Reduction in the Cocoa Spontaneous and Starter Culture Fermentation Time Based on the Antioxidant Profile Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material and Reagents
2.2. Yeast Strain (Saccharomyces cerevisiae) Activation
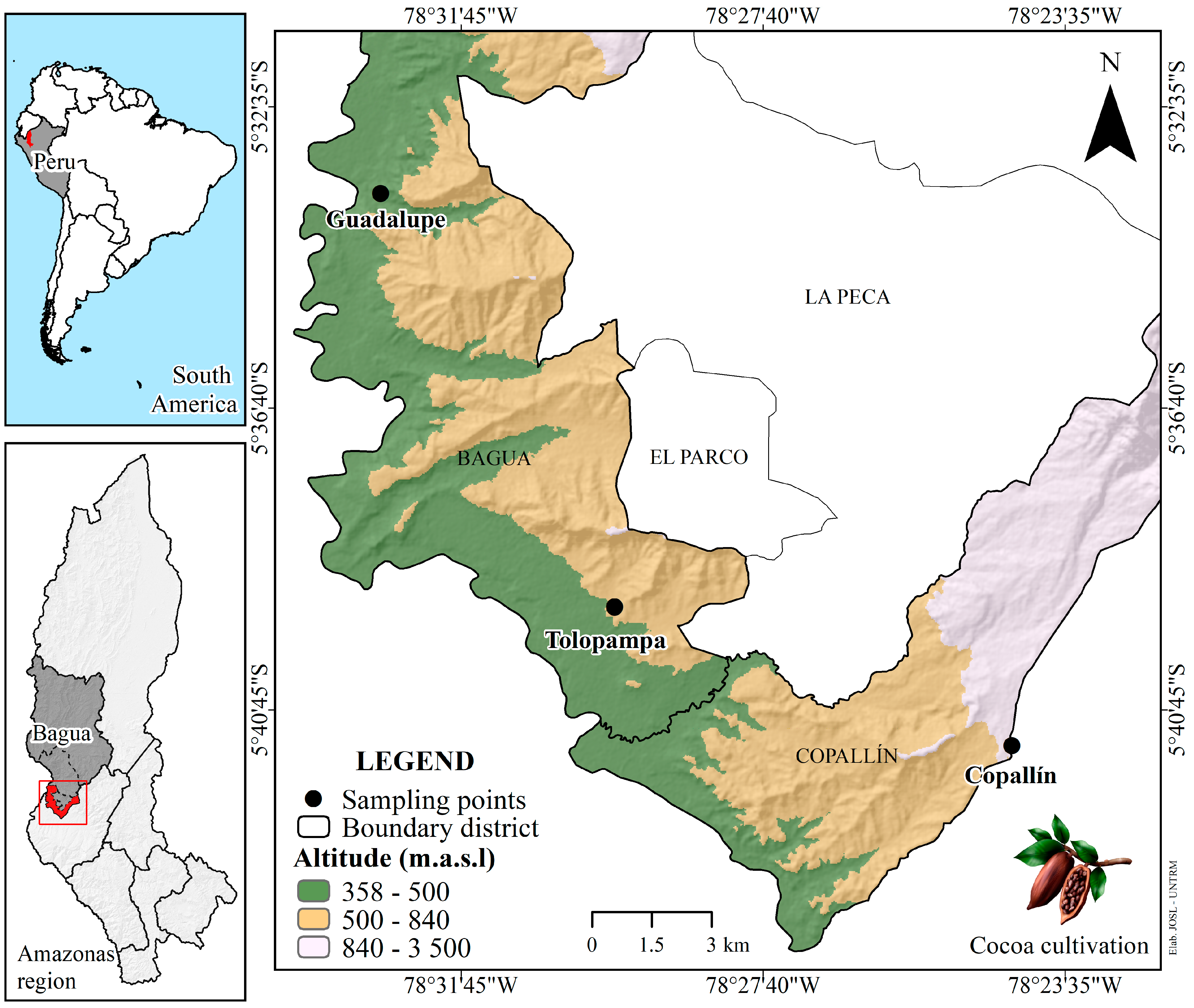
2.3. Criollo Cocoa Fermentation
2.4. Fermentation Monitoring (SF and SC) and Sampling
2.5. Physicochemical Parameters of Fermented Native Cocoa Beans
2.5.1. Water Activity and Moisture of Cocoa Beans
2.5.2. Titratable Acidity and pH of Cocoa Beans
2.5.3. Fermentation Index (FI)
2.6. Cocoa Freeze Drying and Defatting
2.7. Antioxidant Profile of Native Cocoa during Fermentation
2.7.1. Total Polyphenol Content
2.7.2. Total Anthocyanin Content
2.7.3. Antioxidant Activity
- DPPH method: The method described by Gültekin-Özgüven et al. [1] was used based on the reduction capacity of the DPPH radical (2,2-Diphenyl-1-Picrylhydrazyl); for this purpose, cocoa extract (100 μL) and DPPH solution (3.9 mL) were used. The absorbances were measured in a UV-Vis spectrophotometer (SECOMAM, Uv Line 9400, Alès, France) at an absorbance of 517 nm. A standard curve was performed using Trolox (y = −0.0004x + 0.8502, R2 = 0.9993) to determine the antioxidant activity. Finally, the results were expressed in µmol TE/g sample.
- ABTS method: This method is based on ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) free radical scavenging activity, following Floegel et al. [32] and Godočiková et al. [33]. The radical decolorization assay was performed by measuring the absorbance at 734 nm using a UV-Vis spectrophotometer (SECOMAM, Uv Line 9400, Alès, France). A standard curve was performed using Trolox (y = −0.0004x + 0.8502, R2 = 0.9993) to determine the antioxidant activity. Finally, the results were expressed in µmol TE/g sample.
- FRAP method: According to Mihai et al. [31], it is a colorimetric method linked to ferric ion reduction (Fe3+-TPTZ). The FRAP reagent was mixed with acetate buffer (pH 3.6), TPTZ (0.1 M) diluted in hydrochloric acid (0.4 M), and ferric chloride hexahydrate (0.2 M). The mixture was pipetted; then, the extract (90 µL) and distilled water (260 μL) were added; next, the sample was taken to a water bath (Indumelab, BM-10, Lima, Peru) at 30 °C for 4 min, and finally, reading at 593 in a UV-Vis Vis spectrophotometer (EMCLAB, EMC-11-UV, Duisburg, Germany). A standard curve was performed to determine the antioxidant activity (y = 0.0006x + 0.0853, R2 = 0.9949). The results were expressed as μmol Fe2+/100 g sample.
2.7.4. Quantification of Catechin, Epicatechin, Caffeine, and Theobromine
2.8. Aromatic Profile in Native Cocoa Beans during Fermentation
2.8.1. Extraction of Volatile Compounds
2.8.2. Gas Chromatograph Conditions
2.8.3. Identification of Volatile Compounds
2.8.4. Quantification of Volatile Compounds
2.9. Data Analysis
3. Results and Discussion
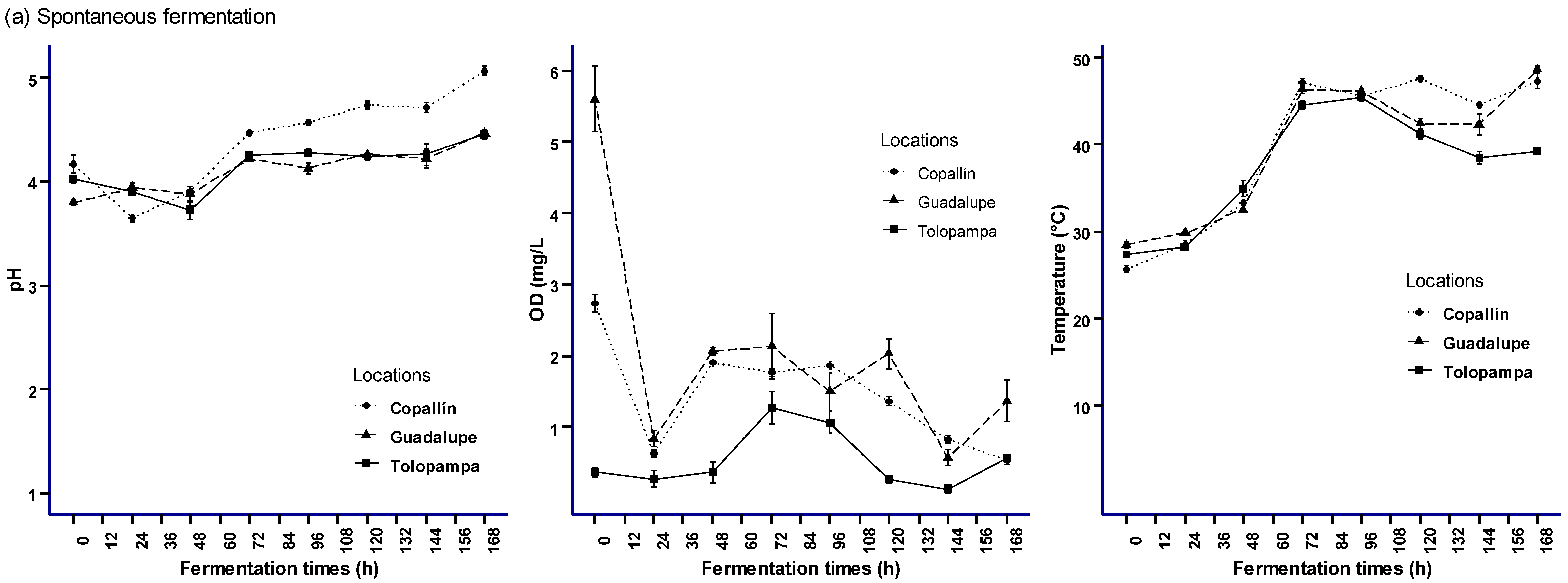
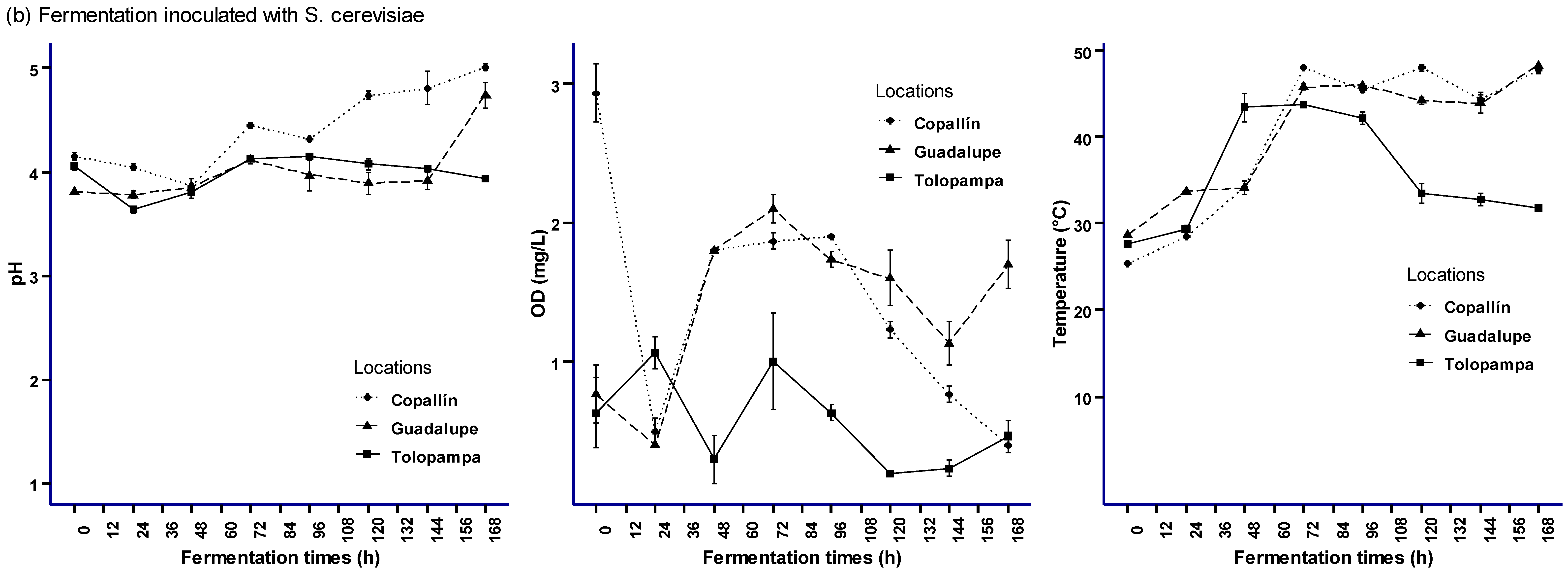
3.1. Fermentation Processes Monitoring (SF and SC) in Cocoa Pulp-Bean Mass
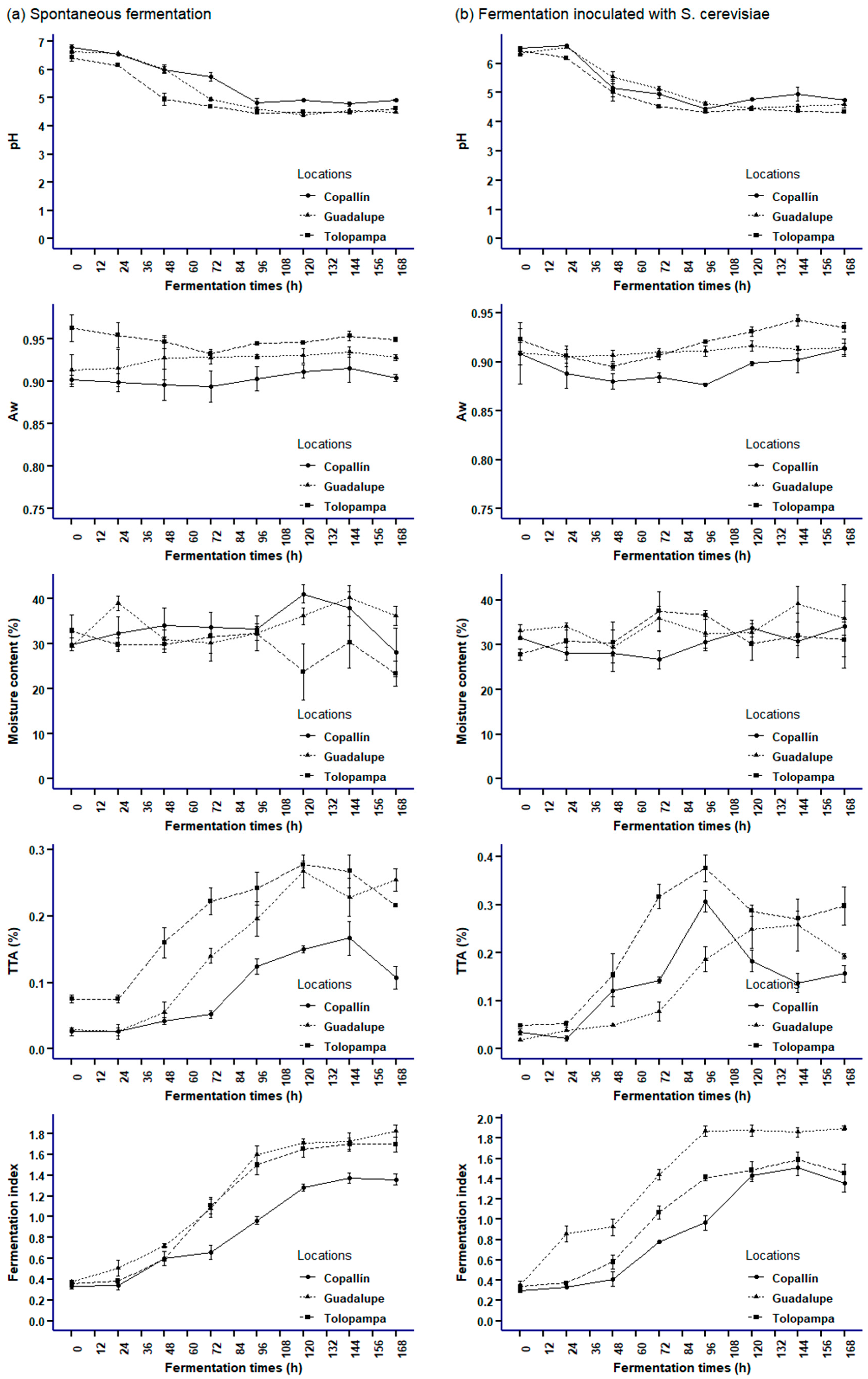
3.2. Physicochemical Parameters in Cocoa Beans during SF and SC
3.3. Antioxidant Profile during Fermentation (SF and SC) in Cocoa Beans
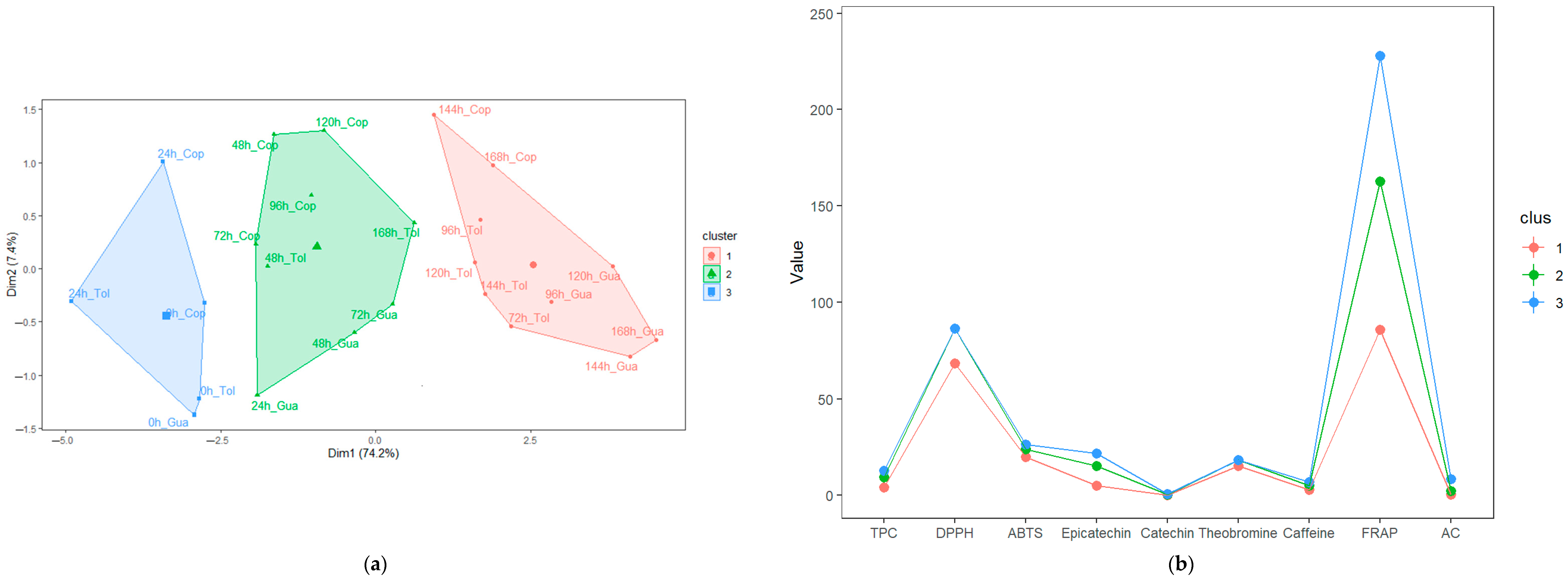
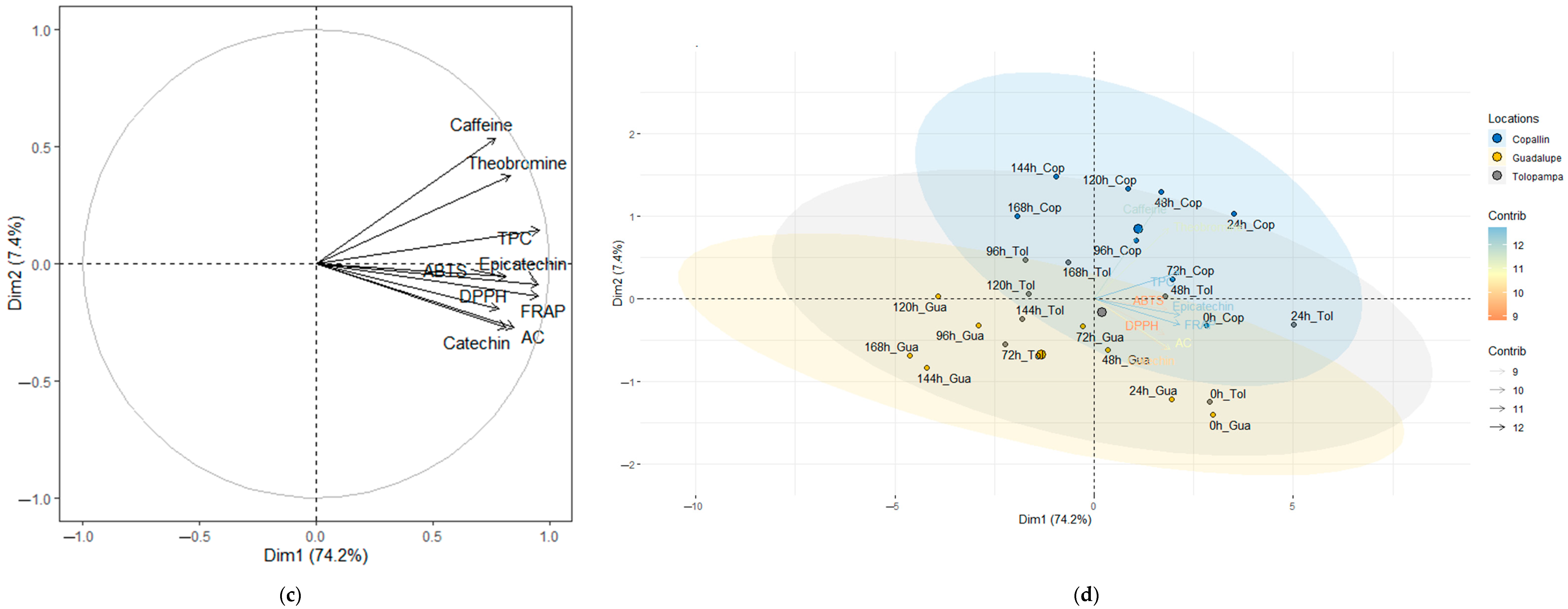
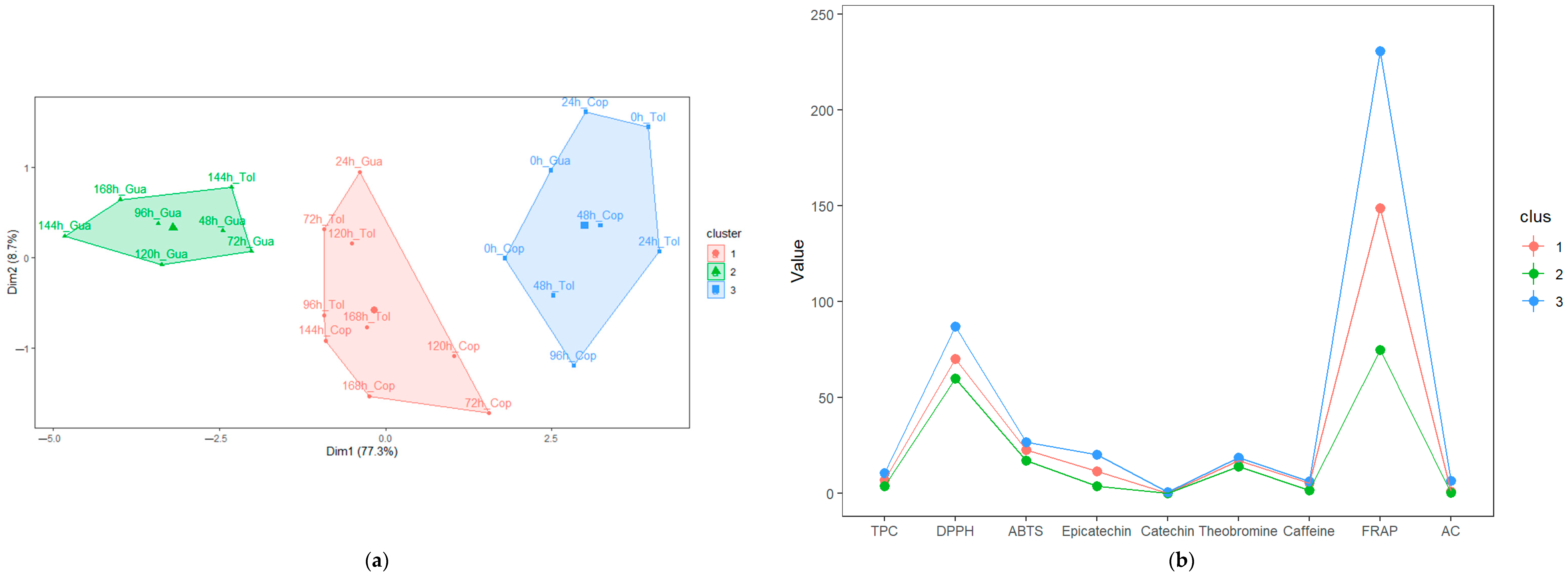
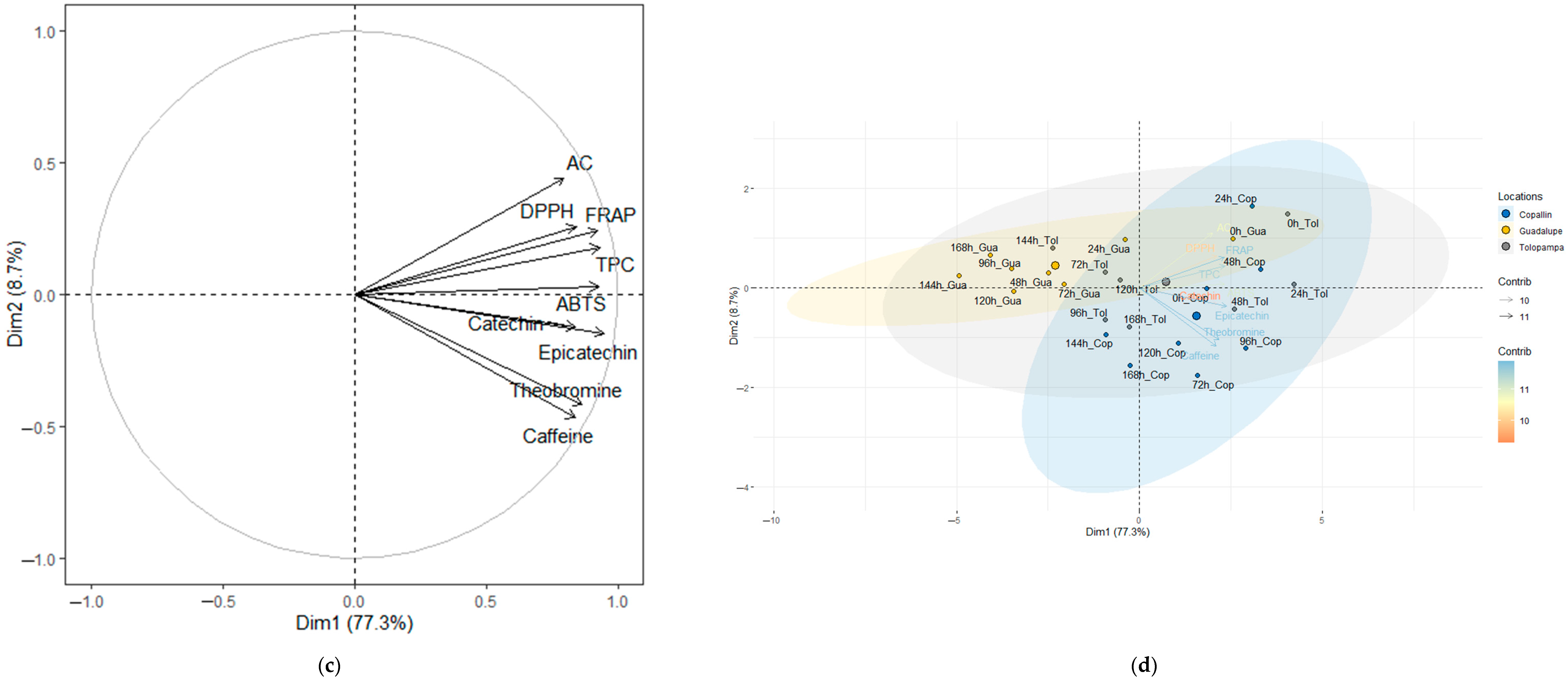
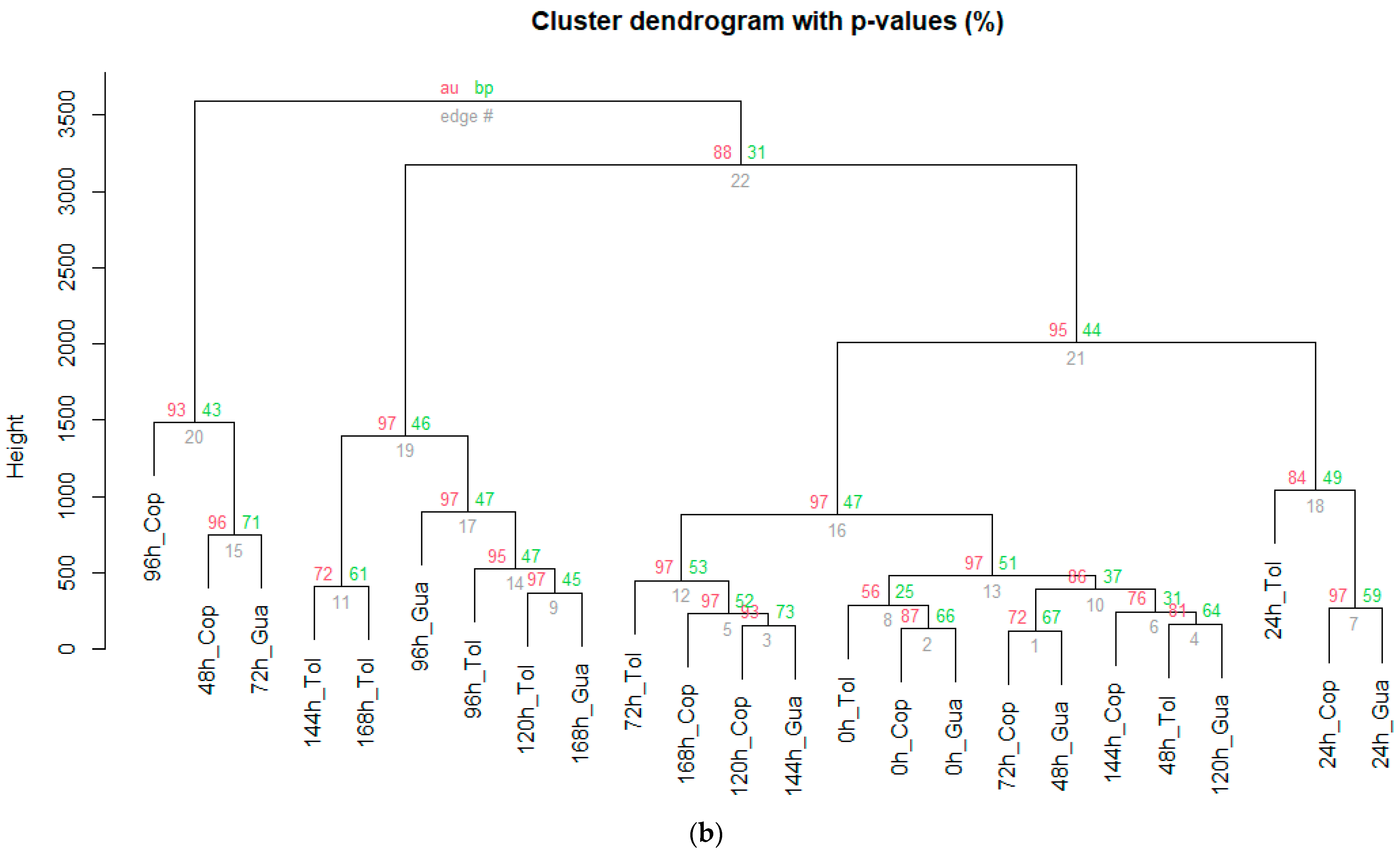
3.4. Characterization of the Stages during Fermentation (SF and SC) of Cocoa Beans
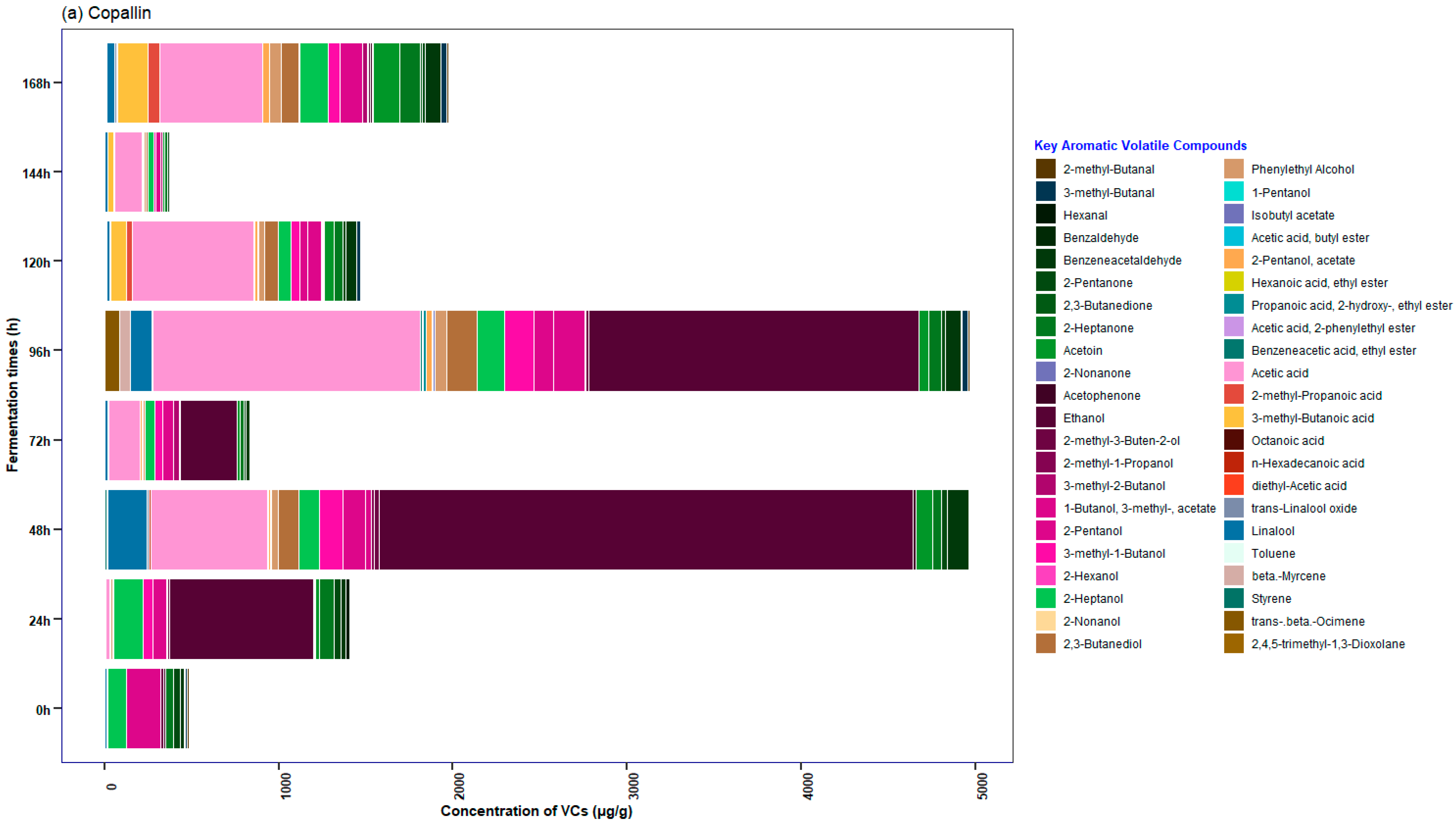
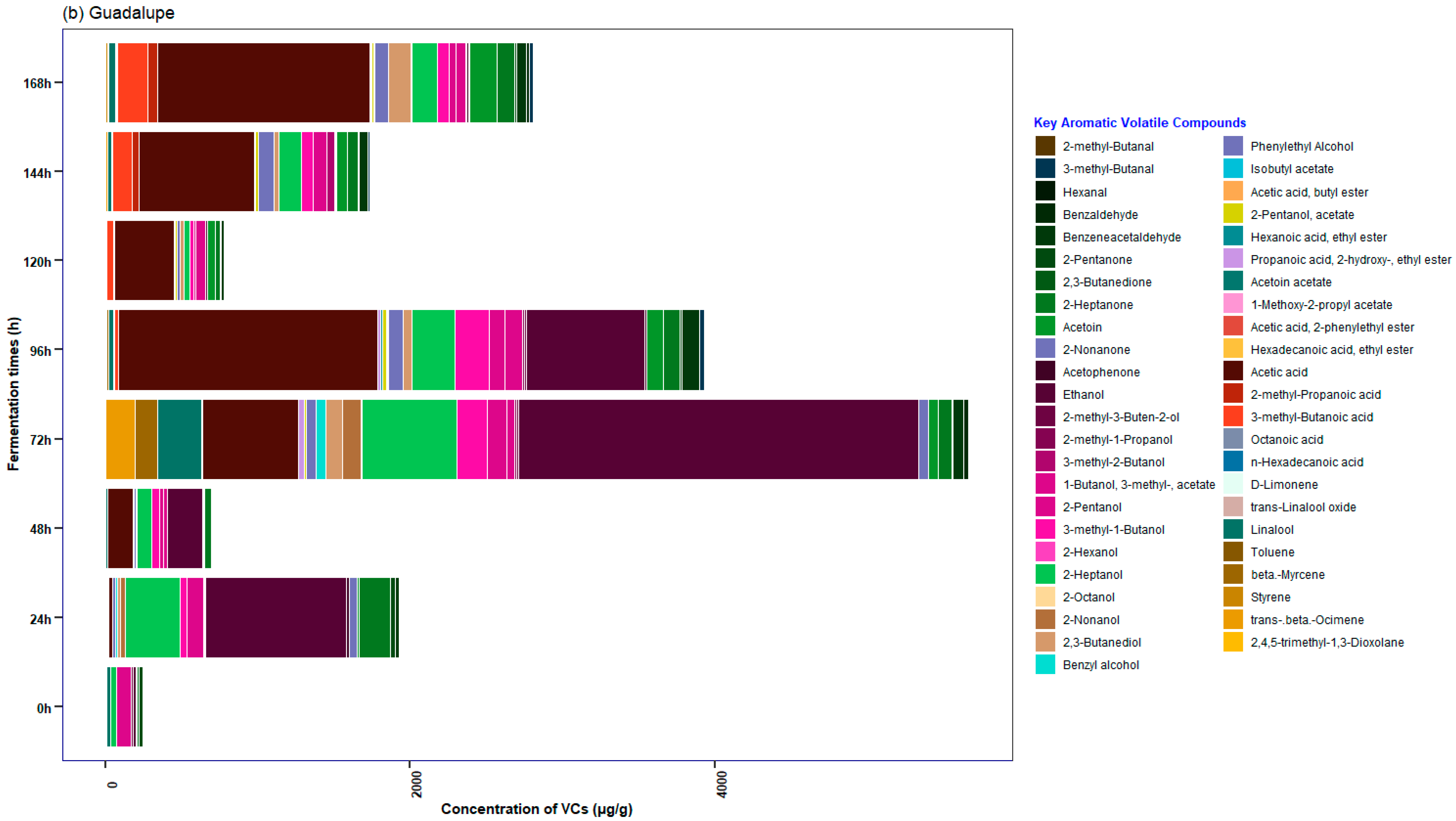
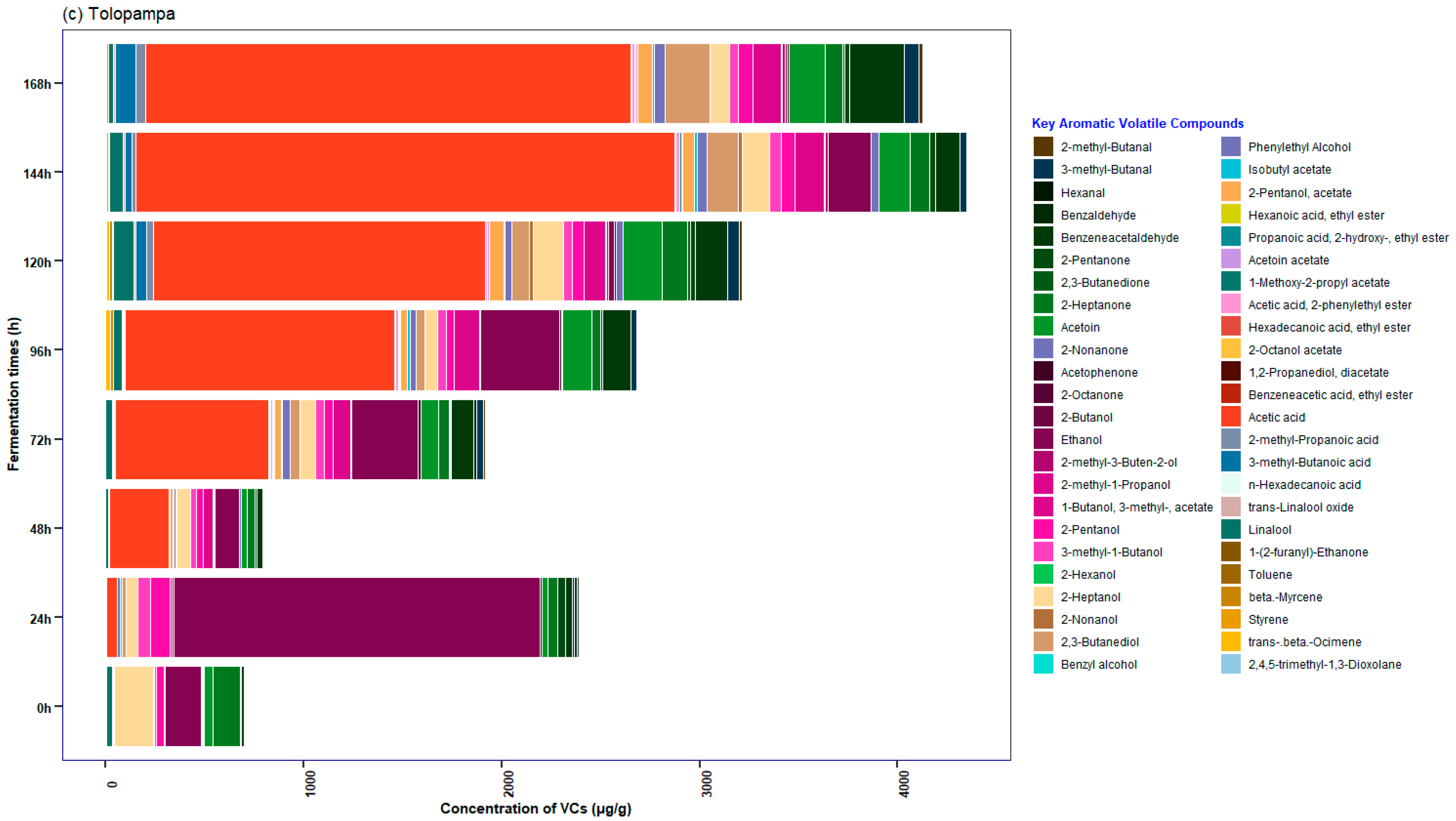
3.5. Characterization of the Profile of Volatile Compounds (VCs) Monitored during Fermentation in Cocoa Beans
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gültekin-Özgüven, M.; Berktaş, İ.; Özçelik, B. Influence of Processing Conditions on Procyanidin Profiles and Antioxidant Capacity of Chocolates: Optimization of Dark Chocolate Manufacturing by Response Surface Methodology. LWT Food Sci. Technol. 2016, 66, 252–259. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Dolatowska-Żebrowska, K.; Wirkowska-Wojdyła, M.; Bryś, J.; Górska, A. Comparison of Thermal Characteristics and Fatty Acids Composition in Raw and Roasted Cocoa Beans from Peru (Criollo) and Ecuador (Forastero). Appl. Sci. 2021, 11, 2698. [Google Scholar] [CrossRef]
- Castro-Alayo, E.M.; Idrogo-Vásquez, G.; Siche, R.; Cardenas-Toro, F.P. Formation of Aromatic Compounds Precursors during Fermentation of Criollo and Forastero Cocoa. Heliyon 2019, 5, e01157. [Google Scholar] [CrossRef]
- Do Carmo, B.d.N.; Campos, R.; da Silva, R.; Abreu, M.B.; Santos, A. Bioactive Amines and Phenolic Compounds in Cocoa Beans Are Affected by Fermentation. Food Chem. 2017, 228, 484–490. [Google Scholar] [CrossRef]
- Hue, C.; Gunata, Z.; Breysse, A.; Davrieux, F.; Boulanger, R.; Sauvage, F.X. Impact of Fermentation on Nitrogenous Compounds of Cocoa Beans (Theobroma cacao L.) from Various Origins. Food Chem. 2016, 192, 958–964. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Functional Role of Yeasts, Lactic Acid Bacteria and Acetic Acid Bacteria in Cocoa Fermentation Processes. FEMS Microbiol. Rev. 2020, 44, 432–453. [Google Scholar] [CrossRef]
- Utrilla-Vázquez, M.; Rodríguez-Campos, J.; Avendaño-Arazate, C.H.; Gschaedler, A.; Lugo-Cervantes, E. Analysis of Volatile Compounds of Five Varieties of Maya Cocoa during Fermentation and Drying Processes by Venn Diagram and PCA. Food Res. Int. 2020, 129, 108834. [Google Scholar] [CrossRef]
- Deus, V.L.; Bispo, E.S.; Franca, A.S.; Gloria, M.B.A. Understanding Amino Acids and Bioactive Amines Changes during On-Farm Cocoa Fermentation. J. Food Compos. Anal. 2021, 97, 103776. [Google Scholar] [CrossRef]
- Mota-Gutierrez, J.; Ferrocino, I.; Giordano, M.; Suarez-Quiroz, M.L.; Gonzalez-Ríos, O.; Cocolin, L. Influence of Taxonomic and Functional Content of Microbial Communities on the Quality of Fermented Cocoa Pulp-Bean Mass. Appl. Environ. Microbiol. 2021, 87, e00425-21. [Google Scholar] [CrossRef]
- Balcázar-Zumaeta, C.R.; Castro-Alayo, E.M.; Cayo-Colca, I.S.; Idrogo-Vásquez, G.; Muñoz-Astecker, L.D. Metabolomics during the Spontaneous Fermentation in Cocoa (Theobroma cacao L.): An Exploraty Review. Food Res. Int. 2023, 163, 112190. [Google Scholar] [CrossRef]
- John, W.A.; Böttcher, N.L.; Behrends, B.; Corno, M.; D’souza, R.N.; Kuhnert, N.; Ullrich, M.S. Experimentally Modelling Cocoa Bean Fermentation Reveals Key Factors and Their Influences. Food Chem. 2020, 302, 125335. [Google Scholar] [CrossRef]
- Rottiers, H.; Sosa, D.A.T.; De Winne, A.; Ruales, J.; De Clippeleer, J.; De Leersnyder, I.; De Wever, J.; Everaert, H.; Messens, K.; Dewettinck, K. Dynamics of Volatile Compounds and Flavor Precursors during Spontaneous Fermentation of Fine Flavor Trinitario Cocoa Beans. Eur. Food Res. Technol. 2019, 245, 1917–1937. [Google Scholar] [CrossRef]
- Escobar, S.; Santander, M.; Zuluaga, M.; Chacón, I.; Rodríguez, J.; Vaillant, F. Fine Cocoa Beans Production: Tracking Aroma Precursors through a Comprehensive Analysis of Flavor Attributes Formation. Food Chem. 2021, 365, 130627. [Google Scholar] [CrossRef]
- Servent, A.; Boulanger, R.; Davrieux, F.; Pinot, M.-N.; Tardan, E.; Forestier-Chiron, N.; Hue, C. Assessment of Cocoa (Theobroma cacao L.) Butter Content and Composition throughout Fermentations. Food Res. Int. 2018, 107, 675–682. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Zhu, F. Changes in the Composition of Methylxanthines, Polyphenols, and Volatiles and Sensory Profiles of Cocoa Beans from the Sul 1 Genotype Affected by Fermentation. J. Agric. Food Chem. 2020, 68, 8658–8675. [Google Scholar] [CrossRef]
- Agyirifo, D.S.; Wamalwa, M.; Otwe, E.P.; Galyuon, I.; Runo, S.; Takrama, J.; Ngeranwa, J. Metagenomics Analysis of Cocoa Bean Fermentation Microbiome Identifying Species Diversity and Putative Functional Capabilities. Heliyon 2019, 5, e02170. [Google Scholar] [CrossRef]
- Viesser, J.A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Rogez, H.; Góes-Neto, A.; Azevedo, V.; Brenig, B.; Aburjaile, F.; Soccol, C.R. Co-Culturing Fructophilic Lactic Acid Bacteria and Yeast Enhanced Sugar Metabolism and Aroma Formation during Cocoa Beans Fermentation. Int. J. Food Microbiol. 2021, 339, 109015. [Google Scholar] [CrossRef]
- Britto, A.; Lins, M.; de Souza, F.A.; Soares, S.E.; Druzian, J.I.; Radomille, L.R.; Oliveira, C.; da Silva, E. Influence of Under-Fermented Cocoa Mass in Chocolate Production: Sensory Acceptance and Volatile Profile Characterization during the Processing. LWT 2021, 149, 112048. [Google Scholar] [CrossRef]
- Lima, C.O.D.C.; Vaz, A.B.M.; De Castro, G.M.; Lobo, F.; Solar, R.; Rodrigues, C.; Pinto, L.R.M.; Vandenberghe, L.; Pereira, G.; da Costa, A.M.; et al. Integrating Microbial Metagenomics and Physicochemical Parameters and a New Perspective on Starter Culture for Fine Cocoa Fermentation. Food Microbiol. 2021, 93, 103608. [Google Scholar] [CrossRef]
- Chagas, G.C.A.; Ferreira, N.R.; Gloria, M.B.A.; Martins, L.H.d.S.; Lopes, A.S. Chemical Implications and Time Reduction of On-Farm Cocoa Fermentation by Saccharomyces cerevisiae and Pichia kudriavzevii. Food Chem. 2021, 338, 127834. [Google Scholar] [CrossRef]
- Díaz-Muñoz, C.; Van de Voorde, D.; Comasio, A.; Verce, M.; Hernandez, C.E.; Weckx, S.; De Vuyst, L. Curing of Cocoa Beans: Fine-Scale Monitoring of the Starter Cultures Applied and Metabolomics of the Fermentation and Drying Steps. Front. Microbiol. 2021, 11, 616875. [Google Scholar] [CrossRef]
- Assi-Clair, B.J.; Koné, M.K.; Kouamé, K.; Lahon, M.C.; Berthiot, L.; Durand, N.; Lebrun, M.; Julien-Ortiz, A.; Maraval, I.; Boulanger, R.; et al. Effect of Aroma Potential of Saccharomyces cerevisiae Fermentation on the Volatile Profile of Raw Cocoa and Sensory Attributes of Chocolate Produced Thereof. Eur. Food Res. Technol. 2019, 245, 1459–1471. [Google Scholar] [CrossRef]
- Castro-Alayo, E.M.; Torrejón-Valqui, L.; Medina-Mendoza, M.; Cayo-Colca, I.S.; Cárdenas-Toro, F.P. Kinetics Crystallization and Polymorphism of Cocoa Butter throughout the Spontaneous Fermentation Process. Foods 2022, 11, 1769. [Google Scholar] [CrossRef]
- Koné, K.M.; Assi-Clair, B.J.; Kouassi, A.D.D.; Yao, A.K.; Ban-Koffi, L.; Durand, N.; Lebrun, M.; Maraval, I.; Bonlanger, R.; Guehi, T.S. Pod Storage Time and Spontaneous Fermentation Treatments and Their Impact on the Generation of Cocoa Flavour Precursor Compounds. Int. J. Food Sci. Technol. 2020, 56, 2516–2529. [Google Scholar] [CrossRef]
- Velásquez-Reyes, D.; Rodríguez-Campos, J.; Avendaño-Arrazate, C.; Gschaedler, A.; Alcázar-Valle, M.; Lugo-Cervantes, E. Forastero and Criollo Cocoa Beans, Differences on the Profile of Volatile and Non-Volatile Compounds in the Process from Fermentation to Liquor. Heliyon 2023, 9, e15129. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis. Available online: https://www.aoac.org/ (accessed on 8 June 2023).
- Hinneh, M.; Semanhyia, E.; Van de Walle, D.; De Winne, A.; Tzompa-Sosa, D.A.; Scalone, G.L.L.; De Meulenaer, B.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; et al. Assessing the Influence of Pod Storage on Sugar and Free Amino Acid Profiles and the Implications on Some Maillard Reaction Related Flavor Volatiles in Forastero Cocoa Beans. Food Res. Int. 2018, 111, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, C.; Viera-Alcaide, I.; Morales-Sillero, A.M.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Bioactive Compounds in Mexican Genotypes of Cocoa Cotyledon and Husk. Food Chem. 2018, 240, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Melo, T.S.; Pires, T.C.; Engelmann, J.V.P.; Monteiro, A.L.O.; Maciel, L.F.; Bispo, E.d.S. Evaluation of the Content of Bioactive Compounds in Cocoa Beans during the Fermentation Process. J. Food Sci. Technol. 2020, 58, 1947–1957. [Google Scholar] [CrossRef]
- Balcázar-Zumaeta, C.R.; Castro-Alayo, E.M.; Medina-Mendoza, M.; Muñoz-Astecker, L.D.; Torrejón-Valqui, L.; Rodriguez-Perez, R.J.; Rojas-Ocampo, E.; Cayo-Colca, I.S. Physical and Chemical Properties of 70% Cocoa Dark Chocolate Mixed with Freeze-Dried Arazá (Eugenia stipitata) Pulp. Prev. Nutr. Food Sci. 2022, 27, 474–482. [Google Scholar] [CrossRef]
- Mihai, R.A.; Abarca, P.A.L.; Romero, B.A.T.; Florescu, L.I.; Catană, R.; Kosakyan, A. Abiotic Factors from Different Ecuadorian Regions and Their Contribution to Antioxidant, Metabolomic and Organoleptic Quality of Theobroma cacao L. Beans, Variety “Arriba Nacional”. Plants 2022, 11, 976. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Godočiková, L.; Ivanišová, E.; Zaguła, G.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A.; Kowalczewski, P.Ł.; Kačániová, M. Antioxidant Activities and Volatile Flavor Components of Selected Single-Origin and Blend Chocolates. Molecules 2020, 25, 3648. [Google Scholar] [CrossRef] [PubMed]
- Valle-Epquín, M.G.; Balcázar-Zumaeta, C.R.; Auquiñivín-Silva, E.A.; Fernández-Jeri, A.B.; Idrogo-Vásquez, G.; Castro-Alayo, E.M. The Roasting Process and Place of Cultivation Influence the Volatile Fingerprint of Criollo Cocoa from Amazonas, Peru. Sci. Agropecu. 2020, 11, 599–610. [Google Scholar] [CrossRef]
- Alvarez-Villagomez, K.G.; Ledesma-Escobar, C.A.; Priego-Capote, F.; Robles-Olvera, V.J.; García-Alamilla, P. Influence of the Starter Culture on the Volatile Profile of Processed Cocoa Beans by Gas Chromatography–Mass Spectrometry in High Resolution Mode. Food Biosci. 2022, 47, 101669. [Google Scholar] [CrossRef]
- Ibitoye, F.O.; Osaloni, A.R.; Adebote, V.T. Effect of Fermentation on Bacteria Isolates and Phytochemical Properties of Cocoa (Theobroma cacao) Beans. South Asian J. Res. Microbiol. 2020, 7, 1–8. [Google Scholar] [CrossRef]
- Racine, K.C.; Lee, A.H.; Wiersema, B.D.; Huang, H.; Lambert, J.D.; Stewart, A.C.; Neilson, A.P. Development and Characterization of a Pilot-Scale Model Cocoa Fermentation System Suitable for Studying the Impact of Fermentation on Putative Bioactive Compounds and Bioactivity of Cocoa. Foods 2019, 8, 102. [Google Scholar] [CrossRef]
- Sande, D.; Passos, R.; Ferreira, T.; Silva, E.d.L.; Reis, A.C.; de Cerqueira, A.B.; Romano, C.C.; da Cruz, D.W.; Teixeira, J.C.; Dias, J. Fermentation in Fine Cocoa Type Scavina: Change in Standard Quality as the Effect of Use of Starters Yeast in Fermentation. Food Chem. 2020, 328, 127110. [Google Scholar] [CrossRef]
- Calvo, A.M.; Botina, B.L.; García, M.C.; Cardona, W.A.; Montenegro, A.C.; Criollo, J. Dynamics of Cocoa Fermentation and Its Effect on Quality. Sci. Rep. 2021, 11, 16746. [Google Scholar] [CrossRef]
- Portillo, E.; de Farinas, L.G. Análisis Químico Del Cacao Criollo Porcelana (Theobroma cacao L.) En El Sur Del Lago de Maracaibo. Rev. Fac. Agron. 2007, 24, 522–546. [Google Scholar]
- Santander, M.; Rodríguez, J.; Vaillant, F.E.; Escobar, S. An Overview of the Physical and Biochemical Transformation of Cocoa Seeds to Beans and to Chocolate: Flavor Formation. Crit. Rev. Food Sci. Nutr. 2020, 60, 1593–1613. [Google Scholar] [CrossRef]
- Kongor, J.E.; Hinneh, M.; de Walle, D.V.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors Influencing Quality Variation in Cocoa (Theobroma cacao) Bean Flavour Profile—A Review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Sarbu, I.; Csutak, O. The Microbiology of Cocoa Fermentation. In Caffeinated and Cocoa Based Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 423–446. ISBN 978-0-12-815864-7. [Google Scholar]
- Rigling, M.; Heger, F.; Graule, M.; Liu, Z.; Zhang, C.; Ni, L.; Zhang, Y. A Robust Fermentation Process for Natural Chocolate-like Flavor Production with Mycetinis scorodonius. Molecules 2022, 27, 2503. [Google Scholar] [CrossRef]
- Herrera-Rocha, F.; Cala, M.P.; Mejía, J.L.A.; Rodríguez-López, C.M.; Chica, M.J.; Olarte, H.H.; Fernández-Niño, M.; Barrios, A.F.G. Dissecting Fine-Flavor Cocoa Bean Fermentation through Metabolomics Analysis to Break down the Current Metabolic Paradigm. Sci. Rep. 2021, 11, 21904. [Google Scholar] [CrossRef]
- Kouamé, C.; Loiseau, G.; Grabulos, J.; Boulanger, R.; Mestres, C. Development of a Model for the Alcoholic Fermentation of Cocoa Beans by a Saccharomyces cerevisiae Strain. Int. J. Food Microbiol. 2021, 337, 108917. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Weckx, S. The Cocoa Bean Fermentation Process: From Ecosystem Analysis to Starter Culture Development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A.; Oyedeji, A.B.; Adebiyi, J.A.; Chinma, C.E.; Oyeyinka, S.A.; Olatunde, O.O.; Green, E.; Njobeh, P.B.; Kondiah, K. Kinetics of Phenolic Compounds Modification during Maize Flour Fermentation. Molecules 2021, 26, 6702. [Google Scholar] [CrossRef] [PubMed]
- Dewandari, K.T.; Rahmawati, R.; Munarso, S.J. The Effect of Techniques and Fermentation Time on Cocoa Beans Quality (Theobroma cacao L.). IOP Conf. Ser. Earth Environ. Sci. 2021, 653, 012046. [Google Scholar] [CrossRef]
- Horta, H.B.; García, M.C.; Ceron, I.X.; Sandoval, A.P. Evaluation of the Fermentation Process and Final Quality of Five Cacao Clones from the Department of Huila, Colombia. Dyna 2019, 86, 233–239. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor Formation and Character in Cocoa and Chocolate: A Critical Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Fowler, M.S.; Fisk, I.D. Hyperspectral Imaging for Non-Destructive Prediction of Fermentation Index, Polyphenol Content and Antioxidant Activity in Single Cocoa Beans. Food Chem. 2018, 258, 343–351. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Zhu, F. Intravariety Diversity of Bioactive Compounds in Trinitario Cocoa Beans with Different Degrees of Fermentation. J. Agric. Food Chem. 2019, 67, 3150–3158. [Google Scholar] [CrossRef]
- Ooi, T.S.; Ting, A.S.Y.; Siow, L.F. Influence of Selected Native Yeast Starter Cultures on the Antioxidant Activities, Fermentation Index and Total Soluble Solids of Malaysia Cocoa Beans: A Simulation Study. LWT 2020, 122, 108977. [Google Scholar] [CrossRef]
- Hernández, M.d.P.L.; Núñez, J.C.; Gómez, M.S.H.; Tovar, M.D.L. Physicochemical and Microbiological Dynamics of the Fermentation of the Ccn51 Cocoa Material in Three Maturity Stages. Rev. Bras. Frutic. 2019, 41, 1–13. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Triboletti, S.; Alessandria, V.; Serio, A.; Sergi, M.; Paparella, A.; Rantsiou, K.; Chaves-López, C. Functional Biodiversity of Yeasts Isolated from Colombian Fermented and Dry Cocoa Beans. Microorganisms 2020, 8, 1086. [Google Scholar] [CrossRef]
- Lima, L.J.R.; Almeida, M.H.; Nout, M.J.R.; Zwietering, M.H. Theobroma cacao L., “The Food of the Gods”: Quality Determinants of Commercial Cocoa Beans, with Particular Reference to the Impact of Fermentation. Crit. Rev. Food Sci. Nutr. 2011, 51, 731–761. [Google Scholar] [CrossRef]
- Ramos, C.L.; Dias, D.R.; Miguel, M.G.D.C.P.; Schwan, R.F. Impact of Different Cocoa Hybrids (Theobroma cacao L.) and S. cerevisiae UFLA CA11 Inoculation on Microbial Communities and Volatile Compounds of Cocoa Fermentation. Food Res. Int. 2014, 64, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Visintin, S.; Ramos, L.; Batista, N.; Dolci, P.; Schwan, F.; Cocolin, L. Impact of Saccharomyces cerevisiae and Torulaspora delbrueckii Starter Cultures on Cocoa Beans Fermentation. Int. J. Food Microbiol. 2017, 257, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Alfonzo, A.; Gaglio, R.; Barbera, M.; Francesca, N.; Moschetti, G.; Settanni, L. Evaluation of the Fermentation Dynamics of Commercial Baker’s Yeast in Presence of Pistachio Powder to Produce Lysine-Enriched Breads. Fermentation 2020, 6, 2. [Google Scholar] [CrossRef]
- Reina, C.A.M.; Vivas, J.G.A.; Delgado, L.M. Evaluación físico-química de granos de cacao (Theobroma cacao L.) provenientes de Mosquera (Nariño), durante su proceso de fermentación y secado. In Proceedings of the XXI Encuentro Nacional y XV Internacional de Semilleros de Investigación, San Juan de Pasto, Colombia, 11–14 October 2018. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. Antioxidant Activity, Total Polyphenol, Flavonoid and Tannin Contents of Fermented Green Coffee Beans with Selected Yeasts. Fermentation 2019, 5, 29. [Google Scholar] [CrossRef]
- Hernández, C.-H.; López-Andrade, P.A.; Ramírez-Guillermo, M.A.; Ramírez, D.G.; Pérez, J.F.C. Evaluation of Different Fermentation Processes for Use by Small Cocoa Growers in Mexico. Food Sci. Nutr. 2016, 4, 690–695. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Miller, K.B.; Rank, C.; Stuart, D.A. Impact of Fermentation, Drying, Roasting, and Dutch Processing on Epicatechin and Catechin Content of Cacao Beans and Cocoa Ingredients. J. Agric. Food Chem. 2010, 58, 10518–10527. [Google Scholar] [CrossRef] [PubMed]
- Schinella, G.; Mosca, S.; Cienfuegos-Jovellanos, E.; Pasamar, M.Á.; Muguerza, B.; Ramón, D.; Ríos, J.L. Antioxidant Properties of Polyphenol-Rich Cocoa Products Industrially Processed. Food Res. Int. 2010, 43, 1614–1623. [Google Scholar] [CrossRef]
- Kishore, G.; Ranjan, S.; Pandey, A.; Gupta, S. Influence of Altitudinal Variation on the Antioxidant Potential of Tartar Buckwheat of Western Himalaya. Food Sci. Biotechnol. 2010, 19, 1355–1363. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Quao, J.; Takrama, F.S.; Budu, A.S.; Saalia, F.K. Changes in Total Polyphenols, o-Diphenols and Anthocyanin Concentrations during Fermentation of Pulp Pre-Conditioned Cocoa (Theobroma cacao) Beans. Int. Food Res. J. 2012, 19, 1071–1077. [Google Scholar]
- Di Mattia, C.D.; Sacchetti, G.; Mastrocola, D.; Serafini, M. From Cocoa to Chocolate: The Impact of Processing on In Vitro Antioxidant Activity and the Effects of Chocolate on Antioxidant Markers In Vivo. Front. Immunol. 2017, 8, 1207. [Google Scholar] [CrossRef]
- Vázquez-Ovando, A.; Ovando-Medina, I.; Adriano-Anaya, L.; Betancur-Ancona, D.; Salvador-Figueroa, M. Alcaloides y polifenoles del cacao, mecanismos que regulan su biosíntesis y sus implicaciones en el sabor y aroma. Arch. Latinoam. Nutr. 2016, 66, 239–254. [Google Scholar]
- Harrington, W.L. The Effects of Roasting Time and Temperature on the Antioxidant Capacity of Cocoa Beans from Dominican Republic, Ecuador, Haiti, Indonesia, and Ivory Coast. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2011. [Google Scholar]
- Pallares, A.P.; Estupiñán, M.R.; Villamil, J.A.P.; Giraldo, L.J.L. Impacto de la fermentación y secado sobre el contenido de polifenoles y capacidad antioxidante del clon de cacao CCN-51. Rev. Ion 2017, 29, 7–21. [Google Scholar] [CrossRef]
- Romanens, E.; Pedan, V.; Meile, L.; Schwenninger, S.M. Influence of Two Anti-Fungal Lactobacillus Fermentum-Saccharomyces cerevisiae Co-Cultures on Cocoa Bean Fermentation and Final Bean Quality. PLoS ONE 2020, 15, e0239365. [Google Scholar] [CrossRef]
- Gutiérrez-Ríos, H.G.; Suárez-Quiroz, M.L.; Hernández-Estrada, Z.J.; Castellanos-Onorio, O.P.; Alonso-Villegas, R.; Rayas-Duarte, P.; Cano-Sarmiento, C.; Figueroa-Hernández, C.Y.; González-Rios, O. Yeasts as Producers of Flavor Precursors during Cocoa Bean Fermentation and Their Relevance as Starter Cultures: A Review. Fermentation 2022, 8, 331. [Google Scholar] [CrossRef]
- Suazo, Y.; Davidov-Pardo, G.; Arozarena, I. Effect of Fermentation and Roasting on the Phenolic Concentration and Antioxidant Activity of Cocoa from Nicaragua. J. Food Qual. 2014, 37, 50–56. [Google Scholar] [CrossRef]
- Dang, Y.K.T.; Nguyen, H.V.H. Effects of Maturity at Harvest and Fermentation Conditions on Bioactive Compounds of Cocoa Beans. Plant Foods Hum. Nutr. 2019, 74, 54–60. [Google Scholar] [CrossRef]
- Septianti, E.; Salengke; Langkong, J. Profile of Bioactive Compounds, Antioxidant and Aromatic Component from Several Clones of Cocoa Beans during Fermentation. IOP Conf. Ser. Earth Environ. Sci. 2020, 575, 012009. [Google Scholar] [CrossRef]
- Cerri, M.; Reale, L.; Zadra, C. Metabolite Storage in Theobroma cacao L. Seed: Cyto-Histological and Phytochemical Analyses. Front. Plant Sci. 2019, 10, 1599. [Google Scholar] [CrossRef]
- Samaniego, I.; Espín, S.; Quiroz, J.; Ortiz, B.; Carrillo, W.; García-Viguera, C.; Mena, P. Effect of the Growing Area on the Methylxanthines and Flavan-3-Ols Content in Cocoa Beans from Ecuador. J. Food Compos. Anal. 2020, 88, 103448. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products—An Overview: Flavor Chemistry of Cocoa. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Wang, S.; Zhu, F. Chemical and Biological Properties of Cocoa Beans Affected by Processing: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8403–8434. [Google Scholar] [CrossRef]
- Quelal-Vásconez, M.A.; Lerma-García, M.J.; Pérez-Esteve, É.; Arnau-Bonachera, A.; Barat, J.M.; Talens, P. Changes in Methylxanthines and Flavanols during Cocoa Powder Processing and Their Quantification by Near-Infrared Spectroscopy. LWT 2020, 117, 108598. [Google Scholar] [CrossRef]
- Bustamante, S.Z.; Tenorio, A.T.; Rojano, B.A. Efecto de la fermentación sobre la actividad antioxidante de diferentes clones de cacao colombiano. Rev. Cuba. Plantas Med. 2013, 18, 391–404. [Google Scholar]
- Gutiérrez, T.J. State-of-the-Art Chocolate Manufacture: A Review: State-of-the-Art Chocolate Manufactur. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1313–1344. [Google Scholar] [CrossRef]
- Brunetto, M.D.R.; Gutiérrez, L.; Delgado, Y.; Gallignani, M.; Zambrano, A.; Gómez, Á.; Ramos, G.; Romero, C. Determination of Theobromine, Theophylline and Caffeine in Cocoa Samples by a High-Performance Liquid Chromatographic Method with on-Line Sample Cleanup in a Switching-Column System. Food Chem. 2007, 100, 459–467. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Zhu, F. Composition of Methylxanthines, Polyphenols, Key Odorant Volatiles and Minerals in 22 Cocoa Beans Obtained from Different Geographic Origins. LWT Food Sci. Technol. 2022, 153, 112395. [Google Scholar] [CrossRef]
- Gomes, P.C.; Bezerra, V.; Santos, A.; de Souza, J.P.I.; Souza, J.R.; Chagas, G.C.A.; Barbosa, P.S. Determination of Theobromine and Caffeine in Fermented and Unfermented Amazonian Cocoa (Theobroma cacao L.) Beans Using Square Wave Voltammetry after Chromatographic Separation. Food Control 2020, 108, 106887. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Casimiro-Gonzales, S.; Fernández-Prior, Á.; Chávez, K.C.; Gómez-Mendoza, J.; de la Fuente-Carmelino, L.; Muñoz, A.M. Colour, Fatty Acids, Bioactive Compounds, and Total Antioxidant Capacity in Commercial Cocoa Beans (Theobroma cacao L.). LWT 2021, 147, 111629. [Google Scholar] [CrossRef]
- Cheng, H.; Wei, K.; Wang, L. The Impact of Variety, Environment and Agricultural Practices on Catechins and Caffeine in Plucked Tea Leaves. In Processing and Impact on Active Components in Food; Elsevier: Amsterdam, The Netherlands, 2015; pp. 597–603. ISBN 978-0-12-404699-3. [Google Scholar]
- Kuhnert, N.; D’souza, R.N.; Behrends, B.; Ullrich, M.S.; Witt, M. Investigating Time Dependent Cocoa Bean Fermentation by ESI-FT-ICR Mass Spectrometry. Food Res. Int. 2020, 133, 109209. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, Y.; Zhu, S.; Luo, C.; Ma, J.; Zhong, F. The Effect of Alkalization on the Bioactive and Flavor Related Components in Commercial Cocoa Powder. J. Food Compos. Anal. 2012, 25, 17–23. [Google Scholar] [CrossRef]
- Bastos, V.S.; Uekane, T.M.; Bello, N.A.; de Rezende, C.M.; Flosi, V.M.; Del Aguila, E.M. Dynamics of Volatile Compounds in TSH 565 Cocoa Clone Fermentation and Their Role on Chocolate Flavor in Southeast Brazil. J. Food Sci. Technol. 2019, 56, 2874–2887. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Gysel, L.; Maridueña-Zavala, M.G.; Molina-Miranda, M.J. Time-Related Changes in Volatile Compounds during Fermentation of Bulk and Fine-Flavor Cocoa (Theobroma cacao) Beans. J. Food Qual. 2018, 2018, 1758381. [Google Scholar] [CrossRef]
- Chen, Q.-C.; Xu, Y.-X.; Wu, P.; Xu, X.-Y.; Pan, S.-Y. Aroma Impact Compounds in Liuyang Douchi, a Chinese Traditional Fermented Soya Bean Product: Aroma Impact Compounds in Liuyang Douchi. Int. J. Food Sci. Technol. 2011, 46, 1823–1829. [Google Scholar] [CrossRef]
- Qin, X.-W.; Lai, J.-X.; Tan, L.-H.; Hao, C.-Y.; Li, F.-P.; He, S.-Z.; Song, Y.-H. Characterization of Volatile Compounds in Criollo, Forastero, and Trinitario Cocoa Seeds (Theobroma cacao L.) in China. Int. J. Food Prop. 2017, 20, 2261–2275. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Contreras-Ramos, S.M.; Orozco-Avila, I.; Jaramillo-Flores, E.; Lugo-Cervantes, E. Effect of Fermentation Time and Drying Temperature on Volatile Compounds in Cocoa. Food Chem. 2012, 132, 277–288. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M.E. Dynamics of Volatile and Non-Volatile Compounds in Cocoa (Theobroma cacao L.) during Fermentation and Drying Processes Using Principal Components Analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Lee, A.H.; Neilson, A.P.; O’Keefe, S.F.; Ogejo, J.A.; Huang, H.; Ponder, M.; Chu, H.S.S.; Jin, Q.; Pilot, G.; Stewart, A.C. A Laboratory-Scale Model Cocoa Fermentation Using Dried, Unfermented Beans and Artificial Pulp Can Simulate the Microbial and Chemical Changes of on-Farm Cocoa Fermentation. Eur. Food Res. Technol. 2019, 245, 511–519. [Google Scholar] [CrossRef]
- Calva-Estrada, S.J.; Utrilla-Vázquez, M.; Vallejo-Cardona, A.; Roblero-Pérez, D.B.; Lugo-Cervantes, E. Thermal Properties and Volatile Compounds Profile of Commercial Dark-Chocolates from Different Genotypes of Cocoa Beans (Theobroma cacao L.) from Latin America. Food Res. Int. 2020, 136, 109594. [Google Scholar] [CrossRef]
- Junior, G.C.C.; Ferreira, N.R.; Andrade, E.H.; Nascimento, L.D.; Siqueira, F.C.; Lopes, A.S. Profile of Volatile Compounds of On-Farm Fermented and Dried Cocoa Beans Inoculated with Saccharomyces cerevisiae KY794742 and Pichia kudriavzevii KY794725. Molecules 2021, 26, 344. [Google Scholar] [CrossRef]
- Sandoval-Lozano, C.J.; Caballero-Torres, D.; López-Giraldo, L.J. Screening Wild Yeast Isolated from Cocoa Bean Fermentation Using Volatile Compounds Profile. Molecules 2022, 27, 902. [Google Scholar] [CrossRef] [PubMed]
- Klis, V.; Pühn, E.; Jerschow, J.J.; Fraatz, M.A.; Zorn, H. Fermentation of Cocoa (Theobroma cacao L.) Pulp by Laetiporus persicinus Yields a Novel Beverage with Tropical Aroma. Fermentation 2023, 9, 533. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 7 July 2023).
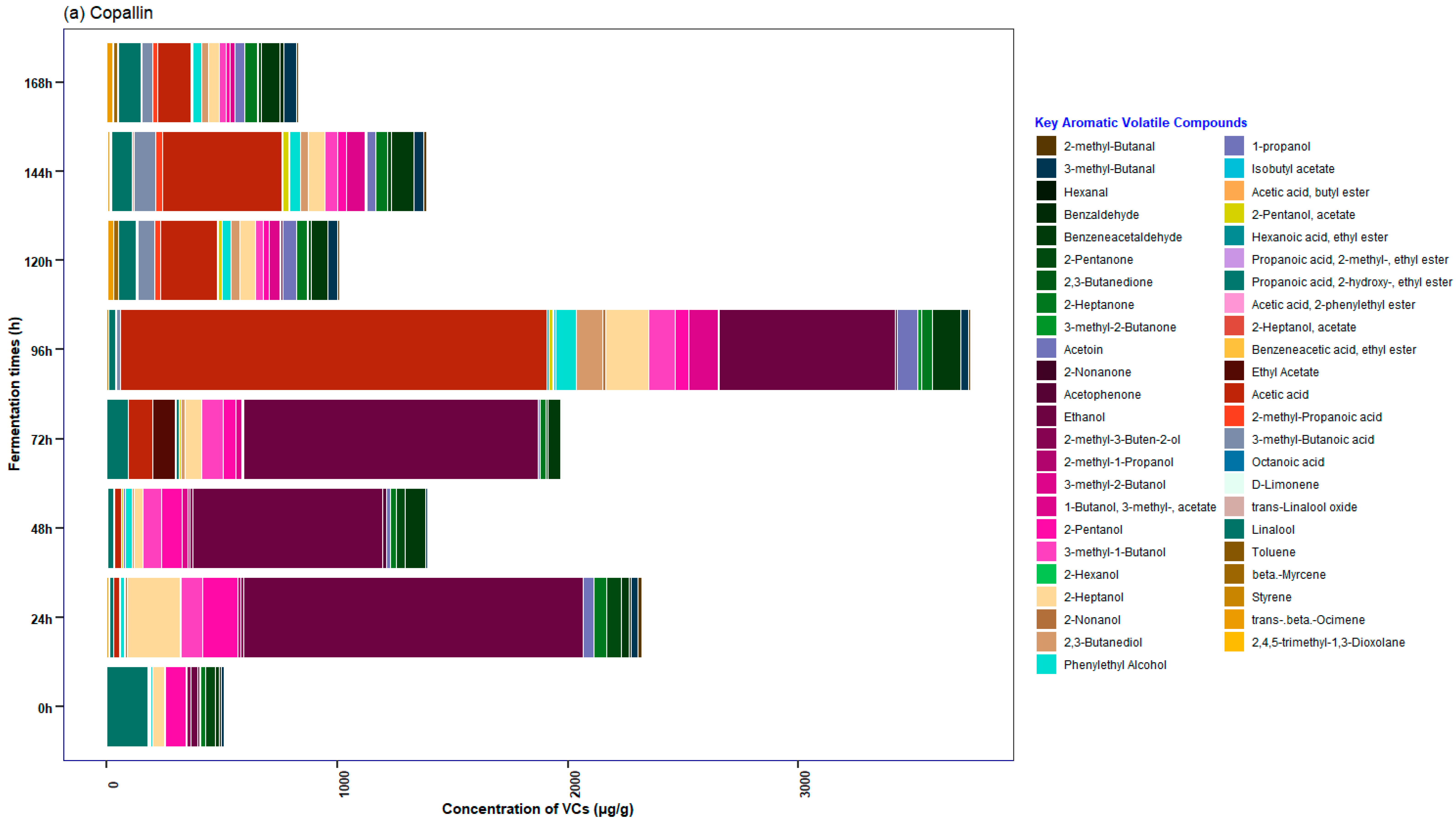
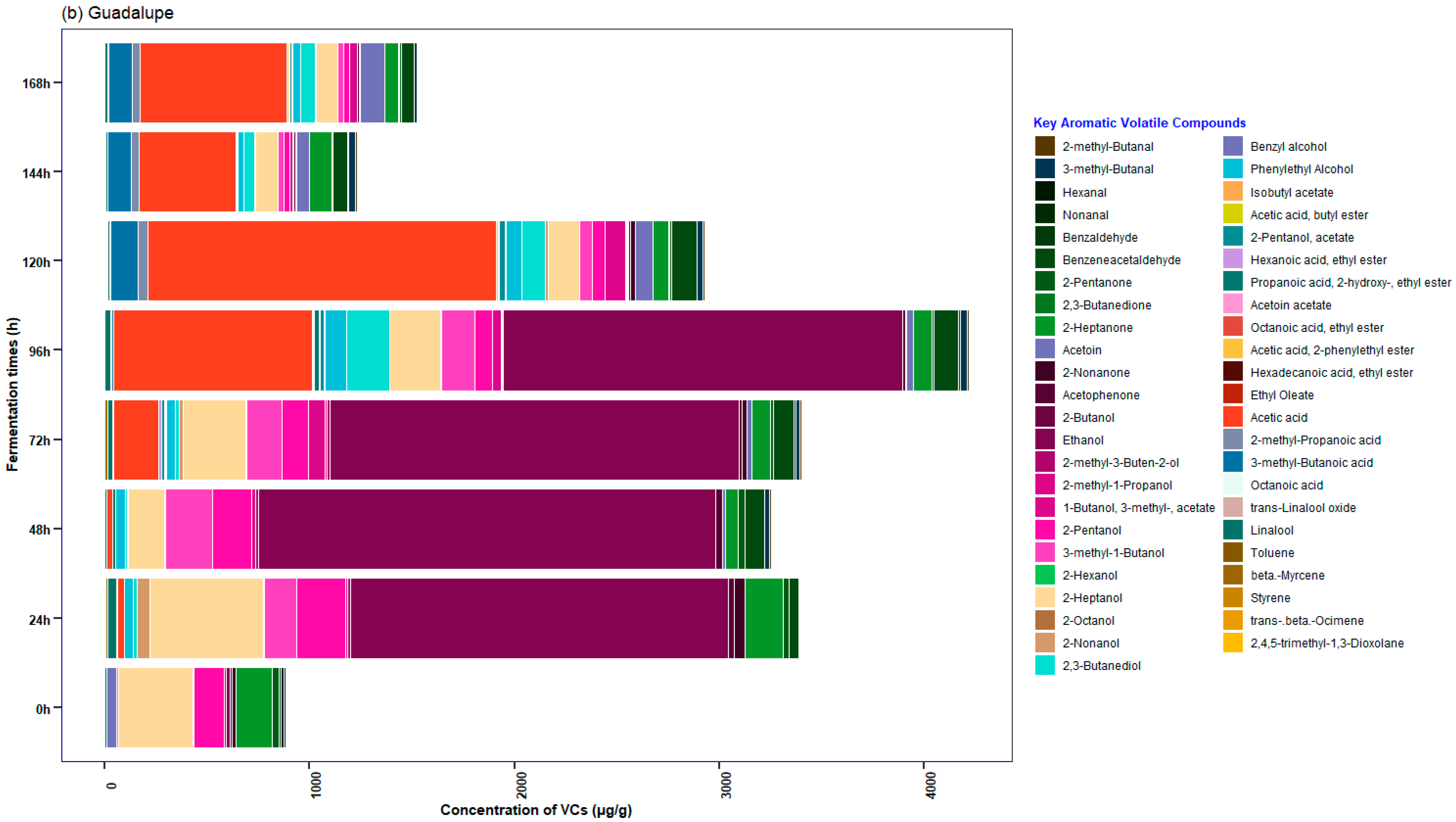
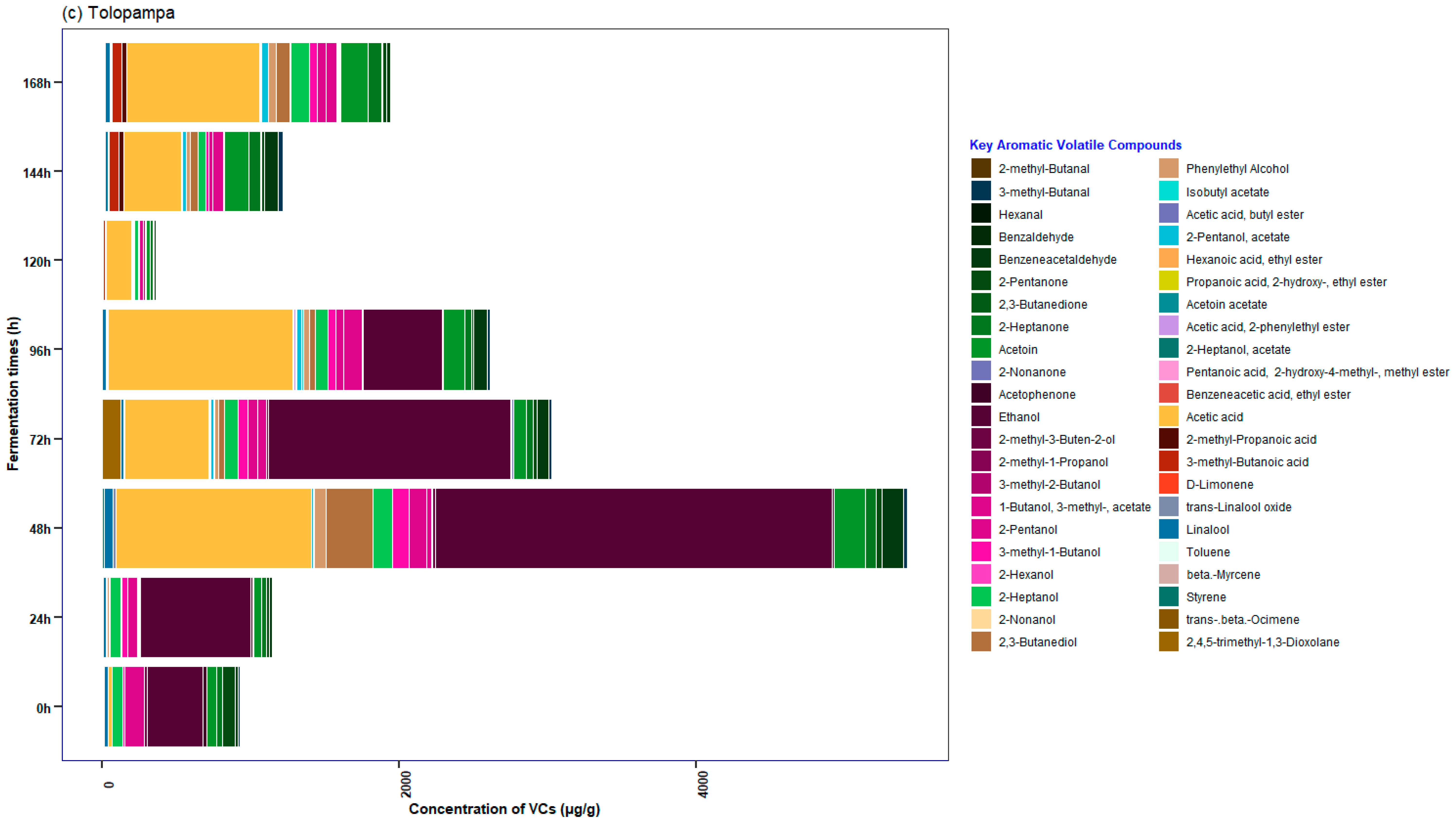
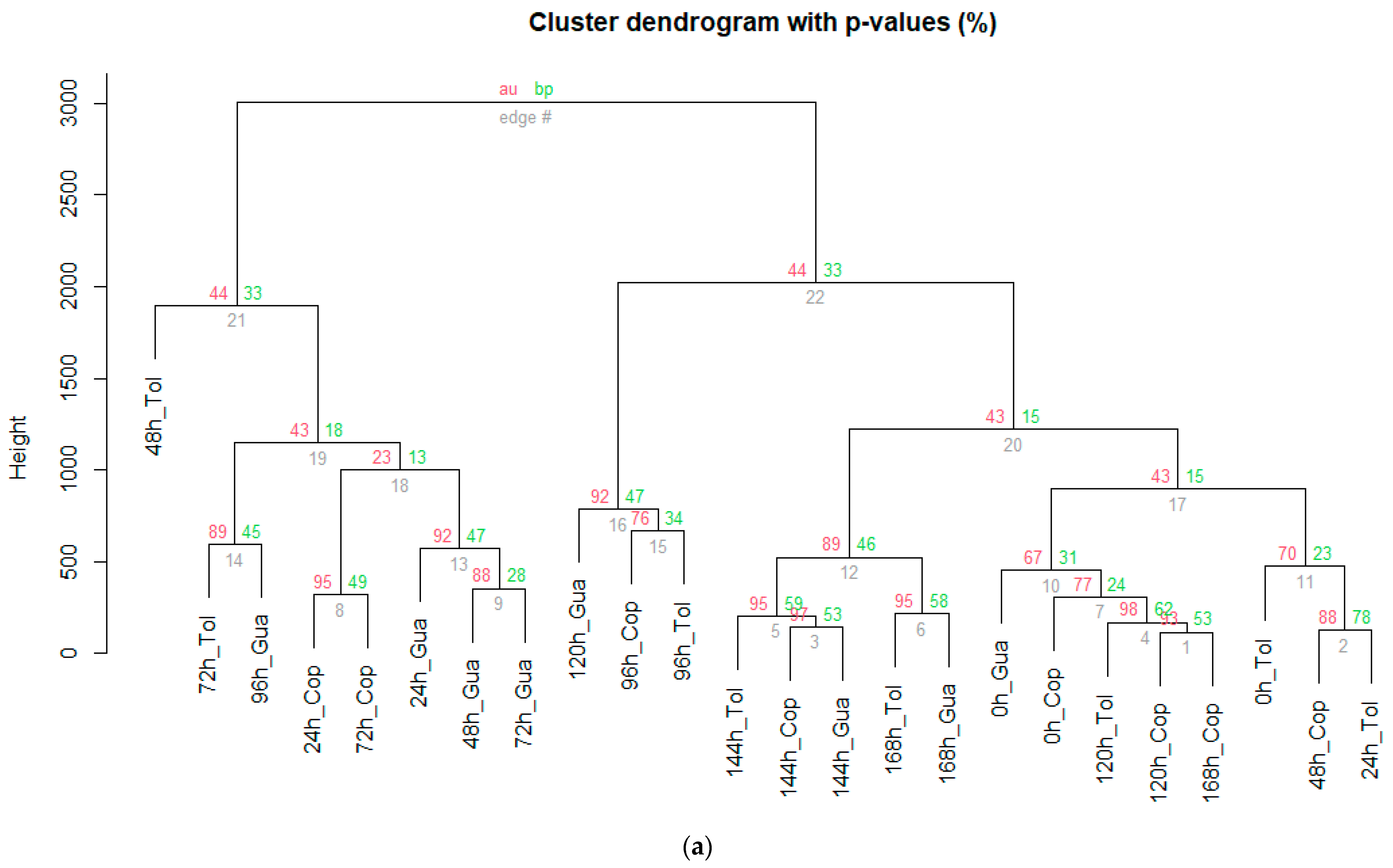
| Locations | Fermentation Time (h) | Total phenol Content 1 | Anthocyanin Content 2 | Antioxidant Activity | Flavan-3-Ols 3 | Methylxanthines 3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| DPPH 4 | ABTS 4 | FRAP 5 | Epicatechin | Catechin | Theobromine | Caffeine | ||||
| Copallín | 0 | 11.70 b [0.21] | 9.49 a [0.08] | 85.50 a [1.99] | 26.90 a [0.14] | 226.00 a [3. 21] | 14.70 ab [1.54] | 0.44 a [0.09] | 17.90 a [0.75] | 7.09 bc [1.05] |
| 24 | 15.50 a [0.34] | 7.82 b [0.06] | 81.50 ab [0.14] | 27.10 a [0.32] | 184.00 bc [11.50] | 21.70 a [4.07] | 0.44 a [0.14] | 18.70 a [1.21] | 9.96 a [0.92] | |
| 48 | 13.00 b [0.84] | 3.18 d [0.02] | 79.80 ab [13.5] | 26.70 a [0.64] | 149.00 d [13.30] | 16.70 ab [4.82] | 0.19 ab [0.05] | 18.80 a [0.13] | 7.36 bc [1.07] | |
| 72 | 12.20 b [0.31] | 3.61 c [0.07] | 92.40 a [0. 83] | 25.30 a [0.50] | 198.00 ab [7.60] | 20.00 a [2.02] | 0.16 ab [0.14] | 18.50 a [1.02] | 5.36 cd [0.27] | |
| 96 | 9.62 c [0.30] | 2.00 e [0.07] | 88.40 a [6. 21] | 23.00 b [0.92] | 170.00 bcd [7.90] | 15.60 ab [0.71] | 0.20 ab [0.03] | 19.10 a [0.08] | 5.56 c [0.36] | |
| 120 | 8.56 c [0.19] | 1.29 f [0.01] | 83.50 a [2. 39] | 22.90 bc [0.90] | 160.00 cd [12.90] | 12.10 bc [0.25] | 0.32 ab [0.05] | 18.00 a [0.15] | 8.85 ab [1.27] | |
| 144 | 6.39 d [0.88] | 0.74 g [0.04] | 66.30 bc [4.78] | 21.10 bc [0.94] | 116.00 e [8.95] | 6.42 c [1.20] | 0.20 ab [0.28] | 18.30 a [0.47] | 6.35 c [0.10] | |
| 168 | 5.25 d [0.41] | 0.80 g [0.08] | 62.40 c [6.19] | 20.90 c [0.90] | 104.00 e [12.90] | 5.42 c [2.57] | 0.00 b [0.00] | 18.20 a [0.25] | 3.16 d [0.33] | |
| Guadalupe | 0 | 10.90 a [0.34] | 6.13 a [0.08] | 83.40 ab [4.65] | 26.90 a [0.81] | 218.00 a [8.99] | 21.70 a [0.11] | 0.96 a [0.05] | 18.40 a [0.39] | 3.94 a [0.72] |
| 24 | 9.32 a [0.14] | 4.63 b [0.08] | 90.40 a [5.41] | 25.20 b [0.37] | 224.00 a [9.21] | 19.00 a [1.86] | 0.53 b [0.08] | 18.20 a [0.70] | 2.78 b [0.39] | |
| 48 | 6.93 b [0.33] | 2.43 c [0.08] | 87.90 ab [11.0] | 26.80 a [0.17] | 144.00 b [4.78] | 9.30 b [3.49] | 0.36 b [0.29] | 17.70 a [0.43] | 2.84 b [0.15] | |
| 72 | 7.13 b [1.12] | 0.71 d [0.01] | 83.60 ab [5.66] | 25.90 ab [0.62] | 110.00 c [6.75] | 12.30 b [2.27] | 0.26 bc [0.10] | 17.40 a [0.16] | 2.35 bc [0.07] | |
| 96 | 4.05 c [0.30] | 0.25 e [0.02] | 74.80 bc [3.68] | 21.40 c [0.21] | 51.60 d [6.83] | 2.76 c [0.57] | 0.00 c [0.00] | 14.90 b [0.06] | 1.61 cd [0.14] | |
| 120 | 3.82 cd [0.62] | 0.22 e [0.00] | 57.10 d [0.66] | 16.40 e [0.68] | 51.00 d [2.51] | 2.90 c [1.41] | 0.00 c [0.00] | 14.80 b [1.07] | 1.20 de [0.21] | |
| 144 | 2.42 de [0.12] | 0.18 e [0.08] | 67.40 cd [1.81] | 15.90 e [0.14] | 49.80 d [7.40] | 2.82 c [0.59] | 0.00 c [0.00] | 13.00 b [0.24] | 0.64 e [0.09] | |
| 168 | 1.01 e [0.75] | 0.11 e [0.04] | 59.30 d [0.90] | 17.90 d [0.41] | 20.80 e [4.36] | 1.46 c [0.73] | 0.00 c [0.00] | 13.00 b [1.63] | 0.52 e [0.19] | |
| Tolopampa | 0 | 11.70 ab [0.63] | 10.30 a [0.08] | 91.70 a [0. 52] | 25.40 a [1.34] | 249.00 a [1.76] | 20.80 b [1.83] | 0.35 bc [0.10] | 17.30 bc [0.44] | 4.90 bc [0.04] |
| 24 | 14.20 a [0.58] | 9.42 b [0.08] | 89.10 ab [1.26] | 26.10 a [1.11] | 263.00 a [0.77] | 30.40 a [1.06] | 0.86 a [0.09] | 19.10 a [0.39] | 9.21 a [0.73] | |
| 48 | 9.96 bc [1.83] | 3.72 c [0.06] | 90.40 ab [2.53] | 22.00 b [0.58] | 199.00 b [10.80] | 20.30 b [0.59] | 0.40 b [0.00] | 18.20 ab [0.48] | 6.14 b [0.63] | |
| 72 | 3.64 d [0.81] | 1.14 d [0.06] | 79.20 ab [0.50] | 21.10 bc [0.37] | 115.00 c [6.64] | 5.02 d [4.09] | 0.00 d [0.00] | 14.20 e [0.49] | 2.92 c [0.21] | |
| 96 | 4.30 d [1.45] | 0.79 e [0.04] | 78.30 b [3.75] | 19.70 cd [0.65] | 117.00 c [0.79] | 6.50 cd [0.40] | 0.00 d [0.00] | 16.10 cd [0.06] | 4.81 bc [1.55] | |
| 120 | 5.36 d [0.38] | 0.77 e [0.02] | 58.80 c [6.30] | 25.70 a [0.19] | 124.00 c [13.30] | 10.10 c [0.58] | 0.09 d [0.00] | 15.30 de [0.74] | 3.34 c [0.78] | |
| 144 | 3.98 d [0.94] | 0.47 f [0.01] | 79.70 ab [5.07] | 19.10 cd [0.93] | 110.00 c [12.40] | 8.60 cd [0.20] | 0.16 cd [0.15] | 15.40 de [0.04] | 3.62 c [0.03] | |
| 168 | 6.50 cd [2.15] | 0.40 f [0.03] | 79.80 ab [8.23] | 18.00 d [0.17] | 112.00 c [0.82] | 11.00 c [1.59] | 0.43 b [0.03] | 18.20 ab [0.90] | 4.54 bc [0.25] | |
| Locations | Fermentation Time (h) | Total Phenol Content 1 | Anthocyanin Content 2 | Antioxidant Activity | Flavan-3-Ols 3 | Methylxanthines 3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| DPPH 4 | ABTS 4 | FRAP 5 | Epicatechin | Catechin | Theobromine | Caffeine | ||||
| Copallín | 0 | 6.97 de [0.18] | 6.90 c [0.08] | 59.90 b [4.35] | 27.80 a [0.11] | 242.00 a [10.40] | 16.00 bcd [3.11] | 0.61 ab [0.28] | 17.60 b [0.29] | 6.30 ab [0.82] |
| 24 | 14.40 a [0.25] | 9.05 a [0.05] | 89.70 ab [0.36] | 27.60 a [0.31] | 258.00 a [1.01] | 14.50 cd [3.41] | 0.16 c [0.02] | 17.50 b [2.26] | 6.81 ab [1.14] | |
| 48 | 11.70 ab [0.56] | 7.10 b [0.07] | 93.90 a [0.52] | 25.70 a [0.73] | 235.00 a [11.10] | 20.80 ab [0.40] | 0.31 bc [0.09] | 20.70 a [0.16] | 6.54 ab [0.41] | |
| 72 | 7.90 cde [0.14] | 1.85 d [0.08] | 74.60 ab [2.19] | 25.30 a [1.92] | 128.00 c [2.30] | 21.80 a [1.21] | 0.43 abc [0.02] | 19.70 ab [0.09] | 8.04 a [0.51] | |
| 96 | 10.50 bc [0.16] | 1.83 d [0.06] | 92.40 ab [1.7] | 26.70 a [0.77] | 171.00 b [11.20] | 19.60 abc [0.62] | 0.70 a [0.06] | 19.80 ab [0.37] | 8.42 a [0.74] | |
| 120 | 9.54 bcd [2.54] | 0.78 e [0.07] | 67.30 ab [24.4] | 25.60 a [0.11] | 160.00 b [12.50] | 16.80 abcd [1.30] | 0.42 abc [0.16] | 18.00 b [0.17] | 6.83 ab [1.20] | |
| 144 | 6.03 e [0.28] | 0.93 e [0.06] | 59.20 b [8.32] | 21.10 b [0.61] | 134.00 c [7.90] | 9.16 e [0.17] | 0.13 c [0.09] | 18.50 ab [0.15] | 4.64 b [0.08] | |
| 168 | 5.71 e [1.72] | 0.85 e [0.01] | 60.70 ab [20.8] | 20.90 b [1.52] | 147.00 bc [3.93] | 12.00 de [1.37] | 0.27 bc [0.13] | 19.00 ab [0.26] | 6.65 ab [0.62] | |
| Guadalupe | 0 | 11.40 a [1.19] | 5.80 a [0.02] | 86.10 a [2.21] | 25.80 a [0.32] | 210.00 a [6.80] | 20.90 a [1.27] | 0.84 a [0.09] | 17.40 a [0.14] | 3.2 a [1.42] |
| 24 | 8.03 b [0.18] | 0.81 b [0.05] | 83.30 ab [4.07] | 26.30 a [1.34] | 173.00 b [7.94] | 6.49 b [2.59] | 0.06 b [0.06] | 15.50 abc [0.49] | 2.75 ab [0.39] | |
| 48 | 4.77 cd [0.64] | 0.59 c [0.01] | 64.20 c [4.08] | 20.70 bc [1.95] | 84.40 c [12.10] | 5.07 bc [1.39] | 0.00 b [0.00] | 14.60 bcd [1.31] | 2.13 abc [0.87] | |
| 72 | 5.80 bc [1.46] | 0.72 b [0.01] | 67.00 bc [6.24] | 21.50 b [1.52] | 83.10 cd [11.00] | 3.23 bc [1.98] | 0.00 b [0.00] | 15.90 ab [0.07] | 2.87 a [0.70] | |
| 96 | 4.04 cd [0.80] | 0.43 d [0.03] | 54.40 cd [11.9] | 17.80 cd [0.39] | 67.20 cde [11.70] | 3.39 bc [2.60] | 0.00 b [0.00] | 13.80 cde [0.20] | 0.91 bc [0.26] | |
| 120 | 3.68 cde [0.66] | 0.2 e [0.01] | 57.70 cd [8.69] | 15.20 de [0.09] | 56.00 de [8.43] | 4.54 bc [1.43] | 0.00 b [0.00] | 15.00 bc [1.43] | 1.54 abc [0.28] | |
| 144 | 1.50 e [0.09] | 0.15 e [0.05] | 45.70 d [1.26] | 12.10 f [0.53] | 41.10 e [9.40] | 1.29 c [0.02] | 0.00 b [0.00] | 12.80 de [0.12] | 0.24 c [0.02] | |
| 168 | 2.52 de [0.03] | 0.16 e [0.04] | 60.00 cd [2.13] | 14.70 ef [0.76] | 62.70 cde [11.10] | 3.11 bc [0.12] | 0.00 b [0.00] | 12.10 e [0.07] | 0.96 bc [0.07] | |
| Tolopampa | 0 | 12.10 a [0.20] | 9.95 a [0.05] | 92.10 a [0.99] | 28.10 a [0.09] | 283.00 a [10.70] | 23.30 a [0.29] | 0.62 ab [0.11] | 18.60 a [0.40] | 5.31 bc [0.30] |
| 24 | 10.20 b [0.95] | 9.32 b [0.05] | 91.60 a [0.97] | 26.60 ab [1.10] | 227.00 b [7.69] | 24.40 a [1.37] | 0.93 a [0.26] | 19.60 a [0.74] | 7.94 a [0.10] | |
| 48 | 9.23 b [0.24] | 2.77 c [0.08] | 89.90 a [1.26] | 25.50 b [0.86] | 220.00 b [11.80] | 22.00 a [0.85] | 0.60 b [0.06] | 19.20 a [0.28] | 6.26 b [0.61] | |
| 72 | 5.84 cd [0.74] | 1.13 d [0.08] | 75.20 b [2.88] | 21.00 cd [0.92] | 145.00 cd [13.20] | 10.10 b [0.33] | 0.28 c [0.05] | 14.80 cd [0.45] | 3.67 de [0.33] | |
| 96 | 5.56 cd [1.00] | 0.85 e [0.04] | 64.80 c [4.26] | 21.00 cd [0.12] | 137.00 cd [10.80] | 9.42 bc [1.86] | 0.19 c [0.08] | 17.00 b [0.13] | 4.92 bcd [0.25] | |
| 120 | 6.38 c [0.57] | 0.80 e [0.04] | 78.80 b [0.21] | 22.70 c [1.43] | 165.00 c [11.80] | 8.00 bc [0.54] | 0.19 c [0.08] | 16.20 bc [0.29] | 4.24 cd [0.77] | |
| 144 | 4.05 d [0.60] | 0.76 e [0.03] | 71.10 bc [6.76] | 19.20 d [0.26] | 131.00 d [5.94] | 6.17 c [1.12] | 0.00 c [0.00] | 13.60 d [0.89] | 2.10 e [0.11] | |
| 168 | 7.09 c [0.10] | 0.75 e [0.01] | 69.60 bc [3.14] | 21.80 c [1.21] | 148.00 cd [13.50] | 11.30 b [2.72] | 0.15 c [0.01] | 17.10 b [0.51] | 6.38 ab [1.20] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balcázar-Zumaeta, C.R.; Pajuelo-Muñoz, A.J.; Trigoso-Rojas, D.F.; Iliquin-Chavez, A.F.; Fernández-Romero, E.; Yoplac, I.; Muñoz-Astecker, L.D.; Rodríguez-Hamamura, N.; Maza Mejía, I.M.; Cayo-Colca, I.S.; et al. Reduction in the Cocoa Spontaneous and Starter Culture Fermentation Time Based on the Antioxidant Profile Characterization. Foods 2023, 12, 3291. https://doi.org/10.3390/foods12173291
Balcázar-Zumaeta CR, Pajuelo-Muñoz AJ, Trigoso-Rojas DF, Iliquin-Chavez AF, Fernández-Romero E, Yoplac I, Muñoz-Astecker LD, Rodríguez-Hamamura N, Maza Mejía IM, Cayo-Colca IS, et al. Reduction in the Cocoa Spontaneous and Starter Culture Fermentation Time Based on the Antioxidant Profile Characterization. Foods. 2023; 12(17):3291. https://doi.org/10.3390/foods12173291
Chicago/Turabian StyleBalcázar-Zumaeta, César R., Alexa J. Pajuelo-Muñoz, Deisy F. Trigoso-Rojas, Angel F. Iliquin-Chavez, Editha Fernández-Romero, Ives Yoplac, Lucas D. Muñoz-Astecker, Nadia Rodríguez-Hamamura, Ily M. Maza Mejía, Ilse S. Cayo-Colca, and et al. 2023. "Reduction in the Cocoa Spontaneous and Starter Culture Fermentation Time Based on the Antioxidant Profile Characterization" Foods 12, no. 17: 3291. https://doi.org/10.3390/foods12173291
APA StyleBalcázar-Zumaeta, C. R., Pajuelo-Muñoz, A. J., Trigoso-Rojas, D. F., Iliquin-Chavez, A. F., Fernández-Romero, E., Yoplac, I., Muñoz-Astecker, L. D., Rodríguez-Hamamura, N., Maza Mejía, I. M., Cayo-Colca, I. S., Chagas-Junior, G. C. A., Maicelo-Quintana, J. L., & Castro-Alayo, E. M. (2023). Reduction in the Cocoa Spontaneous and Starter Culture Fermentation Time Based on the Antioxidant Profile Characterization. Foods, 12(17), 3291. https://doi.org/10.3390/foods12173291










