Effect of Sodium Nitrite, Nisin and Lactic Acid on the Prevalence and Antibiotic Resistance Patterns of Listeria monocytogenes Naturally Present in Poultry
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Sample Processing
2.3. Microbiological Determinations
2.4. Identification of Listeria monocytogenes
2.5. Antibiotic Susceptibility Testing
2.6. Statistical Analysis
3. Results
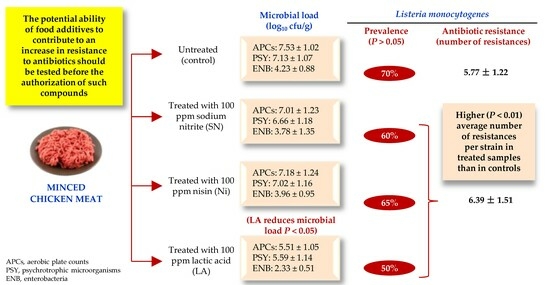
3.1. Microbial Counts
3.2. Prevalence of Listeria monocytogenes
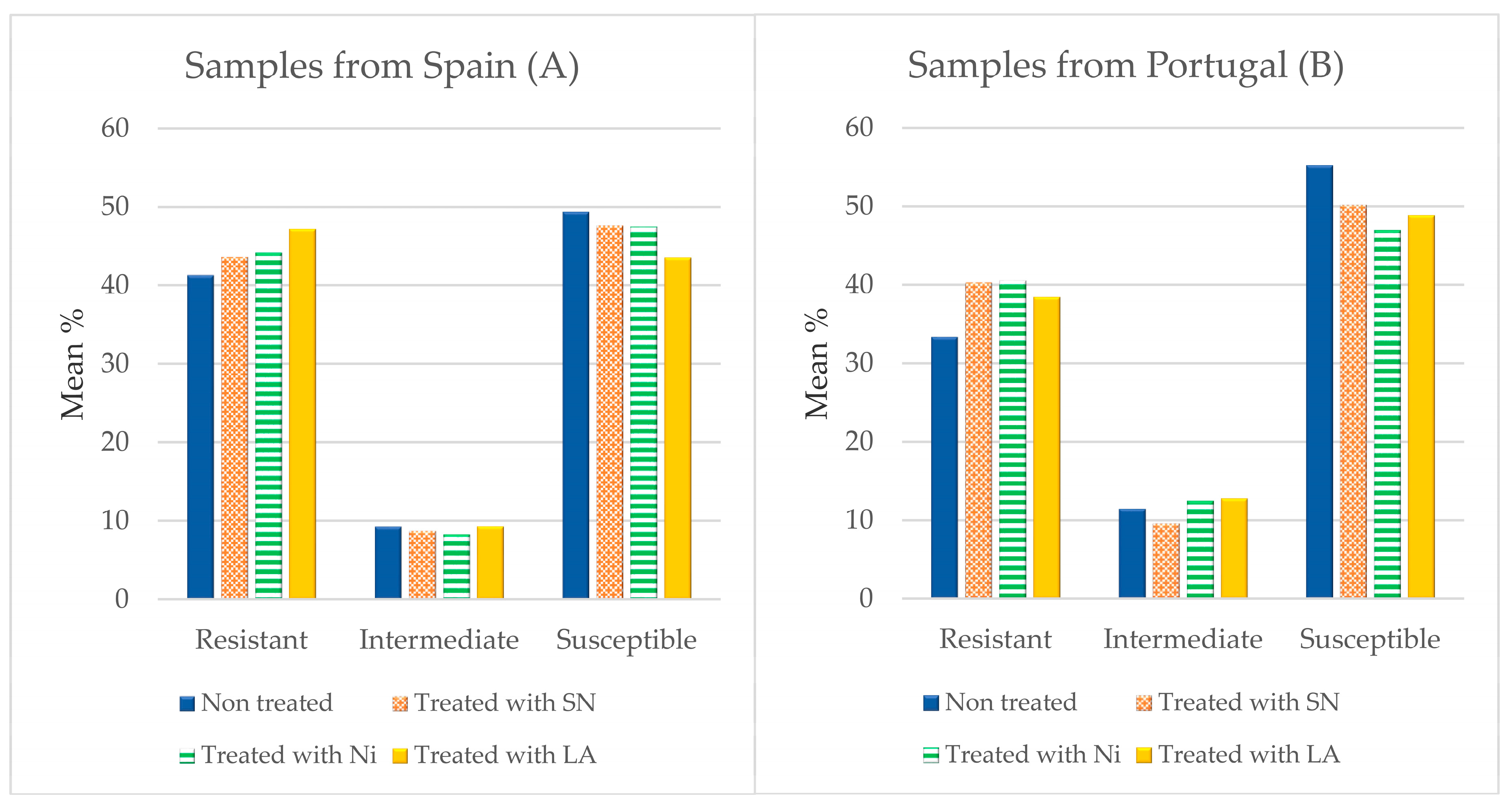
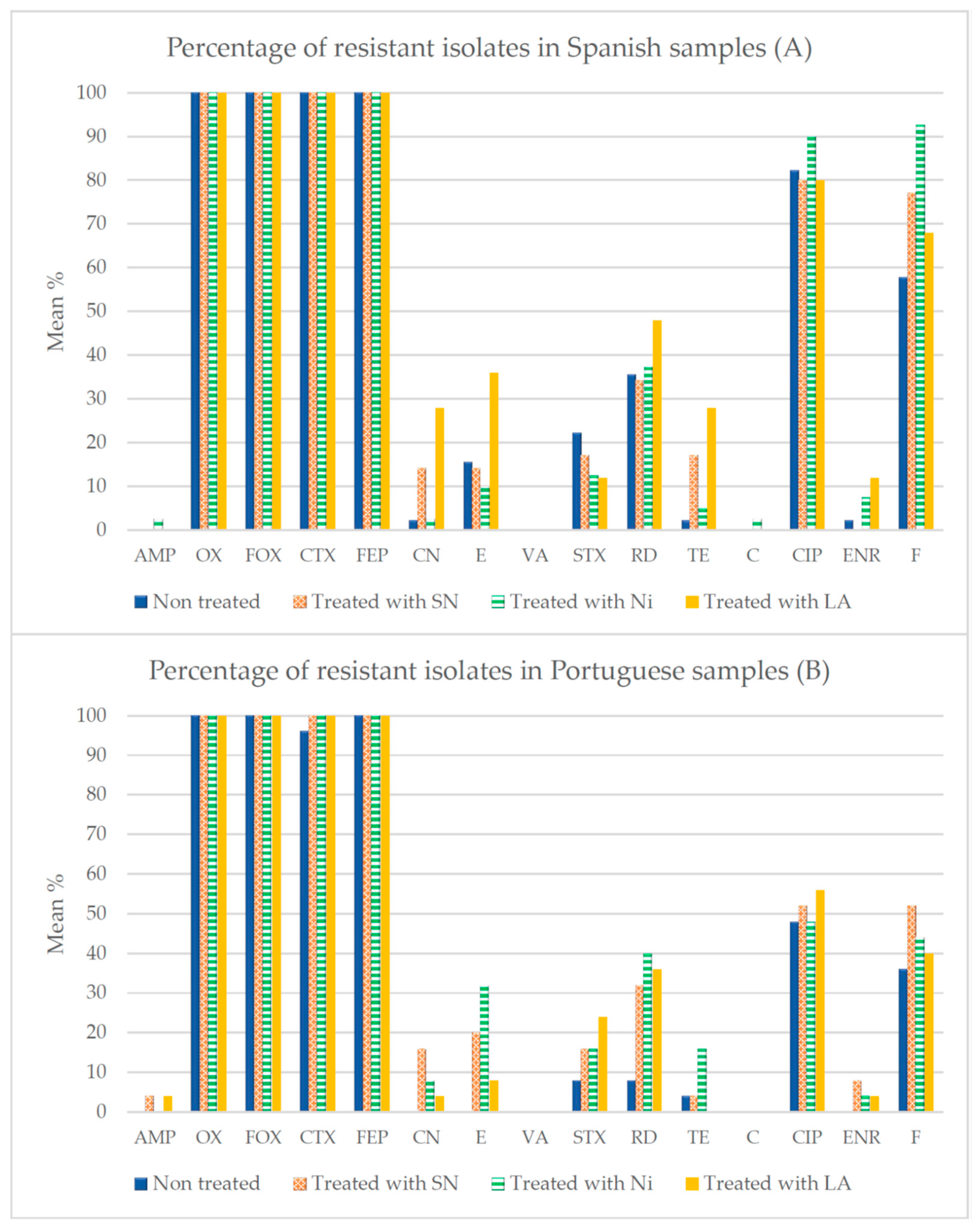
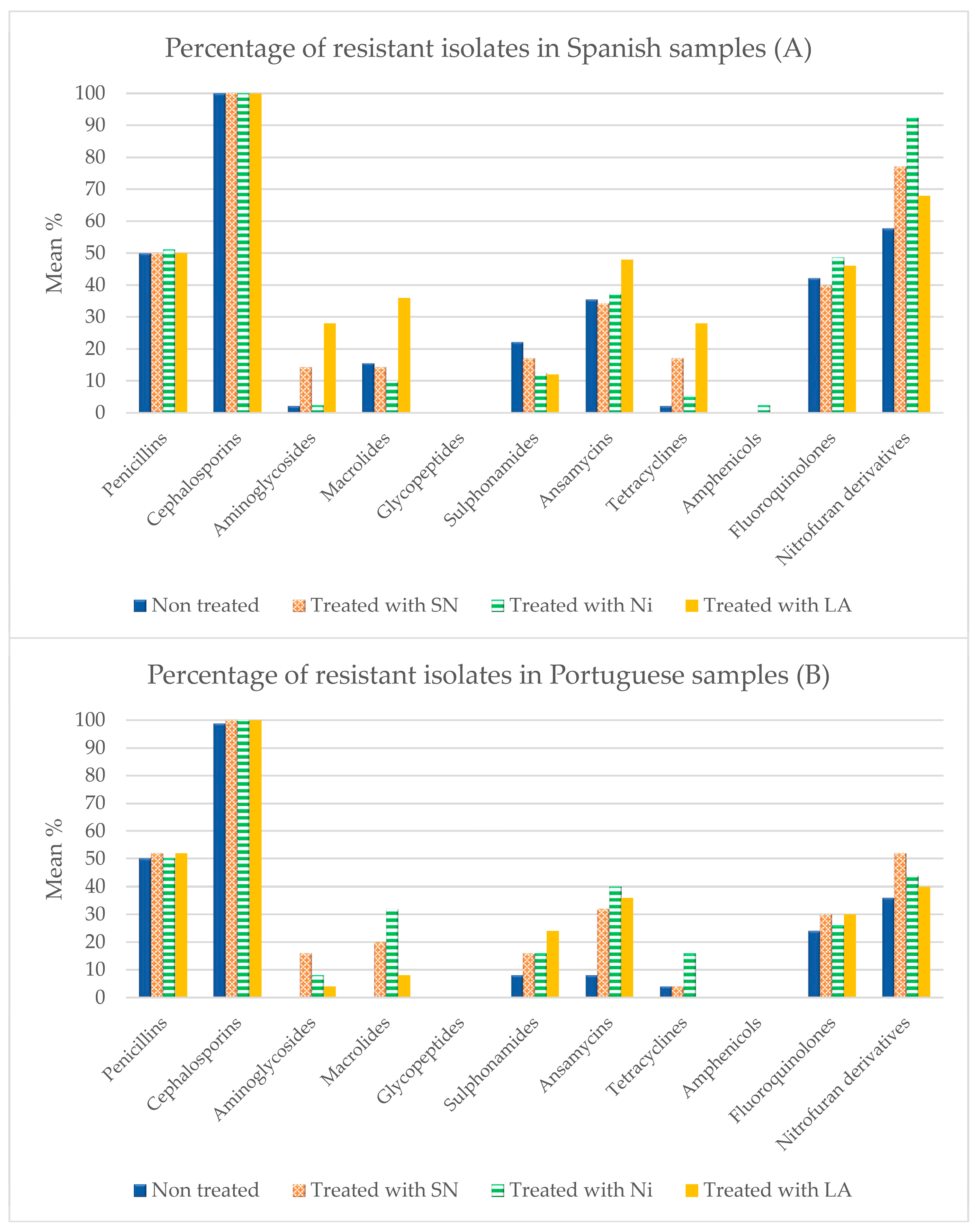
3.3. Antibiotic Susceptibility
4. Discussion
4.1. Microbial Counts
4.2. Prevalence of Listeria monocytogenes
4.3. Susceptibility to Antibiotics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial resistance in bacterial poultry pathogens: A review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef]
- FAOSTAT. Consumo Carne de Pollo. Organización de las Naciones Unidas para la Agricultura y la Alimentación (FAO). 2020. Available online: http://www.fao.org/faostat/es/#data/QL (accessed on 30 March 2023).
- MAPA. Sector Avícola de Carne de España. Ministerio de Agricultura. Pesca y Alimentación (MAPA), Madrid. 2020. Available online: https://www.mapa.gob.es/es/ganaderia/temas/produccion-y-mercados-ganaderos/sectores-ganaderos/avicola-de-carne/ (accessed on 30 March 2023).
- Capita, R.; Castaño-Arriba, A.; Rodríguez-Melcón, C.; Igrejas, G.; Poeta, P.; Alonso-Calleja, C. Diversity, antibiotic resistance, and biofilm-forming ability of enterobacteria isolated from red meat and poultry preparations. Microorganisms 2020, 8, 1226. [Google Scholar] [CrossRef]
- Del Río, E.; Panizo-Morán, M.; Prieto, M.; Alonso-Calleja, C.; Capita, R. Effect of various chemical decontamination treatments on natural microflora and sensory characteristics of poultry. Int. J. Food Microbiol. 2007, 115, 268–280. [Google Scholar] [CrossRef]
- Álvarez-Astorga, M.; Capita, R.; Alonso-Calleja, C.; Moreno, B.; García-Fernández, C. Microbiological quality of retail chicken by-products in Spain. Meat Sci. 2002, 62, 45–50. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Riesco-Peláez, F.; Carballo, J.; García-Fernández, C.; Capita, R.; Alonso-Calleja, C. Structure and viability of 24- and 72-h-old biofilms formed by four pathogenic bacteria on polystyrene and glass contact surfaces. Food Microbiol. 2018, 76, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Melcón, C.; Riesco-Peláez, F.; García-Fernández, C.; Alonso-Calleja, C.; Capita, R. Susceptibility of Listeria monocytogenes planktonic cultures and biofilms to sodium hypochlorite and benzalkonium chloride. Food Microbiol. 2019, 82, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Campos, D.; Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Persistent Listeria monocytogenes isolates from a poultry-processing facility form more biofilm but do not have a greater resistance to disinfectants than sporadic strains. Pathogens 2019, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, 7666. [Google Scholar] [CrossRef]
- AESAN. Aditivos Alimentarios. Agencia Española de Seguridad Alimentaria y Nutrición, Madrid. 2019. Available online: https://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/subdetalle/aditivos_alimentarios.htm#:~:text=Los%20aditivos%20son%20sustancias%20que%20se%20a%C3%B1aden%20a,distintas%20de%20aditivos%20en%20funci%C3%B3n%20de%20sus%20propiedades (accessed on 30 March 2023).
- European Commission. Commission Regulation (EU) No 1130/2011 of 11 November 2011 amending Annex III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives by establishing a Union list of food additives approved for use in food additives, food enzymes, food flavourings and nutrients. Off. J. Eur. Union 2011, 295, 178–204. [Google Scholar]
- Comisión del Codex Alimentarius. Propuestas de nuevas disposiciones para la nisina (SIN 234) en la categoría de alimentos 0.8 “carne y productos cárnicos, incluidos los de aves de corral y caza”. In Codex Alimentarius; CX/FA 13/45/10; Programa conjunto FAO/OMS sobre normas alimentarias comité del Codex sobre aditivos alimentarios; FAO: Rome, Italy, 2013. [Google Scholar]
- European Commission. Commission Regulation (EU) No 101/2013 of 4 February 2013 concerning the use of lactic acid to reduce microbiological surface contamination on bovine carcasses. Off. J. Eur. Union 2013, 34, 1–3. [Google Scholar]
- Capita, R.; Alonso-Calleja, C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Capita, R.; Felices-Mercado, A.; García-Fernández, C.; Alonso-Calleja, C. Characterization of Listeria monocytogenes originating from the Spanish meat-processing chain. Foods 2019, 8, 542. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 30 March 2023).
- OCDE. Antimicrobial Resistance. Tackling the Burden in the European Union. 2019. Available online: https://www.oecd.org/health/health-systems/AMR-Tackling-the-Burden-in-the-EU-OECD-ECDC-Briefing-Note-2019.pdf (accessed on 30 March 2023).
- Capita, R.; Riesco-Peláez, F.; Alonso-Hernando, A.; Alonso-Calleja, C. Exposure of Escherichia coli ATCC 12806 to sublethal concentrations of food-grade biocides influences its ability to form biofilm, resistance to antimicrobials, and ultrastructure. Appl. Environ. Microbiol. 2014, 80, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Molina-González, D.; Alonso-Calleja, C.; Alonso-Hernando, A.; Capita, R. Effect of sub-lethal concentrations of biocides on the susceptibility to antibiotics of multi-drug resistant Salmonella enterica strains. Food Control 2014, 40, 329–334. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Effect of low doses of biocides on the susceptibility of Listeria monocytogenes and Salmonella enterica to various antibiotics of clinical importance. Food Control 2023, 149, 109602. [Google Scholar] [CrossRef]
- Capita, R.; Álvarez-Fernández, E.; Fernández-Buelta, E.; Manteca, J.; Alonso-Calleja, C. Decontamination treatments can increase the prevalence of resistance to antibiotics of Escherichia coli naturally present on poultry. Food Microbiol. 2013, 34, 112–117. [Google Scholar] [CrossRef]
- Jay, J.M. A review of aerobic and psychrotrophic plate count procedures for fresh meat and poultry products. J. Food Prot. 2002, 65, 1200–1206. [Google Scholar] [CrossRef]
- Cousin, M.A.; Jay, J.M.; Vasavada, P.C. Psychrotrophic microorganisms. In Compendium of Methods for the Microbiological Examination of Foods; Downes, F.P., Ito, K., Eds.; American Public Health Association: Washington, DC, USA, 2001; pp. 59–166. [Google Scholar]
- Baird, R.M.; Corry, J.E.J.; Curtis, G.D.W. Pharmacopeia of culture media for food microbiology. Int. J. Food Microbiol. 1987, 5, 221–222. [Google Scholar]
- Ryu, J.; Park, S.H.; Yeom, Y.S.; Shrivastav, A.; Lee, S.H.; Kim, Y.R.; Kim, H.Y. Simultaneous detection of Listeria species isolated from meat processed foods using multiplex PCR. Food Control 2013, 32, 659–664. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; National Committee for Clinical Laboratory Standards: Pennsylvania, PA, USA, 2020; Volume 40, p. M100. Available online: http://em100.edaptivedocs.net/GetDoc.aspx?doc=CLSI%20M100%20ED30:2020&xormat=SPDF&src=BB (accessed on 31 March 2023).
- EUCAST. European Committee on Antimicrobial Susceptibility Testing. V. 13.0. 2023. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_13.0_Breakpoint_Tables.pdf (accessed on 31 March 2023).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Esteves, A.; Panera-Martínez, S.; Capita, R.; Alonso-Calleja, C. Quantification of total and viable cells and determination of serogroups and antibiotic resistance patterns of Listeria monocytogenes in chicken meat from the north-western Iberian Peninsula. Antibiotics 2022, 11, 1828. [Google Scholar] [CrossRef] [PubMed]
- Lerasle, M.; Federighi, M.; Simonin, H.; Anthoine, V.; Rezé, S.; Chéret, R.; Guillou, S. Combined use of modified atmosphere packaging and high pressure to extend the shelf-life of raw poultry sausage. Innov. Food Sci. Emerg. Technol. 2014, 23, 54–60. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338, 1–26. [Google Scholar]
- ICMSF. Microorganisms in Foods: Use of Data for Assessing Process Control and Product Acceptance; Springer: Boston, MA, USA, 2011; Available online: https://link.springer.com/book/10.1007/978-1-4419-9374-8 (accessed on 31 March 2023).
- IFST. Development and use of microbiological criteria for foods. Food Sci. Technol. Today 1997, 11, 137–176. [Google Scholar]
- Buzón-Durán, L.; Capita, R.; Alonso-Calleja, C. Microbial loads and antibiotic resistance patterns of Staphylococcus aureus in different types of raw poultry-based meat preparations. Poult. Sci. 2017, 96, 4046–4052. [Google Scholar] [CrossRef]
- Pascual Anderson, M.R. Microbiología Alimentaria: Metodología Analítica para Alimentos y Bebidas; Díaz de Santos: Madrid, Spain, 1992. [Google Scholar]
- Lee, S.; Lee, H.; Kim, S.; Lee, J.; Ha, J.; Choi, Y.; Oh, H.; Choi, K.-H.; Yoon, Y. Microbiological safety of processed meat products formulated with low nitrite concentration—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 1073–1077. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Stasiak, D.M.; Kęska, P. The influence of different levels of sodium nitrite on the safety, oxidative stability, and color of minced roasted beef. Sustainability 2019, 11, 3795. [Google Scholar] [CrossRef]
- Gunvig, A.; Hansen, F.; Borggaard, C. A mathematical model for predicting growth/no-growth of psychrotrophic C. botulinum in meat products with five variables. Food Control 2013, 29, 309–317. [Google Scholar] [CrossRef]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in cured meats, health risk issues, alternatives to nitrites: A review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef]
- Pini, F.; Aquilani, C.; Giovannetti, L.; Viti, C.; Pugliese, C. Characterization of the microbial community composition in Italian Cinta Senese sausages dry-fermented with natural extracts as alternatives to sodium nitrite. Food Microbiol. 2020, 89, 103417. [Google Scholar] [CrossRef]
- Parthasarathy, D.K.; Bryan, N.S. Sodium nitrite: The “cure” for nitric oxide insufficiency. Meat Sci. 2012, 92, 274–279. [Google Scholar] [CrossRef]
- González, B.; Díez, V. The effect of nitrite and starter culture on microbiological quality of “chorizo”—A Spanish dry cured sausage. Meat Sci. 2002, 60, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Lamas, A.; Miranda, J.M.; Vázquez, B.; Cepeda, A.; Franco, C.M. An evaluation of alternatives to nitrites and sulfites to inhibit the growth of Salmonella enterica and Listeria monocytogenes in meat products. Foods 2016, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Oliver, M.; Escalona-Buendía, H.B.; Ponce-Alquicira, E. Effect of the addition of microcapsules with avocado peel extract and nisin on the quality of ground beef. Food Sci. Nutr. 2020, 8, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zou, L.; Yang, Q.; Xia, J.; Zhou, K.; Zhu, Y.; Han, X.; Pu, B.; Hu, B.; Deng, W.; et al. Antimicrobial activities of nisin, tea polyphenols, and chitosan and their combinations in chilled Mutton. J. Food Sci. 2016, 81, M1466–M1471. [Google Scholar] [CrossRef]
- Wu, J.; Zang, M.; Wang, S.; Zhao, B.; Bai, J.; Xu, C.; Shi, Y.; Qiao, X. Nisin: From a structural and meat preservation perspective. Food Microbiol. 2023, 111, 104207. [Google Scholar] [CrossRef]
- Yuste, J.; Pla, R.; Capellas, M.; Mor-Mur, M. Application of high-pressure processing and nisin to mechanically recovered poultry meat for microbial decontamination. Food Control 2002, 13, 451–455. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Lactic acid concentrations that reduce microbial load yet minimally impact colour and sensory characteristics of beef. Meat Sci. 2017, 129, 169–175. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on the evaluation of the safety and efficacy of lactic acid for the removal of microbial surface contamination of beef carcasses, cuts and trimmings. EFSA J. 2011, 9, 2317–2351. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on evaluation of the safety and efficacy of the organic acids lactic and acetic acids to reduce microbiological surface contamination on pork carcasses and pork cuts. EFSA J. 2018, 16, 5482. [Google Scholar] [CrossRef]
- Ramirez-Hernandez, A.; Brashears, M.M.; Sanchez-Plata, M.X. Efficacy of lactic acid, lactic acid–acetic acid blends, and peracetic acid to reduce Salmonella on chicken parts under simulated commercial processing conditions. J. Food Prot. 2018, 81, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Alakomi, H.-L.; Skytta, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes Gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef]
- Burbano, E.M.; Carrascal, A.K.; Mercado, M.; Poutou, R. Validación de PCR para Listeria monocytogenes en leches. Aliment. Hoy 2007, 10, 14–20. [Google Scholar]
- De la Rosa-Zariñana, A.E.; Crosby-Galván, M.M.; Ramírez-Guzmán, M.E.; Hernández-Sánchez, D.; Mata-Espinoza, M.A. Standardization of PCR technique for detecting Listeria monocytogenes in chicken, beef and pork. Ecosistemas Recur. Agropecu. 2018, 5, 25–34. [Google Scholar] [CrossRef]
- Poutou, R.M.; Burbano, S.; Sierra, K.; Torres, A.; Carrascal, K.; Mercado, M. Estandarización de la extracción de ADN y validación de la PCR múltiple para detectar Listeria monocytogenes en queso, leche, carne de res y pollo. Univ. Sci. 2005, 10, 61–78. [Google Scholar]
- Panera-Martínez, S.; Rodríguez-Melcón, C.; Serrano-Galán, V.; Alonso-Calleja, C.; Capita, R. Prevalence, quantification and antibiotic resistance of Listeria monocytogenes in poultry preparations. Food Control 2022, 135, 108608. [Google Scholar] [CrossRef]
- Alonso-Hernando, A.; Prieto, M.; García-Fernández, C.; Alonso-Calleja, C.; Capita, R. Increase over time in the prevalence of multiple antibiotic resistance among isolates of Listeria monocytogenes from poultry in Spain. Food Control 2012, 23, 37–41. [Google Scholar] [CrossRef]
- Capita, R.; Alonso-Calleja, C.; Moreno, B.; García-Fernández, M.C. Occurrence of Listeria species in retail poultry meat and comparison of a cultural/immunoassay for their detection. Int. J. Food Microbiol. 2001, 65, 75–82. [Google Scholar] [CrossRef]
- Jamshidi, A.; Zeinali, T. Significance and characteristics of Listeria monocytogenes in poultry products. Int. J. Food Sci. 2019, 18, 7835253. [Google Scholar] [CrossRef]
- Ristori, C.A.; Rowlands, R.E.G.; Martins, C.G.; Barbosa, M.L.; Yoshida, J.T.U.; De Melo Franco, B.D.G. Prevalence and populations of Listeria monocytogenes in meat products retailed in Sao Paulo, Brazil. Foodborne Pathog. Dis. 2014, 11, 969–973. [Google Scholar] [CrossRef]
- Kanarat, S.; Jitnupong, W.; Sukhapesna, J. Prevalence of Listeria monocytogenes in chicken production chain in Thailand. Thai J. Vet. Med. 2011, 41, 155–161. [Google Scholar] [CrossRef]
- Kosek-Paszkowska, K.; Bania, J.; Bystroń, J.; Molenda, J.; Czerw, M. Occurrence of Listeria sp. in raw poultry meat and poultry meat products. Bull. Vet. Inst. Pulawy 2005, 49, 219–222. [Google Scholar]
- Schäfer, D.F.; Steffens, J.; Barbosa, J.; Zeni, J.; Paroul, N.; Valduga, E.; Junges, A.; Backes, G.T.; Cansian, R.L. Monitoring of contamination sources of Listeria monocytogenes in a poultry slaughterhouse. LWT-Food Sci. Technol. 2017, 86, 393–398. [Google Scholar] [CrossRef]
- Fallah, A.A.; Saei-Dehkordi, S.S.; Rahnama, M.; Tahmasby, H.; Mahzounieh, M. Prevalence and antimicrobial resistance patterns of Listeria species isolated from poultry products marketed in Iran. Food Control 2012, 28, 327–332. [Google Scholar] [CrossRef]
- Bilir Ormanci, F.S.; Erol, I.; Ayaz, N.D.; Iseri, O.; Sariguzel, D. Immunomagnetic separation and PCR detection of Listeria monocytogenes in Turkey meat and antibiotic resistance of the isolates. Br. Poult. Sci. 2008, 49, 560–565. [Google Scholar] [CrossRef]
- Sugiri, Y.D.; Gölz, G.; Meeyam, T.; Baumann, M.P.O.; Kleer, J.; Chaiswwong, W.; Alter, T. Prevalence and antimicrobial susceptibility of Listeria monocytogenes on chicken carcasses in Bandung, Indonesia. J. Food Prot. 2014, 77, 1407–1410. [Google Scholar] [CrossRef]
- Oliveira, T.S.; Varjão, L.M.; da Silva, L.N.N.; Pereira, R.D.L.; Hofer, E.; Vallim, D.C.; Almeida, R.C.D. Listeria monocytogenes at chicken slaughterhouse: Occurrence, genetic relationship among isolates and evaluation of antimicrobial susceptibility. Food Control 2018, 88, 131–138. [Google Scholar] [CrossRef]
- Soultos, N.; Koidis, P.; Madden, R.H. Presence of Listeria and Salmonella spp. in retail chicken in Northern Ireland. Lett. Appl. Microbiol. 2003, 37, 421–423. [Google Scholar] [CrossRef]
- Osaili, T.M.; Alaboudi, A.R.; Nesiar, E.A. Prevalence of Listeria spp. and antibiotic susceptibility of Listeria monocytogenes isolated from raw chicken and ready-to-eat chicken products in Jordan. Food Control 2011, 22, 586–590. [Google Scholar] [CrossRef]
- Van Nierop, W.; Dusé, A.G.; Marais, E.; Aithma, N.; Thothobolo, N.; Kassel, M.; Stewart, R.; Potgieter, A.; Fernandes, B.; Galpin, J.S.; et al. Contamination of chicken carcasses in Gauteng, South Africa, by Salmonella, Listeria monocytogenes and Campylobacter. Int. J. Food Microbiol. 2005, 99, 1–6. [Google Scholar] [CrossRef]
- Gonçalves-Tenório, A.; Nunes Silva, B.; Rodriguez, V.; Cadavez, V.; Gonzales-Barron, U. Prevalence of pathogens in poultry meat: A meta-analysis of European published surveys. Foods 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Saludes, M.; Troncoso, M.; Figueroa, G. Presence of Listeria monocytogenes in Chilean food matrices. Food Control 2015, 50, 331–335. [Google Scholar] [CrossRef]
- Gudbjörnsdóttir, B.; Suihko, M.L.; Gustavsson, P.; Thorkelsson, G.; Salo, S.; Sjöberg, A.M.; Niclasen, O.; Bredholt, S. The incidence of Listeria monocytogenes in meat, poultry and seafood plants in the Nordic countries. Food Microbiol. 2004, 21, 217–225. [Google Scholar] [CrossRef]
- Kuan, C.H.; Goh, S.G.; Loo, Y.Y.; Chang, W.S.; Lye, Y.L.; Puspanadan, S.; Tang, J.Y.H.; Nakaguchi, Y.; Nishibuchi, M.; Mahyudin, N.A.; et al. Prevalence and quantification of Listeria monocytogenes in chicken offal at the retail level in Malaysia. Poult. Sci. 2013, 92, 1664–1669. [Google Scholar] [CrossRef]
- Gunasena, D.; Kodikara, C.; Ganepola, K.; Widanapathirana, S. Occurrence of Listeria monocytogenes in food in Sri Lanka. J. Natl. Sci. Fund. Sri Lanka 1995, 23, 107–114. [Google Scholar] [CrossRef]
- Vitas, A.I.; Aguado, V.; Garcia-Jalon, I. Occurrence of Listeria monocytogenes in fresh and processed foods in Navarra (Spain). Int. J. Food Microbiol. 2004, 90, 349–356. [Google Scholar] [CrossRef]
- Sakaridis, I.; Soultos, N.; Iossifidou, E.; Papa, A.; Ambrosiadis, I.; Koidis, P. Prevalence and antimicrobial resistance of Listeria monocytogenes isolated in chicken slaughterhouses in Northern Greece. J. Food Prot. 2011, 74, 1017–1021. [Google Scholar] [CrossRef]
- Uyttendaele, M.; De Troy, P.; Debevere, J. Incidence of Listeria monocytogenes in different types of meat products on the Belgian retail market. Int. J. Food Microbiol. 1999, 53, 75–80. [Google Scholar] [CrossRef]
- Zeinali, T.; Jamshidi, A.; Basami, M.; Rad, M. Isolation and identification of Listeria spp. in chicken carcasses marketed in northeast of Iran. Int. Food Res. J. 2017, 24, 881–887. [Google Scholar]
- Antunes, P.; Réu, C.; Sousa, J.C.; Pestana, N.; Peixe, L. Incidence of susceptibility to antimicrobial agents of Listeria spp. and Listeria monocytogenes isolated from poultry carcasses in Porto, Portugal. J. Food Prot. 2022, 65, 1888–1893. [Google Scholar] [CrossRef]
- Elmali, M.; Can, H.Y.; Yaman, H. Prevalence of Listeria monocytogenes in poultry meat. Food Sci. Technol. 2015, 35, 672–675. [Google Scholar] [CrossRef]
- Fate, S.E.; Schweihofer, J.P.; Conklin, T. Assessment of sanitation practices for the control of Listeria monocytogenes at small and very small ready-to-eat meat and poultry processors. J. Food Prot. 2021, 84, 1567–1574. [Google Scholar] [CrossRef]
- Malley, T.J.V.; Butts, J.; Wiedmann, M. Seek and destroy process: Listeria monocytogenes process controls in the ready-to-eat meat and poultry industry. J. Food Prot. 2015, 78, 436–445. [Google Scholar] [CrossRef]
- Tompkin, R.B. Control of Listeria monocytogenes in the food-processing environment. J. Food Prot. 2002, 65, 709–725. [Google Scholar] [CrossRef]
- Myers, K.; Cannon, J.; Montoya, D.; Dickson, J.; Lonergan, S.; Sebranek, J. Effects of high hydrostatic pressure and varying concentrations of sodium nitrite from traditional and vegetable-based sources on the growth of Listeria monocytogenes on ready-to-eat (RTE) sliced ham. Meat Sci. 2013, 94, 69–76. [Google Scholar] [CrossRef]
- Ariyapitipun, T.; Mustapha, A.; Clarke, A.D. Survival of Listeria monocytogenes Scott A on vacuum-packaged raw beef treated with polylactic acid, lactic acid, and nisin. J. Food Prot. 2000, 63, 131–136. [Google Scholar] [CrossRef]
- Solomakos, N.; Govaris, A.; Koidisb, P.; Botsoglou, N. The antimicrobial effect of thyme essential oil, nisin, and their combination against Listeria monocytogenes in minced beef during refrigerated storage. Food Microbiol. 2008, 25, 120–127. [Google Scholar] [CrossRef]
- Raeisi, M.; Tabaraei, A.; Hashemi, M.; Behnampour, N. Effect of sodium alginate coating incorporated with nisin, Cinnamomum zeylanicum, and rosemary essential oils on microbial quality of chicken meat and fate of Listeria monocytogenes during refrigeration. Int. J. Food Microbiol. 2016, 238, 139–145. [Google Scholar] [CrossRef]
- Pawar, D.D.; Malik, S.V.S.; Bhilegaonkar, N.K.; Barbuddhe, S.B. Effect of nisin and its combination with sodium chloride on the survival of Listeria monocytogenes added to raw buffalo meat mince. Meat Sci. 2000, 56, 215–219. [Google Scholar] [CrossRef]
- Samelis, J.; Bedie, G.K.; Sofos, J.N.; Belk, K.F.; Scanga, J.A.; Smith, G.C. Combinations of nisin with organic acids or salts to control Listeria monocytogenes on sliced pork bologna stored at 4 °C in vacuum packages. LWT Food Sci. Technol. 2005, 38, 21–28. [Google Scholar] [CrossRef]
- Sivarooban, T.; Hettiarachchy, N.S.; Johnson, M.G. Inhibition of Listeria monocytogenes using nisin with grape seed extract on turkey frankfurters stored at 4 and 10 °C. J. Food Prot. 2007, 70, 1017–1020. [Google Scholar] [CrossRef]
- Ruiz, A.; Williams, S.K.; Djeri, N.; Hinton, A., Jr.; Rodrick, G.E. Nisin, rosemary, and ethylenediaminetetraacetic acid affect the growth of Listeria monocytogenes on ready-to-eat turkey ham stored at four degrees celsius for sixty-three days. Poult. Sci. 2009, 88, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Lungu, B.; Johnson, M.G. Fate of Listeria monocytogenes inoculated onto the surface of model turkey frankfurter pieces treated with zein coatings containing nisin, sodium diacetate, and sodium lactate at 4 °C. J. Food Prot. 2005, 68, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.E.; Smith, J.V.; Broadbent, J.R. Efficacy of washing meat surfaces with 2% levulinic, acetic, or lactic acid for pathogen decontamination and residual growth inhibition. Meat Sci. 2011, 88, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Hernando, A.; Capita, R.; Prieto, M.; Alonso-Calleja, C. Comparison of antibiotic resistance patterns in Listeria monocytogenes and Salmonella enterica strains pre-exposed and exposed to poultry decontaminants. Food Control 2009, 20, 1108–1111. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; García-Fernández, C.; Carballo, J.; Capita, R. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) for twelve antimicrobials (biocides and antibiotics) in eight strains of Listeria monocytogenes. Biology 2022, 11, 46. [Google Scholar] [CrossRef]
- WHO. Critically Important Antimicrobials for Human Medicine; World and Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/publications/i/item/9789241515528 (accessed on 31 March 2023).
- OIE. OIE list of Antimicrobial Agents of Veterinary Importance. 2021. Available online: https://www.woah.org/app/uploads/2021/06/a-oie-list-antimicrobials-june2021.pdf (accessed on 31 March 2023).
- Rodríguez-Melcón, C.; Serrano-Galán, V.; Capita, R.; Alonso-Calleja, C. Estimation by flow cytometry of percentages of survival of Listeria monocytogenes cells treated with tetracycline, with or without prior exposure to several biocides. Food Microbiol. 2023, 112, 104210. [Google Scholar] [CrossRef]
- De Briyne, N.; Atkinson, J.; Pokludová, L.; Borriello, S.P. Antibiotics used most commonly to treat animals in Europe. Vet. Rec. 2014, 175, 325. [Google Scholar] [CrossRef]
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2020; Trends from 2010 to 2020 Eleventh ESVAC Report; Publications Office of the European Union: Luxembourg, 2021; Available online: file:///C:/Users/Rosa%20Capita/Downloads/sales%20of%20veterinary%20antimicrobial%20agents%20in%2031%20european-TCAE21001ENN.pdf (accessed on 22 July 2023).
- Álvarez-Fernández, E.; Alonso-Calleja, C.; García-Fernández, C.; Capita, R. Prevalence and antimicrobial resistance of Salmonella serotypes isolated from poultry in Spain: Comparison between 1993 and 2006. Int. J. Food Microbiol. 2012, 153, 281–287. [Google Scholar] [CrossRef]
- Condell, O.; Iversen, C.; Cooney, S.; Power, K.A.; Walsh, C.; Burgess, C.; Fanning, S. Efficacy of biocides used in the modern food industry to control Salmonella enterica, and links between biocide tolerance and resistance to clinically relevant antimicrobial compounds. Appl. Environ. Microbiol. 2012, 78, 3087–3097. [Google Scholar] [CrossRef]
- Randall, L.P.; Cooles, S.W.; Coldham, N.G.; Penuela, E.G.; Mott, A.C.; Woodward, M.J.; Piddock, L.J.V.; Webber, M.A. Commonly used farm disinfectants can select for mutant Salmonella enterica serovar Typhimurium with decreased susceptibility to biocides and antibiotics without compromising virulence. J. Antimicrob. Chemother. 2007, 60, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
| Microbial Group | Culture Media | Incubation | References | |
|---|---|---|---|---|
| Time | Temperature (°C) | |||
| Aerobic plate counts (APCs) | PCA 1 | 3 days | 30 °C | [23] |
| Psychrotrophic microorganisms | PCA 1 | 10 days | 7 °C | [24] |
| Enterobacteria | VRBGA 2,3 | 24 h | 37 °C | [25] |
| Gene | Primers | Sequency (5′ → 3′) | Annealing Temperature (°C) (Product Size, bp) | Reference |
|---|---|---|---|---|
| lmo1030 | Lmo1030-F | GCTTGTATTCACTTGGATTTGTCTGG | 62 (509) | [26] |
| Lmo1030-R | ACCATCCGCATATCTCAGCCAACT |
| Microbial Group | Food Additive | |||
|---|---|---|---|---|
| Control (Without Treatment) | Sodium Nitrite (SN) | Nisin (Ni) | Lactic Acid (LA) | |
| Aerobic plate counts | 7.53 ± 1.02 a | 7.01 ± 1.23 a | 7.18 ± 1.24 a | 5.51 ± 1.05 b |
| Psychrotrophic | 7.13 ± 1.07 a | 6.66 ± 1.18 a | 7.02 ± 1.16 a | 5.59 ± 1.14 b |
| Enterobacteria | 4.23 ± 0.88 a | 3.78 ± 1.35 a | 3.96 ± 0.95 a | 2.33 ± 0.51 b |
| Antibiotic Resistance Pattern | Number of Isolates in Control (Non-Treated) Samples | ||
|---|---|---|---|
| Spain | Portugal | Total | |
| OX-FOX-FEP | 0 | 1 | 1 |
| OX-FOX-CTX-FEP | 1 | 7 | 8 |
| OX-FOX-CTX-FEP-SXT | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-CIP | 7 | 6 | 13 |
| OX-FOX-CTX-FEP-F | 3 | 3 | 6 |
| OX-FOX-CTX-FEP-E | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-RD | 1 | 1 | 2 |
| OX-FOX-CTX-FEP-SXT-CIP | 2 | 0 | 2 |
| OX-FOX-CTX-FEP-RD-CIP | 3 | 0 | 3 |
| OX-FOX-CTX-FEP-CIP-F | 10 | 4 | 14 |
| OX-FOX-CTX-FEP-E-RD | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-SXT-CIP-F | 2 | 0 | 2 |
| OX-FOX-CTX-FEP-RD-CIP-F | 5 | 1 | 6 |
| OX-FOX-CTX-FEP-E-CIP-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-E-RD-CIP | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-SXT-TE-CIP-F | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-CN-E-SXT-CIP | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-E-RD-CIP-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-SXT-CIP-ENR-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-SXT-RD-CIP-F | 3 | 0 | 3 |
| OX-FOX-CTX-FEP-E-SXT-RD-TE | 1 | 0 | 1 |
| Antibiotic Resistance Pattern | Number of Isolates in Samples Treated with SN | ||
|---|---|---|---|
| Spain | Portugal | Total | |
| OX-FOX-CTX-FEP | 0 | 2 | 2 |
| OX-FOX-CTX-FEP-CIP | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-F | 3 | 2 | 5 |
| OX-FOX-CTX-FEP-SXT | 2 | 4 | 6 |
| OX-FOX-CTX-FEP-RD | 0 | 2 | 2 |
| OX-FOX-CTX-FEP-CIP-F | 12 | 6 | 18 |
| OX-FOX-CTX-FEP-TE-F | 2 | 0 | 2 |
| OX-FOX-CTX-FEP-RD-CIP | 1 | 0 | 1 |
| AMP-OX-FOX-CTX-FEP-E | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-RD-CIP-F | 6 | 1 | 7 |
| OX-FOX-CTX-FEP-E-CIP-F | 2 | 0 | 2 |
| OX-FOX-CTX-FEP-CN-SXT-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-CN-RD-TE | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-SXT-CIP-F | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-CN-E-RD | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-E-SXT-CIP-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-SXT-RD-CIP-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-RD-CIP-ENR-F | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-CN-E-RD-TE | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-CN-SXT-RD-TE-CIP | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-CN-E-SXT-RD-TE | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-CN-E-SXT-RD-CIP | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-CN-E-SXT-RD-TE-CIP | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-CN-E-SXT-RD-CIP-ENR | 0 | 1 | 1 |
| Antibiotic Resistance Pattern | Number of Isolates in Samples Treated with Ni | ||
|---|---|---|---|
| Spain | Portugal | Total | |
| OX-FOX-CTX-FEP | 0 | 3 | 3 |
| OX-FOX-CTX-FEP-CIP | 3 | 2 | 5 |
| OX-FOX-CTX-FEP-RD | 0 | 3 | 3 |
| OX-FOX-CTX-FEP-F | 3 | 4 | 7 |
| OX-FOX-CTX-FEP-SXT | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-CIP-F | 15 | 3 | 18 |
| OX-FOX-CTX-FEP-SXT-RD | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-E-CIP | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-RD-CIP-F | 8 | 0 | 8 |
| OX-FOX-CTX-FEP-CIP-ENR-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-E-CIP-F | 1 | 1 | 2 |
| OX-FOX-CTX-FEP-SXT-RD-CIP-F | 2 | 0 | 2 |
| OX-FOX-CTX-FEP-E-SXT-CIP-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-RD-CIP-ENR-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-E-RD-CIP-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-RD-C-CIP-F | 1 | 0 | 1 |
| AMP-OX-FOX-CTX-FEP-SXT-TE-F | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-E-RD-TE-CIP | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-CN-E-SXT-RD | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-SXT-RD-CIP-ENR-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-E-RD-TE-CIP-F | 0 | 3 | 3 |
| OX-FOX-CTX-FEP-CN-E-RD-TE-CIP-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-CN-E-SXT-RD-CIP-ENR | 0 | 1 | 1 |
| Antibiotic Resistance Pattern | Number of Isolates in Sampls Treated with LA | ||
|---|---|---|---|
| Spain | Portugal | Total | |
| OX-FOX-CTX-FEP | 0 | 6 | 6 |
| OX-FOX-CTX-FEP-CIP | 4 | 2 | 6 |
| OX-FOX-CTX-FEP-F | 2 | 0 | 2 |
| OX-FOX-CTX-FEP-SXT | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-RD | 0 | 2 | 2 |
| OX-FOX-CTX-FEP-CIP-F | 5 | 3 | 8 |
| OX-FOX-CTX-FEP-ENR-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-RD-CIP | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-SXT-CIP | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-SXT-RD | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-RD-F | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-RD-CIP-F | 3 | 2 | 5 |
| AMP-OX-FOX-CTX-FEP-CIP-F | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-SXT-CIP-F | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-E-CIP-F | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-CN-E-RD-TE | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-SXT-RD-CIP-F | 1 | 1 | 2 |
| OX-FOX-CTX-FEP-E-RD-CIP-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-E-TE-CIP-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-CN-E-RD-TE-CIP | 3 | 0 | 3 |
| OX-FOX-CTX-FEP-CN-E-SXT-RD-CIP-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-CN-E-RD-TE-ENR-F | 1 | 0 | 1 |
| OX-FOX-CTX-FEP-CN-E-SXT-RD-CIP-ENR | 0 | 1 | 1 |
| OX-FOX-CTX-FEP-CN-E-SXT-RD-TE-CIP-ENR-F | 1 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Melcón, C.; Esteves, A.; Carballo, J.; Alonso-Calleja, C.; Capita, R. Effect of Sodium Nitrite, Nisin and Lactic Acid on the Prevalence and Antibiotic Resistance Patterns of Listeria monocytogenes Naturally Present in Poultry. Foods 2023, 12, 3273. https://doi.org/10.3390/foods12173273
Rodríguez-Melcón C, Esteves A, Carballo J, Alonso-Calleja C, Capita R. Effect of Sodium Nitrite, Nisin and Lactic Acid on the Prevalence and Antibiotic Resistance Patterns of Listeria monocytogenes Naturally Present in Poultry. Foods. 2023; 12(17):3273. https://doi.org/10.3390/foods12173273
Chicago/Turabian StyleRodríguez-Melcón, Cristina, Alexandra Esteves, Javier Carballo, Carlos Alonso-Calleja, and Rosa Capita. 2023. "Effect of Sodium Nitrite, Nisin and Lactic Acid on the Prevalence and Antibiotic Resistance Patterns of Listeria monocytogenes Naturally Present in Poultry" Foods 12, no. 17: 3273. https://doi.org/10.3390/foods12173273
APA StyleRodríguez-Melcón, C., Esteves, A., Carballo, J., Alonso-Calleja, C., & Capita, R. (2023). Effect of Sodium Nitrite, Nisin and Lactic Acid on the Prevalence and Antibiotic Resistance Patterns of Listeria monocytogenes Naturally Present in Poultry. Foods, 12(17), 3273. https://doi.org/10.3390/foods12173273