliminary NMR and chemometric study of pine jams used as medicinal remedies. Bulg. Chem. Commun. 2017, 49, 215–220. [Google Scholar]
- Gerginova, D.; Simova, S.; Popova, M.; Stefova, M.; Stanoeva, J.P.; Bankova, V. NMR Profiling of North Macedonian and Bulgarian Honeys for Detection of Botanical and Geographical Origin. Molecules 2020, 25, 4687. [Google Scholar] [CrossRef]
- Tomasina, F.; Carabio, C.; Celano, L.; Thomson, L. Analysis of two methods to evaluate antioxidants. Biochem. Mol. Biol. Educ. 2012, 40, 266–270. [Google Scholar] [CrossRef]
- Benzie, I.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 77–106. [Google Scholar]
- Chaodi, K.; Yingying, Z.; Mingyue, Z.; Jing, Q.; Wentao, Z.; Jin, G.; Wenping, G.; Yingying, L. Screening of specific quantitative peptides of beef by LC–MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 13293. [Google Scholar]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Nieuwoudt, H.; Opara, U.L. Evaluation of biochemical markers associated with the development of husk scald and the use of diffuse reflectance NIR spectroscopy to predict husk scald in pomegranate fruit. Sci. Hortic. 2018, 232, 240–249. [Google Scholar] [CrossRef]
- Fletcher, M.T.; Hungerford, N.L.; Webber, D.; Carpinelli de Jesus, M.; Zhang, J.; Stone, I.S.; Blanchfield, J.T.; Zawawi, N. Stingless bee honey, a novel source of trehalulose: A biologically active disaccharide with health benefits. Sci. Rep. 2020, 10, 12128. [Google Scholar] [CrossRef]
- Hungerford, N.L.; Zhang, J.; Smith, T.J.; Yates, H.S.; Chowdhury, S.A.; Carter, J.F.; Carpinelli de Jesus, M.; Fletcher, M.T. Feeding sugars to stingless bees: Identifying the origin of trehalulose-rich honey composition. J. Agric. Food Chem. 2021, 69, 10292–10300. [Google Scholar] [CrossRef]
- Ng, W.-J.; Sit, N.-W.; Ooi, P.A.-C.; Ee, K.-Y.; Lim, T.-M. Botanical Origin Differentiation of Malaysian Stingless Bee Honey Produced by Heterotrigona itama and Geniotrigona thoracica Using Chemometrics. Molecules 2021, 26, 7628. [Google Scholar] [CrossRef]
- Simova, S. Trehalulose in Stingless Bees; Bulgarian NMR Centre, Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences: Sofia, Bulgaria, 2023; manuscript in preparation. [Google Scholar]
- Ali, H.; Abu Bakar, M.F.; Majid, M.; Muhammad, N.; Lim, S.Y. In vitro anti-diabetic activity of stingless bee honey from different botanical origins. Food Res. 2020, 4, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Wilczyńska, A. Effect of filtration on colour, antioxidant activity and total phenolics of honey. LWT—Food Sci. Technol. 2014, 57, 767–774. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant activity, total phenolic content, individual phenolics and physicochemical parameters suitability for Romanian honey authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. A review on ethnopharmacological applications, pharmacological activities, and bioactive compounds of Mangifera indica (Mango). Evid.-Based Complement. Alternat. Med. 2017, 2017, 6949835. [Google Scholar] [CrossRef]
- Zhang, G.; Shimokawa, S.; Mochizuki, M.; Kumamoto, T.; Nakanishi, W.; Watanabe, T.; Ishikawa, T.; Matsumoto, K.; Tashima, K.; Horie, S.; et al. Chemical constituents of Aristolochia constricta: Antispasmodic effects of its constituents in guinea-pig ileum and isolation of a diterpeno–lignan hybrid. J. Nat Prod. 2008, 71, 1167–1172. [Google Scholar] [CrossRef]
- Pereira, A.O.; Avila, J.M.; do Carmo, G.; Siqueira, F.S.; Campos, M.M.; Back, D.F.; Morel, A.F.; Dalcol, I.I. Chemical composition, antimicrobial and antimycobacterial activities of Aristolochia triangularis Cham. from Brazil. Ind. Crop.Prod. 2018, 121, 461–467. [Google Scholar] [CrossRef]
- Koirala, N.; Modi, B.; Subba, R.K.; Panthi, M.; Xiao, J. Medicinal Plants in Targeting Tuberculosis II. In Medicinal Plants for Lung Diseases; Dua, K., Nammi, S., Chang, D., Chellappan, D.K., Gupta, G., Collet, T., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- May-Canché, I.; Moguel-Ordoñez, Y.; Valle-Mora, J.; González-Cadenas, R.; Toledo-Núñez, B.; Arroyo-Rodríguez, L.; Piana, L.; Rémy Vandame, R. Sensory and physicochemical analysis of honeys of nine stingless bee species of Mexico and Guatemala. J. Food Sci. Technol. 2022, 59, 4772–4781. [Google Scholar] [CrossRef]





| Members | Correct | Tuxtla Chico | Cacahoatán | No Class (YPred ≤ 0) | |
|---|---|---|---|---|---|
| Tuxtla Chico | 12 | 100% | 12 | 0 | 0 |
| Cacahoatán | 12 | 100% | 0 | 12 | 0 |
| No class | 0 | 0 | 0 | 0 | |
| Total | 24 | 100% | 12 | 12 | 0 |
| Fisher’s prob. | 3.7 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerginova, D.; Popova, M.; Chimshirova, R.; Trusheva, B.; Shanahan, M.; Guzmán, M.; Solorzano-Gordillo, E.; López-Roblero, E.; Spivak, M.; Simova, S.; et al. The Chemical Composition of Scaptotrigona mexicana Honey and Propolis Collected in Two Locations: Similarities and Differences. Foods 2023, 12, 3317. https://doi.org/10.3390/foods12173317
Gerginova D, Popova M, Chimshirova R, Trusheva B, Shanahan M, Guzmán M, Solorzano-Gordillo E, López-Roblero E, Spivak M, Simova S, et al. The Chemical Composition of Scaptotrigona mexicana Honey and Propolis Collected in Two Locations: Similarities and Differences. Foods. 2023; 12(17):3317. https://doi.org/10.3390/foods12173317
Chicago/Turabian StyleGerginova, Dessislava, Milena Popova, Ralitsa Chimshirova, Boryana Trusheva, Maggie Shanahan, Miguel Guzmán, Erik Solorzano-Gordillo, Estefhanía López-Roblero, Marla Spivak, Svetlana Simova, and et al. 2023. "The Chemical Composition of Scaptotrigona mexicana Honey and Propolis Collected in Two Locations: Similarities and Differences" Foods 12, no. 17: 3317. https://doi.org/10.3390/foods12173317
APA StyleGerginova, D., Popova, M., Chimshirova, R., Trusheva, B., Shanahan, M., Guzmán, M., Solorzano-Gordillo, E., López-Roblero, E., Spivak, M., Simova, S., & Bankova, V. (2023). The Chemical Composition of Scaptotrigona mexicana Honey and Propolis Collected in Two Locations: Similarities and Differences. Foods, 12(17), 3317. https://doi.org/10.3390/foods12173317